ABSTRACT
Background
Recent studies have shown that circulating branched-chain amino acids (BCAAs) are elevated in obese, insulin-resistant individuals. However, it is not known if supplementation of additional BCAAs will further impair glucose metabolism.
Objectives
The aim of this pilot study was to determine the effects of BCAA supplementation on glucose metabolism in obese, prediabetic individuals.
Methods
This is a randomized crossover study involving 12 obese individuals with prediabetes. Participants were randomly assigned to receive a daily supplement containing either 20 g BCAA or protein low in BCAAs for 4 wk with a 2-wk washout in between. At each visit, an oral-glucose-tolerance test (OGTT) was performed. Collected blood samples were used to measure glucose, insulin, and insulin resistance–associated biomarkers.
Results
BCAA supplementation tended to decrease the plasma glucose area under the curve (AUC) measured by the OGTT (AUC percentage change from supplementation baseline, BCAA: −3.3% ± 3%; low-BCAA: 10.0% ± 6%; P = 0.08). However, BCAA supplementation did not affect plasma insulin during OGTT challenge (BCAA: −3.9% ± 8%; low-BCAA: 14.8% ± 10%; P = 0.28). The plasma concentrations of nerve growth factor (BCAA: 4.0 ± 1 pg/mL; low-BCAA: 5.7 ± 1 pg/mL; P = 0.01) and monocyte chemoattractant protein-1 (BCAA: −0.4% ± 9%; low-BCAA: 29.0% ± 18%; P = 0.02) were significantly lowered by BCAA supplementation compared to low-BCAA control. Plasma interleukin 1β was significantly elevated by BCAA supplementation (BCAA: 231.4% ± 187%; low-BCAA: 20.6% ± 33%; P = 0.05). BCAA supplementation did not affect the circulating concentrations of the BCAAs leucine (BCAA: 9.0% ± 12%; low-BCAA: 9.2% ± 11%), valine (BCAA: 9.1% ± 11%; low-BCAA: 12.0% ± 13%), or isoleucine (BCAA: 2.5% ± 11%; low-BCAA: 7.3% ± 11%).
Conclusions
Our data suggest that BCAA supplementation did not impair glucose metabolism in obese, prediabetic subjects. Further studies are needed to confirm the results seen in the present study. This study was registered at clinicaltrials.gov as NCT03715010.
Keywords: branched-chain amino acid (BCAA), insulin resistance, prediabetes, obesity, OGTT, glucose metabolism
Introduction
Type 2 diabetes mellitus (T2DM) affects 30 million people in the United States (1), and this number is projected to increase to >55 million in the next 10 y (2). T2DM, which is strongly associated with obesity, is associated not only with increased risks for cardiovascular disease and cancer, but also with increased healthcare costs. Efforts to understand the pathophysiology of T2DM have focused on the interaction between lipid metabolism and glucose metabolism (3). However, there is increasing evidence suggesting that insulin resistance and T2DM are also associated with dysregulation of branched-chain amino acid (BCAA) metabolism, resulting in elevated circulating BCAAs (4). Whether elevated circulating BCAAs are a contributing cause or a secondary effect of T2DM remains unclear.
The BCAAs leucine, valine, and isoleucine are essential amino acids that play important roles in signaling pathways. For example, leucine is one key regulator of the mammalian target of rapamycin signaling, which is the central component of a complex signaling network for protein synthesis, cell proliferation, and insulin signaling (5). BCAAs are usually metabolized in the skeletal muscle by branched-chain aminotransferase to form first branched-chain α-keto acids (BCKAs) and then branched-chain ketoacid dehydrogenases (BCKDs), to form metabolites that participate in anaplerosis. Recent studies have demonstrated that increased circulating BCAA concentrations, likely due to BCKD dysfunction, are positively associated with obesity and T2DM in humans (6–9). Animal and human studies have also demonstrated that dietary BCAAs could be a contributing cause to impaired glucose metabolism (10–12). However, other studies reported a negative association between dietary BCAA intake and T2DM risk (13), and that dietary protein intake does not necessarily have a positive correlation with insulin resistance. Therefore, studies of BCAAs and T2DM demonstrate inconsistent associations between BCAAs and glucose metabolism. Whey protein, rich in BCAAs, is widely used to control or lose weight in this population, making studies of BCAAs in obesity and prediabetes clinically significant.
In our current crossover study, obese, prediabetic subjects were supplemented with 20 g/d of either BCAAs or rice protein low in BCAAs (4 g BCAAs/d for 4 wk in a randomized sequence, with a 2-wk washout period in between). An oral-glucose-tolerance test (OGTT) was performed before and after each supplementation. The primary outcome measures were plasma glucose and insulin in response to the OGTT, whereas the secondary outcome measures were plasma insulin resistance–associated biomarkers, plasma BCAAs and BCKAs, body weight, body fat, muscle mass, and vitals including blood pressure and heart rate.
Methods
Study design
We conducted a randomized, 2-arm, open-label crossover study with 12 obese, prediabetic subjects at the Center for Human Nutrition, University of California, Los Angeles, CA. The study was carried out in accordance with the guidelines of the Human Subjects Protection Committee of the University of California, Los Angeles. The clinical protocol was approved by the Internal Review Board of the University of California, Los Angeles. All subjects gave written informed consent before the study began. This study was registered at clinicaltrials.gov as NCT03715010.
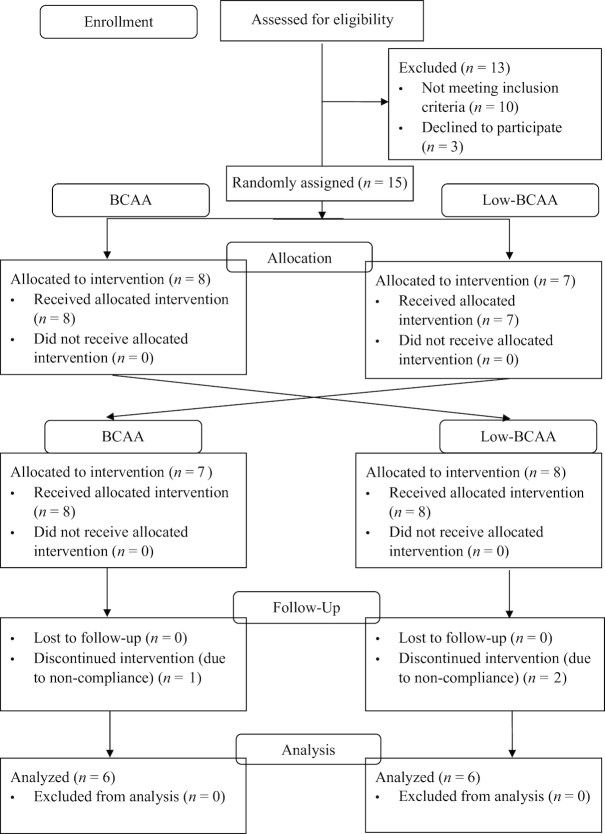
The study (Figure 1) consisted of a 2-wk run-in phase before the baseline. Twelve participants were randomly assigned to be supplemented with either BCAA powder (20 g BCAAs/serving) or rice protein low in BCAAs (4 g BCAAs/serving), as control, and were asked to consume 1 serving/d for 4 wk. Our randomization algorithm involved sampling probability values from the uniform distribution without replacement, and allocating drawn probabilities to the group assignment. Permuted block design was used with sampling of 8 per block for a total number of 2 blocks. After the 4-wk supplementation phase, the participants went into a 2-wk washout phase, during which they stopped consuming BCAA or low-BCAA supplements. After the washout period, the participants were then provided with the other supplement in a crossover fashion, i.e., either low-BCAA or BCAA supplements for another intervention of 4 wk. The participants were also asked to maintain a consistent diet, especially consumption of BCAA-rich products, throughout the entire study period. OGTTs and body composition measurements were performed before and after the 4-wk interventions (at weeks 0, 4, 6, and 10).
FIGURE 1.
Screening, enrollment, random assignment, follow-up, and analysis of samples of the study participants. BCAA, branched-chain amino acid.
The BCAA powder used was LifAmino Vegan Instantized BCAA powder (Scientific Living). NutriBiotic Vanilla Rice Protein powder (NutriBiotic) served as the control low-BCAA supplement. Nutrient information for both powders is shown in Table 1.
TABLE 1.
Nutrient composition of the BCAA and low-BCAA (rice protein powder) supplements1
| Supplement | Serving size, g | Calories, kcal | Protein, g | Carbohydrate, g | Fat, g | Leucine, g | Isoleucine, g | Valine, g | Soy lecithin, g | Total BCAA |
|---|---|---|---|---|---|---|---|---|---|---|
| BCAA powder | 20 | 78 | 20 | 0 | 0 | 10 | 5 | 5 | ≤ 1 | 20 |
| Rice protein powder | 25 | 100 | 20 | 3.3 | 0 | 1.82 | 1.167 | 1.2 | 0 | 4.19 |
1BCAA, branched-chain amino acid.
Study participants
Eligible subjects were between 20 and 65 y of age at screening, had fasting blood glucose concentrations of ≥100 but <126 mg/dL or glycated hemoglobin ≥5.7% but ≤6.4%, and waist circumferences of ≥40 inches (101.6 cm) for men and ≥35 inches (88.9 cm) for women. A medical history, blood chemistry, and hematology profile were obtained at screening to exclude subjects with diabetes mellitus, uncontrolled hypertension, liver, kidney, and cardiovascular disease, taking medications that interfere with glucose metabolism, and anyone who is unable to follow protocol.
Three individuals dropped out due to noncompliance. Their data were not included in the statistical evaluation. The remaining subjects consisted of 12 obese, prediabetic patients (5 men and 7 women), who completed both phases of the crossover study. The patients were a heterogeneous population (7 white, 4 African American, and 1 Asian) and lived in Los Angeles, California. In general, no adverse events or side effects were observed. The initial recruitment date was July 1, 2016 and the study ended January 9, 2018.
Body composition
Body composition was measured with a Tanita BC418 body-fat analyzer (Tanita Corp.). Body composition was tracked at baseline and after 4 wk of BCAA supplementation and at baseline of the second supplementation and after 4 wk of low-BCAA supplementation.
OGTT
After an overnight fast (10–12 h), a basal blood draw was obtained as 0 min, followed by a 75-g glucose cola administration to the participants. Subsequent blood samples were obtained 30, 60, and 120 min afterwards. Plasma was separated from whole blood by centrifugation (910 × g, 15 min at 15 °C), frozen, and kept at −80 °C. The insulin resistance index assessed by HOMA-IR was calculated as follows (14): [fasting blood glucose (mg/dL) × fasting plasma insulin (µIU/mL)]/405.
Blood biochemical analysis
Blood samples were collected and coded to protect patient confidentiality. Plasma glucose and insulin were measured in the UCLA Biomarker Laboratory. Plasma glucose was determined with a glucose assay kit (Cayman Chemical Company). Plasma insulin, nerve growth factor (NGF), leptin, TNF-α, IL-1β, IL-6, IL-8, and monocyte chemoattractant protein-1 (MCP-1) were determined with a MILLIPLEX map kit (EMD Millipore), and data were captured and processed with a Luminex 200 instrument with xPonent software.
Plasma was also used to determine circulating concentrations of BCAAs (leucine, isoleucine, and valine) and BCKAs, such as α-ketoisocaproate (KIC), α-ketoisovalerate (KIV), and α-ketomethylvalerate (KMV). Following derivatization of BCAA with a Waters AccQ-Fluor Reagent Kit, and BCKA with 4-nitro-1,2-phenylenediamine, these compounds were analyzed by HPLC as described by Zhang et al. (15).
Statistical analysis
The estimated sample size for our intervention was n = 12. Our sample size calculation was based on Pal and Ellis (16), who studied whey supplementation containing >20 g BCAA/d in 22 subjects with the use of a crossover design to compare whey with turkey and tuna supplementation. We employed the difference between the AUC observed for their whey and turkey meals to construct our power calculation which included accounting for the crossover. Given the difference they observed and the SD derived from the SE, 12 subjects was a sufficient sample size to provide 83% power (α = 0.05) to observe a similar difference between supplementations in a crossover design with 2 different supplementations, i.e., our BCAA and low-BCAA supplementations.
Data were entered into Microsoft Excel and imported into SAS version 9.4. Data were structured to reflect the crossover design where BCAA or low-BCAA supplementation occurred in the first supplementation period, followed by the corresponding supplementation. We captured the timed measurements of glucose and insulin at 4 points, i.e., 0, 30, 60, and 120 min following challenge at baseline, and at the end of each supplementation period. The remaining outcome variables were assessed with the use of baseline means and means following the supplementation period. Evaluations were conducted within subjects and between groups. Model constructs were developed for supplementation, period, and sequence. Supplementation effects were evaluated with the use of both ANOVA and repeated-measures ANOVA for a 2-period crossover design. Repeated-measures models evaluated the OGTT outcome values over the 4 time points at baseline and at the end of intervention. Both sets of models were adjusted for period and sequence. All estimates presented are adjusted. We evaluated the hypotheses for equal-outcome mean differences according to supplementation with the use of the F statistic at a 2-tailed significance level (17).
Results
Subject characteristics
Figure 1 shows a flow diagram of the participants according to the CONSORT (Consolidated Standards of Reporting Trials) guidelines. Table 2 shows the baseline and final characteristics of the participants in the study. All baseline differences according to assigned supplementation sequence were nonsignificant. We observed no significant differences for body weight, fat mass, muscle mass, BMI, fasting blood glucose, fasting insulin, and HOMA-IR after either BCAA or low-BCAA supplementation (Table 2). BCAA supplementation tended to decrease plasma glucose AUC as measured by the OGTT (P = 0.08), whereas systolic blood pressure was significantly increased after BCAA supplementation (P = 0.02).
TABLE 2.
Effects of BCAA supplementation on metabolic parameters in obese, prediabetic subjects1
| Low-BCAA | BCAA | Baseline P values | Postintervention P values | |||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Baseline | Week 4 | |||
| Body weight, kg | 97.2 ± 3.1 | 97.3 ± 3.1 | 97.1 ± 3.0 | 97.3 ± 3.1 | 0.98 | 0.64 |
| Body fat, kg | 38.6 ± 2.6 | 39.1 ± 2.9 | 39.0 ± 2.8 | 39.0 ± 2.9 | 0.93 | 0.45 |
| Muscle mass, kg | 56.7 ± 3.0 | 56.7 ± 3.3 | 56.6 ± 3.2 | 56.8 ± 3.3 | 0.99 | 0.95 |
| BMI, kg/m2 | 34.3 ± 1.0 | 34.3 ± 1.0 | 34.2 ± 1.0 | 34.3 ± 1.0 | 0.99 | 0.87 |
| Systolic blood pressure, mmHg | 116.5 ± 3.8 | 118.1 ± 4.2 | 120.1 ± 3.8 | 130.8 ± 3.6 | 0.16 | 0.02 |
| Diastolic blood pressure, mmHg | 76.3 ± 2.3 | 79.1 ± 2.5 | 77.7 ± 2.9 | 81.5 ± 3.1 | 0.49 | 0.28 |
| Fasting blood glucose,2 mg/dL | 97.6 ± 4.73 | 96.9 ± 2.3 | 96.2 ± 2.2 | 96.8 ± 4.4 | 0.64 | 0.08 |
| Fasting insulin,2 µIU/mL | 17.1 ± 2.53 | 32.4 ± 16.6 | 24.0 ± 7.4 | 18.3 ± 4.9 | 0.82 | 0.28 |
| HOMA-IR2 | 4.2 ± 0.73 | 8.2 ± 4.4 | 6.0 ± 2.0 | 4.8 ± 1.7 | 0.69 | 0.33 |
1Data are expressed as mean ± SE (n = 12). Statistical comparisons were performed with the use of ANOVA with a 2-period crossover design to compare within-subject and between-subject supplementation of either low-BCAA or BCAA powder supplements. P values are 2-sided and presented for mean group differences at baseline and postintervention by the BCAA group. BCAA, branched-chain amino acid; OGTT, oral-glucose-tolerance test.
2Baseline and postintervention P values from repeated-measures analysis of variance models with 2-period crossover design based on outcomes derived from the OGTT (at 0, 30, 60, 120 min).
3OGTT means at time 0 for baseline and at time 0 after intervention.
Plasma glucose and insulin
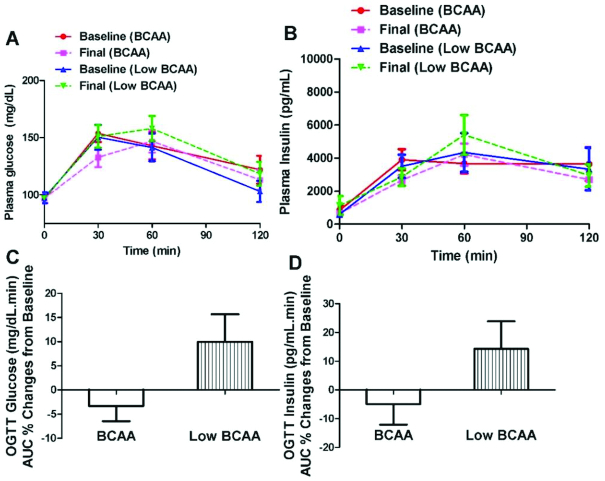
An OGTT was conducted at baseline and after 4 wk of supplementation with either BCAA or low-BCAA (Figure 2A–D). Serum glucose during the OGTT was evaluated from the AUC at 0, 30, 60, 90, and 120 min. BCAA supplementation tended to decrease plasma glucose AUC determined as the percentage change from baseline (BCAA: −3.3% ± 3%; low-BCAA: 10.0% ± 6%; P = 0.08) (Figure 2C). However, BCAA supplementation did not affect plasma insulin during the OGTT (BCAA: −3.9% ± 8%; low-BCAA: 14.8% ± 10%; P = 0.28) (Figure 2D).
FIGURE 2.
BCAA supplementation tended to improve glucose metabolism in obese, prediabetic subjects. OGTT was performed before and after supplementation of BCAA and low-BCAA interventions in a crossover fashion. (A) Plasma glucose response during OGTT. (B) Plasma insulin response during OGTT. (C) Plasma glucose AUC percentage changes normalized to the supplementation baseline. (D) Plasma insulin AUC percentage changes normalized to the supplementation baseline. Data are mean ± SE (n = 12). P values were derived from repeated-measures ANOVA with the use of a 2-period crossover design with P < 0.05 representing the test for significance. (C) BCAA supplementation tended to decrease the plasma glucose AUC (as percentage change normalized to supplementation baseline) during OGTT (P = 0.08). BCAA, branched-chain amino acid; OGTT, oral-glucose-tolerance test.
Plasma adipokine concentrations
Adipose tissue inflammation is an important contributor to obesity-associated insulin resistance and we were interested in exploring whether BCAA supplementation would improve insulin resistance by mediating adipose tissue inflammatory status. We measured plasma adipokine and inflammatory cytokine concentrations of leptin, NGF, TNF- α, IL-1β, IL-6, IL-8, and MCP-1. A portion of the cytokines were reduced by BCAA supplementation but not all. The plasma NGF concentration was significantly lowered by BCAA supplementation compared with low-BCAA supplementation (BCAA: 4.0 ± 1 pg/mL; low-BCAA: 5.7 ± 1 pg/mL; P = 0.01) (Figure 3). In addition, plasma MCP-1 was significantly decreased by BCAA supplementation (BCAA: −0.4% ± 9%; low-BCAA: 29.0% ± 18%, P = 0.02) (Figure 4). Plasma IL-1β was significantly elevated by BCAA supplementation (BCAA: 231.4% ± 187%; low-BCAA: 20.6% ± 33%; P = 0.05) (Figure 5). Plasma leptin, IL-6, IL-8, and TNF-α were not significantly affected by BCAA supplementation (Supplemental Figures 1–4).
FIGURE 3.
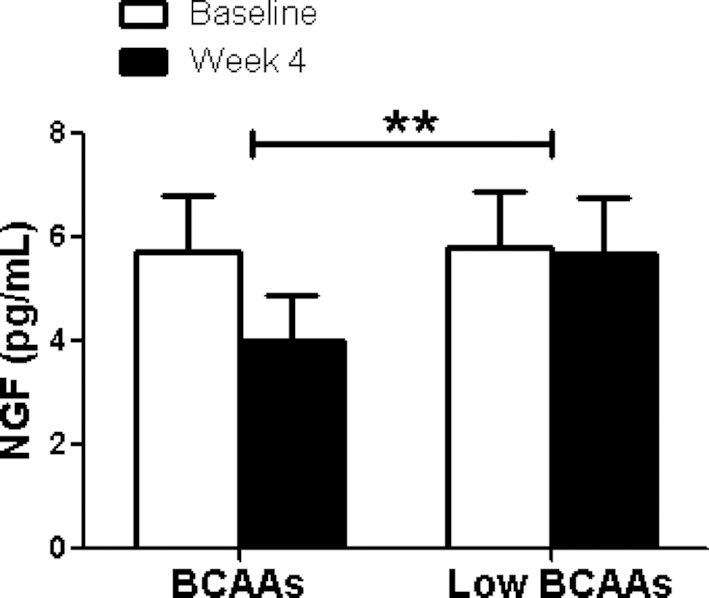
BCAA supplementation resulted in significantly decreased plasma NGF concentrations. The figure shows the fasting plasma NGF concentration before (baseline) and after (final) BCAA supplementation with BCAA and low-BCAA powder supplements. Data are mean ± SE (n = 12). P values were derived from ANOVA with the use of a 2-period crossover design, P < 0.01. BCAA, branched-chain amino acid; NGF, nerve growth factor.
FIGURE 4.
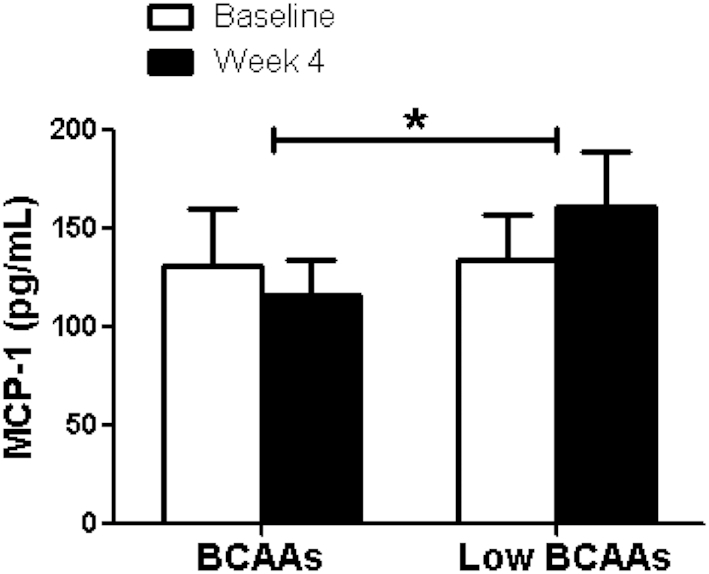
BCAA supplementation resulted in significantly decreased plasma MCP-1 concentrations. The figure shows the fasting plasma MCP-1 concentration before (baseline) and after (final) BCAA supplementation with BCAA and low-BCAA powder supplements. Data are mean ± SE (n = 12). P values were derived from ANOVA with the use of a 2-period crossover design, P < 0.05. BCAA, branched-chain amino acid; MCP-1, monocyte chemoattractant protein-1.
FIGURE 5.
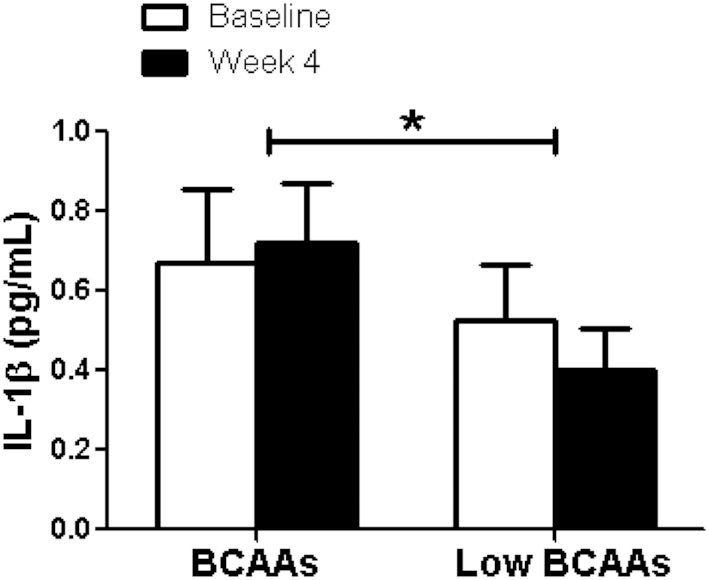
BCAA supplementation resulted in significantly increased plasma IL-1β concentrations. The figure shows the fasting plasma IL-1β concentration before (baseline) and after (final) BCAA supplementation with BCAA and low-BCAA powder supplements. Data are mean ± SE (n = 12). P values were derived from ANOVA with the use of a 2-period crossover design, P < 0.05. BCAA, branched-chain amino acid.
Plasma concentrations of BCAAs and related metabolites
Plasma concentrations of BCAAs (leucine, valine, and isoleucine) and associated metabolites, i.e., the BCKAs KIC, KIV, and KMV, were measured. BCAA supplementation did not affect the circulating concentrations of the BCAAs leucine (BCAA: 9.0% ± 12%; low-BCAA: 9.2% ± 11%), valine (BCAA: 9.1% ± 11%; low-BCAA: 12.0% ± 13%), isoleucine (BCAA: 2.5% ± 11%; low-BCAA: 7.3% ± 11%) (Figure 6) or the BCKAs KIC (BCAA: 11.2% ± 5%; low-BCAA: 10.9% ± 6%), KIV (BCAA: 13.0% ± 5%; low-BCAA: 14.9% ± 7%), and KMV (BCAA: 1.9% ± 7%; low-BCAA: 12.9% ± 7%) (Figure 7).
FIGURE 6.
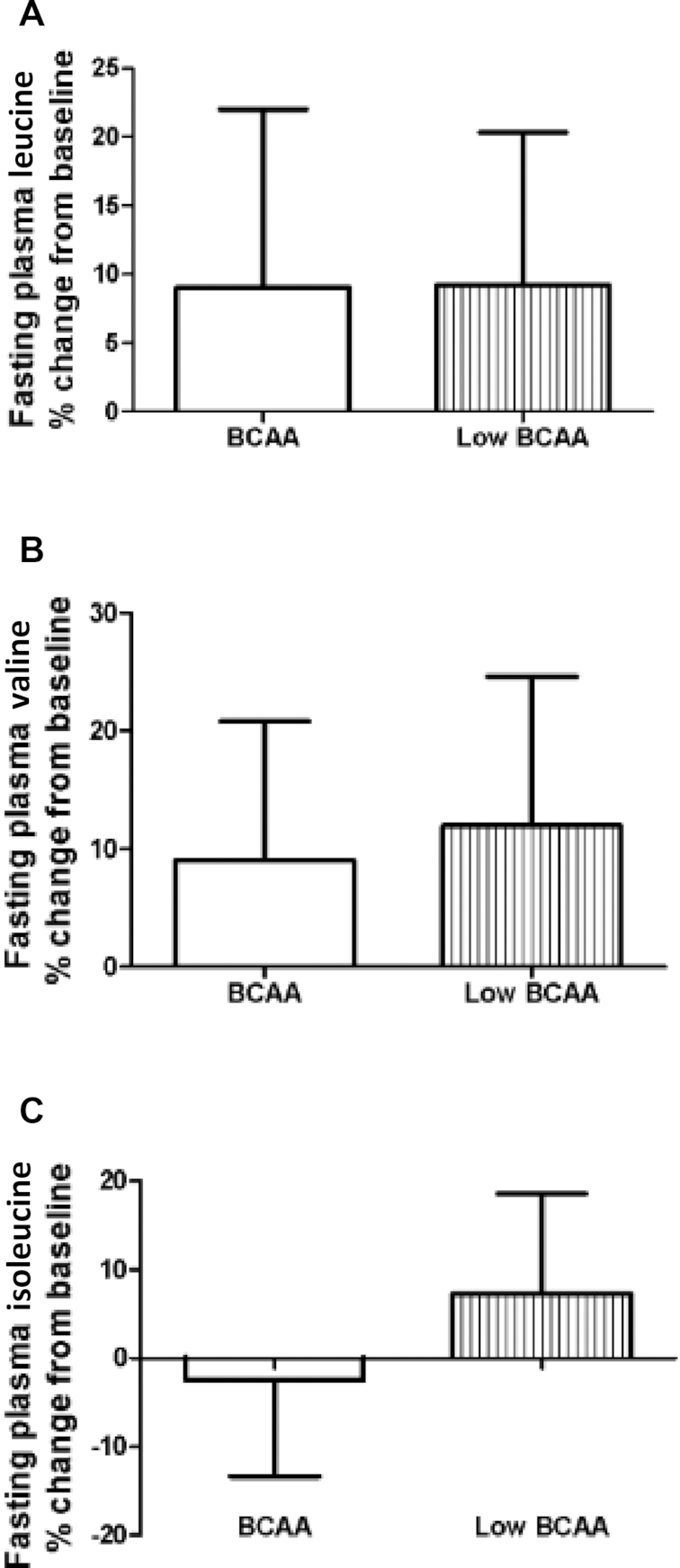
There were no significant differences in plasma BCAA concentrations after BCAA supplementation. The figure shows the fasting plasma BCAAs (A) leucine, (B) valine, and (C) isoleucine concentrations as percentage change normalized to the supplementation baseline. Data are mean ± SE (n = 12). BCAA, branched-chain amino acid.
FIGURE 7.
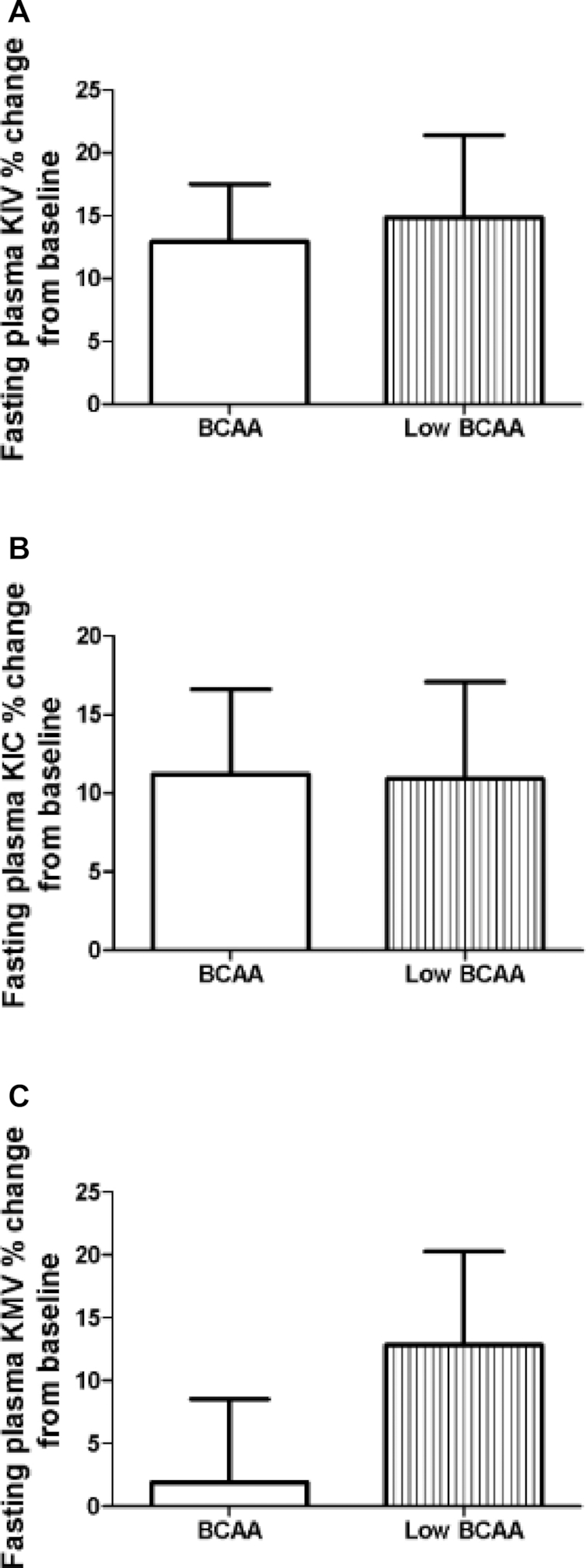
There were no significant differences on plasma BCKA concentrations after BCAA supplementation. The figure shows the fasting plasma BCKAs (A) KIV, (B) KIC, and (C) KMV concentrations as percentage change normalized to the supplementation baseline. Data are mean ± SE (n = 12). BCAA, branched-chain amino acid; BCKA, branched-chain α-keto acid; KIC, α-ketoisocaproate; KIV, α-ketoisovalerate; KMV, α-keto-methylvalerate.
Discussion
Recent studies have reported that circulating BCAAs and associated metabolites were positively associated with HOMA-IR (18) and an increased risk of T2DM (19). Increased circulating BCAAs may be one cause of insulin resistance, but this hypothesis remains unclear despite several mechanistic studies (6, 20). Our study design—increasing BCAA consumption in the diet of obese, prediabetic patients—was intended to examine the interaction between BCAAs and insulin resistance. We observed that dietary supplementation of BCAAs tended to decrease plasma glucose elevation during an OGTT.
BCAAs also decreased circulating NGF concentrations. NGF is regarded as an adipokine (21), and can be upregulated in obese individuals with type 2 diabetes (22). Our data showed that BCAA supplementation induced a decrease in fasting circulating NGF, in conjunction with lowered glucose and insulin concentrations, during an OGTT. This suggested an inverse association between circulating NGF and glucose metabolism. In support of this, BCAA supplementation has been found to lead to decreased NGF expression from the hippocampus in rats (23). Interestingly, there is evidence demonstrating that rosiglitazone, an antidiabetic peroxisome proliferator-activator receptor γ agonist, when used for in vitro treatment of adipocytes, can lead to a significant decrease in NGF expression in a dose-dependent manner (24). This may indicate that BCAA supplementation acts via a similar mechanism to that of rosiglitazone in regulating glucose metabolism. Nevertheless, the mechanisms of how BCAAs improve glucose metabolism by regulating NGF remain to be further studied.
In addition, we also showed that there was a significant decrease in circulating MCP-1 following BCAA supplementation. MCP-1 contributes to chronic inflammation and insulin resistance via macrophage recruitment. Decreasing MCP-1 in animals reduces insulin resistance (25). The improvement of glucose metabolism from BCAA supplementation may have been partially due to a significant decrease in circulating MCP-1 concentrations. In support of this, prior evidence shows that BCAA supplementation in obese mice downregulates MCP-1 mRNA expression in adipose tissue (26).
Although it has been proposed that elevated circulating BCAA concentrations in insulin-resistant individuals result from increased dietary consumption of BCAA-rich proteins, we demonstrated that supplementation of additional dietary BCAA did not cause an increase in circulating BCAAs, indicating that circulating BCAA concentrations are tightly regulated, and that dietary BCAA consumption is not likely the cause of elevated BCAAs in insulin-resistant individuals. In addition, clinical studies suggest that dietary protein has only a small association with the risk of developing T2DM, and dietary protein intake does not correlate with HOMA-IR in diabetic subjects (11, 12). It is interesting to note that circulating BCAA concentrations could also be affected by the gut microbiome. For example, the gut microbiota has the ability to utilize/metabolize amino acids, including BCAAs, to synthesize bacterial-derived short-chain fatty acids, and may regulate the health status of the host (27). Therefore, it is plausible that the obese, prediabetic subjects in our study may have a different gut microbiome that could regulate circulating BCAAs. This might also explain the improvement in glucose metabolism caused by BCAA supplementation in those subjects.
It has been suggested that dysfunction of BCKDs in adipose tissue could contribute to increased plasma BCAA concentrations in individuals with insulin resistance (28), and BCKDHA has been described as a susceptibility gene for T2DM (29). The dysfunction of BCKDs could lead to the formation of BCKA metabolites, i.e., KIC, KIV, and KMV (metabolites upstream of BCKDs). These metabolites could promote stress-activated mitochondrial dysfunction as has been demonstrated in individuals with maple syrup urine disease (5, 30). Increased amounts of BCKAs have also been demonstrated in insulin-resistant individuals, and may contribute to insulin resistance by causing mitochondrial dysfunction (5). In the present study, the BCKAs KIC, KIV, and KMV were not elevated in the plasma of the subjects after BCAA supplementation. This observation may explain why BCAA supplementation did not impair glucose metabolism in the study subjects. The metabolic phenotype, in terms of BCAA metabolism, of the prediabetic subjects in our study appears not to be consistent with studies reporting that individuals with T2DM have dysfunctional BCAA catabolism. In support of this, insulin-resistant patients do not universally demonstrate elevated circulating BCKA concentrations (28).
Our observation of BCAA supplementation effects on glucose metabolism differs from that of several comparable studies. For example, Fontana et al. (10) demonstrated that mice fed a diet with reduced BCAA concentrations showed improved glucose tolerance compared to mice fed a control diet only, although Cummings et al. (31) reported improved insulin resistance in animals fed a diet low in BCAAs. Newgard et al. (6) reported that BCAA addition to a high-fat diet did not improve glucose metabolism in mice but induced insulin resistance. It is likely that the deleterious effects of BCAAs require the presence of a high-fat diet. This is supported by the observation that high-fat BCAA feeding causes accumulation of incompletely oxidized lipid acylcarnitines metabolites that may contribute to insulin resistance compared with animals fed standard-diet BCAAs (6). There is also evidence showing that skeletal muscle 3-hydroxy-isobutyrate, a valine catabolite, could act as a paracrine regulator to promote fatty acid uptake, and lipid accumulation in muscle cells, resulting in insulin resistance (32). As for human observational studies, 2 studies reported a positive association between dietary BCAA intake and T2DM risk (33, 34), although Nagata et al. (13) reported a negative association between dietary BCAA intake and T2DM risk in a Japanese population. In an even more recent study, Asghari et al. (35) reported that high intakes of dietary BCAAs are associated with the development of insulin resistance. It is worth noting that those studies were conducted in different ethnic groups, suggesting that genetic variation could explain these varying observations.
Clearly, there remain controversies regarding the relation between BCAAs and insulin resistance. The current theory mostly points towards a positive association between circulating BCAAs and insulin resistance development/progression. The observations in our study could be a result of limitations in our study design. To improve our study design, a bolus of BCAAs could be administered to subjects immediately before performing an OGTT in order to elevate circulating BCAAs. This approach might result in a glucose metabolism of obese, prediabetic subjects different to that reported in the current study. In addition, future studies with larger patient populations and longer supplementation periods will also be helpful in validating the results of the current study. Nevertheless, the relation between BCAAs and insulin resistance remains to be further elucidated as this is a possible developmental area of novel interventions for managing T2DM.
In conclusion, the present study provided evidence that additional BCAA supplementation in obese, prediabetic patients did not impair glucose metabolism; instead, glucose metabolism tended to be improved after daily additional BCAA supplementation for 4 wk. This is likely because BCAA supplementation did not promote an elevation in circulating BCAAs and BCKAs; alternatively, BCAAs might act by decreasing circulating NGF concentrations to improve glucose metabolism. However, the mechanisms of how BCAA supplementation brings about these effects remain to be elucidated.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Center for Human Nutrition, UCLA for sponsoring the study.
The authors’ responsibilities were as follows—ZL, YW, DH, and SMH: designed the research; SLW, MH, AY, IG, GT, and AR: performed the research and data collection; SLW, JY, MH, LZ, RL, CLC, and JH: analyzed the data; SLW: wrote the article; ZL, YW, DH, SMH, and CC: edited and reviewed the manuscript; ZL: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by the Center for Human Nutrition, UCLA.
Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BCAA, branched-chain amino acid; BCKA, branched-chain α-keto acid; BCKD, branched-chain keto acid dehydrogenase; KIC, α-ketoisocaproate, KIV, α-ketoisovalerate; KMV, α-ketomethylvalerate; MCP-1, monocyte chemoattractant protein-1; NGF, nerve growth factor; OGTT, oral-glucose-tolerance test; T2DM, type 2 diabetes mellitus.
References
- 1. CDC. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2017. [Internet]. 2017. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. [Google Scholar]
- 2. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S.. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients. 2016;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch CJ, Adams SH.. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA et al.. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB et al.. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen T, Ni Y, Ma X, Bao Y, Liu J, Huang F, Hu C, Xie G, Zhao A, Jia W et al.. Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep. 2016;6:20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knebel B, Strassburger K, Szendroedi J, Kotzka J, Scheer M, Nowotny B, Mussig K, Lehr S, Pacini G, Finner H et al.. Specific metabolic profiles and their relationship to insulin resistance in recent-onset type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2016;101:2130–40. [DOI] [PubMed] [Google Scholar]
- 10. Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, Cava E, Spelta F, Tosti V, Syed FA et al.. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Nielen M, Feskens EJ, Mensink M, Sluijs I, Molina E, Amiano P, Ardanaz E, Balkau B, Beulens JW, Boeing H et al.. Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct Case-Cohort Study. Diabetes Care. 2014;37:1854–62. [DOI] [PubMed] [Google Scholar]
- 12. Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ et al.. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T.. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am J Epidemiol. 2013;178:1226–32. [DOI] [PubMed] [Google Scholar]
- 14. Allard P, Delvin EE, Paradis G, Hanley JA, O'Loughlin J, Lavallee C, Levy E, Lambert M.. Distribution of fasting plasma insulin, free fatty acids, and glucose concentrations and of homeostasis model assessment of insulin resistance in a representative sample of Quebec children and adolescents. Clin Chem. 2003;49:644–9. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Yin B, Li R, He P.. Determination of branched-chain keto acids in serum and muscles using high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Molecules. 2018;23:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pal S, Ellis V.. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr. 2010;104:1241–8. [DOI] [PubMed] [Google Scholar]
- 17. Walker GA. Common statistical methods for clinical research. Cary (NC): SAS Institute Inc; 2002. [Google Scholar]
- 18. Wurtz P, Soininen P, Kangas AJ, Ronnemaa T, Lehtimaki T, Kahonen M, Viikari JS, Raitakari OT, Ala-Korpela M.. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C et al.. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A et al.. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang B, Jenkins JR, Trayhurn P.. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–740. [DOI] [PubMed] [Google Scholar]
- 22. Bullo M, Peeraully MR, Trayhurn P, Folch J, Salas-Salvado J.. Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur J Endocrinol. 2007;157:303–10. [DOI] [PubMed] [Google Scholar]
- 23. Scaini G, Mello-Santos LM, Furlanetto CB, Jeremias IC, Mina F, Schuck PF, Ferreira GC, Kist LW, Pereira TC, Bogo MR et al.. Acute and chronic administration of the branched-chain amino acids decreases nerve growth factor in rat hippocampus. Mol Neurobiol. 2013;48:581–9. [DOI] [PubMed] [Google Scholar]
- 24. Peeraully MR, Jenkins JR, Trayhurn P.. NGF gene expression and secretion in white adipose tissue: regulation in 3T3-L1 adipocytes by hormones and inflammatory cytokines. Am J Physiol Endocrinol Metab. 2004;287:E331–339. [DOI] [PubMed] [Google Scholar]
- 25. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K et al.. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terakura D, Shimizu M, Iwasa J, Baba A, Kochi T, Ohno T, Kubota M, Shirakami Y, Shiraki M, Takai K et al.. Preventive effects of branched-chain amino acid supplementation on the spontaneous development of hepatic preneoplastic lesions in C57BL/KsJ- db/db obese mice. Carcinogenesis. 2012;33:2499–506. [DOI] [PubMed] [Google Scholar]
- 27. Neis EP, Dejong CH, Rensen SS.. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7:2930–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN et al.. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tiffin N, Adie E, Turner F, Brunner HG, van Driel MA, Oti M, Lopez-Bigas N, Ouzounis C, Perez-Iratxeta C, Andrade-Navarro MA et al.. Computational disease gene identification: a concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic Acids Res. 2006;34:3067–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, Shellmer D, Moser AB, Morton DH.. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cummings NE, Williams EM, Kasza I, Konon EN, Schaid MD, Schmidt BA, Poudel C, Sherman DS, Yu D, Arriola Apelo SI et al.. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596:623–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A et al.. A branched chain amino acid metabolite drives vascular transport of fat and causes insulin resistance. Nat Med. 2016;22:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, Wolpin BM, Hu FB, Qi L.. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016;45:1482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isanejad M, LaCroix AZ, Thomson CA, Tinker L, Larson JC, Qi Q, Qi L, Cooper-DeHoff RM., Phillips LS, Prentice RL et al.. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women's Health Initiative. Br J Nutr. 2017;117:1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Asghari G, Farhadnejad H, Teymoori F, Mirmiran P, Tohidi M, Azizi F.. High dietary intake of branched-chain amino acids is associated with an increased risk of insulin resistance in adults. J Diabetes. 2018;10:357–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.