ABSTRACT
Background
Dementia and late-life cognitive decline are leading causes of death and disability in the United States. Prevention of these diseases, by maintaining brain health throughout the life course, is essential. Diet and lifestyle changes are the chief strategies aimed at primary prevention for many of the risk factors of cognitive decline.
Objective
The aim of this study was to examine the potential impact of dietary factors on cognitive function.
Methods
This prospective cohort study followed 516 young adults through midlife. The Youth/Adolescent Questionnaire was used to collect habitual nutrition data (mean age: 32.03 ± 5.96 y) at baseline. Scores from a neurocognitive battery were used to assess cognitive function (mean age: 49.03 ± 4.86 y) at follow-up and were transformed to z scores. Separate multivariable-adjusted linear regression models were fitted. The trend across quintiles for each dietary variable was assessed.
Results
Vitamin B-6, whole grains, processed meats, and foods fried at home all displayed significant linear trends in their relation with cognitive function. Dietary intake of vitamin B-6 and whole grains was directly associated with better cognitive function after adjustment for age, race, sex, and total calorie intake (β coefficient from linear regression and SE: 1.755 ± 0.621, P = 0.005, and 0.001 ± 0.000, P = 0.018, respectively). Processed meat and foods fried at home consistently displayed inverse associations with cognitive function across crude and adjusted models (linear trend P values were 0.05 and <0.0001, respectively).
Conclusions
Our findings suggest that dietary consumption in young adulthood may affect cognitive function in midlife. Strong associations between dietary intake and cognition were observed in our analysis, but as with all observational studies, the possibility of residual confounding cannot be excluded.
Keywords: diet, nutrition, cognition, brain health, cognitive function
Introduction
Brain health throughout the life course is critical to successful aging. It has been well documented that diet influences the development and management of chronic diseases, specifically hypertension and type 2 diabetes (1, 2), which are associated with increased risk of late-life cognitive decline and dementia (3, 4). Given that there is no cure for late-life cognitive decline or dementia, strategies for prevention, before cognitive decline becomes apparent, are essential. The latent onset period for dementia lasts decades, suggesting that prevention must begin in early and midlife (5, 6). Several epidemiological studies examining cognitive decline have identified hypertension, smoking, diabetes mellitus, obesity, atherosclerotic disease, atrial fibrillation, and metabolic syndrome as risk factors (7-9).
Diet and lifestyle changes are the cornerstone of strategies aimed at primary prevention for many of these risk factors for cognitive decline. In the Prevención con Dieta Mediterránea randomized controlled trial, participants randomly assigned to the Mediterranean diet intervention with nut or extra virgin olive oil supplementation had higher cognition scores than those randomly assigned to a low-fat diet after 6.5 years of follow-up (10). Fruit and vegetable intake has also been shown to be a positive correlate of cognition in some studies (11, 12). In contrast, refined carbohydrates and simple sugars, such as sucrose and fructose, when not consumed as part of whole fruits and vegetables, have been hypothesized to be detrimental. In particular, high-fructose corn syrup found in baked goods and sugar-sweetened beverages has been linked to health concerns because of its adverse effect on postprandial serum triglycerides and its potential to increase insulin resistance (13). Other putative dietary factors that may negatively affect cognitive health include processed meats, fried foods, soft drinks, and refined carbohydrates, which are common components of the Western dietary pattern (14). A prospective study examining dietary and cognitive patterns in adolescence demonstrated that the Western dietary pattern was associated with lower cognitive performance scores after 3 years compared to other dietary patterns (14).
The results from observational studies exploring the relation between dietary factors and cognition have been inconsistent, with few data available for middle-aged individuals prior to the onset of clinically apparent cognitive dysfunction (15-17). Most available data are predominantly drawn from older white or European Americans and include few African American individuals and/or those living in a rural rather than urban setting (18-21). Therefore, we took advantage of data from the well-characterized, midlife, biracial Bogalusa Heart Study cohort in a semi-rural setting to examine the potential impact of dietary factors on cognitive function.
Methods
Study population
The Bogalusa Heart Study is a long-term epidemiological study exploring the natural history of cardiovascular disease in Bogalusa, Louisiana. The study began following children in 1973–1974 and is still collecting data today. From this semi-rural, biracial community, African American (35%) and white (65%) children aged 5–14 years were recruited (22). For this analysis, the baseline dietary data were collected between 1998 and 2001 on 1970 individuals (mean age: 32.03 ± 5.96 y). The follow-up data, including the cognitive function testing, were collected between 2013 and 2016 for 1298 individuals (mean age: 49.03 ± 4.86 y). There were 782 individuals with data from both the baseline and follow-up periods. Among those, 79 individuals with a dietary intake of <500 calories or >3200 calories were excluded due to unrealistic values, and 7 participants with a history of cardiovascular disease and stroke at baseline were excluded from the study. In addition, 174 individuals were excluded due to incomplete cognitive data. Finally, 6 individuals were excluded due to missing covariate data. A total of 516 participants were included in the final analysis (Supplemental Figure 1). For the fully adjusted model, 441 participants were included; 75 individuals were excluded from this portion of the analysis due to incomplete salary information. All of the data were managed using REDcap electronic data capture tools hosted at Tulane University (23). Informed consent was obtained for every participant, and the protocols for the studies were approved by the institutional review board of the Tulane University Health Science Center.
Dietary data
The Youth/Adolescent Questionnaire (YAQ) is a semiquantitative, validated, 151-item food frequency questionnaire used to collect longitudinal nutrition data on children and adolescents (24). Participants were an average age of 32.03 ± 5.96 y in 1998–2001 when dietary nutrient intake data were collected. The YAQ was found to be reflective of food commonly consumed by this young adult population (25). Although the average age is older than that generally accepted as “youth,” investigators chose to maintain the use of this food frequency questionnaire to enhance comparability with previous exams. The Nutrition Data System for Research developed by the Nutrition Coordinating Center at the University of Minnesota was used to calculate the specific nutrient information (26). Processed meat and whole grain scores were created by converting each food item in the category into grams, using standard portion sizes, and then adding the different food grams together. The foods included in the whole grain variable were whole-grain cereal, cooked oats, dark bread, and other grains such as kasha, couscous, and bulgur. The foods included in the processed meat variable were the meat portion of hot dogs, turkey or chicken sandwiches, roast beef or ham sandwiches, and deli meat sandwiches such as salami and bologna. The category of foods fried at home was used as a categorical frequency variable. The question assessing fried food intake at home was “How often do you eat food that is fried at home, like fried chicken?” with response options including “Never/less than once per week,” “1–3 times per week,” “4–6 times per week,” and “Daily.”
Cognitive function assessment
Scores from a standardized neurocognitive battery were used to assess cognitive function. These tests included the Wechsler Adult Intelligence Scale, from this, Digit Span forward and backward, vocabulary, and digit symbol coding. In addition, tests were derived from the Wechsler Memory Scale IV, including Logical Memory I and Logical Memory II and Recognition. Letter and word reading were assessed using the Wide Range Achievement Test IV. Finally, Trail Making Test Parts A and B were included to assess executive function. The raw scores of the components of the cognitive function assessment were transformed to z scores with a mean of zero and an SD of 1.0. These standardized scores were combined to formulate a global cognitive function assessment score.
Covariates
Information on education was collected at follow-up along with the cognitive assessment. Education level was categorized into three groups: less than high school and high school education, some college and associate's degree, and bachelor's degree or higher. Salary level was classified into 9 categories: >$5,000, $5,000–$11,999, $12,000–$15,999, $16,000–$24,999, $25,000–$34,999, $35,000–$49,999, $50,000–$74,999, $75,000–$99,999, and ≥$100,000. The 10-item Center for Epidemiologic Studies Depression Scale Revised was used to determine self-reported depressive symptoms for the participants at follow-up. Any score ≥10 was considered depressed (27). Diabetes was defined by having a fasting blood glucose level >125 mg/dL or taking medication to control diabetes. Hypertension was defined by having an average systolic blood pressure ≥140 mm Hg or an average diastolic blood pressure ≥90 mm Hg or taking medication to control hypertension at baseline. BMI (in kg/m2) was collected at follow-up and was categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25–29.9), and obese (≥30).
Physical activity was assessed at baseline. Two questions were considered: “Compared to other people your age and sex, how would you rate your physical activity at work during the past year?” and “Compared to other people your age and sex, how would you rate your physical activity outside of work during the past year?” Responses these questions ranged from 1, meaning inactive, to 5, meaning very active. For physical activity at work, participants could respond with 0 to indicate not applicable. This questionnaire was validated and demonstrated differences between different groups of Bogalusa Heart Study participants with insulin resistance syndrome (28). The scores for physical activity were added together; thus, the overall score could range from 1, being least active, to 10, being most active.
Statistical analysis
Univariate analyses were performed to assess normality. Bivariate analyses were performed with ANOVA and chi-square to test for significance. Separate linear regression models were fitted for the different dietary parameters. The models were then adjusted for age, race, sex, and total calorie intake. Then, adjusted model 1 was adjusted for age, race, sex, education, BMI, hypertension, diabetes, depressive symptoms, word reading score, physical activity, and total calorie intake. The final model, adjusted model 2, was adjusted for age, race, sex, education, BMI, hypertension, diabetes, depressive symptoms, word reading score, physical activity, total calorie intake, and salary. The trend across quintiles for each dietary variable was assessed for linearity. Because this is a hypothesis-generating study, where the information is expected to be used to test future dietary interventions that may improve cognitive function, multiple comparison procedures are not appropriate (29). In addition, we conducted stratification-based sensitivity analyses for the outcome of interest, structuring the models by levels of socioeconomic status (SES), race, salary, and depression status, based on significant interaction terms determined a priori (considering the main effect of the dietary exposures, and covariates, in all models). All statistical analyses were generated using SAS/STAT software (version 9.4; SAS Institute). Two-tailed values of P = 0.05 or less were considered statistically significant.
Results
Table 1 displays the baseline characteristics of the study participants by quintile of cognitive function. The average age at baseline dietary assessment was 32.03 ± 5.96 y. There was no difference between age, sex, BMI, physical activity, diabetes, or alcohol consumption across quintiles of cognitive function. Higher standardized cognition scores were associated with higher level of education and employment. Those in the lowest quintiles of cognitive function reported being disabled or unemployed most often. The overall prevalence of depressive symptoms was 30.4% (P-value of 0.08). Individuals in the lower quintiles of cognitive function were also more frequently hypertensive (P = 0.07). Vitamin B-6, fructose, processed meat, and the frequency of consumption of foods fried at home differed significantly across quintiles of cognitive function. Those in the highest quintiles of cognitive function consumed the greatest amount of vitamin B-6 and reported the least processed meat and fried food intake at home. Those in the median quintile of cognitive function consumed the most fructose.
TABLE 1.
Participant characteristics by quintile of cognitive score
| Characteristic | Overall (n = 516) | Q1 (n = 103) | Q2 (n = 103) | Q3 (n = 104) | Q4 (n = 103) | Q5 (n = 103) | P 1 |
|---|---|---|---|---|---|---|---|
| Age, y2 | 50.10 (45.92, 53.09) | 50.2 (45.98, 52.86) | 50.59 (45.85, 53.57) | 49.47 (45.94, 52.88) | 49.94 (46.05, 53.27) | 49.76 (45.53, 52.95) | 0.9105 |
| Sex, n (%) | |||||||
| Male | 193 (37.40) | 42 (40.78) | 39 (37.86) | 39 (37.50) | 40 (38.83) | 33 (32.04) | 0.7603 |
| Female | 323 (62.60) | 61 (59.22) | 64 (62.14) | 65 (62.50) | 63 (61.17) | 70 (67.96) | |
| Race, n (%) | |||||||
| White | 374 (72.06) | 48 (46.60) | 61 (59.22) | 78 (75.00) | 90 (87.38) | 94 (91.26) | <0.0001 |
| Black | 145 (27.94) | 55 (53.40) | 42 (40.78) | 26 (25.00) | 13 (12.62) | 9 (8.74) | |
| BMI, kg/m22 | 31.24 (25.90, 35.53) | 31.02 (26.22, 35.92) | 30.00 (25.99, 34.62) | 31.51 (26.35, 36.23) | 29.68 (25.64, 36.35) | 28.87 (25.45, 33.29) | 0.2627 |
| Education, n (%) | |||||||
| High school or less | 264 (50.87) | 92 (89.32) | 68 (66.02) | 52 (50.00) | 34 (33.01) | 17 (16.50) | <0.0001 |
| Some college | 125 (24.08) | 6 (5.83) | 23 (22.33) | 25 (24.04) | 38 (36.89) | 32 (31.07) | |
| Bachelor's or more | 130 (25.05) | 5 (4.85) | 12 (11.65) | 27 (25.96) | 31 (30.10) | 54 (52.43) | |
| Depression, n (%) | |||||||
| CESD-R-10 ≥10 | 158 (30.44) | 41 (39.81) | 35 (33.98) | 29 (27.88) | 26 (25.24) | 25 (24.27) | 0.0785 |
| CESD-R-10 <10 | 361 (69.56) | 62 (60.19) | 68 (66.02) | 75 (72.12) | 77 (74.76) | 78 (75.73) | |
| Diabetes, n (%) | 15 (2.9) | 4 (3.88) | 2 (1.94) | 4 (3.85) | 3 (2.91) | 2 (1.94) | 0.8523 |
| Hypertension, n (%) | 75 (14.53) | 22 (21.36) | 19 (18.45) | 12 (11.54) | 12 (11.65) | 10 (9.71) | 0.0748 |
| Employment, n (%) | |||||||
| Working | 180 (34.88) | 28 (27.18) | 46 (44.66) | 41 (39.42) | 37 (35.92%) | 28 (27.18%) | <0.0001 |
| Keeping house | 242 (46.90) | 37 (35.92) | 42 (40.78) | 48 (46.15) | 56 (54.37) | 59 (57.28) | |
| School | 26 (5.04) | 3 (2.91) | 4 (3.88) | 5 (4.81) | 2 (1.94) | 12 (11.65) | |
| Disabled | 22 (4.26) | 13 (12.62) | 4 (3.88) | 2 (1.92) | 2 (1.94) | 1 (0.97) | |
| Unemployed | 38 (7.36) | 19 (18.45) | 7 (6.80) | 6 (5.77) | 4 (3.88) | 2 (1.94) | |
| Other | 8 (1.55) | 3 (2.91) | 0 (0.00) | 2 (1.92) | 2 (1.94) | 1 (0.97) | |
| Physically active, n (%) | 216 (41.86) | 38 (36.89) | 49 (47.57) | 45 (43.27) | 40 (38.83) | 44 (42.72) | 0.5698 |
| Alcohol, g | 0.89 (0, 3.71) | 0 (0, 3.71) | 0 (0, 3.71) | 1.03 (0, 3.72) | 0.88 (0, 3.18) | 0.88 (0, 2.67) | 0.5426 |
| Total calories | 1824 (1448, 2298) | 1781 (1349, 2382) | 1730 (1434, 2232) | 1977 (1542, 2453) | 1815 (1449, 2294) | 1851 (1501, 2197) | 0.3578 |
| Vitamin C, mg3 | 83 (53, 122) | 86 (57, 122) | 84 (58, 135) | 85 (49, 121) | 734 (50, 107) | 82 (54, 125) | 0.278 |
| Vitamin B-6, mg3 | 1.45 (1.27, 1.71) | 1.38 (1.25, 1.59) | 1.42 (1.23, 1.66) | 1.41 (1.25, 1.58) | 1.54 (1.36, 1.82) | 1.55 (1.30, 1.82) | 0.0068 |
| Fructose, g3 | 25.99 (17.66, 36.62) | 24.99 (16.47, 35.356) | 27.53 (19.31, 42.01) | 30.42 (20.90, 38.91) | 24.07 (15.05, 32.81) | 24.45 (17.49, 35.24) | 0.01 |
| Sucrose, g3 | 51.95 (41.54, 64.56) | 49.89 (41.08, 60.41) | 57.06 (43.20, 67.25) | 53.37 (42.78, 66.23) | 50.88 (42.01, 62.37) | 49.82 (39.13, 64.59) | 0.1297 |
| ω-3, g3 | 0.09 (0.07, 0.13) | 0.10 (0.07, 0.14) | 0.10 (0.06, 0.13) | 0.09 (0.07, 0.12) | 0.10 (0.06, 0.13) | 0.09 (0.07, 0.12) | 0.6973 |
| Whole grains, g3 | 477 (174, 971) | 383 (158, 944) | 439 (153, 1023) | 326 (97, 751) | 546 (227, 1128) | 655 (220, 1161) | 0.2315 |
| Processed meat, g3 | 682 (453, 969) | 764 (543, 1076) | 712 (497, 1050) | 591 (380, 904) | 599 (422, 949) | 625 (451, 909) | 0.0035 |
| Foods fried at home per week, n (%) | |||||||
| Never/less than once | 174 (33.72) | 22 (21.36) | 22 (21.36) | 27 (25.96) | 48 (46.60) | 55 (53.40) | <0.0001 |
| 1–3 times | 292 (56.59) | 64 (62.14) | 63 (61.17) | 69 (66.35) | 51 (49.51) | 45 (43.69) | |
| 4–6 times | 35 (6.78) | 9 (8.74) | 13 (12.62) | 6 (5.77) | 4 (3.88) | 3 (2.91) | |
| Daily | 15 (2.91) | 8 (7.77) | 5 (4.85) | 2 (1.92) | 0 (0.00) | 0 (0.00) | |
1 P value: ANOVA test for continuous variables and chi-square test for categorical variables. The median and IQRs (Q25, Q75) were reported for continuous variables because they are not normally distributed. CESD-R-10, 10-item version of the Center for Epidemiologic Studies Depression Scale Revised.
2At follow-up.
3Per 2000 calories.
Mean cognitive score across dietary quintiles of nutrients are displayed in Figure 1. Trends for vitamin B-6, whole grains, processed meats, and foods fried at home were significantly linear (P < 0.05). Vitamin C, fructose, and omega-3 fatty acid did not show statistically significant trends. Consumption of sucrose was the only component to demonstrate a significant quadratic trend, with the middle quintile having the highest value for cognitive score and the top and bottom quintiles with lower values for cognitive score.
FIGURE 1.
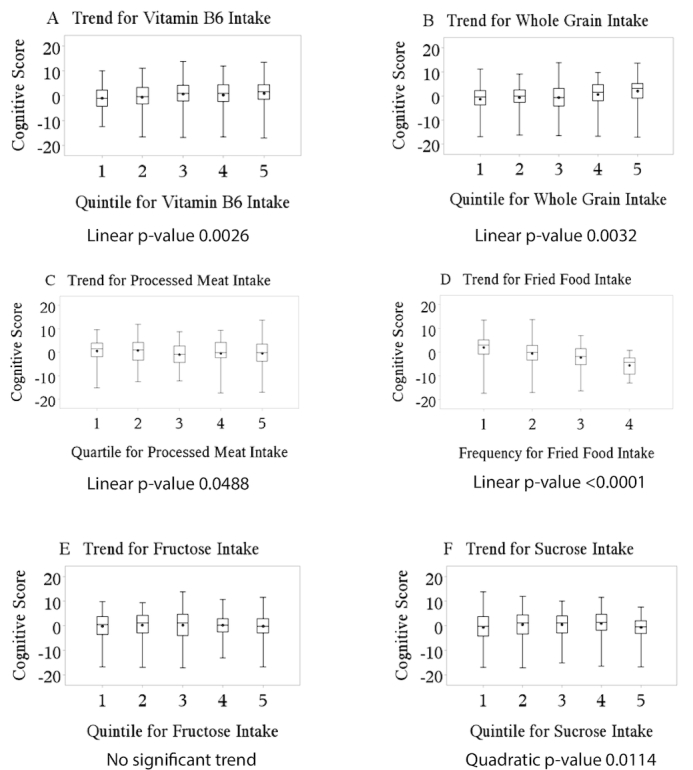
Trends for dietary variables: (A) vitamin B-6, (B) whole grains, (C) processed meat, (D) foods fried at home, (E) fructose, and (F) sucrose.
The results of the individual regression analysis of the different dietary determinants influence on the standardized cognitive score outcome are displayed in Table 2. The regression coefficients for the crude associations demonstrate that vitamin B-6, whole grains, processed meats, and foods fried at home were positively associated with cognitive function. The age, race, and sex adjusted models showed similar results. For the adjusted models 2 and 3, the frequency of intake of foods fried at home was significantly inversely associated with cognitive function scores. In adjusted model 2, intake of processed meats was only marginally, inversely significant, with a P value of 0.065. These 2 dietary factors consistently displayed inverse associations with cognitive function scores. In adjusted model 3, processed meat was significantly inversely associated with cognitive function, with a P value of 0.021. Although there was a positive association between fructose intake and cognitive scores in the adjusted model 2 (P = 0.027), there was no statistically significant linear, quadratic, or cubic trend across quintiles of fructose. In addition, there is no significant association between fructose and cognitive scores in adjusted model 3, with a P value of 0.236. Significant interactions for vitamin B-6 and depression status were observed. Furthermore, a depression status-stratified sensitivity analysis revealed that the independent association between vitamin B-6 and cognitive score was only significant in nondepressed individuals (β: −2.16; SE = 1.45; P = 0.03) compared to depressed individuals (β: −1.74; SE = 2.2; P = 0.4). No significant interactions were observed between the dietary exposures and SES, race, and salary.
TABLE 2.
Dietary associations with standardized cognitive score1
| Crude association | Adjusted 1 | Adjusted 2 | Adjusted 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 516) | (n = 516) | (n = 516) | (n = 441) | |||||||||
| Dietary correlates | β | SE | P | β | SE | P | β | SE | P | β | SE | P |
| Vitamin C, mg | −0.004 | 0.005 | 0.418 | 0.008 | 0.004 | 0.062 | 0.005 | 0.003 | 0.128 | 0.005 | 0.003 | 0.154 |
| Vitamin B-6, mg | 2.334 | 0.652 | 0.001 | 1.755 | 0.621 | 0.005 | −0.035 | 0.429 | 0.936 | −0.147 | 0.442 | 0.739 |
| Fructose, g | −0.019 | 0.018 | 0.289 | 0.005 | 0.017 | 0.761 | 0.026 | 0.012 | 0.027 | 0.014 | 0.012 | 0.236 |
| Sucrose, g | −0.008 | 0.016 | 0.594 | −0.012 | 0.015 | 0.402 | 0.013 | 0.010 | 0.209 | 0.007 | 0.010 | 0.522 |
| ω-3, g | 1.469 | 5.276 | 0.781 | 5.203 | 4.971 | 0.296 | −0.216 | 3.327 | 0.948 | −0.948 | 3.404 | 0.781 |
| Whole grains, g | 0.001 | 0.000 | 0.023 | 0.001 | 0.000 | 0.018 | 0.000 | 0.000 | 0.206 | 0.000 | 0.000 | 0.327 |
| Processed meats, g | −0.002 | 0.001 | 0.004 | −0.001 | 0.001 | 0.013 | −0.001 | 0.000 | 0.065 | −0.001 | 0.000 | 0.021 |
| Foods fried at home | ||||||||||||
| Never/less than once per week2 | ||||||||||||
| 1–3 times per week | −2.934 | 0.501 | <.0001 | −2.153 | 0.496 | <.0001 | −0.800 | 0.348 | 0.022 | −0.831 | 0.355 | 0.020 |
| 4–6 times per week | −4.689 | 0.958 | <.0001 | −3.272 | 0.952 | 0.001 | −1.262 | 0.669 | 0.060 | −1.148 | 0.695 | 0.099 |
| Daily | −8.461 | 1.403 | <.0001 | −6.654 | 1.378 | <.0001 | −1.835 | 0.994 | 0.065 | −3.459 | 1.489 | 0.021 |
1Crude association: adjusted for total calorie intake; adjusted 1: adjusted for total calorie intake, sex, age at follow-up, and race; adjusted 2: same as adjusted 1 plus physical activity, diabetes, hypertension, BMI at follow-up, word score, and education; and adjusted 3: same as adjusted 2 plus depressive symptoms.
2Reference group.
Discussion
Our findings suggest that diet in young adulthood may be an important indicator of future cognitive function in middle-aged adults. As the burden of dementia is increasing with population increases in life expectancy, and there is no known cure, preventative strategies must be an essential component of public health initiatives to reduce disease. This prospective study provides evidence that diet in young adulthood may be a key component to maintaining brain health and cognitive function in midlife and later. Because diet is a universal exposure, adopting even minor changes at a population level could potentially lessen the burden of dementia over time.
A Western dietary pattern includes consumption of processed meats, refined carbohydrates, and fried foods. A previous study in adolescents found that the Western dietary pattern was associated with poorer cognitive performance (14). Our study findings are in agreement with these results and extend them to a biracial population of young adults living in a semi-rural area. Consumption of processed meat and frequency of consuming fried foods at home were significantly and inversely associated with cognitive scores across quintiles. Those who reported consuming fried foods at home never or less than once a week scored higher on cognitive tests than those who reported eating fried foods at home 1–3 times a week, 4–6 times a week, or daily. The Reasons for Geographic and Racial Differences in Stroke study reported results indicating that diet patterns including fried foods and processed meats were associated with lower scores on cognitive tests (30). Furthermore, fried food consumption has been found to be positively associated with BMI (31). A review of the relation between obesity and mental disorders including cognitive function found that both diseases share pathophysiological mechanisms (32). It has been proposed that the Western diet pattern may be associated with higher levels of inflammation, which could play a role in brain health (33). In a prospective study, higher intakes of red meat, processed meat, and fried food and lower intake of whole grains were associated with higher levels of IL-6. This study also found that an inflammatory diet was associated with faster cognitive decline. These associations were stronger in participants younger than age 56 y (33).
In our study, we found a significant, positive, and linear association between whole-grain intake and cognitive function. Those who reported consuming the most whole grains had the highest cognitive function score. Results from the Women's Health Study also suggest that whole-grain intake may positively influence cognitive function (34). Although a few other studies have shown similar results between the relation of whole grains and cognitive function (33, 35), ours is the first to extend this finding to young adulthood and to an underserved (rural) population with substantial minority representation. Whole grains are an abundant source of dietary fiber, a high consumption of which is associated with lower blood pressure and lower blood glucose values (36-38). Dietary fiber aids in slowing the rate of nutrient absorption, thus keeping blood glucose at a consistent level for a longer period of time (36, 37), and it is also thought to play a role in the prevention of hypertension (38). It is possible that through these mechanisms, dietary fiber may contribute to the maintenance of healthy cognitive function in midlife and throughout aging.
In many studies, vitamin C is used as an indicator of fruit and vegetable intake (39). World Health Organization recommendations for fruit and vegetable intake at 400 g/d have been associated with better cognitive function in a disadvantaged Brazilian elderly population (11). Although our results did not show a significant association between vitamin C intake and cognitive function, this may reflect differences in the populations studied. In 1 of the few prospective studies to examine this relation, individuals who consumed the most vitamin C from food sources only had slower rates of cognitive decline compared to those who consumed the least vitamin C after a 3-year follow-up. However, there was no association after a 7-year follow-up (40). In our analysis, we did find significant associations between vitamin B-6 intake and cognitive function scores. This may be because vitamin B-6 is a component of the homocysteine cycle, which is thought to play a role in brain health and function (41).
We did not find any significant associations between fructose or sucrose and cognitive function across models, with the exception of adjusted model 2. This finding is consistent with a study investigating sugar intake and cognitive function among middle-aged and older Puerto Ricans, in whom natural sources of fructose had no effect on cognitive function (42). Natural sources of fructose are present in the diet through fruits and vegetables. Conversely, fructose, when consumed as high-fructose corn syrup, is thought to be detrimental because of its adverse effect on postprandial serum triglycerides and potential to increase insulin resistance (13). In a meta-analysis examining sugar consumption, sucrose consumption did not have an effect on cognitive performance in children (43).
In our study, ω-3 fatty acid intake was not a significant predictor of cognitive function. These results are consistent with previous findings in midlife adults, despite ω-3 fatty acid being commonly advertised with the claim of cognitive benefits (44). Similar to what we found, a systematic review of ω-3 supplementation identified no significant impact on 9 different domains of cognitive performance in adults (45). However, there is evidence that ω-3 fatty acid intake is an important preventative factor in early life. EPA and DHA are proposed to be essential for the growing brain, and clinical trials have shown that supplementing breast milk with EPA and/or DHA increased cognitive development in infants and young children (46, 47).
Although the prevalence of depressive symptoms (30.4%) was high in our population, it is important to note that the Center for Epidemiologic Studies Depression instrument is a self-reported screening measure and not a diagnostic interview. This value was on par with those seen in the Jackson Heart Study (JHS), which included 3309 African American participants in nearby Jackson, Mississippi. In the JHS, 738 participants (22.3%) had depressive symptoms at baseline (48). The Bogalusa cohort is rural and of lower average SES compared with the JHS cohort; both of these characteristics have been associated with increased depressive symptoms.
The strengths of this study include its comprehensive neurocognitive assessment, prospective cohort design with the dietary exposure information collected prior to the cognitive assessment, and strong participation of African Americans. By examining nutrition in early adulthood, we avoid the potential for subclinical brain changes that occur decades before the period of latency during which clinical dementia develops.
This study also has a number of limitations. We assessed cognition function at a single follow-up only; therefore, changes in cognitive function could not be assessed. As with all self-reported dietary data, measurement error is an important issue. Individuals who are overweight are more likely to under-report nutrient intake (49). In this analysis, dietary data were collected at 1 point in time. In addition, high-fructose corn syrup was unable to be assessed separately from total fructose. Therefore, we were unable to determine the relation between high-fructose corn syrup and cognitive function separate from total fructose. All participants in this study were white or African American, reflecting the racial makeup of the community. Therefore, our results may not be generalizable to other populations and races or ethnicities, such as those of Latino and Hispanic origin. Last, as with all observational studies, residual confounding may affect the results, and determining the causality of associations ultimately requires randomized controlled trials.
Our findings strongly suggest that further research is warranted to explore the role of diet in young adulthood as it relates to midlife cognitive function and the potential maintenance of cognitive performance through healthy aging. Additional studies with repeated dietary assessments and imaging data to explore brain structure and function would contribute substantially to our understanding of the role of dietary intake in brain health.
In conclusion, as the burden of dementia continues to increase with the aging of the US and the global population, the importance of behavioral intervention strategies that have the potential to influence cognitive function at a population level grows. Our findings are some of the first to suggest that moderating consumption of fried foods and processed meats, and encouraging consumption of fruits, vegetables, and whole grains, in young adulthood in an underserved, biracial population may contribute to brain health throughout the life course.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—NCF: had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; NCF and LAB: drafted and revised the manuscript; LAB: provided critical oversight of the project; and all authors: edited and provided revisions to the manuscript. None of the authors reported a conflict of interest related to this study.
Notes
The investigators and work presented here were supported by grants P20GM109036, R01HD069587, R01AG041200, R01AG016592, R01HL071981, R01HL034594, R21HL126024, R01DK115679, R01DK091718, R01DK100383, and R01DK078616.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
References
- 1. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miler ER et al.. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 2. Salas-Salvado J, Bullo M, Babio N, Martinez-Gonzalez MA, Ibarrola-Jurado N, Basora J, Estruch R, Covas M, Corella D, Aros F et al.. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2010;34(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease pathophysiology, clinical consequences, and medical therapy: part 1. Circulation. 2003;108(12):1527–32. [DOI] [PubMed] [Google Scholar]
- 4. Dedette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, Wolf PA, DeCarli C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh-Manoux A, Kivimäki M. The importance of cognitive ageing for understanding dementia. Age. 2010;32(4):509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. [DOI] [PubMed] [Google Scholar]
- 8. Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes?. J Neurol Sci. 2012;322:141–7. [DOI] [PubMed] [Google Scholar]
- 9. Kalaria RN, Ihara M.. Dementia: vascular and neurodegenerative pathways—will they meet?. Nat Rev Neurol. 2013;9:487–8. [DOI] [PubMed] [Google Scholar]
- 10. Martinez-Lapiscina EH, Clavero P, Toledo E, Julian BS, Sanchez-Tainta A, Corella D, Lamuela-Raventos RM, Martinez JA, Martinez-Gonzalez MA. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized trial. J Nutr Health Aging. 2013;17(6):544–52. [DOI] [PubMed] [Google Scholar]
- 11. Pastor-Valero M, Furlan-Viebig R, Menezes PR, Silva SA, Vallada H., Scazufca M.. Education and WHO recommendations for fruit and vegetable intake are associated with better cognitive function in a disadvantaged Brazilian elderly population: a population-based cross-sectional study. PLoS One. 2014;9(4):e94042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiang X, Huang J, Song D, Deng R, Wei J, Zhang Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: meta-analysis. Front Aging Neurosci. 2017;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bantle JP. Dietary fructose and metabolic syndrome and diabetes. J Nutr. 2009;139(6):1263S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nyaradi A, Foster JK, Hickling S, Li J, Ambrosini GL, Jacques A, Oddy WH. Prospective associations between dietary patterns and cognitive performance during adolescence. J Child Psychol Psychiatry. 2014;55(9):1017–24. [DOI] [PubMed] [Google Scholar]
- 15. Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martínez-Lage P. Diet, cognition, and Alzheimer's disease: food for thought. Eur J Nutr. 2013;53(1):1–23. [DOI] [PubMed] [Google Scholar]
- 16. Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia. Epidemiology. 2013;24(4):479–89. [DOI] [PubMed] [Google Scholar]
- 17. Rafnsson SB, Dilis V, Trichopoulou A. Antioxidant nutrients and age-related cognitive decline: a systematic review of population-based cohort studies. Eur J Nutr. 2013;52(6):1553–67. [DOI] [PubMed] [Google Scholar]
- 18. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin J, Kronmal RA, Kuller LH, Manolio T, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. [DOI] [PubMed] [Google Scholar]
- 19. Gibson EL, Barr S, Jeanes YM. Habitual fat intake predicts memory function in younger women. Front Hum Neurosci. 2013;7:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gardener S, Gu Y, Rainey-Smith SR, Keogh J, Clifton PM, Mathieson SL, Taddei K, Mondal A, Ward VK, Scarmeas N et al.. Adherence to a Mediterranean diet and risk of incident cognitive impairment in an Australian population. Transl Psychiatry. 2012;2e:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97(2):369–76. [DOI] [PubMed] [Google Scholar]
- 22. Berenson GS. Bogalusa Heart Study: a long-term community study of a rural biracial (black/white) population. Am J Med Sci. 2001;322(5):267–74. [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rockett HR, Breitenbach M, Frazier A, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a Youth/Adolescent Food Frequency Questionnaire. Prev Med. 1997;26(6):808–16. [DOI] [PubMed] [Google Scholar]
- 25. Deshmukh-Taskar PR, O'Neil C, Nicklas T, Yang SJ, Liu Y, Gustat J, Berenson GS. Dietary patterns associated with metabolic syndrome, socio-demographic, and lifestyle factors in young adults: the Bogalusa Heart Study. Public Health Nutr. 2009;12:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products—a research perspective. J Food Comp Anal. 2001;14:315–22. [Google Scholar]
- 27. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 28. Gustat J, Srinivasan SR, Elkasabany A, Berenson GS. Relation of self-rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults. J Clin Epidemiol. 2002;55(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 29. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 30. Pearson KE, Wadley VG, McClure LA, Shikany JM, Unverzagt FW, Judd SE. Dietary patterns are associated with cognitive function in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. J Nutr Sci. 2016;5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, Liming L, Curhan GC, Pasquale LR, Wiggs JL et al.. Fried food consumption, genetic risk, and body mass index: gene–diet interaction analysis in three US cohort studies. BMJ. 2014;348:g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agustí A, García-Pardo MP, López-Almela I, Campillo I, Maes M, Romaní-Pérez M, Sanz Y. Interplay between the gut–brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner E. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr. 2015;34::506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age: results from the Women's Health Study. Epidemiology. 2013;24(4):490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA.. Prospective study of Dietary Approaches to Stop Hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr. 2013;98(5):1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jenkins DJ, Jenkins AL. Dietary fiber and the glycemic response. Proc Soc Exp Biol Med. 1985;180:422–31. [DOI] [PubMed] [Google Scholar]
- 37. Potter JG, Coffman KP, Reid RL, Krall JM, Albrink MJ. Effect of test meals of varying dietary fiber content on plasma insulin and glucose response. Am J Clin Nutr. 1981;34:328–34. [DOI] [PubMed] [Google Scholar]
- 38. Streppel MT, Arends LR, Van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med. 2005;165(2):150–6. [DOI] [PubMed] [Google Scholar]
- 39. Proteggente AR, Pannala AS, Paganga G, Buren LV, Wagner E, Wiseman S, van de Put F, Dacombe C, Rice-Evans CA. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. 2009;36(2):217–33. [DOI] [PubMed] [Google Scholar]
- 40. Wengreen HJ, Munger RG, Corcoran CD, Zandi P, Hayden KM, Fotuhi M, Skoog I, Norton MC, Tschanz J, Breitner JCS et al.. Antioxidant intake and cognitive function of elderly men and women: the Cache County study. J Nutr Health Aging. 2007;11:230–7. [PubMed] [Google Scholar]
- 41. Bhargava S, Srivastava LM. Homocysteine—marker of the millennium: a review of its evolution and clinical implication. J Med Sci. 2001;4(2):104–16. [Google Scholar]
- 42. Ye X, Gao X, Scott T, Tucker KL. Habitual sugar intake and cognitive function among middle-aged and older Puerto Ricans without diabetes. Br J Nutr. 2011;106:1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolraich ML, Wilson DB, White W. The effect of sugar on behavior or cognition in children: a meta-analysis. JAMA. 1995;274(20):1617–21. [DOI] [PubMed] [Google Scholar]
- 44. Omega-3 MD. 100% pure omega3, clinically proven the highest quality supplement available—UltimateOmega3 [Internet]. n.d. [cited 1 April, 2018]. Available from http://www.omega-3md.com. [Google Scholar]
- 45. Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. Omega-3 polyunsaturated fatty acid supplementation and cognition: a systematic review and meta-analysis. J Psychopharmacol. 2015;29(7):753–63. [DOI] [PubMed] [Google Scholar]
- 46. McCann JC, Ames BN. Is docosahexaenoic acid, an n–3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. 2005;82(2):281–95. [DOI] [PubMed] [Google Scholar]
- 47. Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, Gronn M, Solberg R, Moen A, Nakstad B et al.. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121(6):1137–45. [DOI] [PubMed] [Google Scholar]
- 48. O'Brien EC, Greiner MA, Sims M, Hardy NC, Wang W, Shahar E, Hernandez AF, Curtis LH. Depressive symptoms and risk of cardiovascular events in blacks: findings from the Jackson Heart Study. Circ Cardiovasc Qual Outcomes. 2015;8(6):552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65(4):1203S–9S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


