Significance
Social organisms that share resources must identify their kin to avoid exploitation by nonself competitors; however, underlying mechanisms to explain discrimination are lacking. Myxobacteria, which aggregate into tissue-like groups, use a 2-step self-identification mechanism in which cells interact by a highly variable cell surface receptor that catalyzes cellular cargo exchange. This cargo includes polymorphic toxins that poison nonclonal cells, which lack specific immunity genes. Here, we identified 6 unique families of toxins that are strikingly numerous in myxobacterial genomes. Together, arrays of toxins form what we describe as self-identity barcodes that exquisitely distinguish clonal cooperators from nonself. This work highlights how selfish and discriminating genes, which expand in vast combinations in bacterial genomes, help to diversify and insulate social groups.
Keywords: kin recognition, polymorphic toxins, outer membrane exchange, myxobacteria
Abstract
Myxobacteria are an example of how single-cell individuals can transition into multicellular life by an aggregation strategy. For these and all organisms that consist of social groups of cells, discrimination against, and exclusion of, nonself is critical. In myxobacteria, TraA is a polymorphic cell surface receptor that identifies kin by homotypic binding, and in so doing exchanges outer membrane (OM) proteins and lipids between cells with compatible receptors. However, TraA variability alone is not sufficient to discriminate against all cells, as traA allele diversity is not necessarily high among local strains. To increase discrimination ability, myxobacteria include polymorphic OM lipoprotein toxins called SitA in their delivered cargo, which poison recipient cells that lack the cognate, allele-specific SitI immunity protein. We previously characterized 3 SitAI toxin/immunity pairs that belong to 2 families. Here, we discover 4 additional SitA families. Each family is unique in sequence, but share the characteristic features of SitA: OM-associated toxins delivered by TraA. We demonstrate that, within a SitA family, C-terminal nuclease domains are polymorphic and often modular. Remarkably, sitA loci are strikingly numerous and diverse, with most genomes possessing >30 and up to 83 distinct sitAI loci. Interestingly, all SitA protein families are serially transferred between cells, allowing a SitA inhibitor cell to poison multiple targets, including cells that never made direct contact. The expansive suites of sitAI loci thus serve as identify barcodes to exquisitely discriminate against nonself to ensure populations are genetically homogenous to conduct cooperative behaviors.
Multicellular organisms or groups of social cells need to identify clonal cells to coordinate specific behaviors and allow resources to be directed toward them. Central to understanding these fundamental processes is identifying the proteins involved in self/nonself-recognition and the mechanisms individuals use to discriminate against nonkin to form cohesive and harmonious populations. Myxobacteria represent tractable model systems to study how kin recognition evolves and functions at a molecular level. Myxobacterial cells typically live in social groups in the soil, where they move and feed on prey microbes. When nutrients are depleted, they undergo a synchronized, cooperative developmental program culminating in the formation of a multicellular fruiting body that harbors dormant spores. Cooperating with kin cells while excluding incompatible individuals is imperative for them to maintain a viable social network. During vegetative growth, cells maintain close contacts as they move past one another by gliding motility. Upon each physical contact, cells monitor the identity of their neighbors by homotypic interactions of a highly polymorphic cell surface receptor called TraA, along with its partner protein TraB (1–3). When neighboring cells have identical or matching TraA receptors, they exchange large amounts of cell envelope material in a process called outer membrane exchange (OME). OME can be directly visualized microscopically by rapid and efficient cell-to-cell transfer of outer-membrane (OM) fluorescent reporters (4, 5). TraA/B are dynamic OM proteins, and, when 2 compatible cells touch, multiple receptor complexes from each cell coalesce into distinct foci that bridge the boundary between the 2 cells. This transient interaction culminates in an apparent membrane fusion and bidirectional transfer of proteins and lipids before cells separate by gliding motility (5–7). This striking and robust behavior is thought to help rejuvenate and maintain homeostasis of the cell envelope in a population that ages or encounters insults in constantly fluctuating environments (8, 9).
In nutrient-rich soils, myxobacteria populations are numerous and diverse (10, 11). Local strains compete with each other and must establish and maintain a group identity by recognizing and cooperating with kin while excluding nonkin. TraA serves as one self-recognition determinant by binding to cells with matching receptors (2, 12). Sequence polymorphisms within the TraA variable domain, which determines recognition specificity, is high, and prior studies with a limited allele set experimentally determined or predicted >60 distinct TraA recognition groups (3). However, analysis of TraA allele variation between Myxococcus xanthus strains that are colocalized in the soil revealed that some divergent strains are in fact compatible for OME (2, 13). In other words, TraA is not always sufficient to discriminate between clonal cells and competitors. This suggests that myxobacteria have additional mechanisms to identify clonemates. Indeed, to increase specificity of OME beyond TraA–TraA interactions, there is a second authentication or discrimination step. OM-localized polymorphic toxins are included among the wide array of cell envelope cargo that is delivered during OME (14).
Polymorphic toxin/immunity pairs are ubiquitous in microbial genomes and provide a means to exclude nonkin from clonal populations (1, 15). Toxins typically consist of a domain that facilitates delivery of a C-terminal (CT) toxin domain, which causes growth inhibition or death of a susceptible cell that receives it. Immunity genes, almost always encoded next to the toxin, provide allele-specific protection from the toxic activity. These systems can diversify by amino acid changes in residues involved in the molecular recognition between the toxin and the immunity proteins, resulting in polymorphisms and the formation of new toxin/immunity specificity pairs (16). As microbial strains diversify, so too do their toxin repertoires, and horizontal gene transfer (HGT) plays a major role in toxin/immunity dissemination and diversification between populations (15, 17, 18). Further, toxins involved in interstrain warfare often have a modular architecture in that diverse toxin domains are found at the C terminus of a particular delivery domain and appear to be mixed and matched by recombination between various delivery systems (15). In consequence, organisms can encode an array of unique toxin/immunity pairs that together facilitate intergenomic conflict. A number of functionally diverse delivery mechanisms are known, such as the type-6 secretion system (T6SS) and contact-dependent inhibition (CDI), which deliver their toxic domains via a needle-like secretion machine or a large, extended filament structure, respectively (19–21). Additionally, new polymorphic toxins and delivery systems continue to be discovered (14, 22–27).
We recently described a polymorphic toxin system in myxobacteria that is delivered by OME between cells with compatible TraA receptors (Fig. 1A). These proteins, called SitA, are lipoproteins that reside in the OM and contain CT nuclease domains (14). Once delivered to the target cell OM by OME, SitA must traverse the cell envelope, by an unknown mechanism, to reach the cytoplasm. There, the CT nuclease causes target cell death. Target cells that express the allele-specific, cognate immunity protein, called SitI, are protected (14). sitA and sitI genes are always adjacent in myxobacterial genomes. SitA, or swarm inhibition toxin A, is so named because it was discovered as an effector that prevented outward swarming in a recipient strain during a 2-strain coculture (7, 28). That is, nonmotile M. xanthus mutants that express SitA inhibit the outward swarming of susceptible motile cells that lack the corresponding SitI (14). Swarm inhibition is therefore caused by poisoning of the motile strain before outward swarming occurs. A ∆traA mutation in either the nonmotile inhibitor or the motile target strain blocks toxin transfer, and therefore swarm expansion is restored to the motile strain. Thus, SitA transfer is traA-dependent. Furthermore, the SitA proteins are polymorphic, and some myxobacteria encode multiple sitA alleles per genome. The requirement that a SitA recipient have a compatible traA allele suggests that SitA functions as a kin discrimination factor. Therefore, sitAI loci act as a verification step to ensure OME is occurring among clonal cells (14). Unique among delivery systems, SitA transfer is infectious within a population because the toxins can be serially transferred between cells by multiple OME events. Consequently, a single SitA-producing cell can kill many target cells as the toxin pool rapidly spreads throughout the population (14).
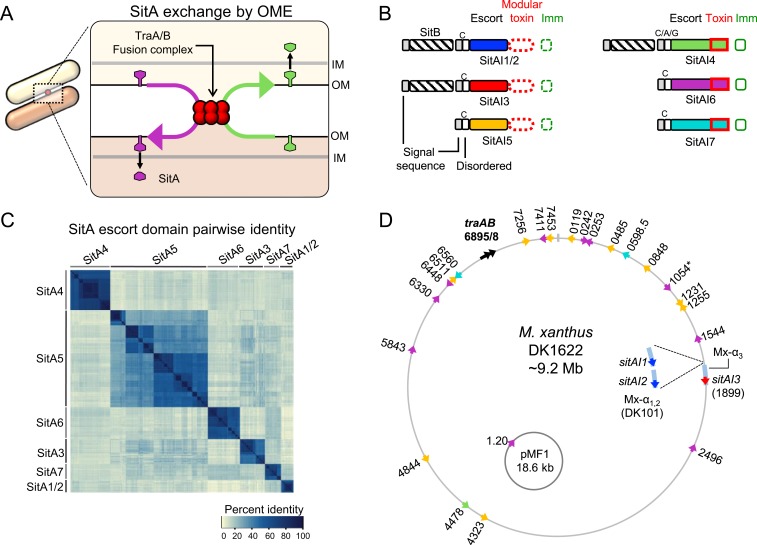
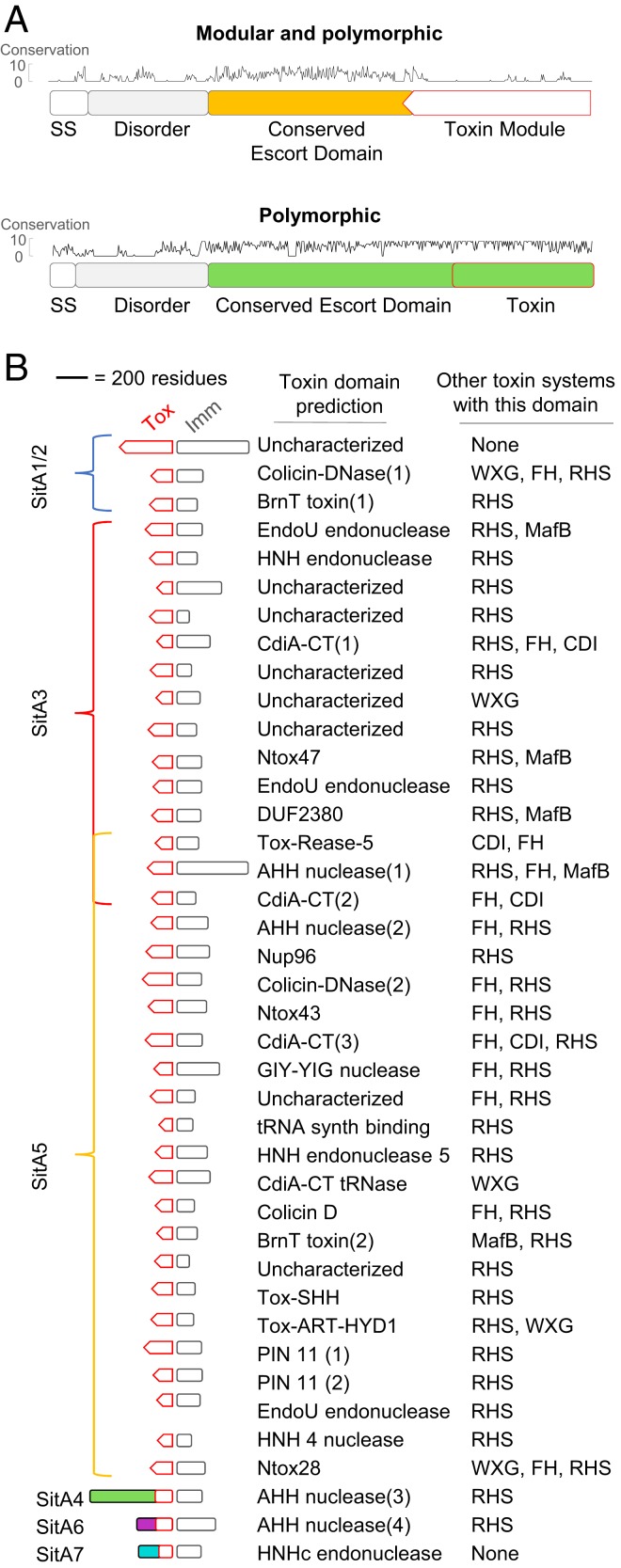
Fig. 1.
Four newly discovered SitA families. (A) Model for exchange of SitA by OME. Two adjacent cells with compatible TraA receptors form a TraAB OM fusion junction. Subsequently, transient membrane fusion allows the passive diffusion of SitA toxins between cells. SitA then traverses the cell envelope to access the cytoplasm, where they either act as nucleases or are inactivated by their allele-specific SitI immunity protein. (B) Overview of the domain organization of SitBAI and SitAI families. SitA families are classified into 2 groups by whether they have a modular toxic CTD (red dashed boxes) or a single polymorphic toxin domain (solid red boxes). Colors correspond to unique escort domains belonging to each family. Invariant lipobox cysteine (“C”) residues or alternative residues (in SitA4) are shown. Genes not to scale; Fig. 5 provides more detail. (C) Pairwise percent identity matrix of Myxococcaceae proteins from each SitA family. SitA proteins belonging to one family share homology with each other but not with proteins of a different SitA family. (D) Locations of sitA loci on the lab strain M. xanthus chromosome and the pMF1 plasmid from M. fulvus 124B02. DK101 is a parent strain of DK1622 that contains a ∼200-kb region of Mx-alpha that was spontaneously lost during the construction of DK1622, which harbors 2 additional sitA alleles (Inset). Numbers correspond to MXAN locus tags. Colors indicate SitA family based on the color scheme in B. Each sitA gene contains a cognate downstream sitI gene (not pictured). *MXAN_1054 has a frame-shift mutation.
In our prior study, 3 sitAI loci were found in the domesticated lab strain (SitA1, SitA2, and SitA3) (14). Interestingly, all 3 loci reside in similar relative locations on 3 tandem but divergent repeats of ∼100-kb prophage-like elements (∼300 kb total) (14, 28). Historically named “Mx-alpha,” these defective prophage produce nonvirulent transducing particles, suggesting that HGT plays a role in acquiring new sitAI loci (14, 29). SitA1 and SitA2 share homologous central “escort” domains, but have CT nuclease modules that share no sequence similarity. The escort domains are thought to facilitate CT toxin delivery into the cytoplasm following delivery to the OM of the target cell. sitA3 is similar to the sitA1/2 genes in that it encodes a lipoprotein toxin delivered by OME, contains a modular CT toxin domain, and is associated with a downstream sitI3 immunity gene. Interestingly, however, SitA3 shares no sequence homology with SitA1 and SitA2. Each of the 3 sitA loci have an overlapping homologous upstream open reading frame (ORF) called sitB, whose gene product enhances the ability of SitA to kill target cells, but is not essential for SitA function (14). SitB contains a signal sequence, and is predicted to form a β-barrel in the OM, but has no clear homology to any characterized domains. Since SitA1/2 and SitA3 are not homologous, it raises the possibility that there are other SitA proteins that share similar function and delivery mechanism but do not necessarily share sequence homology. Here, we describe the discovery and characterization of 4 additional families of SitAI that belong to the overarching SitA class of proteins. We demonstrate that SitAI4, SitAI5, SitAI6, and SitAI7 are OME-dependent toxin families that are strikingly numerous and diverse within myxobacterial genomes predicted to contain functional TraAB proteins. Remarkably, some myxobacterial genomes contain >80 total sitAI loci. Within each family, CT toxin domains and SitI immunity proteins are highly diverse and are often modular. Many of these toxin and immunity domains are previously uncharacterized, but, also, many are conserved in other polymorphic toxin delivery systems, such as CDI and RHS (30), found in diverse taxa. Our discovery greatly expands the known myxobacterial toxic SitA arsenal that contributes to kin discrimination following the mutual decision of partner cells to engage in OME. We suggest that the plethora of polymorphic SitA toxins act as self-identify barcodes to help sequester the cooperative behavior of OME to clonal cells.
Results
Identification of Six Families of SitAI Proteins.
The previously characterized sitA gene families (sitA1/2 and sitA3) are each typically associated with an overlapping upstream sitB gene. Although these SitA families have negligible homology to each other, their upstream SitBs are homologous. To investigate whether other sitBAI loci reside in myxobacterial genomes, we performed BLAST analysis and determined that there is another family of genes associated with sitB homologs. This family, designated SitA4, also contains a lipoprotein signal sequence, an AHH nuclease CT domain found in other toxin systems, and a downstream putative immunity gene. Its central escort domain, however, shared no homology with SitA1/2 or SitA3. This suggested that SitA4 may be a new, independent family of sitA genes that may share a similar function.
To determine if myxobacteria encode other potential OME-dependent toxins, we used various HHM profiles (Pfams [31]) of known toxin or immunity domains (15, 32) as queries to probe publically available Myxococcales genomes for remote homology. Further, we searched for putative toxins that contain lipoprotein signal sequences, a hallmark of OME cargo. These efforts returned multiple conserved hypothetical gene families with similarity to conserved nucleases and immunity domains or otherwise conspicuous gene pairs. We further narrowed our search by focusing on gene families that were well-conserved in a subset of myxobacteria yet had obvious polymorphic regions when aligned by sequence homology. Many of these resulting gene candidates encode N-terminal lipoboxes within their signal sequence. Since OME delivers cell envelope proteins, we focused our attention on only those containing signal sequences. From the NCBI (33) and IMG databases (34), the ORF start codon was frequently incorrectly assigned by automated algorithms, and therefore many signal sequences were only found by manual curation for the correct start codon. In total, these efforts returned 3 additional families of potential SitAI toxin/immunity pairs that we predicted function like other SitAs. However, these families were never found with an upstream sitB gene. Hereafter, we refer to these gene families as SitA5, SitA6, and SitA7, representing the order in which they were discovered. An overview of the characteristic gene and domain architecture of the predicted SitAI families is shown in Fig. 1B. We define a “family” of SitA as a group of genes that share conservation in their central escort domain (colored domains in Fig. 1B). Importantly, the escort domain of a SitA family does not share significant homology with the escort domain of another family (Fig. 1C), again suggesting that there may be multiple SitA gene families that share a similar function, but no sequence homology. Each family had multiple representative genes in myxobacterial genomes. For example, the parent of the lab strain M. xanthus DK1622 has 25 total sitAI loci, which represent all 6 families (Fig. 1D). Additionally, we found a sitAI6 cassette on the only known replicating plasmid in myxobacteria, called pMF1 (35), harbored within strain Myxococcus fulvus 124B02 (Fig. 1D), a loci that was previously shown to be a toxin/immunity pair (Fig. 1D) (36). Interestingly, this gene pair is the only annotated feature on one strand of the plasmid DNA. The other strand codes for the remaining 21 predicted ORFs (37).
We previously showed that the SitA1/2 and SitA3 families have modular CT domains, meaning diverse and distinct toxin domain modules are found at the C terminus, following the conserved escort domain (Fig. 1B). By comparing genes within the newly discovered families, we found that SitA5 proteins are also modular. SitA4, 6, and 7 proteins are not modular; instead, they are polymorphic over the length of the proteins. A more detailed analysis of domain architecture and conserved domains of the 6 SitA families is described later. Our analysis brings the total number of putative SitA families to 6, with SitA1 and SitA2 constituting a single family, “SitA1/2,” based on their homologous escort domains.
The Six Families of SitAI Are OM-Associated Toxins Delivered by OME.
To experimentally probe the function of the 4 putative SitA toxin families, we cloned representative sitAI cassettes from M. fulvus HW-1 (sitAMf) into the M. xanthus chromosome. Importantly, each of these gene cassettes was divergent from those found in the M. xanthus genome. For example, the closest homolog of SitA5 from M. fulvus in M. xanthus was 44.4% identical. Each of these constructed inhibitor strains were then competed with a WT (“target”) strain, which lacked the corresponding sitAI cassette, but contained identical traA alleles, in a one-to-one coculture on solid agar growth media. CFUs of the target strain were determined following 0, 6, and 24 h of coincubation. In all cases, the CFUs of the target strain were dramatically lowered compared to mock-inhibitor control at the 6- and 24-h time points (Fig. 2A). In order to test if these proteins were dependent on OME for delivery, we repeated the CFU competitions using an isogenic ∆traA mutant as the target strain. As mentioned, a traA mutation in one or both strains abolishes transfer because OME is a mutual decision where both cells must have compatible TraA receptors. Importantly, ∆traA targets were all immune from antagonism, as their CFU output was similar to the control (Fig. 2A). Taken together, these data suggest that, like SitA1/2 and 3, the additional families of SitA are toxins delivered by OME.
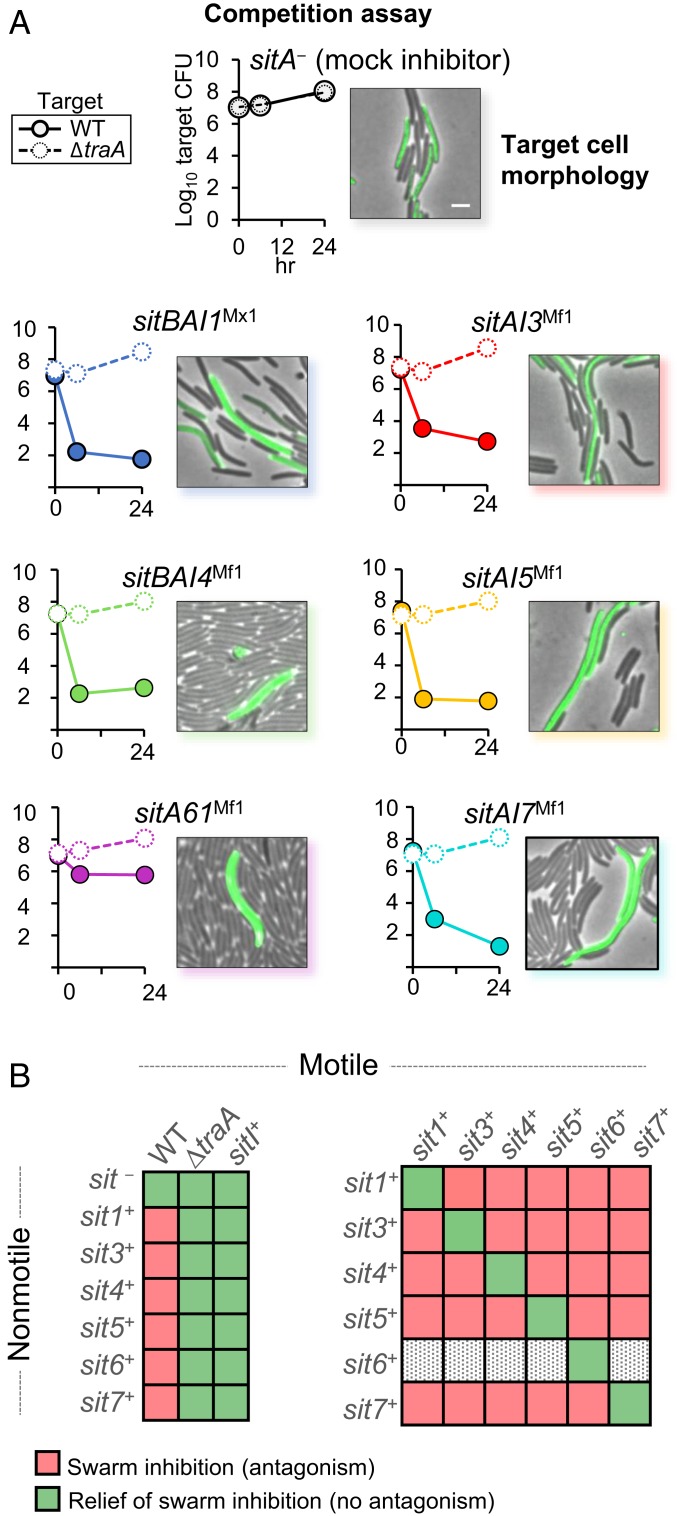
Fig. 2.
Expression of heterologous sitAI cassettes results in traA-dependent target cell death. (A) CFU time course of target cells from agar plates when competed against SitAI-expressing inhibitors. Open circles and dashed lines indicate that target cells are ∆traA; closed circles and solid lines indicate that target cells are traA+. Adjacent image shows the morphology (filamentation) of fluorescently labeled traA+ target cells after coincubation with SitA+ inhibitor cells. (Scale bar, 5 μm.) (B) (Left) Results of swarm inhibition coculture experiments in which a nonmotile inhibitor strain expresses a unique sitAI cassette and susceptible motile target cells are traA+, ∆traA, or SitI+. Strains were mixed at a 1:1 ratio with the exception of SitA6Mf1 inhibitors, which were mixed at 5:1. (Right) Results of swarm inhibition cocultures of SitAI-expressing nonmotile and SitAI-expressing motile strains at an 8:1 cell ratio. SitAI6Mf1-expressing nonmotile inhibitors were excluded because they were always killed by the opposing motile strain and never caused swarm inhibition. SI Appendix, Fig. S1, provides micrographs of these results. All strain, plasmid, and primer details are given in SI Appendix, Tables S1–S3.
Prior work showed that SitA antagonism leads to morphological changes in the target cells (14). To observe target cells during coincubation, we competed the above inhibitor strains with a fluorescently labeled target strain on agarose pads. Along with causing cell lysis, each coincubation resulted in dramatic filamentation, enlargement, and lysis of the fluorescent target cells (Fig. 2A). Mean cell lengths of control cells were 6.05 µm (SD = 1.38, n = 50), whereas the mean cell length of SitA-poisoned cells was 14.38 µm (SD = 4.58, n = 200). These results further suggest that delivery of the newly discovered SitA proteins is toxic to recipient cells that lack the matching SitI immunity protein.
As noted earlier, one outcome of SitA-mediated antagonism is the ability of a nonmotile, SitA-producing strain to inhibit the outward swarming of a SitA-susceptible motile strain when the 2 strains are cultured together on an agar surface. To test whether, like SitA1/2 and 3, the newly described SitA families caused a swarm inhibition phenotype, we expressed the various SitAI loci from M. fulvus in a nonmotile M. xanthus strain. Resulting cocultures between the nonmotile inhibitor and susceptible motile targets that lacked immunity showed swarm inhibition of the motile strain that was dependent on TraAB (Fig. 2B and SI Appendix, Fig. S1A). SitA6 inhibitors exhibited the weakest antagonistic phenotype. It was therefore necessary to increase the nonmotile to motile cell ratio to 5:1 to achieve swarm inhibition in these mixtures. All other assays were conducted at a 1:1 ratio.
SitI proteins have predicted immunity functions, and we previously showed that SitI1, SitI2, and SitI3 selectively protect target cells from death by their cognate SitA (14). To probe the function of SitI4 to SitI7, we repeated the swarm inhibition assay using motile target strains that expressed the same SitAI operon as the nonmotile inhibitor strain. Expression of the operon resulted in relief of swarm inhibition and hence protected the motile strain from toxicity (Fig. 2B and SI Appendix, Fig. S1A). To test for specificity between the various SitAI systems, we then competed all combinations (except as noted later) of nonmotile and motile strains together. Unlike the previous assay, these combinations can involve reciprocal antagonism. Therefore, we gave the nonmotile strain a starting advantage with a cell ratio of 8:1. The motile strain in these combinations escaped swarm inhibition only when both strains expressed the same sitAI cassette. Otherwise, when strains expressed different cassettes, there was reciprocal antagonism and complete swarm inhibition (Fig. 2B and SI Appendix, Fig. S1B). The nonmotile SitAI6 inhibitor was excluded from this assay because the SitA6Mf1 toxins were too weak and thus the motile strain easily outcompetes and escapes swarm inhibition, even at the 8:1 starting ratio. Together, these experiments demonstrate that the newly described SitAI cassettes code for toxic proteins delivered by OME and that expression of these cassettes confer gene-specific immunity in target strains.
As mentioned earlier, a sitAI6 cassette was found on the 18.6-kb autonomously replicating plasmid pMF1 from M. fulvus 124B02 (aka pMF1.20 and pMF1.19 genes). We cloned and recombined this operon into the genome of M. xanthus and found that this resulting strain also reduces CFUs of a target strain in a TraA-dependent manner (SI Appendix, Fig. S2). Although the pMF1.20 was noted previously to be a toxin family distributed in myxobacteria (36), we suggest adopting the name SitAI6 based on these proteins having a similar function and delivery mechanism as other SitA family members.
Interestingly, although all other SitA proteins have an invariant cysteine residue in their lipobox and a sorting sequence that indicates OM localization, many sitA4 alleles do not, revealing that SitA4 proteins are not strictly lipoproteins. For example, 3 of 5 sitA4 genes in M. fulvus HW-1 do not have a cysteine in their signal sequence. Nevertheless, SitA4Mf1 is functional and TraA-dependent (Fig. 2). To this point, OME has been observed for OM-localized proteins and lipids, including those that contain a type I signal sequence and hence are not lipoproteins (4, 38). To confirm whether other SitA4 proteins that do not have a lipobox are functional, we cloned and expressed a separate sitAI4 locus from M. fulvus HW-1 (sitAI4Mf2), whose SitA also lacks a cysteine in its signal sequence. This resulting strain also reduced target strain CFUs in a TraAB-dependent manner (SI Appendix, Fig. S2), suggesting that SitA4 proteins that are not lipidated are nevertheless transferred. Further experiments are needed to test the subcellular localization of SitA4 proteins and whether they are soluble periplasmic proteins that are delivered by OME. In addition, sitAI4Mf2 does not have a cognate sitB gene upstream. Therefore, like SitA1 and SitA3, a cognate SitB4 protein is not required for SitA4 function (14). Results from competition experiments with 3 additional heterologous sitAI cassettes are also shown in the SI Appendix, Fig. S2.
Serial Transfer of SitA Toxins.
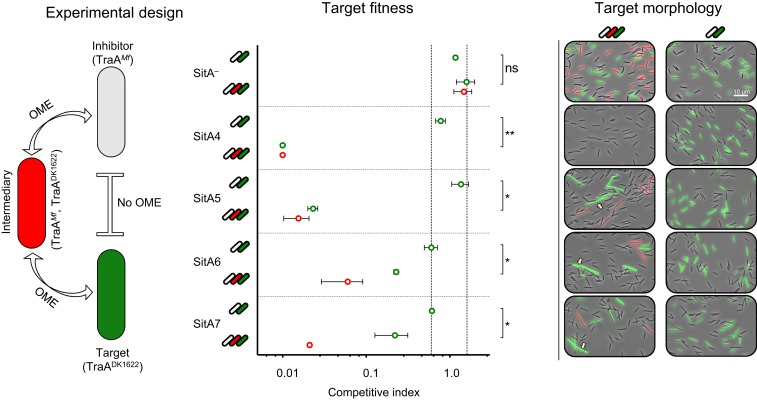
We previously showed that the unique delivery system of OME led to serial transfer of SitA1 (14). In other words, cells infected with SitA proteins in their OM by OME act as carriers that spread the toxin to neighboring cells by subsequent OME events. In this way, toxin-producing cells poison target cells that they never made direct contact with. We therefore tested whether this attribute applies to the families of SitA described here. To test this, we used a 3-strain coculture composed of an isogenic inhibitor and target strain that have incompatible traA alleles and a third-party intermediary strain that expresses both traA alleles (Fig. 3). Therefore, for the inhibitor strain to deliver SitA to the target strain, OME must first occur with the traA merodiploid intermediary that acts a conduit for SitA delivery to the target. Importantly, we previously demonstrated that TraA itself does not transfer since it is apparently anchored to the cell envelope (5, 14), and therefore serial transfer cannot be explained by exchange of TraA mediating direct delivery between producer and target. As a control, for each SitA inhibitor strain, we competed the inhibitor and target without the intermediary strain and found that there was no antagonism and no morphological changes to the target cells because OME cannot occur between these stains (Fig. 3). In contrast, when the intermediary strain was included, the competitive index of the target cells drops dramatically, and target cells become filamentous and lyse (Fig. 3). Therefore, as was shown with SitA1, the newly characterized SitAs were all serially transferred, leading to target cell death dependent on the presence of the intermediary strain.
Fig. 3.
New SitA families are serially transferred. Experimental design: cell types are labeled where the SitAI+ inhibitor cell expresses an incompatible traA allele with the target cell and therefore cannot engage in OME. The intermediary cell expresses both traA alleles and thus can engage in OME with both strains to serve as a conduit for serial transfer. Intermediary cell is also susceptible to the inhibitor cell toxin. Target fitness: competitive index of target (green data points) and intermediary cells (red data points) in the 2-strain and 3-strain cocultures when the inhibitor cells express one of the SitA toxin families or is an isogenic SitA− control. Competitive index is a measure of the target-to-inhibitor cell ratio at 24 h normalized to the ratio at 0 h as enumerated by fluorescent microscopy. Starting ratios are 1:1 and 1:1:1 with the exception of SitA6 inhibitors, which were mixed at 3:1 and 3:1:1 ratios. Area between vertical dashed lines indicates experiments in which target cells were not killed and were not filamentous as observed by the target morphology panel. Error bars indicate SE, and significance indicators show results from a 2-tailed nonparametric t test. ns, not significant (*P < 0.05, **P < 0.01). Target morphology: concurrent micrographs of the quantified experiments show that, in the 3-strain coculture, green fluorescent target cells are either eliminated or show filamentation and morphological defects (arrows). In the 2-strain cocultures, green cells appear normal. (Scale bar, 10 µm.)
Phylogenetic Distribution of SitAI Proteins.
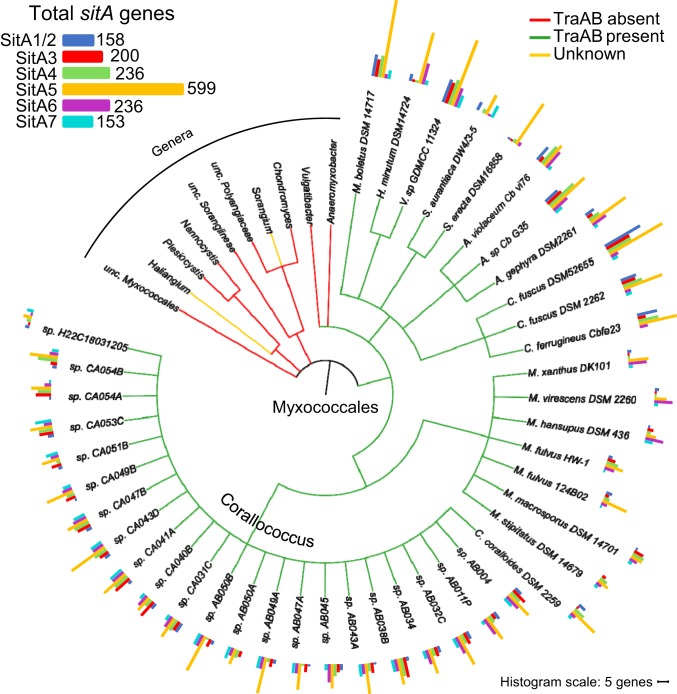
To determine the distribution of SitA toxins, we used representative SitA proteins as queries to BLAST the NCBI nonredundant database. We collected sequences that aligned over the conserved central domain of the query SitA and organized the sequences based on phylogeny. We found that SitA proteins were restricted to the Cystobacterineae suborder of the Myxococcales and were not found outside of myxobacteria (Fig. 4). Further, genomes that are predicted to contain functional traAB genes all contained a collection of sitA genes (Fig. 4, green branches [3]). No sitA genes were found in suborders of Myxococcales that do not have TraAB homologs (Fig. 4, red branches). The cooccurrence of TraAB and SitA further supports that SitAs are delivered by OME and function as a kin discrimination determinant to differentiate OME partners. Distant TraAB homologs are found in clades outside of the Cystobacterineae (Fig. 4, yellow branches), but OME has not been demonstrated with these strains or TraAB homologs (3). Consistent with this, no sitA genes were found in these genomes, suggesting that these distant TraAB homologs may have a different function from OME. Interestingly, the majority of genomes contain sequences for a striking number of distinct sitA genes, with the average number of loci per genome being 38 and the number of genes per genome ranging from 13 in Corallococcus sp. H22C18031205 to 83 in Cystobacter fuscus DSM 52655 (Fig. 4 and SI Appendix, Table S4).
Fig. 4.
sitA genes are numerous in myxobacteria that contain functional TraAB proteins. Tree depicting the NCBI taxonomic organization of sequenced Myxococcales genomes and the distribution of TraAB and SitA. Species that have TraAB homologs are depicted with green branches, whereas genera that do not have TraAB or have distant TraAB homologs that may not function in OME are depicted with red and yellow branches, respectively. Further detail is provided in the text. Number and family designation of all sitA alleles are depicted by colored histograms adjacent to the species names. Scale bar indicates a histogram bar length of 5 genes per genome. SI Appendix, Table S4, provides corresponding numbers of genes per genome. Total number of loci in 41 SitA-containing genomes is shown at upper left.
Sequence Analysis of SitAI Toxin and Immunity Domains.
As noted earlier, the SitA1/2 and SitA3 family toxins contain a conserved escort domain and a polymorphic and modular CT toxin domain. When distinct SitAs do share a modular CT toxin domain, they contain polymorphisms. To characterize all of the domains within SitAI families, we undertook a comprehensive analysis of their domain architectures using the described collection of Myxococcales sequences. First, we aligned the sequences of each family and looked for modularity at the C terminus. We found that, in addition to SitA1/2 and SitA3 families, SitA5 contains a modular C-terminal domain (CTD), as conservation clearly drops following the conserved escort domain (Fig. 5A). SitA4, SitA6, and SitA7 were not modular. For example, unlike the modular SitAs, all SitA4 sequences align at their CT but are enriched in substitutions along the length of the protein. It is therefore likely that polymorphisms at the CT domains are responsible for the specificity of interaction between these SitAs and their cognate SitI proteins. To illustrate these fundamental differences between modular and nonmodular toxins, alignments of representative alleles of SitA4 and SitA5 are shown in Fig. 5A. Also of note in this figure, between the signal sequence and the conserved escort domain, we typically found a nonconserved region that is predicted to be intrinsically disordered.
Fig. 5.
Domain organization and bioinformatic prediction of SitAI functions. (A) Domain organization of representative sequences of a modular and polymorphic SitA family (SitA5) and a polymorphic family (SitA4). Note that the SitA5 family has a nonconserved CTD, i.e., different domain modules compared to the conserved CTD of SitA4. Conservation scores are based on an algorithm that takes into account conservation of physicochemical properties of individual residues (52). Proteins not shown to scale. (B) Functional prediction of all uniquely clustering toxin domains found in Myxococcales sitA alleles by HHpred. The top relevant domain hit is shown, with preference given to Pfam domains deposited in comprehensive domain analyses (15, 32). Search criteria are described in Materials and Methods, and additional details, including HHpred probability score, domain size, and SitI functional predictions, are provided in SI Appendix, Table S5. Domains are shown to scale. Domains followed by a number are to distinguish those that share a common domain prediction despite having very little sequence similarity.
To characterize the toxin and immunity domains of each modular SitA family, we clustered their sequences by similarity and found they formed distinct groups with <20% amino acid identity between clusters (Fig. 5B). Therefore, each cluster is considered an independent toxin module. Consistent with this, each member of a CTD module cluster is associated with a similar but distinct downstream immunity protein (SI Appendix, Table S5). Interestingly, 3 toxin/immunity domain modules were shared between SitA3 and SitA5 families (Fig. 5B), suggesting that recombination occurred between sitA3 and sitA5 loci and/or these domains were acquired independently from common ancestral sequences.
Next, we used HHpred (39) as a sensitive search tool to find homology to conserved domains in the PDB (40), Pfam (31), and NCBI CDD (41) databases for each of the CTD and immunity protein domains. Most of the toxin domains had remote homology to conserved nuclease domains (Fig. 5B). Since HHpred could detect remote homology between SitA-CT and conserved domains, direct sequence similarity was not always conserved, and often sequences were only partially aligned over the SitA CT query. Therefore, although some SitA CT and immunity domains returned hits to conserved domain superfamilies, they appear to belong to more distinct subclasses of these domains. For this reason, although some CT/immunity domains have the same name, they are, in fact, phylogenetically very distinct (Fig. 5B and SI Appendix, Table S5). In addition, a number of the SitA CT and SitI domains did not return hits and were thus uncharacterized. To determine if these uncharacterized CT module domains and their corresponding immunity proteins were found in other organisms, we used BLAST to search against sequences outside of the Myxococcales order (E < 10−05 over 80- to 200-residue cutoff). Interestingly, nearly all of the CTD and immunity sequences were found adjacent to one another in other genomes. Further, these CTD sequences were always found as CTD toxin modules to characterized toxin systems such as RHS, CdiA, WXG, MafB, and filamentous hemagglutinin (FH) in diverse taxa (Fig. 5B). Our sequence searches also returned other uncharacterized toxins that had toxin processing sites such as HINT, PsrW, and pretoxin-TG domains. SitAI1/2 and SitA7 CTDs were the only cases in which the toxin and immunity domains were exclusively found in myxobacteria, but these SitAs are numerous and were experimentally characterized (14). Many toxin CTDs and their corresponding immunity proteins were only found once or twice in SitA sequences, but numerous homologous domains were found at the CT of other toxin systems in organisms quite distant from myxobacteria (SI Appendix, Table S5). This implies that these are in fact functional domains conserved in a wide range of phylogenies, despite being either rare in SitA proteins or simply underrepresented in publically available myxobacterial genomes. A number of the domains we identified were listed as domains of unknown function (DUF), and this study thus provides insight into their function. The conserved escort domains did not show strong similarity to any conserved domains, with the exception of SitA6, which was classified as TIGR02269 (PF14412), previously recorded as a family with 9 paralogous lipoproteins in M. xanthus, all of which we classified here as SitA6 family members (SI Appendix, Table S5). DUF2380 also describes a group of paralogous lipoproteins with AHH nuclease domains (SitA4 and 6 and some SitA5 modules; Fig. 5B and SI Appendix, Table S5).
Variation of sitA Genes between Related Genomes Establishes Self-Identity Barcodes.
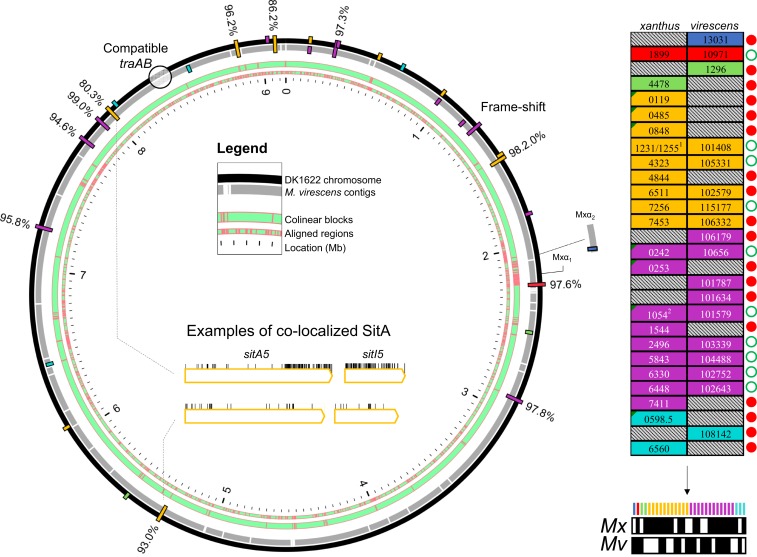
The finding that myxobacterial genomes possess high numbers of sitA genes suggests that any 2 myxobacteria that employ this system are unlikely to possess immunity to one another’s full complement of toxins. As a result, 2 strains or species that are compatible for OME would likely inhibit one another upon contact. To explore this concept, we conducted whole-genome comparisons of sitA genes between M. xanthus DK1622 and a related strain, Myxococcus virescens DSM 2260. We chose these 2 genomes because their traA alleles are highly similar (97.36% amino acid identity) and are predicted to be compatible for OME (3). Despite having different species designations, these strains are in fact closely related, with 99.93% identity at the 16S rRNA locus. We used progressiveMauve (42) to align the 2 genomes and looked at sitAI loci variation. Twelve sitAI loci were shared between the 2 strains, meaning that they are found on aligned regions with perfect gene synteny (Fig. 6). Ten of these 12 loci were very similar in sequence, ranging from 93 to 99% amino acid identity. At one such matching locus, sitA6 of DK1622 had acquired a frame-shift mutation, but the downstream sitI was intact and 97% identical to its SitI ortholog in M. virescens. In contrast, 2 sitAI5 loci were perfectly colocalized between the 2 strains but had a significant number of substitutions enriched in the CTD of SitA and in SitI (example shown in Fig. 6). Beyond the orthologous matching loci, there were 15 individual sitAI operon pairs that were located on genomic islands present in one genome but not the other. Together, these results suggest that a small proportion of sitAI loci diversify much faster than others, and that incompatibilities between strains are mostly derived from horizontal acquisition of new loci and gene loss rather than diversification at existing loci.
Fig. 6.
Comparative analysis of sitA genes from Myxococcus strains that are compatible for OME. (A) Whole-genome alignment depicts conserved and unique SitA toxins between 2 related Myxococcus genomes. Aligned regions are shown in green with red boundaries (“Legend”). Bars represent sitAI loci on the chromosomes. Bars spanning both genomes are colocalized sitA loci in regions of gene synteny. Inner-facing bars are unique M. virescens loci, whereas outer-facing bars are unique DK1622 loci. Amino acid percent identity between colocalized SitA alleles is shown. Inside the circular plot is an amino acid substitution analysis of one example of colocalized and conserved alleles (Lower) between the 2 genomes and colocalized alleles that have diverged at the SitA-CTD and SitI (Upper). Vertical lines indicate substitutions. (B) Total full-length sitA genes between the 2 genomes. MXAN and SAMN4488504 locus tag numbers are shown. Alleles that are predicted to be shared by both genomes (≥93% amino acid identity), i.e., reciprocal immunity, are placed side by side and marked with an open green circle. Genes unique to one genome are marked with a red circle. 1Although MXAN_1231 is an unmatched allele, its sitI is nearly identical to MXAN_1255 and so is not considered a unique allele, as M. virescens has a matching sitI downstream of SAMN4488504_101408. 2MXAN_1054 has acquired a frame-shift mutation, even though its SitI remains intact. The schematic (Bottom Right) is an overview of the total sitA loci from both genomes, whose presence in either genome is indicated by a black fill underneath the color-coded legend. Note that sitA1 and 2 are in the DK101 draft genome (Fig. 1), which is identical to DK1622 but contains 2 additional Mx-alpha regions that are not in the DK1622 genome and are not pictured here.
Importantly, this analysis indicates that, if populations of these 2 strains were to interact, OME between them will result in the transfer of 20 unique toxins: 8 delivered to M. xanthus and 12 delivered to M. virescens (Fig. 6B). Based on the present and prior work (14), subsequent cell death and boundary formation between swarms will ensue, establishing barriers between these competitors and blocking further OME. Therefore, the sitAI loci in these genomes together constitute a self-identity code that distinguishes social groups.
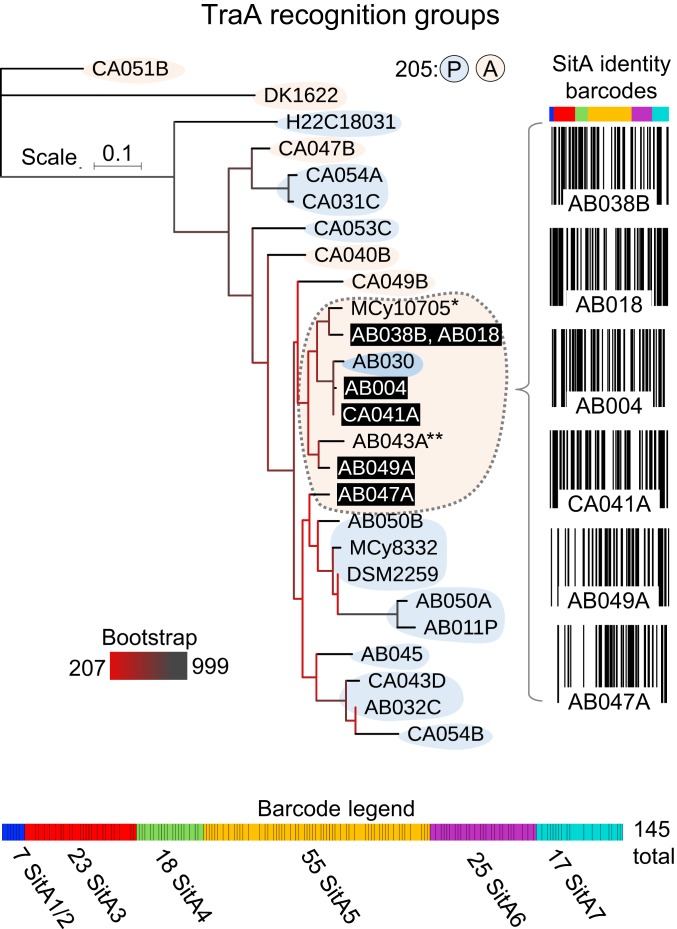
To investigate the extent and diversity of sitAI loci between genomes of related myxobacteria, we performed a similar analysis of sitAI operons in a recently deposited group of genomes from the genus Corallococcus (43). First, TraA sequences were collected, and each isolate was parsed into predicted TraA recognition groups based on the sequence of the region that determines kin specificity, i.e., the variable domain (VD), using criteria from a prior study (3). Of the 27 alleles, we predicted 15 distinct TraA recognition groups (SI Appendix, Fig. S3), which is illustrated in a maximum likelihood tree based on the amino acid sequence of their VD with the lab strain M. xanthus DK1622 serving as the outgroup (Fig. 7). The largest predicted recognition group was composed of 7 Corallococcus isolates, for 6 of which genomic sequence was publically available (Fig. 7). We then examined the SitA repertoires contained in this group. By comparing only the variable CT toxin domains of each SitA sequence, we clustered them into unique groups based on a stringent cutoff of >95% amino acid identity. This analysis concluded that the 224 total SitA-CT sequences belonged to 145 distinct specificity groups (Fig. 7). To visually represent the social compatibility of these strains, we created SitA barcodes that displayed the sequence analysis with vertical black lines that represent the presence or absence (no line) of each of the 145 SitA groups in each genome (Fig. 7). Of the 14 possible interactions among these 6 isolates, the range of unique or discriminating SitA toxins that could be transferred by OME is 29 to 76. These data highlight the precise and extensive nonself-discrimination power that these expansive SitA families enable when 2 TraA-compatible cells interact. The outcome from such OME encounters determines whether individuals are poisoned or remain viable to undergo cooperative interactions as validated clonemates.
Fig. 7.
SitA loci define molecular barcodes that discriminate kin between cells in a TraA recognition group. TraA and SitA analysis within the Corallococcus genus from Fig. 4. Maximum likelihood tree depicts the relationship between TraA variable domains from 27 genomes. Colored shape backdrops indicate the predicted recognition groups of traA alleles based on their sequence identity and the presence of proline or alanine at relative position 205 as described (3). The largest recognition group is denoted by the dashed outline, excluding the allele with 205P. The 6 available genomes from this group (black bars, white text) encode 224 total sitAI loci, which clustered into 145 distinct groups. Barcode diagrams show the presence (black line) or absence (white line) of each SitA group for the 6 genomes. The barcode legend (Bottom) shows the total sitA loci used to generate the barcodes (color-coded cells separated by black borders). The thickness of each cell is proportional to the number of strains that encode the corresponding locus. *MCy1075 belongs to recognition group 13 (SI Appendix, Fig. S3), but genomic sequence for sitA analysis was not available. **AB043A was not included in recognition group 13 because its VD percent identity fell below the 90% threshold in 4 of 8 cases with other family members.
Discussion
In-depth analysis of myxobacterial genomes revealed 6 families of SitA toxins that share a similar function but are not related by sequence. Interestingly, however, 3 families can be associated with a homologous sitB gene. This suggests that SitBAI1/2, SitBAI3, and SitBAI4 share a common origin and subsequently diverged. The sitA5, 6, and 7 genes are never associated with sitB and thus may have evolved in a convergent fashion to adopt a similar function. We classified SitA proteins into distinct families defined by their conserved escort domains. Based on prior studies of bacteriocins (44) and CdiA (45) toxins, we hypothesize that these SitA escort domains facilitate entry of the toxin module into the target cell cytoplasm. Because these escort domains are unique to each family, we further hypothesize that they exploit distinct cellular proteins to gain cytoplasmic entry after they are delivered to the OM by OME. Our future work will examine this question. Possessing an arsenal of SitA toxins with different cytoplasmic entry pathways is advantageous for this system, as it is unlikely that a target cell will evolve resistance by blocking entry to a divergent panel of toxins without mutations to TraA or TraB (46). In this regard, it is interesting to note that we found no evidence of degenerate TraAB mutant sequences from >100 myxobacterial genomes, probably owing to cooperative social fitness gains OME plays, for example in membrane homeostasis and repair (5, 9).
Our results show that the newly discovered SitA families of proteins, and, by extension, other classes of cargo proteins, can be serially transferred between cells. A model for the mechanism of serial transfer was described in a prior study (14). Previously, we suspected that SitB may be required for serial transfer because we showed that ∆sitB inhibitor cells could poison intermediary cells in a serial transfer assay, but not secondary target cells (14). However, here we demonstrated serial transfer of SitA without a cognate SitB protein, suggesting that SitB is not always required for serial transfer, but instead enhances SitA delivery to primary cells, which in turn enhances serial transfer. Finally, although our serial transfer assay was artificially designed by constructing a traA merodiploid strain, serial transfer in nature nevertheless likely occurs between 3 or more distinct genotypes that all contain compatible traA alleles for OME.
Importantly, we found that sitAI genes are expansive in myxobacteria, with several genomes possessing >50 loci. While there is inherent selective pressure to retain immunity genes to any toxin to avoid poisoning by neighboring clonal cells, the reason to retain so many toxins is less clear. For example, in some bacterial toxin systems, arrays of immunity genes remain intact despite not retaining their cognate toxins (15). However, from our analysis, in nearly all cases, complete sitAI operons are retained despite there being multiple genes of any one family present in their genomes. In fact, all 11 heterologous sitAI cassettes that we cloned into M. xanthus were functional. We suggest that one major advantage of containing a vast array of toxins is to allow stringent discrimination against OME partners. A second reason for many sitAI loci is that it provides a competitive advantage over related cells. For instance, if an individual cell within a clonal population gains a novel sitAI by HGT, that cell can now efficiently poison and outcompete its siblings through OME. Similarly, there is selective pressure to retain sitAI cassettes because, if a cell loses a cassette, it would be eliminated by its siblings. Such HGT and exploitation schemes were likely repeated many times over during evolution, such that some strains acquired and retained a large arsenal of sitAI loci.
The large array of sitAI cassettes that cells harbor likely results in differential expression of these loci in response to environmental and developmental cues. A previous study on SitA1 and SitA2 supports the notion that these toxins are constitutively expressed during swarming on solid agar surfaces (28), but future studies should examine the regulation and natural expression levels of the SitA protein families in more detail. Although expression levels of certain SitA toxins are high enough to display a strong killing phenotype (28), it is possible that endogenous expression levels at other loci are not, and therefore low levels of multiple toxins may act in concert to kill target cells by multiple mechanisms of action.
Genome sequences of myxobacterial isolates that were colocated in the soil have demonstrated that many strains that live in close proximity possess identical traA alleles, but nevertheless are incompatible and antagonize one another upon physical contact (13, 47, 48). Although it is beyond the scope of this work, we found that these environmental isolates have different repertoires of sitAI loci (14) despite their genomes being very similar and containing identical traA alleles. These observations suggest that the plethora of SitA toxins contained in these cells play a key role in kin discrimination. Future studies from our lab will investigate this hypothesis. Myxobacteria also possess other antagonistic, polymorphic kin discrimination systems such as the T6SS (49, 50). A fundamental difference of these systems is that SitA delivery requires matching sequence identity at another locus, traA, whereas the T6SS can indiscriminately inject toxins into neighboring cells. Therefore, in the case of SitA, discrimination does not function at a broad level of interstrain or interspecies antagonism, but instead functions to establish a narrow relatedness threshold to guard against OME with related but nonkin genotypes.
Our comparative genomic analysis of M. xanthus and M. virescens provides clues to how SitA proteins were acquired and diversified. For instance, we found that sitA loci that are exclusively found in one strain were all present on either small genetic islands, where sitAI makes up the majority of the island, or on islands that also contain mobile genes, e.g., prophage. This implies that these sitAI loci were acquired by HGT and, in some cases, are components of prophage elements. Similarly, a sitAI locus was found on the only known natural myxobacterial plasmid, again suggesting a role in the selfish element expansion and retention. Our previous study found that SitA1, 2, and 3 all reside on Mx-alpha prophages located in the same chromosomal region of the lab strain (14). In contrast, the 4 families of sitAI loci described here are found at diverse positions around the genome, including associations with different and smaller types of prophage elements. These findings suggest that the global diversity of SitA families arises, at least in part, from different phage elements that carry them. In contrast, there are also sitAI loci positioned in the same chromosomal location in M. xanthus and M. virescens strains, and these loci have retained high pairwise sequence identity. This is despite significant divergence between these strains, including the fact that they were isolated from different geographic locations (M. xanthus DK1622, Iowa [28]; M. virescens DSM 2260, Ontario, Canada [51]). These 10 sitAI loci are thus diversifying slowly, whereas there were only 2 colocalized sitAI loci with lower sequence identity, suggesting they diversified rapidly (Fig. 6). Taken together, this suggests that SitAI repertoire, and thus identity barcode, of any one strain is primarily changed by the acquisition of new loci by HGT as opposed to diversifying selection at existing loci.
To gain a more comprehensive grasp of the role SitAI proteins play in kin discrimination, we analyzed a relatively large group of Corallococcus strains (43). To initiate this analysis, we predicted that these Corallococcus strains contained 15 distinct TraA recognition groups, of which 13 are new and supplement the 42 recognition groups previously demonstrated or predicted from the suborder Cystobacterineae (3). Although the complete collection of Cystobacterineae isolates that exist in nature obviously contains many more than 55 TraA recognition groups, there is nevertheless a finite number of specificities one receptor family can offer, which is clearly much smaller than the number of compatible social groups found in nature (13). However, SitA diversity adds another layer, with increased resolution and specificity, to discriminate nonkin among natural isolates. Indeed, our analysis of one TraA recognition group, consisting of 6 genotypes, revealed a large constellation of diverse sitAI loci (145 distinct groups predicted). This small sampling of genomes highlights that, within the Cystobacterineae suborder, there is a vast pool of sitAI loci with unique specificities. Further, these loci can be mixed and matched in different genomes in astronomically large possibilities of combinations to serve as exquisitely specific kin discrimination barcodes. In turn, discrimination by a suite of sitAI loci protects against exploitation of OME by nonclonemates. Based on the findings that sitAI loci are numerous and frequently found on genomic islands, it is likely that SitAI diversity has played a major role in the genetic isolation and diversification of myxobacteria social groups (48).
Methods and Methods
Strains, plasmids, and primers used in this study are described in SI Appendix, Tables S1–S3, respectively. Bacterial growth conditions, cloning, strain construction, competition experiments, and sequence analysis are described in SI Appendix, Material and Methods.
Data Availability.
All data and protocols are described in this manuscript, SI Appendix, or references therein. Strains, plasmids, and other reagents or information are available upon request.
Supplementary Material
Acknowledgments
We thank Wei Hu and Yue-Zhong Li for M. fulvus 124B02 and the pMF1 plasmid. This work was supported by National Institutes of Health Grant GM101449 (to D.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912556116/-/DCSupplemental.
References
- 1.Wall D., Kin recognition in bacteria. Annu. Rev. Microbiol. 70, 143–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pathak D. T., Wei X., Dey A., Wall D., Molecular recognition by a polymorphic cell surface receptor governs cooperative behaviors in bacteria. PLoS Genet. 9, e1003891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao P., Wei X., Awal R. P., Müller R., Wall D., A highly polymorphic receptor governs many distinct self-recognition types within the Myxococcales order. MBio 10, e02751-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei X., Pathak D. T., Wall D., Heterologous protein transfer within structured myxobacteria biofilms. Mol. Microbiol. 81, 315–326 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Cao P., Wall D., Direct visualization of a molecular handshake that governs kin recognition and tissue formation in myxobacteria. Nat. Commun. 10, 3073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troselj V., Cao P., Wall D., Cell-cell recognition and social networking in bacteria. Environ. Microbiol. 20, 923–933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathak D. T., et al. , Cell contact-dependent outer membrane exchange in myxobacteria: Genetic determinants and mechanism. PLoS Genet. 8, e1002626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassallo C., et al. , Cell rejuvenation and social behaviors promoted by LPS exchange in myxobacteria. Proc. Natl. Acad. Sci. U.S.A. 112, E2939–E2946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassallo C. N., Wall D., Tissue repair in myxobacteria: A cooperative strategy to heal cellular damage. BioEssays 38, 306–315 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X. W., et al. , Myxobacterial community is a predominant and highly diverse bacterial group in soil niches. Environ. Microbiol. Rep. 6, 45–56 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Vos M., Velicer G. J., Genetic population structure of the soil bacterium Myxococcus xanthus at the centimeter scale. Appl. Environ. Microbiol. 72, 3615–3625 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao P., Wall D., Self-identity reprogrammed by a single residue switch in a cell surface receptor of a social bacterium. Proc. Natl. Acad. Sci. U.S.A. 114, 3732–3737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wielgoss S., Fiegna F., Rendueles O., Yu Y. N., Velicer G. J., Kin discrimination and outer membrane exchange in Myxococcus xanthus: A comparative analysis among natural isolates. Mol. Ecol. 27, 3146–3158 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Vassallo C. N., et al. , Infectious polymorphic toxins delivered by outer membrane exchange discriminate kin in myxobacteria. eLife 6, e29397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D., de Souza R. F., Anantharaman V., Iyer L. M., Aravind L., Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol. Direct 7, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aakre C. D., et al. , Evolving new protein-protein interaction specificity through promiscuous intermediates. Cell 163, 594–606 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Melderen L., Saavedra De Bast M., Bacterial toxin-antitoxin systems: More than selfish entities? PLoS Genet. 5, e1000437 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Cruz F., Davies J., Horizontal gene transfer and the origin of species: Lessons from bacteria. Trends Microbiol. 8, 128–133 (2000). [DOI] [PubMed] [Google Scholar]
- 19.Russell A. B., et al. , Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacIntyre D. L., Miyata S. T., Kitaoka M., Pukatzki S., The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U.S.A. 107, 19520–19524 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki S. K., et al. , A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468, 439–442 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamet A., Nassif X., Characterization of the Maf family of polymorphic toxins in pathogenic Neisseria species. Microb. Cell 2, 88–90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamet A., et al. , A widespread family of polymorphic toxins encoded by temperate phages. BMC Biol. 15, 75 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitney J. C., et al. , A broadly distributed toxin family mediates contact-dependent antagonism between gram-positive bacteria. eLife 6, e26938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Z., Casabona M. G., Kneuper H., Chalmers J. D., Palmer T., The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nat. Microbiol. 2, 16183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Bayona L., Guo M. S., Laub M. T., Contact-dependent killing by Caulobacter crescentus via cell surface-associated, glycine zipper proteins. eLife 6, e24869 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza D. P., et al. , Bacterial killing via a type IV secretion system. Nat. Commun. 6, 6453 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Dey A., et al. , Sibling rivalry in Myxococcus xanthus is mediated by kin recognition and a polyploid prophage. J. Bacteriol. 198, 994–1004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starich T., Zissler J., Movement of multiple DNA units between Myxococcus xanthus cells. J. Bacteriol. 171, 2323–2336 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskiniemi S., et al. , Rhs proteins from diverse bacteria mediate intercellular competition. Proc. Natl. Acad. Sci. U.S.A. 110, 7032–7037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Gebali S., et al. , The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang D., Iyer L. M., Aravind L., A novel immunity system for bacterial nucleic acid degrading toxins and its recruitment in various eukaryotic and DNA viral systems. Nucleic Acids Res. 39, 4532–4552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayers E. W., et al. ; NCBI Resource Coordinators , Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 46, D8–D13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen I. A., et al. , IMG/M v.5.0: An integrated data management and comparative analysis system for microbial genomes and microbiomes. Nucleic Acids Res. 47, D666–D677 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J. Y., et al. , Discovery of the autonomously replicating plasmid pMF1 from Myxococcus fulvus and development of a gene cloning system in Myxococcus xanthus. Appl. Environ. Microbiol. 74, 1980–1987 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y. J., et al. , A post-segregational killing mechanism for maintaining plasmid pMF1 in its Myxococcus fulvus host. Front. Cell. Infect. Microbiol. 8, 274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X. J., et al. , The complete genome sequence and analysis of a plasmid-bearing myxobacterial strain Myxococcus fulvus 124B02 (M 206081). Stand. Genomic Sci. 11, 1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pathak D. T., Wall D., Identification of the cglC, cglD, cglE, and cglF genes and their role in cell contact-dependent gliding motility in Myxococcus xanthus. J. Bacteriol. 194, 1940–1949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimmermann L., et al. , A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 430, 2237–2243 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Berman H. M., et al. , The protein data bank. Nucleic Acids Res. 28, 235–242 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchler-Bauer A., et al. , CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45, D200–D203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darling A. E., Mau B., Perna N. T., progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5, e11147 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livingstone P. G., Morphew R. M., Whitworth D. E., Genome sequencing and pan-Genome analysis of 23 Corallococcus spp. strains reveal unexpected diversity, with particular plasticity of predatory gene sets. Front. Microbiol. 9, 3187 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakes K. S., Cramer W. A., Border crossings: Colicins and transporters. Annu. Rev. Genet. 46, 209–231 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Willett J. L., Gucinski G. C., Fatherree J. P., Low D. A., Hayes C. S., Contact-dependent growth inhibition toxins exploit multiple independent cell-entry pathways. Proc. Natl. Acad. Sci. U.S.A. 112, 11341–11346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dey A., Wall D., A genetic screen in Myxococcus xanthus identifies mutants that uncouple outer membrane exchange from a downstream cellular response. J. Bacteriol. 196, 4324–4332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vos M., Velicer G. J., Social conflict in centimeter-and global-scale populations of the bacterium Myxococcus xanthus. Curr. Biol. 19, 1763–1767 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wielgoss S., et al. , A barrier to homologous recombination between sympatric strains of the cooperative soil bacterium Myxococcus xanthus. ISME J. 10, 2468–2477 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troselj V., Treuner-Lange A., Søgaard-Andersen L., Wall D., Physiological heterogeneity triggers sibling conflict mediated by the type VI secretion system in an aggregative multicellular bacterium. MBio 9, e01645-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Y., et al. , A nuclease-toxin and immunity system for kin discrimination in Myxococcus xanthus. Environ. Microbiol. 20, 2552–2567 (2018). [DOI] [PubMed] [Google Scholar]
- 51.McCurdy H. D., Studies on the taxonomy of the Myxobacterales. I. Record of Canadian isolates and survey of methods. Can. J. Microbiol. 15, 1453–1461 (1969). [DOI] [PubMed] [Google Scholar]
- 52.Livingstone C. D., Barton G. J., Protein sequence alignments: A strategy for the hierarchical analysis of residue conservation. Comput. Appl. Biosci. 9, 745–756 (1993). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and protocols are described in this manuscript, SI Appendix, or references therein. Strains, plasmids, and other reagents or information are available upon request.