Significance
The ability to withstand heat is fundamental to an organism’s ecology, and variation in heat tolerance affects the geographic range of species. Many insects have obligate relationships with heat-sensitive bacterial symbionts, raising the question of whether variation in heat sensitivity among symbionts underlies variation in heat sensitivity among their host species. This study compared aphid species with different abilities to withstand heat and showed that titers of the bacterial symbiont Buchnera drop rapidly following heat exposure of heat-sensitive aphid species, whereas Buchnera titers in heat-tolerant aphid species are unaffected or show delayed responses. While heat-induced responses of aphid hosts themselves also may contribute, symbiont ability to withstand heat appears to be a central determinant of host thermal range.
Keywords: symbiosis, aphididae, thermal adaptation, RNA-Seq, heat shock proteins
Abstract
The thermal tolerance of an organism limits its ecological and geographic ranges and is potentially affected by dependence on temperature-sensitive symbiotic partners. Aphid species vary widely in heat sensitivity, but almost all aphids are dependent on the nutrient-provisioning intracellular bacterium Buchnera, which has evolved with aphids for 100 million years and which has a reduced genome potentially limiting heat tolerance. We addressed whether heat sensitivity of Buchnera underlies variation in thermal tolerance among 5 aphid species. We measured how heat exposure of juvenile aphids affects later survival, maturation time, and fecundity. At one extreme, heat exposure of Aphis gossypii enhanced fecundity and had no effect on the Buchnera titer. In contrast, heat suppressed Buchnera populations in Aphis fabae, which suffered elevated mortality, delayed development and reduced fecundity. Likewise, in Acyrthosiphon kondoi and Acyrthosiphon pisum, heat caused rapid declines in Buchnera numbers, as well as reduced survivorship, development rate, and fecundity. Fecundity following heat exposure is severely decreased by a Buchnera mutation that suppresses the transcriptional response of a gene encoding a small heat shock protein. Similarly, absence of this Buchnera heat shock gene may explain the heat sensitivity of Ap. fabae. Fluorescent in situ hybridization revealed heat-induced deformation and shrinkage of bacteriocytes in heat-sensitive species but not in heat-tolerant species. Sensitive and tolerant species also differed in numbers and transcriptional responses of heat shock genes. These results show that shifts in Buchnera heat sensitivity contribute to host variation in heat tolerance.
Thermal tolerance is a basic determinant of an organism’s ecology: The ability to survive and to reproduce in the face of variation in temperature affects geographic range, competitive ability, and population dynamics. Many animals and plants engage in intimate symbiotic interactions with microorganisms, and these symbionts can impact thermal tolerance. When hosts are dependent on symbionts that are themselves heat sensitive or cold sensitive, the host’s temperature range and geographic distribution may be curtailed by the symbiosis. For example, the leaf-cutter ant Atta texana extends its geographic range into colder regions by adopting cold-tolerant mutualistic fungi (1). Often, symbionts appear to be less heat tolerant than hosts. Thus, corals are subject to heat-induced elimination of their obligate algal symbionts, and if symbionts are killed by warming, the corals then bleach and die (2). Similarly, gut symbionts of stinkbugs are heat sensitive: Warming suppresses these symbionts causing stinkbug fitness to drop (3).
Many insect groups maintain obligate symbioses with maternally transmitted bacteria that provide essential nutrients to their hosts (4, 5), raising the question of whether this dependence on heritable symbionts curtails temperature tolerances and geographic ranges (6, 7). Heritable symbionts may be particularly heat sensitive due to long-term accumulation of deleterious mutations that cause rapid evolution of proteins and loss of many genes (5, 8). The resulting extreme genome reduction reflects clonality and limited genetic population sizes over millions of years of vertical transmission within hosts (5). Among the consequences are deleterious amino acid replacements, including replacements that affect translational machinery itself, leading to decline in protein quality (9).
Aphids and their intracellular bacterial associates Buchnera aphidicola are a widely studied model of obligate symbiosis. Buchnera has diversified with aphids through maternal transmission for >100 million years; their tiny genomes encode only 354–587 proteins (10) but retain genes underlying production of amino acids needed for host nutrition (11). Several observations suggest that Buchnera is heat sensitive. First, Buchnera proteins show reduced thermal stability compared to homologous proteins of related free-living bacteria (12). Second, as in other insect endosymbionts (6), Buchnera shows constitutively elevated expression of heat shock proteins including GroEL and DnaK; high expression of these chaperones rescues misfolded proteins in a destabilized proteome (13). Functionality of Buchnera enzymes expressed in Escherichia coli requires overexpression of GroEL (14) or growth at lower temperature (15). Third, in the pea aphid, Ac. pisum, some Buchnera genotypes are especially susceptible to heat due to a recurring mutation in the heat shock promoter of ibpA, which encodes a universal small heat shock protein (16, 17). Aphids with this haplotype lose all or most Buchnera following heat exposure and suffer drastic reductions in fecundity; experimental replacement of this haplotype improves heat tolerance (18). Fourth, the facultative symbiont, Serratia symbiotica, which ameliorates the negative effects of heat on Ac. pisum (19, 20) appears to shield hosts by buffering Buchnera populations, possibly by producing protective metabolites (21). Finally, at least, in some cases, levels of Buchnera within aphids appear to decline at higher temperatures (22).
Our central questions in this study was whether Buchnera limits the heat tolerance of aphid hosts and whether variation among aphid species in heat tolerance is linked to variation in Buchnera. Aphid species vary widely in heat sensitivity (23, 24). We documented aphid survivorship and reproduction following heat challenge in 5 widely distributed aphid species chosen on the basis of apparent differences in thermal tolerance, partly reflected in geographic ranges (25) (SI Appendix, Table S1). Aphis craccivora and Ap. gossypii are generalist crop pests distributed worldwide in warm temperate and tropical regions, whereas Ap. fabae is a generalist that is most common in cool regions, including northern Europe and Canada. Ac. kondoi and Ac. pisum specialize on alfalfa and favor cool environments. We included a second Ac. pisum line in which Buchnera bears a single nucleotide deletion in the ibpA heat shock promoter, increasing heat sensitivity (16, 18). We quantified symbiont titers following heat exposure, visualized bacteriocytes with and without heat exposure, and explored the genomic and transcriptional underpinnings of heat responses in both aphid host and Buchnera.
Results
Effects of Heat Exposure on Aphid Survival, Developmental Time, and Fecundity.
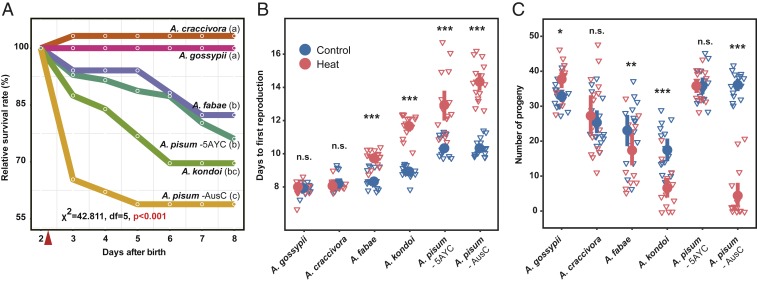
We first measured effects of second-day sublethal exposure to 38 °C on later survival. As expected, we found significant differences among the 6 aphid lines (Fig. 1A, χ2 = 42.811, df = 5, P < 0.001). Ap. craccivora and Ap. gossypii had zero mortality following heat exposure, while Ap. fabae survival dropped to 82.4%. Ac. pisum AusC in which Buchnera carries the ibpA promoter mutation had the lowest survival rate at 58.9% at the end of the trial, significantly lower than that of Ac. pisum 5AYC at 76.1% (P = 0.048). Controls showed no mortality except for 1 Ap. craccivora individual, resulting in its heat treatment survival score over 100% after control calibration.
Fig. 1.
Effects of heat exposure on aphid fitness. (A) Relative daily survival rate of 6 aphid lines after sublethal 38 °C on day 2. Survival calibrated by mortality of controls at constant 20 °C. The red triangle indicates time of heat exposure. The different letters following each aphid line indicate significant differences (P < 0.05) among lines with post hoc pairwise χ2 tests. (B) Time from birth to first reproduction and (C) number of progeny during first 7 d of reproduction. The bars above and below the dots indicate the SEs. The asterisks indicate significant differences between control and heat treatments (*P < 0.05, **P < 0.01, ***P < 0.001, and n.s. nonsignificant).
The aphid lines also differed in how heat exposure affected time to maturity and fecundity (Fig. 1 B and C). Strikingly, Ap. gossypii performed better under heat exposure; it did not delay first reproduction (developmental time: W = 105.5, P = 0.604; fecundity: W = 51.5, P = 0.012). Ap. craccivora was also tolerant to heat exposure, which had no significant effects on developmental time or fecundity. In contrast, all Acyrthosiphon lines were negatively affected. Ac. kondoi suffered both delayed maturity and reduced fecundity (developmental time: W = 0, P < 0.001; fecundity: W = 212.5, P < 0.001). Both lines of Ac. pisum showed delayed maturity after heat shock (developmental time: 5AYC: W = 10, P < 0.001; AusC: W = 0, P < 0.001). However, the Ac. pisum lines differed sharply in the effect of heat on fecundity. Ac. pisum AusC with the heat-sensitive Buchnera ibpA genotype showed a steep drop in fecundity (W = 225, P < 0.001); whereas Ac. pisum 5AYC with the relatively heat-tolerant Buchnera ibpA genotype showed no change in fecundity (W = 110, P = 0.933).
Effect of Heat Exposure on Buchnera Titer.
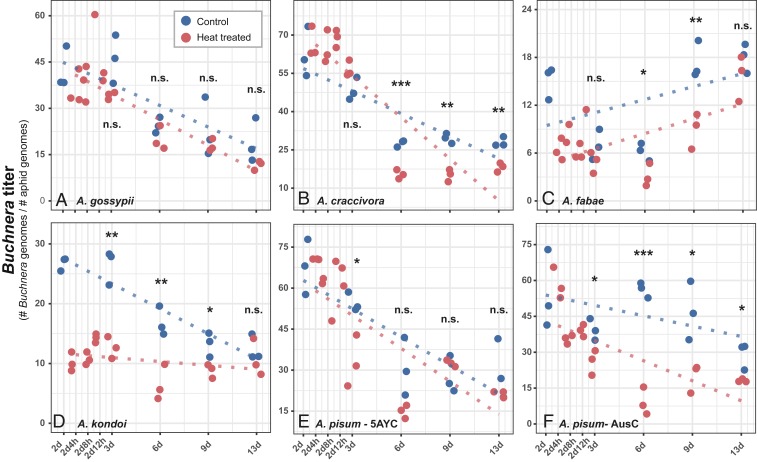
To address whether the effects of heat on hosts reflect impacts on obligate symbionts, we measured effects of heat exposure on the Buchnera titer in each of the aphid lines (Fig. 2 and SI Appendix, Table S2). Buchnera of Ap. gossypii was least affected, and Buchnera titers were unaffected by heat exposure at any time point. Buchnera titers in the 3 Aphis lines were not affected at Day 3 (24 h after exposure); but Buchnera titers in heat-exposed Ap. craccivora and Ap. fabae were reduced at Days 6 and 9 (Fig. 2 A–C). In contrast to all Aphis lines, Buchnera in all Acyrthosiphon lines underwent reductions within 24 h after heat exposure (Day 3) (Fig. 2 D–F). The effect on Ac. pisum AusC was especially persistent as heat exposure reduced titers at all sampling points.
Fig. 2.
Effect of heat exposure on the Buchnera titer for 6 aphid lines: (A) Ap. gossypii, (B) A.p craccivora, (C) Ap. fabae, (D) Ac. kondoi, (E) Ac. pisum-5AYC, and (F) Ac. pisum-AusC. Heat exposure occurred on Day 2. Each dot represents a biological replicate, at least, 10 aphids for each sampling timing. The asterisks indicate significant differences between control and heat treatments (*P < 0.05, **P < 0.01, ***P < 0.001, and n.s. nonsignificant).
Titers were measured as the ratio of Buchnera genome copies to aphid genome copies in the entire aphid and, therefore, reflected changes in bacteriocytes of both mother and embryos during development. As expected, aphid age has a large effect on this ratio (SI Appendix, Table S2). The average titer and the shifts in titer with age vary across aphid species, reflecting species-specific differences in developmental patterns. Overall, species in which Buchnera declined in response to heat exposure were species in which heat caused subsequent reductions in fitness.
Fluorescent in situ Hybridization Visualization of Effects of Heat Exposure on Buchnera in Whole Aphids.
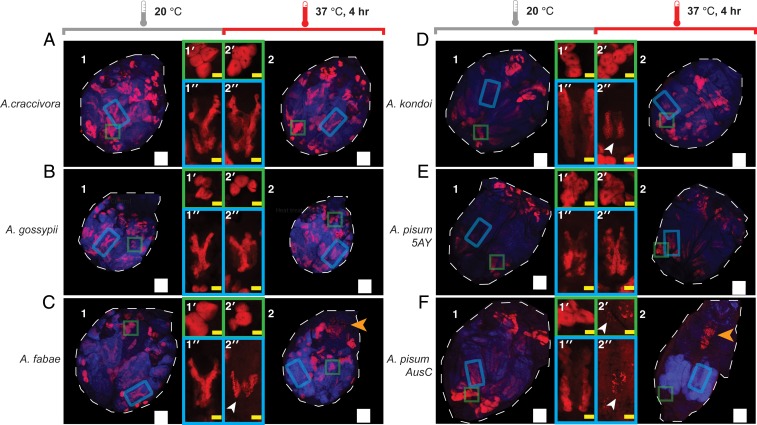
The Buchnera titer alone may not capture how heat disrupts bacteriocytes and does not allow discrimination between effects on maternal and embryonic bacteriocytes. Heat exposure had no visible effect on the bacteriocytes of heat-tolerant aphid species Ap. craccivora and Ap. gossypii (Fig. 3 A and B). The embryonic bacteriomes of heat-sensitive species Ap. fabae and Ac. kondoi, however, were consistently smaller and irregularly shaped after heat treatment, and adult aphid body size was also reduced after heat exposure (Fig. 3 C and D). In Ac. pisum 5AYC, heat treatment had no visible effect on bacteriocytes (Fig. 3E). In contrast, heat caused dramatic disruption in both maternal and embryonic bacteriocytes in Ac. pisum AusC in which heat reduced aphid body size and lowered Buchnera titers (Fig. 3F). Surprisingly, Ac. pisum AusC and Ap. fabae both gave a signal indicating Buchnera rRNA in or on their midguts after heat exposure; the basis for this signal is not clear (Fig. 3, C2, F2). Generally, the FISH results correlate well with qPCR-based measures of the titer, and they reveal that effects on embryonic bacteriocytes are particularly severe.
Fig. 3.
Laser confocal images of whole-mount fluorescent in situ hybridization (FISH) of aphid lines in control (1) and after treatment of 4 h at 37 °C as juveniles (2). Whole aphid images are maximum intensity projects of merged DAPI (blue) and Buchnera (red) channels (see Materials and Methods for full display information). Insets 1′ and 2′ are enlarged maternal bacteriocytes (green squares), and 1′′ and 2′′ are enlarged embryonic bacteriocytes (blue rectangles). Species shown include Ap. craccivora (A), Ap. gossypii (B), Ap. fabae (C), Ac. kondoi (D), Ac. pisum-5AYC (E), and Ac. pisum-AusC (F). Images are representative of 4 to 5 aphids inspected for each condition. The white arrowheads indicate maternal or embryonic bacteriocytes showing gross deformity after heat treatment (C, D, and F). The orange arrowheads indicate observed Buchnera DNA in the midgut of Ap. fabae and Ac. pisum-AusC after heat treatment. The white Scale bars are 150 microns, and the yellow Scale bars are 50 microns.
Gene Expression Differentiation and Ontology Enrichment.
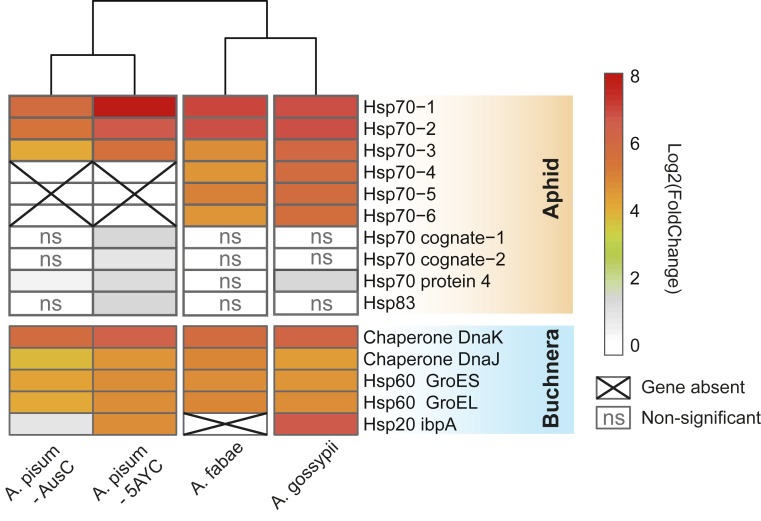
Comparison of transcriptional responses to heat exposure was based on a total of 379,988,517 clean reads from 24 libraries derived from 4 aphid lines, Ap. gossypii, Ap. fabae, Ac. pisum AusC, and Ac. pisum 5AYC (SI Appendix, Table S3). Of these, 10–25% of reads mapped to aphid or Buchnera protein-coding genes; most other reads came from aphid rRNA that was not effectively removed by the animal rRNA depletion kit. Among all significantly up-regulated genes, the heat shock proteins of both aphids and Buchnera showed the most prominent responses in all aphid lines (Fig. 4 and SI Appendix, Table S4). Within the Hsp70 superfamily of the aphids, 1 orthologous gene Hsp68-like cluster and 1 Hsp70 A1-like cluster of genes were strikingly up-regulated in all lines. However, lines differed in the numbers of paralogous copies present with 6 paralogs of these heat-responsive genes in both Ap. gossypii and Ap. fabae and only 3 paralogs in the Ac. pisum lines. The other 2 orthologous genes (Hsp70 cognates and Hsp70 protein 4) in the Hsp70 superfamily showed little or no response to heat exposure with a maximum fold change of 2 in the Ac. pisum 5AYC line.
Fig. 4.
Transcriptional responses following heat exposure of aphid and Buchnera genes for heat shock proteins in 4 aphid lines.
In Buchnera, genes encoding heat shock proteins, including groEL, groES, dnaK, and dnaJ, were the top up-regulated genes in all lines. The small heat shock protein encoded by ibpA (Hsp20) showed strong responses in Buchnera of both Ap. gossypii and Ac. pisum 5AY. Unexpectedly, this locus was absent from the genome of Buchnera of Ap. fabae. PCR and Sanger sequencing spanning the flanking genes (hslU-fpr) were used to verify the absence of ibpA in further samples from our experimental laboratory line (originating from Belgium) and from additional Ap. fabae collections from Germany (SI Appendix, Table S1), indicating that this gene loss is widespread in Buchnera of Ap. fabae. Buchnera of the Ac. pisum AusC line had an intact ibpA but showed little ibpA transcriptional response. In Ac. pisum AusC, ibpA transcripts increased only 1.9 fold after heat exposure in contrast to a 27.3 fold increase in Ac. pisum 5AYC. This lack of response in Ac. pisum AusC corresponds to the presence of a single base deletion in the ibpA promoter, resulting in a reduced spacer length between the −10 and the −35 binding sites for the heat shock promoter and, consequently, a reduced responsiveness to heat (16). We verified the difference in an ibpA promoter between Buchnera of Ac. pisum AusC and Ac. pisum 5AYC using PCR and Sanger sequencing with DNA from our experimental aphid lines.
By clustering the profiles of up-regulated aphid genes to ontology enrichment terms, the 2 Aphis species were found to have many up-regulated genes with terms related to chaperone binding (e.g., GO:0031072) and chaperone-mediated protein folding (e.g., GO: 0061077); these terms were absent from genes up-regulated in Ac. pisum (SI Appendix, Fig. S1). The Ac. pisum 5AYC line was enriched in neurotransmitter and signal release ontology terms, suggesting differences in responses to heat stimuli among aphid species.
Discussion
Aphid species differ dramatically in the effects of brief heat exposure on later survivorship, time to maturity, and fecundity (Fig. 1). Our results reveal that heat sensitivity of their obligate symbiont Buchnera is key in determining the impact of heat on aphid fitness. At 1 extreme, Buchnera populations in Ap. gossypii were unaffected by heat exposure, and hosts also suffered no negative effects: Survivorship or time to maturity were unaffected, and fecundity even increased. At the other extreme, the Buchnera of Ac. kondoi and of Ac. pisum AusC dropped within a day of exposure and remained significantly depressed a week after exposure (Fig. 2), and aphids in both of these lines suffered delayed maturity and sharply reduced fecundity (Fig. 2).
Ac. pisum AusC provides a striking contrast to Ac. pisum 5AYC in heat tolerance of both Buchnera and host. The Buchnera of these lines differ in the presence of a single base deletion that reduces the transcriptional response of ibpA to heat exposure, and that increases the sensitivity of Buchnera to heat with populations in AusC but not 5AYC being dramatically depressed. A similar negative effect of this mutation on Buchnera titers following heat exposure was reported previously for different combinations of Ac. pisum and Buchnera (16, 18). This suggests that this mutation, rather than other genotypic differences of symbiont or host, underlies the sensitivity of AusC to heat, but some effect of other genotypic differences cannot be entirely ruled out. While both lines have reduced survivorship and delayed maturity following heat, 5AYC fecundity is unaffected, whereas AusC fecundity is drastically reduced, and many AusC individuals failed to reproduce. IbpA is a small heat shock protein found in almost all organisms, including prokaryotes and eukaryotes; it acts within the cytoplasm to bind misfolded proteins and prevent them from forming irreversible aggregates (26). Thus, the heat-induced drop in Buchnera numbers in the AusC line represents an impact first on Buchnera with a consequent impact on aphid fecundity.
Previous studies have documented distinct responses to heat in different clones of Ac. pisum from field populations (27). Potentially this variation reflects the ability of Buchnera populations to withstand heat due to genotypic differences in either Buchnera (exemplified by the ibpA promoter mutation) or aphid.
Whereas heat has no negative effect on any component of fitness in 2 Aphis species, it has negative effects for survivorship, developmental time, and fecundity in Ap. fabae (Fig. 1). Ap. fabae also undergoes a drop in Buchnera numbers following heat (Fig. 2), unlike the other 2 Aphis species. A potential factor contributing to heat sensitivity of the Ap. fabae Buchnera is the loss of ibpA, which has otherwise been retained in sequenced Buchnera within the Aphidinae (the largest aphid subfamily, containing most aphid species) (10). This loss likely contributes to the heat sensitivity of this Buchnera and, in turn, of Ap. fabae. Ap. fabae also displays an unusual Buchnera titer, measured as the ratio of Buchnera to aphid genome copies in the whole aphid. This value decreased during development to adulthood in all species except Ap. fabae in which it increased possibly reflecting interspecies developmental differences.
In general, the heat sensitivities of aphids (Fig. 1) and their Buchnera (Fig. 2) correspond to the seasonal and geographic distributions of these aphid species. We note that geographic distribution as indicated by collection records is not a simple indicator of thermal range in the field since aphids are highly mobile and can colonize geographic regions for limited periods during favorable conditions, migrating long distances among regions. For example, Ac. pisum and Ac. kondoi can be found in warm locations, such as Texas and southern Arizona (USA) but only during the winter months, whereas Ap. craccivora and Ap. gossypii are abundant in the same locations during hot summer months. Our results would suggest that Ap. fabae cannot live in hot places; however, this might only apply to particular subspecies of Ap. fabae, which is widespread, may include subspecies or sibling species, and is often misidentified due to its resemblance to the related black species of Aphis, such as Ap. craccivora and Aphis rumicis (25). Our Ap. fabae line originated in northern Europe, and the other Ap. fabae samples confirmed as lacking ibpA were from northern Germany (SI Appendix, Table S1). Thus, at least, some widespread matrilines of Ap. fabae are likely confined to relatively cool geographic regions due, at least, in part, to their Buchnera undergoing an irreversible loss of the gene encoding this small heat shock protein. While its geographic range is difficult to ascertain from published records due to misidentification or possible sibling species, Ap. fabae appears to be largely absent from warm tropical or subtropical regions, whereas Ap. gossypii and Ap. craccivora are common in warm regions worldwide (25).
The 2 lines that had little or no ibpA response, Ac. pisum AusC and Ap. fabae, showed an unexpected similarity in Buchnera localization following heat shock. In both, heat caused Buchnera titers to decline in both maternal and embryonic bacteriocytes, coinciding with the qPCR results showing a drop in the overall titer. And in both, the midgut region acquired a Buchnera signal following heat shock (Fig. 3). Because the FISH probe hybridizes with Buchnera rRNA and because RNA molecules are not expected to be intact once cells are disrupted, the signal should indicate the presence of intact Buchnera cells. However, the basis for this midgut signal is not clear.
Our results indicate that obligate endosymbionts are sometimes more sensitive to heat than their hosts and that they can represent an “Achilles heel” limiting host ability to live under hot conditions as observed for gut symbionts of stinkbugs (3). However, obviously, many insect species with obligate endosymbionts do live in hot places as exemplified by 2 aphid species in our study: Ap. gossypii and Ap. craccivora. In Ap. gossypii, Buchnera experienced no decline following heat exposure, which boosted rather than suppressed host fecundity. One interesting and unresolved question is whether this resilience to heat exposure represents adaptation by Buchnera itself or adaptation by the host to protect and support its Buchnera population. In Ap. craccivora, the Buchnera titer did drop several days after heat exposure, but this reduction in symbiont numbers did not affect host fitness, suggesting that this host is adapted to be resilient in the face of temperature-induced fluctuations in Buchnera populations.
Continuous selection to maintain fitness under hot environments will select for ability to cope with heat on the part of both symbiont and host; failure of either to adapt will result in extinction or range contraction. However, Buchnera appears to often be more heat sensitive than hosts, suggesting that its small asexual genome is less able to respond to selection for maintaining thermal tolerance. When Buchnera heat sensitivity reflects a gene loss as appears to be the case with ibpA in Ap. fabae, then the loss of heat tolerance may be irreversible as Buchnera genomes do not regain genes (10). Thus, dependence on obligate endosymbionts could impose inflexibility on thermal adaptation such that temporary relaxation of selection for heat tolerance, for example, during a cool period or during movement to a cooler location, might result in permanent shifts. Most aphid species are confined to north temperate regions, and the lack of aphid species in the tropics and even in temperate regions in the Southern Hemisphere has been hypothesized to reflect an inability of aphid lineages in north temperate regions to colonize in tropical latitudes (28). Potentially, Buchnera sensitivity to heat contributes to this constraint (29). Interestingly, the few cases of permanent loss of Buchnera are in aphid species living in unusually warm climates, including several losses within Cerataphidini species in subtropical and tropical Asia (30) and 1 within Geopemphigus in Mexico (31). In both cases, Buchnera is replaced with a novel symbiont type. Replacement of an ancient heritable symbiont, such as Buchnera, is a potential evolutionary route to escape the limitations of a poorly functioning partner (e.g., ref. 32). However, such replacements are rare, occurring only a few times in the >200 million-year history of the sap-feeding homopteran insects (33).
Materials and Methods
Additional details of Methods are included in the SI Appendix.
Aphid Clonal Lines and Rearing.
We used 6 clonal lines representing 5 aphid species, including Ac. pisum, Ac. kondoi, Ap. fabae, Ap. craccivora, and Ap. gossypii (SI Appendix, Table S1). All lines were initiated with 1 field-collected female and maintained in the laboratory at constant 20 °C with a 16L/8D daily light cycle. Aphid lines were maintained on plants in 4-in pots and enclosed by an inverted plastic cup modified with a mesh top.
We included 2 Ac. pisum strains: 5AYC (heat resistant Buchnera genotype) and AusC (heat-sensitive Buchnera genotype). Ap. fabae, Ap. craccivora, and Ac. pisum were maintained on Vicia faba, Ac. kondoi were maintained on Medicago sativa (alfalfa), and Ap. gossypii were maintained on Cucumis melo (melon). We verified that all these aphid clones harbor Buchnera but lack secondary symbionts as confirmed by PCR based on universal primers spanning the 16S-23S ribosomal RNA spacer (34).
Thermal Fitness Measurement.
To determine aphid species survivorship following a heat treatment, we exposed second-day nymphs to 38 °C for 3 h in a thermal incubator, then monitored aphid survival daily for a week. To reduce impacts of plants and soil on the microclimate within cup cages, 2 to 3 aphid adults were transferred to seedlings in plastic screen cages (10 × 4 × 25 cm) to reproduce for 1 day at 20 °C. After heat treatment on the second day, the nymphs were moved to intact host plants in cup cages for further development. For each line, about 30 nymphs were treated, and about 30 nymphs were kept at constant 20 °C as controls.
To determine developmental time and fecundity following heat exposure, we used a similar setup and procedure for a second set of aphids, exposing them to 37 °C for 4 h, a treatment that stresses but does not kill the aphids. We then recorded survivorship, developmental time (days from birth to first reproduction), and fecundity (total offspring during the first 7 d of reproduction). Twelve to 15 adults were tested for treatment and for control in each line, respectively.
Real-Time qPCR of the Buchnera Titer.
To estimate dynamics of Buchnera populations following heat exposure, the ratio of Buchnera genome copies to aphid genome copies was determined by real-time qPCR using single-copy genes from both genomes. Aphids were heat exposed as described above (37 °C for 4 h on the second day) and sampled at 4, 8, 12, and 24 h (equivalent to the third day), sixth day, ninth day, and 13th day. Control aphids were kept at 20 °C for the duration of the experiment. At least, 10 aphids were collected as a replicate in each time point; at least, 30 individuals were sampled in the early stages due to the small size of young nymphs. For each time point, 3 biological replicates were prepared; for each biological replicate, we performed 3 technical replicates. PCR primers, and conditions are in SI Appendix, Table S2.
FISH to Determine Effects of Heat on Bacteriocytes.
Whole-mount in situ hybridization followed published protocols (35, 36). Control and heat-exposed aphids were treated as described above. Newly matured (Day 1) adults were sampled and fixed.
Buchnera Genome Sequencing.
Buchnera genomes from Ap. fabae and Ap. gossypii were sequenced de novo and assembled to be used as reference genomes for this study.
Transcriptional Responses to Heat Exposure.
To explore transcriptional responses to heat exposure in both aphids and Buchnera, we sampled 2-d-old nymphs with and without exposure to 37 °C for 4 h. The treated aphids were allowed to recover at 20 °C for 2 h before sampling. About 40–50 nymphs were collected for each replicate and then flash frozen in liquid nitrogen before storing at −80 °C prior to RNA extraction. Three biological replicates each for control and heat treatment were prepared for 4 aphid lines: Ap. gossypii, Ap. fabae, and the 2 lines of Ac. pisum.
Statistical Analyses.
All statistical analyses were performed using R 3.5.1 (37). We used the Cox proportional hazard model in the “survival” package to compare the survival curves after heat shock among aphid lines. Because none of the lines dropped survival to 50% within 7 d, we also compared the survival values on the last day by χ2 distribution in the “rcompanion” package. Pearson’s χ2 test and post hoc pairwise χ2 tests are presented in Fig. 1 with the significance threshold of P < 0.05. To test aphid fitness after heat exposure, we used generalized linear models for developmental time and fecundity measurements with aphid lines and treatment as fixed effects. When treatment was significant as a main effect, we followed this test with unpaired 2-sample Wilcoxon rank sum tests. Because data were not normally distributed, we used the continuity correction between control and heat treatment for each aphid line in the “dplyr” package. Additionally, tests for effects of heat exposure on the Buchnera titer were conducted using ANOVA under a general linear model, and independent T tests were used to compare control and treatment for each time point.
Data Availability.
All sequence data are available at National Center for Biotechnology Information. Buchnera genome sequences for Ap. fabae and Ap. gossypii are deposited under accession numbers CP042426 and CP042427. RNA-Seq data are deposited under BioProject ID PRJNA577642, SRA accession numbers SRR10291791–SRR10291814.
Supplementary Material
Acknowledgments
We thank Thomas Smith, Eli Powell, Julie Perreau, and Xuenong Xu for comments and assistance; Raymond Huey for comments on the paper; and Kim Hammond for aphid care, laboratory assistance, and help with the paper preparation. Funding came from the US National Science Foundation (Award DEB 1551092 to N.A.M.), and the China Scholarship Council and the Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences to B.Z.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/ in the Nucleotide repository (accession nos. CP042426 and CP042427) and in BioProject (ID PRJNA577642).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915307116/-/DCSupplemental.
References
- 1.Mueller U. G., et al. , Evolution of cold-tolerant fungal symbionts permits winter fungiculture by leafcutter ants at the northern frontier of a tropical ant-fungus symbiosis. Proc. Natl. Acad. Sci. U.S.A. 108, 4053–4056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampayo E. M., Ridgway T., Bongaerts P., Hoegh-Guldberg O., Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl. Acad. Sci. U.S.A. 105, 10444–10449 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikuchi Y., et al. , Collapse of insect gut symbiosis under simulated climate change. MBio 7, e01578-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner P., Endosymbiosis of Animals with Plant Microorganisms (Interscience, New York, 1965). [Google Scholar]
- 5.Moran N. A., McCutcheon J. P., Nakabachi A., Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42, 165–190 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Kupper M., Gupta S. K., Feldhaar H., Gross R., Versatile roles of the chaperonin GroEL in microorganism-insect interactions. FEMS Microbiol. Lett. 353, 1–10 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Wernegreen J. J., Mutualism meltdown in insects: Bacteria constrain thermal adaptation. Curr. Opin. Microbiol. 15, 255–262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran N. A., Bennett G. M., The tiniest tiny genomes. Annu. Rev. Microbiol. 68, 195–215 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Melnikov S. V., van den Elzen A., Stevens D. L., Thoreen C. C., Söll D., Loss of protein synthesis quality control in host-restricted organisms. Proc. Natl. Acad. Sci. U.S.A. 115, E11505–E11512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong R. A., Park H., Moran N. A., Genome evolution of the obligate endosymbiont Buchnera aphidicola. Mol. Biol. Evol. 36, 1481–1489 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Shigenobu S., Watanabe H., Hattori M., Sakaki Y., Ishikawa H., Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407, 81–86 (2000). [DOI] [PubMed] [Google Scholar]
- 12.van Ham R. C. H. J., et al. , Reductive genome evolution in Buchnera aphidicola. Proc. Natl. Acad. Sci. U.S.A. 100, 581–586 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fares M. A., Ruiz-González M. X., Moya A., Elena S. F., Barrio E., Endosymbiotic bacteria: GroEL buffers against deleterious mutations. Nature 417, 398 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Huang C.-Y., Lee C.-Y., Wu H.-C., Kuo M.-H., Lai C.-Y., Interactions of chaperonin with a weakly active anthranilate synthase from the aphid endosymbiont Buchnera aphidicola. Microb. Ecol. 56, 696–703 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Price D. R. G., Wilson A. C. C., A substrate ambiguous enzyme facilitates genome reduction in an intracellular symbiont. BMC Biol. 12, 110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunbar H. E., Wilson A. C. C., Ferguson N. R., Moran N. A., Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5, e96 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke G. R., McLaughlin H. J., Simon J. C., Moran N. A., Dynamics of a recurrent Buchnera mutation that affects thermal tolerance of pea aphid hosts. Genetics 186, 367–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran N. A., Yun Y., Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. U.S.A. 112, 2093–2096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montllor C. B., Maxmen A., Purcell A. H., Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27, 189–195 (2002). [Google Scholar]
- 20.Russell J. A., Moran N. A., Costs and benefits of symbiont infection in aphids: Variation among symbionts and across temperatures. Proc. Biol. Sci. 273, 603–610 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke G., Fiehn O., Moran N., Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 4, 242–252 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Chen C.-Y., Lai C.-Y., Kuo M.-H., Temperature effect on the growth of Buchnera endosymbiont in Aphis craccivora (Hemiptera: Aphididae). Symbiosis 49, 53–59 (2009). [Google Scholar]
- 23.Ma G., Rudolf V. H., Ma C. S., Extreme temperature events alter demographic rates, relative fitness, and community structure. Glob. Change Biol. 21, 1794–1808 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Pritchard J. E., Vickers L. H., “Aphids and stress” in Aphids as Crop Pests, van Emden H. F., Harrington H., Eds. (Wallingford Oxfordshire, ed. 2, 2017), pp. 132–147. [Google Scholar]
- 25.Blackman R. L., Eastop V. F., Aphids on the World’s Crops: An Identification and Information Guide (John Wiley & Sons, Chichester, UK, ed. 2, 2000). [Google Scholar]
- 26.Narberhaus F., α-crystallin-type heat shock proteins: Socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 66, 64–93 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb R. J., MacKay P. A., Gerber G. H., Are development and growth of pea aphids, Acyrthosiphon pisum, in North America adapted to local temperatures? Oecologia 72, 170–177 (1987). [DOI] [PubMed] [Google Scholar]
- 28.Heie O. E., Why are there so few aphids in the temperate areas of the southern hemisphere? Eur. J. Entomol. 91, 127–133 (2013). [Google Scholar]
- 29.Perovsky E., Wegierek P., Aphid-Buchnera-ant symbiosis; or why are aphids rare in the tropics and very rare further south? Earth Environ. Sci. Trans. R. Soc. Edinburgh 107, 297–310 (2018). [Google Scholar]
- 30.Fukatsu T., Aoki S., Kurosu U., Ishikawa H., Phylogeny of Cerataphidini aphids revealed by their symbiotic microorganisms and basic structure of their galls: Implications for host-symbiont coevolution and evolution of sterile soldier castes. Zool. Sci. 11, 613–623 (1994). [Google Scholar]
- 31.Chong R. A., Moran N. A., Evolutionary loss and replacement of Buchnera, the obligate endosymbiont of aphids. ISME J. 12, 898–908 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koga R., Moran N. A., Swapping symbionts in spittlebugs: Evolutionary replacement of a reduced genome symbiont. ISME J. 8, 1237–1246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett G. M., Moran N. A., Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc. Natl. Acad. Sci. U.S.A. 112, 10169–10176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandström J. P., Russell J. A., White J. P., Moran N. A., Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10, 217–228 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Koga R., Tsuchida T., Fukatsu T., Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl. Entomol. Zool. 44, 281–291 (2009). [Google Scholar]
- 36.Koga R., Meng X. Y., Tsuchida T., Fukatsu T., Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacteriocyte-embryo interface. Proc. Natl. Acad. Sci. U.S.A. 109, E1230–E1237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.RStudio Team , RStudio: Integrated Development for R (Version 0.98.1074, RStudio, Inc., Boston, MA, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequence data are available at National Center for Biotechnology Information. Buchnera genome sequences for Ap. fabae and Ap. gossypii are deposited under accession numbers CP042426 and CP042427. RNA-Seq data are deposited under BioProject ID PRJNA577642, SRA accession numbers SRR10291791–SRR10291814.