Abstract
Background:
In recent years, nanotechnology with modern advances in the macromolecular design of nano-carriers has proved to be helpful in the development of drugs delivery systems. This research represents a novel co-administration of nano-vehicles, known as liposomes. Liposomes efficiently encapsulate curcumin and BR in a polymer structure, which results in enhanced aqueous solubility of the mentioned hydrophobic agents and higher bioavailability of the drugs.
Methods:
Preparation of curcumin and BR liposomes were carried out by the thin film method, and the amounts of purified drug and its release were analyzed. After dose determination, the human lung cancer cells (QU-DB) were exposed to BR and curcumin liposomes for 12, 24, and 48 h. Then the viability and apoptosis assays were carried out by using MTT and flow cytometry technique, respectively.
Results:
In this research, in vitro anti-cancer effects of former nano-formulations on lung cancer cells was confirmed, and no cytotoxicity effects of these nano-preparations were observed in the normal cells (HFLF-PI5).
Conclusion:
Our findings suggest the nano-liposomal drugs as effective anti-cancer agents; however, additional clinical examinations are required.
Key Words: Apoptosis, Bromocriptine, Curcumin
INTRODUCTION
The most common cancer in the world is lung cancer[1,2]. Despite numerous developments in surgery, chemotherapy, and radiotherapy over the past decades, the fatality rate of lung cancer has still continued largely unaffected, which is mostly because of its metastasis[3]. New treatment approaches for lung cancer patients are highly demanded because of the overall poor prognosis. Meanwhile, due to the low bioavailability of curcumin, its biological activity is severely limited, though the curcumin therapeutic index is promising[4].
For handling drug delivery issues, the most important nanoparticle systems are liposomes, polymer conjugates, polymeric micelles, dendrimers, nano-shells, proteins, and nucleic acid-based nanoparticles. Among these tools, liposomes and polymer-based nano-formulations create a common nanoparticle therapeutic agent, available for the clinical use[5]. Liposomes are artificially constructed vesicles consisting of a phospholipid bilayer and have the ability to adjust the bio-distribution of drugs[6]. The current trials for various liposome constructions and a large number of commercially presented therapeutics seem to be promising[7]. The hydrophobic properties of curcumin has made it as a good candidate for liposome integration and encapsulation in the lipid layer of the liposomes[8].
Studies have suggested that the integration of curcumin into liposomes significantly augmentes its bio-availability. Moreover, the activity of liposomal curcumin is more favorable than that of curcumin alone[9]. A primary therapeutic drug for adenomas is BR. BR binds to the dopamine D2 receptors on pituitary epithelial cells to prevent prolactin secretion. Nowadays, BR is used to treat various diseases such as Parkinson, acromegaly, addiction, hyperprolactinemia, high growth hormone-secreting disorders, cyclical mastalgia, and type 2 diabetes[6]. BR stimulates dopamine receptors and has anti-mitotic and anti-tumor properties. D2 receptor mRNA has been identified in all BR-sensitive tumors[5,10]. The results of a previous research showed that significant changes occur in the expression of D2-like dopamine gene receptor in NSCLC[11].
In the present research, in vitro apoptosis occurrence by BR was examined in a lung adenocarcinoma cell line. The primary goal of this study was to estimate the possible curcumin and BR partitioning into the liposomes and adjustment of curcumin and BR encapsulation in liposomes. Also, we tried to assess the effect of curcumin-loaded and BR-loaded liposomes on lung cancer cells. To achieve this goal, DLS examination, anti-proliferative effects study using a MTT-based assay, and flow cytometry were applied.
MATERIALS AND METHODS
Chemicals and cells
Curcumin and BR were obtained as a gift from Genetics Department of Tarbiat Modares University (Tehran, Iran). Soybean phospholipids with 75% phosphatidyl-choline, 2-distearoyl-sn-glycero-3-phosphoethanol-amine, and sodium salt (DSPE-mPEG-2000) were procured from Lipoid GmbH (Ludwigshafen, Germany). Cholesterol was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and solvents used in this investigation were of analytical grade. Human lung carcinoma cell line, QU_DB, and human lung cell line, HFLF-PI5, (as a control) were provided from the National Cell Bank of Pasteur Institute of Iran, Tehran.
Preparation and characterization
Curcumin and BR liposomes were prepared by the thin film hydration-sonication method. Hydration was performed with 1300 μL deionized water at 65 °C for 60 minutes, using a rotary instrument (Heidolph, Germany). The resulting vesicles were then sonicated to reduce the mean size. The size distribution and polydispersity index of the drug-loaded liposome were evaluated using the DLS technique. All the measurements were carried out in triplicates at room temperature, and their mean value was reported.
Drug entrapment efficiency
Free unentrapped drugs were separated from drug-loaded liposomes using the dialysis membrane (cut off: 12-14 kDa). After digesting the liposomal vesicles with isopropanol (99% purity), the amounts of liposomal drug entrapped were analyzed by a UV spectrophoto-meter at 422 nm[12].
Release assay
The release of drug from liposomes against PBS was monitored by dialysis at pH 7 at 37 °C for 96 hours. The calculation of drug release was performed at different times in the PBS solution. Further, drug release calculation was conducted using a calibration curve by a UV spectrophotometer.
MTT assay
The cell proliferation effects of BR and curcumin liposomes were examined by the MTT assay[11,13]. The cells were seeded at 104 cells/well (0.1 ml) in triplicates in a 96-well plate and treated with varying doses of BR (12.5-25 µM) and curcumin liposomes (12-20 µm), after 12-, 24-, and 48-h incubation at 37 °C.
Flow cytometry
The QU-DB cells were treated with different concentrations of BR (12.5-25 µM) and curcumin liposomes (12-20 µm) for 12, 24, and 48 h, and then Annexin-V-Fluos staining kit (Roche, Germany) was used for detection of apoptosis[14]. The treated cell pellets were resuspended in 100 μl of Annexin-V-Fluos solution, and after 15-min incubation at 25 °C, they were analyzed on a FACSCalibur machine (BD Biosciences, CA, USA).
RESULTS
Nanoparticle size
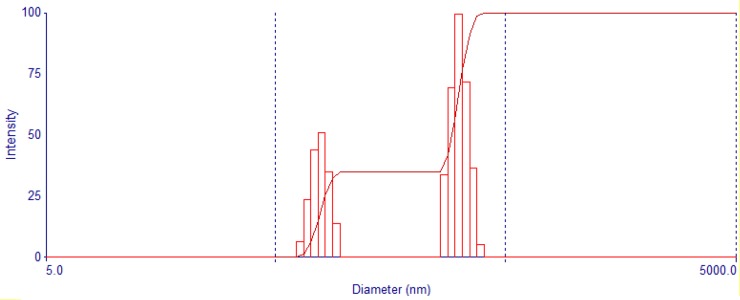
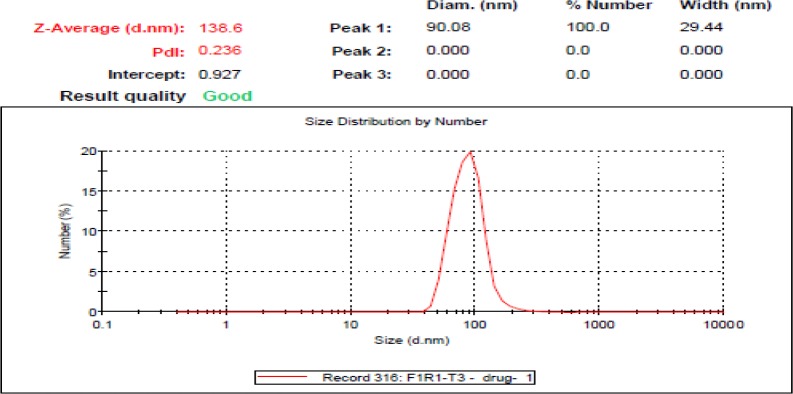
The DLS of liposome-curcumin is presented in Figure 1. Around 20% of the particles are in the range of 100 nm. More analysis indicating that the remaining particles were about 80 nm in size. Figure 2 illustrates the intensity of liposome-BR, where the diameters of nanoparticles are less than 50 nm.
Fig. 1.
DLS of liposome-curcumin nanoparticles
Fig. 2.
The intensity of liposome-BR
Drug release assay
Figure 3 demonstrate the rate of drug release from liposomes. As revealed in the Figure, the extent of drug release of liposome-BR nanoparticles is greater than that of liposome-curcumin nanoparticles at the same time. In particular, during 10 h, 60% of the liposome-BR nanoparticles and only 20% of the liposome-curcumin nanoparticles demonstrated the drug release.
Fig. 3.
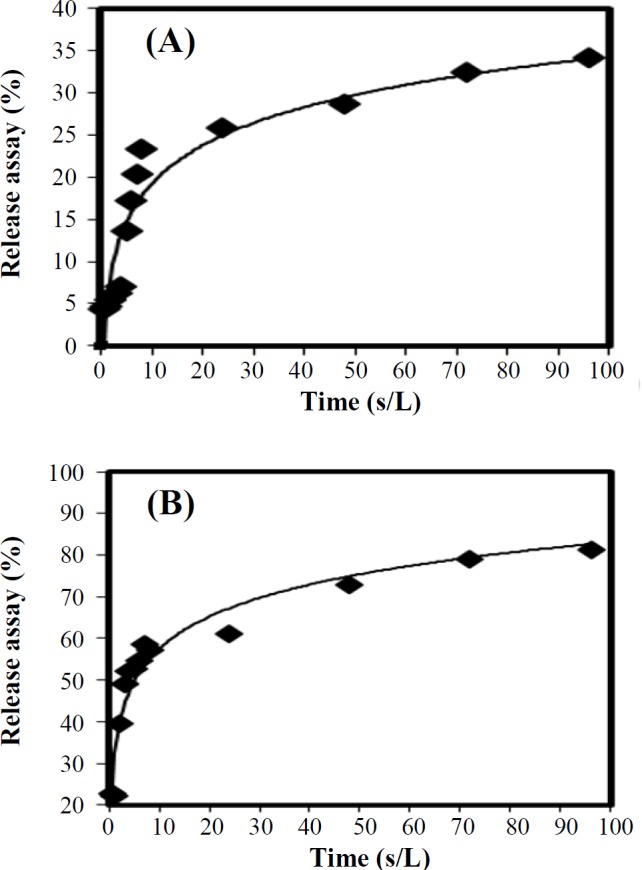
Release of liposome-curcumin (A) and liposome-BR (B) nanoparticles
Drug entrapment efficiency and stability test
Entrapment efficiencies were calculated as 90.89% and 80.41% for the liposome-curcumin and liposome-BR nanoparticles, respectively. Also, the stability of the liposomal suspensions was evaluated after three months of storage at room temperature at 4 °C. The particle size supply, morphology, and drug encapsulation efficiencies of the samples were determined as a function of the storage time. The results indicated that only 15% of the liposomal suspensions efficacy was diminished.
The effect of prepared liposomes on cell viability
Different concentrations of BR (12.5-25 µM) and curcumin (12-20 µM) liposomes were applied on the QU-DB cells in triplicate for 12, 24, and 48 h, and MTT assay was performed after the treatment. According to Figure 4, the proliferation of cancer cells reduced significantly at the concentrations of 20 to 25 µM for BR and at 16 to 20 µM for curcumin. In addition, the results of our study indicated that BR liposome had a greater antiproliferative effect than curcumin liposome, within the optimum time of 24 hours.
Fig. 4.
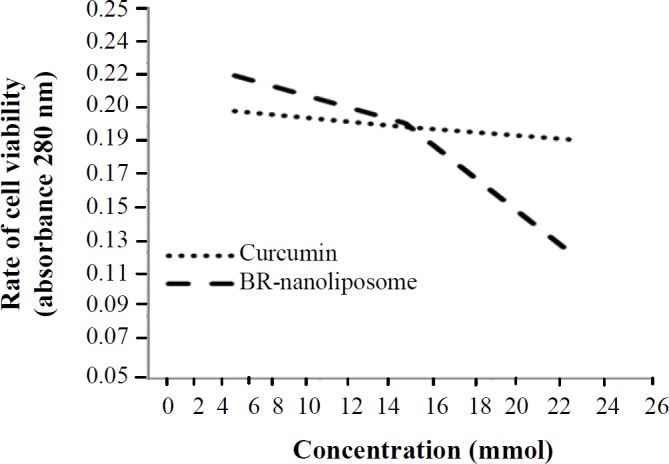
Effect of various doses of curcumin and BR nano-liposomes on QU-DB cells
Flow cytometry analysis of apoptotic cancer cells
The maximum number of apoptotic cells (27.82%) was detected at the concentration of 20 µM for curcumin liposomes, 47.30% at the concentration of 25 µM of BR liposome, and 49.72% in their co-administration by reducing the number of necrotic cells. Data are shown in Figure 5.
Fig. 5.
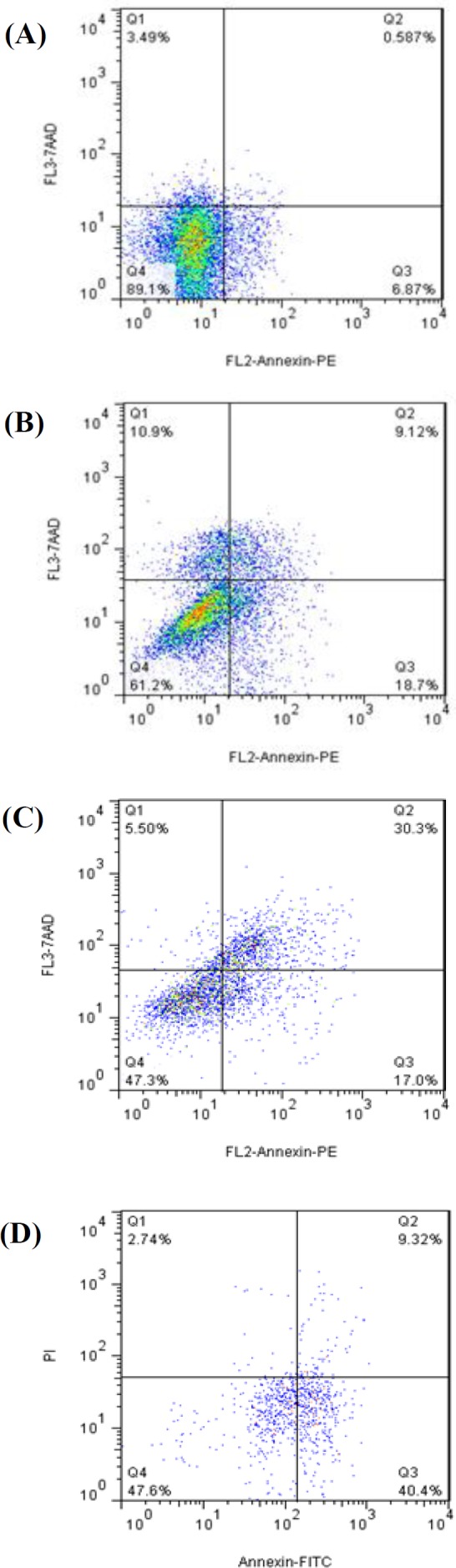
Flow cytometry analysis of apoptotic cancer cells. (A) untreated cells, (B) QU-DB cells treated by curcumin liposome, (C) bromocriptine liposome, and (D) curcumin and bromo-criptine liposomes
DISCUSSION
The delivery of chemotherapy agents to solid tumors and raising their bioavailability have been a key challenge in the recent biomedical research. Medical imaging and targeted drug delivery using nanotechnology-based tools are rapidly growing to repond this challenge[15,16].
The liposomal curcumin prompted in vitro apoptosis of human pancreatic cells and down-regulated NF-kB machinery. The results of a study on A549/DDP multidrug-resistant human lung adenocarcinoma cells showed that curcumin stimulates apoptosis through a miRNA signaling pathway[17]. Peng et al.[18] have reported increased cell apoptosis via Akt-Bad signaling pathway in U2OS cells by curcumin-loaded nanoparticles. Other researchers have used a combination of 2-hydroxypropyl-γ-cyclodextrin and liposomes to enhance the curcumin bioavailability and aqueous solubility and encapsulation efficiency, in comparison to the sole use of liposome as the drug delivery vehicle. Their results indicated both in vivo and in vitro anticancer capacity for 2-hydroxypropyl-γ-cyclodextrin/curcumin-liposome complex against KHOS cell line[19]. Koshkina et al.[20] employed 9-nitrocamptothecin liposome aerosol against osteo-sarcoma lung metastases in mice. Their results showed a significant decrease in the number of tumor foci and the size of tumor nodules in the lung. An early investigation has shown the usefulness of dopaminergic agonists in treatment of lung cancer[21]. BR acts mainly via D2 receptors, through binding to to adenylyl cyclase and reducing intracellular cAMP. By suppressing the cAMP levels, peptide secretion would be inhibited in a dose-dependent manner [22]. More studies have investigated the effects of dopamine neurotransmitter agonists on cancer cells[23,24].
Previous findings have highlighted a quantitatively significant difference for D2-like dopamine receptor genes expression in the NSCLC, among all kinds of dopamine receptor genes[11,14,25]. Such significant changes could be used to diagnose, treat, and monitor NSCLC[11]. In supplementary studies, cell proliferation and of D2 receptors expression studies were performed before and after treating cancer cells by BR[11,14,25]. It has also been discovered that BR-induced apoptosis in lung carcinoma cells, by activating D2 dopamine receptors and plasma membrane changes, occurs in the early stages of apoptosis[14,25]. Fadok et al.[26] have found that during the expansion of apoptosis, macrophages specifically recognize PS exposed to the surface of lymphocytes. In this case, phagocytosis of cells and apoptotic bodies are performed, and the organisms are protected from inflammation, leading to the exposure of cellular compositions. Therefore, according to the results of our previous studies, we designed and constructed two nanoliposomes, which efficiently were encapsulated in a polymer structure[11,14]. This design led to the enhanced aqueous solubility of the mentioned hydrophobic agents and the bioavailability of drugs.
In this research, anti-cancer potency of nano-formulations without cytotoxicity effects on normal cells was confirmed by co-administration of curcumin and BR nano-liposomes in lung cancer cells. Moreover, BR can be suggested as a valuable agent for use in nano-liposomal drugs for medical management of lung cancer.
CONFLICT OF INTEREST.
None declared.
References
- 1.Brenner DR, Boffetta P, Duell EJ, Bickeböller H, Rosenberger A, McCormack V, Muscat JE, Yang P, Wichmann HE, Brueske-Hohlfeld I, Schwartz AG, Cote ML, Tjønneland A, Friis S, Le Marchand L, Zhang ZF, Morgenstern H, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Foretova L, Janout V, Bencko V, Schejbalova M, Brennan P, Mates IN, Lazarus P, Field JK, Raji O, McLaughlin JR, Liu G, Wiencke J, Neri M, Ugolini D, Andrew AS, Lan Q, Hu W, Orlow I, Park BJ, Hung RJ. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. American journal of epidemiology. 2012;176(7):573–585. doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coté ML, Liu M, Bonassi S, Neri M, Schwartz AG, Christiani DC, Spitz MR, Muscat JE, Rennert G, Aben KK, Andrew AS, Bencko V, Bickeböller H, Boffetta P, Brennan P, Brenner H, Duell EJ, Fabianova E, Field JK, Foretova L, Friis S, Harris CC, Holcatova I, Hong YC, Isla D, Janout V, Kiemeney LA, Kiyohara C, Lan Q, Lazarus P, Lissowska J, Le Marchand L, Mates D, Matsuo K, Mayordomo JI, McLaughlin JR, Morgenstern H, Müeller H, Orlow I, Park BJ, Pinchev M, Raji OY, Rennert HS, Rudnai P, Seow A, Stucker I, Szeszenia-Dabrowska N, Dawn Teare M, Tjønnelan A, Ugolini D, van der Heijden HF, Wichmann E, Wiencke JK, Woll PJ, Yang P, Zaridze D, Zhang ZF, Etzel CJ, Hung RJ. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. European journal of cancer. 2012;48(13):1957–1968. doi: 10.1016/j.ejca.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risch A, Plass C. Lung cancer epigenetics and genetics. International journal of cancer. 2008;123(1):1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmaco-dynamics. Molecules. 2012;17(5):5972–5987. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campa D, Zienolddiny S, Lind H, Ryberg D, Skaug V, Canzian F, Haugen A. Polymorphisms of dopamine receptor/transporter genes and risk of non-small cell lung cancer. Lung cancer. 2007;56(1):17–23. doi: 10.1016/j.lungcan.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 6.de Leeuw van Weenen JE, Auvinen HE, Parlevliet ET, Coomans CP, Schröder-van der Elst JP, Meijer OC, Pijl H. Blocking dopamine D2 receptors by haloperidol curtails the beneficial impact of calorie restriction on the metabolic phenotype of high‐fat diet-induced obese mice. Journal of neuroendocrinology. 2011;23(2):158–167. doi: 10.1111/j.1365-2826.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, Kondos S, Takeuchi J, Morimura T. Induction of apoptosis in murine ACTH‐secreting pituitary adenoma cells by bromocriptine. FEBS letters. 1994;339(1-2):73–75. doi: 10.1016/0014-5793(94)80387-0. [DOI] [PubMed] [Google Scholar]
- 8.Attari F, Sepehri H, Delphi L, Goliaei B. Apoptotic and necrotic effects of pectic acid on rat pituitary GH3/B6 tumor cells. Iranian biomedical journal. 2009;13(4):229–236. [PubMed] [Google Scholar]
- 9.Chen C, Johanston TD, Jeon H, Gedaly R, McHugh PP, Burke TG, Ranjan D. An in vitro study of liposomal curcumin: stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. International journal of pharmaceutics. 2009;366(1-2):133–139. doi: 10.1016/j.ijpharm.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Trouillas J, Chevallier P, Remy C, Rajas F, Cohen R, Calle A, Hooghe-Peters EL, Rousset B. Differential actions of the dopamine agonist bromocriptine on growth of SMtTW tumors exhibiting a prolactin and/or a somatotroph cell phenotype: relation to dopamine D2 receptor expression. Endocrinology. 1999;140(1):13–21. doi: 10.1210/endo.140.1.6450. [DOI] [PubMed] [Google Scholar]
- 11.Shaikhpoor M, Ahangari G, Sadeghizadeh M, Khosravi A, Derakhshani Deilami G. Significant changes in D2-like dopamine gene receptors expression associated with non-small-cell lung cancer: Could it be of potential use in the design of future therapeutic strategies? Current Cancer therapy reviews. 2012;8(4):304–310. [Google Scholar]
- 12.Esposito E, Mariani P, Ravani L, Contado C, Volta M, Bido S, Drechsler M, Mazzoni S, Menegatti E, Morari M, Cortesi R. Nanoparticulate lipid dispersions for bromocriptine delivery: characterization and in vivo study. European journal of pharmaceutics and biopharmaceutics. 2012;80(2):306–314. doi: 10.1016/j.ejpb.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta histochemica. 2012;114(8):785–796. doi: 10.1016/j.acthis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Sheikhpour M, Ahangari G, Sadeghizadeh M, Deezagi A. A novel report of apoptosis in human lung carcinoma cells using selective agonist of D2-like dopamine receptors: a new approach for the treatment of human non-small cell lung cancer. International journal of immunopathology and pharmacology. 2013;26(2):393–402. doi: 10.1177/039463201302600212. [DOI] [PubMed] [Google Scholar]
- 15.Vde Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the α2-adrenergic receptors in beta cells. Biochemical pharmacology. 2010;79(12):1827–1836. doi: 10.1016/j.bcp.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Salvador A, Dubreuil D, Denouel J, Millerioux L. Sensitive method for the quantitative determination of bromocriptine in human plasma by liquid chromatography–tandem mass spectrometry. Journal of chromatography B: analytical technologies in the biomedical and life sciences. 2005;820(2):237–242. doi: 10.1016/j.jchromb.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang T, Ti X, Shi J, Wu C, Ren X, Yin H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochemical and biophysical research communications. 2010;399(1):1–6. doi: 10.1016/j.bbrc.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH, Chen FA, Shieh PC, Yang JS. Curcumin-loaded nanoparticles enhance apoptotic cell death of U2OS human osteosarcoma cells through the Akt-Bad signaling pathway. International journal of oncology. 2014;44(1):238–246. doi: 10.3892/ijo.2013.2175. [DOI] [PubMed] [Google Scholar]
- 19.Dhule SS, Penfornis P, Frazier T, Walker R, Feldman J, Tan G, He J, Alb A, John V, Pochampally R. Curcumin-loaded γ-cyclodextrin liposomal nano-particles as delivery vehicles for osteosarcoma. Nanomedicine. 2012;8(4):440–451. doi: 10.1016/j.nano.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koshkina NV, Waldrep JC, Roberts LE, Golunski E, Melton S, Knight V. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clinical cancer research. 2001;7(10):3258–3262. [PubMed] [Google Scholar]
- 21.Ishibashi M, Fujisawa M, Furue H, Maeda Y, Fukayama M, Yamaji T. Inhibition of growth of human small cell lung cancer by bromocriptine. Cancer research. 1994;54(13):3442–3446. [PubMed] [Google Scholar]
- 22.Farrell WE, Clark AJ, Stewart MF, Crosby SR, White A. Bromocriptine inhibits pro-opiomelanocortin mRNA and ACTH precursor secretion in small cell lung cancer cell lines. Journal of clinical investigation. 1992;90(3):705–710. doi: 10.1172/JCI115941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norsa A, Martino V. Somatostatin, retinoids, melatonin, vitamin D, bromocriptine, and cyclophosphamide in advanced non-small-cell lung cancer patients with low performance status. Cancer Biotherapy and radiopharmaceuticals. 2006;21(1):68–73. doi: 10.1089/cbr.2006.21.68. [DOI] [PubMed] [Google Scholar]
- 24.Norsa A, Martino V. Somatostatin, retinoids, melatonin, vitamin D, bromocriptine, and cyclophosphamide in advanced non–small-cell lung cancer patients with low performance status. Cancer biotherapy and radiopharmaceuticals. 2006; 21(1):68–73. doi: 10.1089/cbr.2006.21.68. [DOI] [PubMed] [Google Scholar]
- 25.Babaei E, Sadeghizadeh M, Hassan ZM, Feizi MA, Najafi F, Hashemi SM. Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. International immunopharmacology. 2012;12(1):226–234. doi: 10.1016/j.intimp.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. The journal of immunology. 1992;149(12):4029–4035. [PubMed] [Google Scholar]