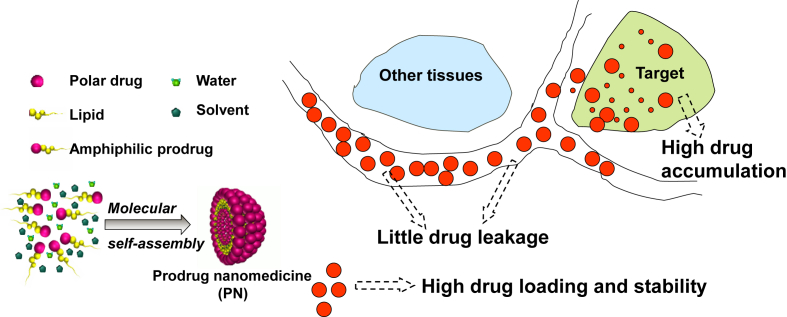
Abstract
Drug delivery systems (DDS) are defined as methods by which drugs are delivered to desired tissues, organs, cells and subcellular organs for drug release and absorption through a variety of drug carriers. Its usual purpose to improve the pharmacological activities of therapeutic drugs and to overcome problems such as limited solubility, drug aggregation, low bioavailability, poor biodistribution, lack of selectivity, or to reduce the side effects of therapeutic drugs. During 2015–2018, significant progress in the research on drug delivery systems has been achieved along with advances in related fields, such as pharmaceutical sciences, material sciences and biomedical sciences. This review provides a concise overview of current progress in this research area through its focus on the delivery strategies, construction techniques and specific examples. It is a valuable reference for pharmaceutical scientists who want to learn more about the design of drug delivery systems.
KEY WORDS: Pharmaceutics, Drug delivery system, Basic research, Application, Delivery strategy
Graphical abstract
This review makes a concise overview of current progress in the research of drug delivery systems that focused on the delivery strategies, construction techniques and specific representatives.
1. Introduction
Drug delivery systems (DDS) are used to transport therapeutic drugs in the body as needed to safely achieve the desired therapeutic effect. Such systems are usually designed to i) improve aqueous solubility and chemical stability of active agents, ii) increase pharmacological activity, and iii) reduce side effects. Modern drug delivery systems have undergone continuous progress since the 1950s, when the first sustained release formulation Dexedrine was introduced1. The goal of any drug delivery system is to provide and maintain therapeutic concentrations of drug at the target biological site.
Among present drug delivery systems, nanoparticles as carriers have shown great potential in recent years. The encapsulation of drugs in nanoparticles, including micelles, liposomes, dendrimers, nanocapsules, nanospheres and others, improves the therapeutic index and reduces the adverse side effects. For example, liposome drug delivery systems can improve bioavailability, increase efficacy and reduce toxicity. Several successful liposome-based drugs have been approved by the U. S. Food and Drug Administration (FDA), such as liposomal doxorubicin (Doxil®)2 and liposomal amphotericin B (Ambisome®)3.
This review focuses on the recent advances of drug delivery systems reported within 2015–2018. An extensive literature review was performed mainly using PubMed. In this review, we highlight the emerging strategies for drug delivery, and also emphasize the current construction techniques for delivery systems; specifically, several representative inorganic materials for delivery system and the delivery of biomacromolecules are also discussed.
2. Emerging strategies for drug delivery
2.1. Stimuli-responsive strategy
Currently, stimuli-responsive delivery has been the most attractive strategy in the field of drug delivery. This strategy has been actively explored in order to achieve the tumor-specific delivery and controlled release of their cargoes, where endogenous or exogenous triggers can be employed. The endogenous triggers including pH-sensitive4, ROS (reactive oxygen species)-sensitive5, redox-sensitive6, enzyme-sensitive7 and temperature-sensitive delivery strategies towards some disease sites like tumors8. Exogenous triggers include light-triggered9 and temperature-triggered10 strategies induced by exogenous methods. Magnetic-triggered11, and X-ray triggered12 delivery strategies have also been used in the design of stimuli-responsive systems. Ultrasound can also serve as a trigger for remote control of drug release deeply within the body. The use of focused ultrasound has the advantage of delivering spatially localized heat, thus improving site-specific controlled release by destabilizing the structure of such delivery systems10.
Many stimuli-responsive systems containing sensitive segments can be used for delivering drugs to target tissue and achieving an on-demand drug release. The surface properties and nanostructures of stimuli-responsive nanoparticles can also be modulated through intrinsic or extrinsic stimuli for improving cellular uptake and enhancing penetration ability.
2.1.1. Controlled drug release by stimuli-responsive systems
In many circumstances, controlled release of drug at the target site is required for proper therapeutic effect and safety. This can be achieved by various stimuli. For instance, ultrasound-triggered release has been useful for on-demand control of local pain, as specific sonosensitizer can be activated to produce ROS. These products react with liposomal membranes through peroxidation of unsaturated membrane lipids to release drugs, allowing patients to self-manage the intensity and duration of local anesthesia. No systemic toxicity or tissue damage was detected13. It was shown that ultrasonic energy transferred to liposomes would cause a sonosensitizer to release ROS, peroxidating unsaturated lipids in the bilayers, leading to local release of anesthetics. An amphiphilic copolymer (PB-PEG) composed of pendant phenylboronic acid and methoxy poly(ethylene glycol) was designed to efficiently load doxorubicin (DOX), epirubicin (EPI), or irinotecan (IR) via donor–receptor coordination between the boron and nitrogen atoms14. The PB-PEG nanoparticles with a drug loading level of up to 49% exhibited an enhanced cytotoxicity as compared to traditional micelles through hydrophobic interaction. In another case, a prodrug of DOX (iPDOX) was prepared by attaching iRGD and DOX to pluronic P85 copolymer through a matrix metalloproteinase-2 (MMP-2)-labile peptide, and iPDOX was further encapsulated in the micelles consisting of acid-responsive poly(ethylene glycol)-b-poly(2-(hexamethyleneimino)ethyl methacrylate) (PEG-b-PHMA)15. The resulting nanoparticles were able to release iPDOX in tumoral acidic microenvironment (pH ∼6.8), and then iRGD facilitated subsequent tumor penetration and intracellular uptake of released DOX through peptide cleavage, thereby leading to preferable anticancer efficacy against MCF-7/ADR tumor-bearing nude mice15. Li et al.16 employed poly(etherimide)-poly(lactic-co-glycolic acid) (PEI-PLGA) copolymer to load antiplatelet antibody R300 and DOX within the nanoparticles, which were further modified by a lipid shell layer inserted with matrix metalloproteinase 2 (MMP2)-cleavable peptides. These nanoparticles were found to deplete the platelet to enhance tumor permeability for effective DOX delivery. Cysteine-based poly-(disulfide amide) nanoparticles were designed to scavenge glutathione to inhibit detoxification of active Pt species, leading to the enhanced therapeutic index of Pt drugs against cisplatin-resistant tumors17. In addition to polymeric micelles, polymeric vesicles as a drug vehicle have also been explored to provide hypoxia-responsive or redox-responsive drug delivery18, 19.
For drugs like photosensitizers, specific stimuli could be used to directly trigger their therapeutic effect. Recently, Guo et al.20 fabricated the self-assembled nanoparticles consisting of PEGylated platinated boron dipyrromethene (Bodiplatin) with ultralow radiative transition. Upon single-wave light exposure, these nanoparticles generate both singlet oxygen through preferable singlet-to-triplet transition and photothermal conversion through nonradioactive decay, thus providing synergistic effect between photodynamic and photothermal treatments for tumor ablation. To further regulate the photoconversion of photosensitizers for cooperative cancer phototherapy, trimeric boron dipyrromethene (tri-BDP) within the nanoparticles were prepared to enhance non-radiative transition together with moderate singlet-to-triplet transition21. Thus, the polymeric tri-BDP nanoparticles caused dramatic tumor ablation through the dominant late apoptosis and moderate early apoptosis upon light irradiation21.
2.1.2. Enhanced uptake and penetration by stimuli-responsive systems
The surface charge and moiety of nanoparticles can influence the interaction of delivery systems with cells and blood components22, thus improving cellular uptake and penetration. To reduce opsonin adsorption and reticuloendothelial systems (RES) recognition, PEG is widely used as a surface layer of nanoparticles. However, PEG also reduces cellular uptake. To resolve this problem, one strategy the development of detachable PEG. After distribution into tumor, the PEG can be detached in acidic conditions23, high level of GSH3 and overexpressed enzymes24. The inner nanoparticle layer can be modified with ligands, such as cell penetrating peptides, to further improve cellular uptake25. Surface coating with charge-reversible groups is another method for improving drug delivery26. Particles with neutral or negative charge display long blood circulation time, whereas particles with positive charge show high cellular uptake.
As a basic rationale of nanoparticle-based drug delivery, the enhanced permeability and retention (EPR) effect is greatly influenced by the particle size and morphology. Generally, larger particles possess better retention but display less penetration, whereas smaller particles have poor retention but more penetration27, 28. To achieve both higher tumor penetration and retention, various kinds of size-changeable nanoparticles have been developed. Shrinkable nanoparticles were fabricated by the small-sized nanoparticles with degradable crosslinkers or cores. For example, small-sized gold nanocluster (AuNC) and dendrigraft poly-lysine (DGL) with hyaluronic acid (HA) were used to form nanoparticles about 200 nm29, 30. After extravasating tumor from microvessels, the nanoparticles can be retained around tumor vessels with minimal backflow to vessels. Subsequently, high levels of tumor-bearing hyaluronidase can degrade the HA thereby releasing the small-sized AuNC and DGL. These smaller particles can then deliver drug cargoes into deep tumor with good penetration.
For the size-shrinkable strategy, it would be useful to develop small-sized aggregable nanoparticles with good initial penetration capacity. The smaller size of the nanoparticles facilitates extravasation from tumor vessels into the deep tumor. The aggregation is thought to occur through click cycloaddition and the size is increased, limiting flow back to tumor vessels, with good retention31. Still, there are concerns for the protein corona generated on the surface of the nanoparticles when administered in vivo, which might influence nanoparticle aggregation32 and have an effect on the final application of this strategy.
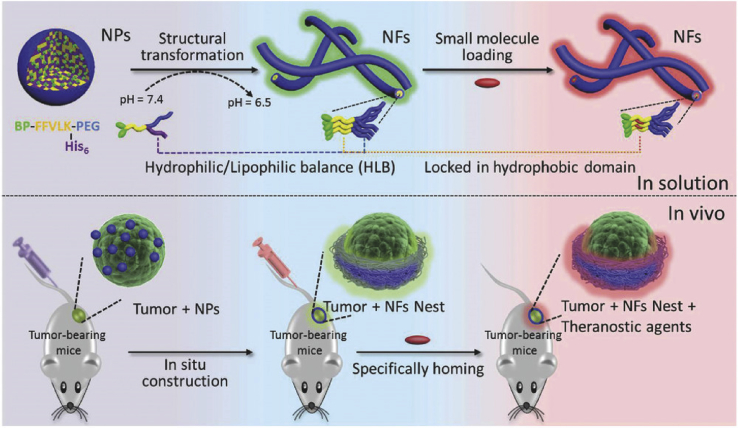
Alterations in the morphology of nanoparticles has also been used to improve tumor targeting for drug delivery. Normally, spherical particles display better tissue penetration, whereas nanotubes or fibers show better retention. Therefore, changing the particles from spherical to tubular would be useful in drug delivery. A peptide-based material, BP-KLVFF-His6-PEG was developed with the capacity of forming nanoparticles in phosphate buffer solution33 (Fig. 1). However, when the aqueous environment acidifies, the His6 becomes hydrophilic, resulting in particle shift rearrangement to form nanofibers, showing long retention time up to 96 h.
Figure 1.
The schematic diagram of pH-triggered morphological transformation from self-assembled nanoparticles (NPs) to nanofibers (NFs) and a pre-nested host in a tumor where thermally sensitive drugs are located (Adapted from Ref. 33 with permission. Copyright © 2017 Wiley).
To date, many polymeric nanoparticles have been constructed for cancer-targeted drug delivery, and a few clinical studies of these nanoparticles have been reported. Genexol® has been explored as a commercialized polymeric formulation for treatments of breast cancer, ovarian cancer, and non-small-cell lung cancer. Several polymeric micelles encapsulating chemotherapeutic drugs are also being evaluated under clinical trials. Subbiah et al.34 developed a polymeric formulation of NC-6004 consisting of PEG-b-poly(α,β-aspartic acid) and cisplatin, which are being investigated under a phase III trial for the treatment of advanced metastatic pancreatic cancer. Although clinical studies of the polymeric nanoparticles still suffer from limited success, it is highly desired to focus on translational studies of these new therapeutic formulations for cancer nanomedicine.
2.2. Co-delivery strategy and theranostics
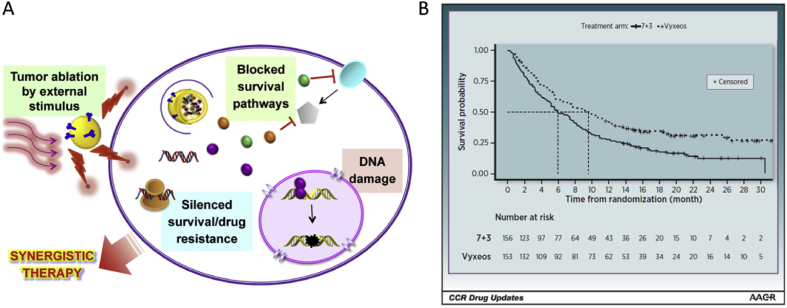
Combination therapy with various drugs is a common clinical practice in many diseases, but the optimization of PK profiles of the combined drugs for the best synergistic effect is not well demonstrated. Due to varying PK behaviors within the combinations, it is hard to obtain an optimal dose ratio that is often optimized by the cell-based tests35. Various delivery systems with identical PK properties (e.g., liposomes) can be useful for co-delivery of drug combinations. Recently, the co-delivery strategy has made substantial progress, as evidenced by the landmark approval of liposomal combination (VYXEOS®) with co-encapsulation of daunorubicin and cytarabine in 2017 (Fig. 2). A potential application of co-delivery combination in cancer therapy is to overcome drug resistance36, 37 and metastasis38.
Figure 2.
(A) The nanoparticle-based combination therapy. (B) Plasma drug concentrations after i.v. injection of the VYXEOS® liposomes and the free combo drugs in mice (Adapted from Ref. 39 with permission. Copyright © 2016, Elsevier and Ref. 40 with permission. Copyright © 2019, American Association for Cancer).
Co-delivery of photosensitizer and chemotherapeutic agent has also been widely studied for improved therapeutic effect. Light-responsive polymeric nanoparticles have been assembled from tellurium-containing block polymer (PEG-PUTe-PEG), indocyanine green (ICG), and cisplatin through the coordination of platinum and tellurium41. Upon light exposure, ICG generated 1O2 to oxidize the tellurium for rapid drug release, and simultaneously produced photothermal effect, together affording synergistic thermo-chemotherapy. Aiming at synergistic cancer therapy, Zhang et al.42 synthesized a prodrug of camptothecin and 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-α (HPPH) via a GSH-sensitive spacer. The polymeric micelles loading CPT-HPPH prodrug can efficiently accumulated at HCT116 tumors, demonstrating synergistic efficacy of chemotherapy and photodynamic therapy.
Nanoparticles have been used as co-delivery strategies for specific and personalized theranostics43, combining therapeutic effects with diagnostics and prognostics, as well as image-guided therapy, etc. For example, Tang's group44 developed a pH-responsive bone-targeting drug delivery system. In this system, zoledronic acid and plumbagin were co-delivered with upconversion material gadolinium(III) in a mesoporous silica nanoparticle system, which could detect and treat early bone metastasis of breast cancer. Still, challenges exist for the co-delivery strategies for theranostics, such as their ability to provide high signal-to-background images45. Dysregulated pH, as a result of aberrant cancer metabolism, is emerging as a ubiquitous characteristic of cancer46, and has been one of the highlights of DDS for generating high signal-to-background images for diagnostics and theranostics. Gao's group47 pioneered the development of an ultra-pH sensitive (UPS) micellar theranostic nanoplatform with several unique properties, including tunable pH transition (pHt spanning from 4.4 to 7.4), sharp pH responsiveness (ΔpHoff/on < 0.25), ultrahigh fluorescence signal activation (up to 100-fold), and ultrafast pH response (<5 ms). This UPS theranostic technology has been successfully adopted for the imaging of a broad range of tumors by amplifying the tumor acidosis signals48. Moreover, a pH-activatable ICG-encoded nanosensor (PINS) based on the UPS systems has been fabricated for precise delineation of tumor margin. The real-time tumor-acidosis guided detection and surgery of solid tumors significantly prolonged mice survival after tumor resection49. The pH-responsive nanoplatform has also been applied for the efficient cytosolic delivery of antitumor agents, e.g., anti-PD-1 antibody, for enhanced cancer therapy50, 51.
In addition to the conventional antitumor agents, photothermal therapy (PTT)52, 53 and photodynamic therapy (PDT)54 are also widely adopted in theranostics. To avoid the poor signal-to-noise or even “false positive” results in conventional imaging strategies, Yan's group55 designed a dual-stimuli-responsive and reversibly activatable nanoprobe. Asymmetric cyanine and glycosyl-functionalized gold nanorods (AuNRs) served as the two building blocks linked by matrix metalloproteinases (MMPs)-specific peptide to achieve MMPs/pH synergistic and pH reversible activation. This nanoprobe could be activated only in tumor sites with negligible background and be implemented in fluorescence-guided photothermal therapy. During the PDT process, oxygen would be consumed to generate ROS, thus the cancer cells in hypoxic tumors are remarkably resistant to PDT. To overcome this limitation, an oxygen and Pt(II) self-generating multifunctional nanocomposite was proposed to reverse the hypoxia. Pt(IV) and chlorin e6 were loaded in the nanocomposite, induced by the near infrared (NIR) light to release Pt(II) and oxygen for the production of cytotoxic ROS and synergistic photo-chemo therapy to enhance antitumor efficacy56. Several other oxygen co-delivery PDT systems have been designed for enhanced anticancer efficacy57.
Recently, great attention has been paid in the development of multifunctional and multimodality theranostic strategies for simultaneous cancer imaging, therapy and post-therapy monitoring, etc58. For example, a three-in-one system based on the porphyrin/camptothecin-floxuridine triad microbubbles (PCF-MBs) was established to act as not only a contrast agent for ultrasonic/fluorescence bimodal imaging but also a multimodal therapeutic agent for synergistic chemo-photodynamic therapy59. In addition, hollow manganese dioxide (H-MnO2), as a biodegradable nanomaterial, was utilized to load Ce6 and DOX for tumor microenvironment (TME)-specifically imaging and modulating the hypoxic TME, which led to comprehensive anti-tumor efficacy and immune responses33.
Mechanistic understanding of tumor biology has propelled the development of a variety of multifunctional strategies for the precise cancer theranostics60, 61, 62. Hence, it is of great importance to design stimuli-responsive drug delivery system dependent on the TME and external stimuli to selectively release therapeutic and imaging agents at the appropriate intervals and locations.
2.3. Biomimetic delivery strategy
Biomimetic drug delivery system (BDDS) is a novel strategy for nanosystems which works by mimicking the unique structures, functions and biosynthetic pathways of biological systems (the whole cells, structures or composition of cell membrane, and the natural budding processes of exosome)63, 64, 65. BDDS has advantages of high biocompatibility, low immunogenicity, long systematic circulation and lesion targeting64, 65, 66. It has been widely exploited for biomedical applications, including targeted delivery of therapeutics and diagnosis of diseases, tissue regeneration and wound healing, as well as cell microreactor and blood detoxification67, 68, 69, 70, 71. The development of such biomimetic systems is mainly focused on cell membrane-camouflaged nanoparticles, extracellular vesicles, lipoprotein-coated nanoparticles, virus-like nanoparticles, and others.
Cell membrane-coated nanoparticles are developed by cloaking natural cell membrane on synthetic nanoparticles, which preserves the properties of donor cells including long circulation, disease site targeting and immune evasion. Zhang's group72 exploited erythrocyte membrane-coated nanogels to co-deliver paclitaxel and interleukin-2 for combinatorial immunochemotherapy. The biomimetic nanogels could responsively release drugs, improve drug penetration, and remodel the tumor microenvironment with enhanced antitumor effect. Cancer cell membrane-cloaked nanoparticles exhibited specific homologous-targeting property of cancer cells for targeted drug delivery and reserved tumor associated antigens for inducing multiantigenic immunity73, 74. Inspired by the targeting ability of inflammatory neutrophils towards circulating tumor cells (CTCs), this neutrophil-mimicking nanoplatform showed selective depletion of CTCs and prevention of the formation of metastatic niches75.
Extracellular vesicles (EVs) are nano-sized membrane vesicles mainly classified into exosomes (30–100 nm) and microvesicles (100–1000 nm, MVs), which are produced in different pathways. Both can mediate intercellular communication and regulate the occurrence and development of diseases. EVs have multiple advantages as drug delivery vesicles owing to their biocompatibility, low immunogenicity and intrinsic cell targeting properties. Wu et al.76 constructed dendritic cells derived antigenic MVs packed with low-dose chemotherapeutic drug, which remarkably depressed the murine melanoma and hepatic ascites growth by the combination of direct cytotoxicity and increased infiltration of immune cells. Usman et al.77 encapsulated CRISPR/Cas9 genome editing systems and microRNA into red blood cell derived EVs and demonstrated significantly robust inhibition of target gene in leukemia and breast cancer models.
Lipoprotein-coated nanoparticles have also been extensively investigated with high biocompatibility and biomimetic properties. Nie's group16 fabricated PEGylated phospholipid-enveloped polymeric nanoparticles for the depletion of tumor-associated platelets to enhance the vascular permeability and promote drug accumulation in tumor. Kuai et al.78 fabricated high density lipoprotein-mimicking nanodiscs for efficient co-delivery of neo-antigens and TLR9 agonist CpG-ODN to lymphoid nodes which induced robust adaptive immunity with remarkable tumor growth inhibition.
Virus-like nanoparticles generated from viruses were also investigated as distinct advantages in immunomodulation for their similar characteristics with antigen and adjuvant79. Similarly, empty cowpea mosaic virus-derived vesicles could be utilized as immunostimulatory reagents for activating neutrophils and eliciting enhanced antitumor immune responses with striking antitumor efficacy against various cancers80.
BDDS has shown bright prospects in the field of biomedical application. However, just like all new-born technologies, there is still a multitude of problems to be solved before wide clinical application. These include the retention of biomimetic function or biological information, poor control of the encapsulation of chemical molecules or synthetic nanoparticles, the negative influence of in vitro modification or drug loading on biomimetic system itself, as well as difficulties in establishing a standardized protocol for their preparation, purification and storage63, 66, 68. In addition, it is noteworthy that allogeneic or xenogeneic biological components involved in this kind of nanovesicles formulation may cause immunogenic and/or safety concerns.
2.4. Ligand-modified target drug delivery strategy
Ligand-mediated target drug delivery strategy could provide improvements in various characteristics including permeation across physiologic barriers81, penetration into target sites82, internalization by target cells83, and specific subcellular locations84, etc. Originating from this strategy, the antibody–drug conjugate (ADC) has seen several marketed products, such as brentuximab vedotin85, gemtuzumab ozogamicin86 and trastuzumab emtansine87, demonstrating the scientific significance and clinical value of ligand-mediated targeting.
The optimization of DDS has pursued the miniaturization, stability, efficiency and safety of the current design of the targeting moiety, paralleling the de novo optimization of the lead compound, with in-depth and interdisciplinary cooperation of chemicobiology, computational chemistry and structural biology. For example, Giralt and Teixidó's groups88 reported the optimization of apamin for brain-targeting drug delivery. Apamin is a nature-derived bicyclic neurotoxin with proven ability to cross the blood–brain barrier (BBB). Based on the structure–function relationship, a minimized MiniAp-4 molecule was developed, with the toxic sites replaced by substituents, modifications of the bicyclic structure, and disulfide bond replaced by a lactam bond. The optimized molecule possessed even superior BBB-penetration ability vs. that of the parental peptide, with lowered toxicity, improved tolerance of proteases and easy preparation.
After entering systemic circulation, drug delivery systems commonly interact with various plasma macromolecules, resulting in the as-reported “protein corona”. Therefore, the influence of ligand modification on their surface properties must be considered in the optimal design of targeting moieties. Guan et al.89 reported that stabilized DCDX ligand of nAChR was liable for IgM absorption in blood circulation, inducing rapid liposome clearance. With computer-aided peptide design, a short and stable peptidomimetic ligand D8 was developed with reduced positive net charge, attenuated IgM absorption, enhanced immunocompatibility, prolonged circulation and preserved bioactivity.
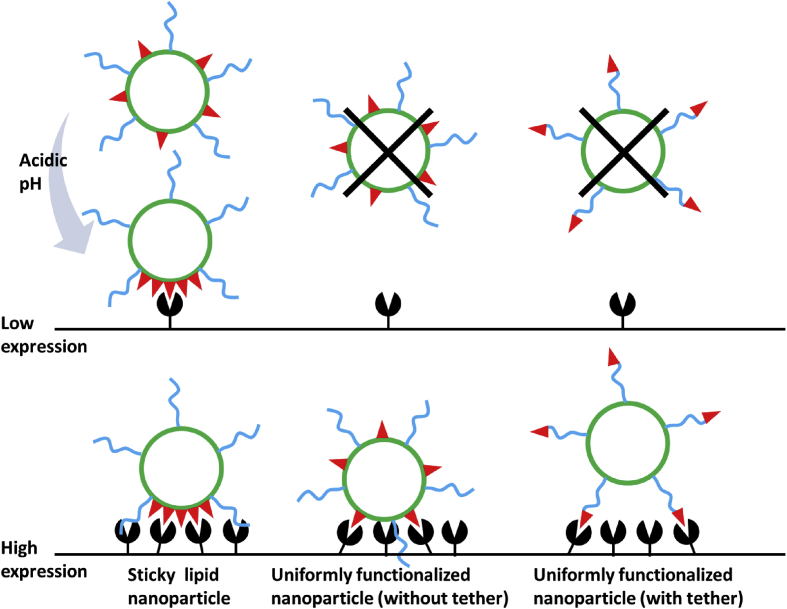
In most conventional ligand-modified delivery systems, the targeting moieties distribute uniformly on the vehicle surface, with only a small proportion participating in the interaction with target cells, causing limited recognition ability as well as incomplete utilization of ligand materials. Sempkowski et al.90 reported when aiming at target cells with relatively low receptor expressions (<2 × 105 copies/cell), or at target tissues with heterogeneity (e.g., tumor), the increased requirement of ligand density would largely restrict the application of targeted delivery. This group's approach was to construct “sticky” lipid nanoparticles with ligands changing into clusters in response to acidic pH, providing significantly enhanced recognition of target cells (Fig. 3). Lateral phase separation of lipids such as DPPS exhibited environmentally responsive lipid heterogeneities, forming clustered microdomains of ligands conjugated to these lipids. This increased ligand density and recognition in target tissues in acidic environments, and reduced specific interactions with normal cells with low receptor expressions.
Figure 3.
Schematic diagram of pH-tunable sticky vesicles, conventional uniformly functionalized nanoparticle with or without tether (PEG), and their affinity towards target cells with different receptor expressions.
Besides direct modification on the vehicle surface, a new strategy has been developed for the presentation of ligands. In this approach, the cavity formed by molecular imprinting acts as a recognition molecule to match the target receptor. Taking p32 receptor as template molecule, Zhang et al.91 used conformational epitope imprinting to fabricate a targeted delivery system via one-pot synthesis with the fabricated cavity as stable targeting moiety, providing strong affinity towards the target protein as well as controllable modification density. As a mature and controllable technology, molecular imprinting might see further application in targets which could hardly be recognized. The folate receptor (FR) is highly expressed in various malignancies, with intense research and application in targeted delivery. However, endogenous folate in human body imposes restrictions on FR-targeted delivery, e.g., folate-free medium and low folate diet are usually required in the in vitro and in vivo experiments to avoid competitive binding. With molecular imprinting, Liu et al.92 adopted the non-folate-binding sites in FR as templates, giving rise to optimized ligands with high affinity and unaffected by endogenous molecules. This study provides new strategies for the targeted delivery system based on endogenous ligands.
3. Emerging techniques for drug delivery
Advances in other specific technologies have also improved modern drug delivery systems. Several of these are discussed below.
3.1. 3D printing-based drug delivery technology
3D printing is a layer-by-layer process technology capable of producing 3D drug delivery formulations from digital designs. 3D printing has been termed as an “additive manufacturing” technology by the American Society of Mechanical Engineers93. Sachs and Cima94 published the first 3D printing drug paper in 1996. As compared with conventional technology, 3D printing possesses unique advantages for fabrication of complex, personalized and on-demand products. These advantages are crucial to improve the safety, efficacy, and accessibility of medicines95. In the past 3 years, 3D printing technology has made breakthroughs in the field of personalized medicine.
Fused deposition modeling (FDM) is the most widely used 3D printing technology for the preparation of special drug dosage forms. Goyanes et al.96 first proposed the concept of FDM 3D printing tablet in 2014. Thereafter, FDM-based 3D printing technology has been applied for the fabrication of various drug delivery formulations including fast or controlled release tablets97, 98, time delayed capsules99, multilayer capsules100, T type intrauterine implant system101, personalized percutaneous patch102, and drug-eluting stents or implants103. For example, Gaisford's group104 reported the fabrication of fast release tablets using FDM 3D printing. The drug release profiles of the tablets could be readily modified by adjusting the printing parameters.
For fabrication of controlled drug delivery formulations, Pan's group105 reported a double-chamber drug delivery device by combining FDM 3D printing with HME. They formulated glipizide (anti-diabetes drug)-loaded drug delivery tablets with a dual-nozzle 3D printer, and formulated a double-chamber device composed of a tablet embedded within a larger tablet, each chamber contains different amount of glipizide. The double-chamber design can facilitate both fast and delayed drug release by reasonably adjusting drug distribution in the chambers, which is promising for on-demand drug delivery. Similarly, Rantanen's group106 reported a customized approach to modify the drug release kinetics for flexible dosing and precision medication. They first co-extruded nitrofurantoin, Metolose® and polylactic acid into filaments with up to 40% Metolose® content, and subsequently 3D printed the filaments into model disk geometries. Nitrofurantoin release from the disks could be coordinately adjusted by Metolose® loading, with increased drug release for higher Metolose® loads. This study demonstrated the potential of drug-loaded feed materials for 3D printing of precision drug products with tailored drug release characteristics.
For the fabrication of drug-loaded eluent, Sandler's group103 reported drug-containing T-shaped prototypes of intrauterine system (IUS) by using 3D printing. The drug release profiles from the printed devices were faster than that from the corresponding filaments due to decreased drug crystallinity in IUS, and the differences in the external/internal structure and geometry between the products. This study demonstrated that 3D printing is applicable for developing drug-containing IUS and might open new avenues for the fabrication of controlled release implantable devices.
Overall, 3D printing is unique for the preparation of personalized biomedical devices, which is emerging as a novel process technology for development of novel drug delivery formulations and drug-loaded devices on demand. Up to May of 2016, FDA had approved more than 85 personalized medical products including one orally disintegrating 3D printing drug, levetiracetam tablets (trade name of SPRITAM®)107. On 5th of December, 2017, the FDA released the world's first technical guideline “Technical Guideline for Additive Manufactured Medical Devices” for 3D printing of biomedical products. In the “Made in China 2025” Plan issued by the State Council of China, extensive efforts have been devoted to 3D printing, particularly, in the fields of biomedicine and high-performance biomedical devices. Given the unique advantage of 3D printing to develop complex products, 3D printing should be of wide application for personalized medicine.
3.2. Microneedle-based transdermal drug delivery technology
Microneedle (MN) technology has greatly accelerated the development of transdermal drug delivery system (TDDS). Due to the microscale size of the needles, the microneedle is able to penetrate the stratum corneum barrier of the transdermal delivery systems without reaching the nerve fibres and blood vessels. Therefore, the MN is painless and convenient in its application with the ability to avoid hepatic first pass metabolism as well as realize sustained release108. MNs are classified into solid MN, coated MN, dissolving MN and hollow MN according to the shape and use of MNs. Each of the MN has pros and cons. A solid MN punctures the surface of the skin, and then drugs can be applied to the skin for slow diffusion of drug through the pores and into the body. Although it can prevent pathogenic infection, the drug delivery effect is low. Coated MN is used to coat a water-soluble drug. The MN is attached to the skin, the drug is quickly delivered to the skin, and then the MN is removed. It is suited to deliver a very small fixed amount of drug, but the remaining MN is dangerous because it can infect other people109. Dissolving MN is suitable for many biomacromolecules and vaccines, since sustained release of antigen from MN could induce a better cellular immune response than fast release110. Dissolving MN improves the disadvantages of the coated MN by delivering large doses of drug, but it is difficult to transfer a fixed amount of drug111. Many techniques have been developed on the basis of dissolving MN, such as tip-concentrated MN, rapidly dissolving MN, double-layered MN, according to specific requirements112. Hollow-shaped MN has a hollow core inside the needles where the drug is released. Owing to precision control for drug delivery, hollow MN has been a promising strategy for both bolus and basal insulin administration113. Several hollow microneedle-based/assisted products have been approved and produced commercially used for clinic such as BD Soluvia™ and MicronJet 600™ as influenza vaccines114. The current fourth-generation self-adjustable TDDS based on MN technology could precisely control the amount and duration of drug release in response to the pathophysiological condition of patient.
Some coated MN can self-regulate through the environment, and two strategies can be used for developing self-adjustable MNs, mainly on the basis of the dissolving MN. One is represented by Kim115, 116, in which wearable biosensors and advanced transdermal delivery schemes are seamlessly integrated. This kind of design is not really “smart” and the drug release relies on external stimuli control. The other is closed-loop drug delivery strategies, also known as smart patch, which can intelligently govern the drug release kinetics in response to the fluctuation of physiological parameters. The following are several examples of glucose-responsive insulin smart patches. One imitating the function of pancreatic cells loaded with insulin and glucose oxidase (GOx) enzyme has been developed with hypoxia-sensitive hyaluronic acid conjugated with 2-nitroimidazole. Under hypoxic conditions, 2-nitroimidazole is converted to hydrophilic 2-aminoimidazoles through bioreduction and thereby induces insulin release117. Another smart patch uses dual-responsive (hypoxia and H2O2) vesicles fabricated by self-assembly of hypoxia and dual-sensitive diblock co-polymers, which shows a pulsatile release of insulin and efficiently regulates the blood glucose in type I diabetic mice for 10 h118. Another patch reported by Tong et al.119 uses a system which integrates glucose- and H2O2-responsive polymeric vehicles. After transdermal administration to diabetic rats, the insulin release was controlled from glucose- and H2O2-responsive elements. A smart thrombin-responsive MN system was described in which heparin release was successfully triggered by thrombin-cleavable peptides interlinked within hyaluronic acid hydrogel scaffolds in the presence of rising thrombin levels120.
Although a large amount of literature claimed safety and non-irritation of MNs, several clinical phase I studies reported that skin irritation and patch application uniformity are still major obstacles121, 122. The MN applicator and its parameter settings have crucial roles for MN penetration and subsequent dissolution into skin. The next-generation transdermal drug delivery system will plausibly be a single device of multi-functional integration. In addition, patient-tailored transdermal drug delivery will be very likely available in the foreseeable future with the development of co-application between sensing and therapy.
3.3. Nanocrystals
Unlike polymeric nanoparticle formulations, nanocrystal suspensions are generally stabilized by surfactants or polymeric steric stabilizers, and their particles are composed of 100% active pharmaceutical ingredients (APIs) without any carriers or vehicles. A reduction in particle size leads to an increase in dissolution rate due to an increased surface area and an enhancement in saturation solubility of the drug. Therefore, the nanosuspension approach can be very advantageous for poorly water-soluble drugs with size-related dissolution limitations. Several clinical available products using nanocrystals technique have been approved, such as the Rapamune, TriCor, Emend, Megace ES, Triglide, Cesamet, Theodur, Naprelan, and Invega sustenn123.
The use of nanocrystals substantially reduces the amount of excipients needed in the formulation compared to other nanocarriers, and thus ultra-high loading capacity is easily achieved. Therefore, nanocrystals are considered as the ideal candidates for i.v. delivery, and several poorly water-soluble drugs have been formulated as nanocrystals for i.v. administration124, 125, 126, 127, 128. However, during the development of drug nanocrystals for i.v. administration, one must keep in mind that the nanosuspensions are usually surface modified just by stabilizer adsorption, so the stabilizer may rapidly shed off from the nanocrystal surface and the hydrophobic interface would be exposed upon enter the blood stream, resulting in rapid recognition of opsonin proteins and rapid elimination via phagocytic cells.
With the aid of better adhesion to mucosal surfaces, nanocrystals are frequently applied to mucosal route of administration, including pulmonary and ophthalmic129, 130, 131, 132. For pulmonary drug delivery, rapid drug dissolution following inhalation is necessary; otherwise the atomized particles could be cleared away through mucociliary transport. Optimization of local bioavailability of inhaled nanoparticles requires consideration of dissolution, particle aerodynamics and tissue penetration. Because the aerodynamic diameter of the respirable particles ranges from 1 to 5 μm, recently nano-in-microparticles are designed for deep lung deposition, with the purpose of reconciling the advantages of nanocrystals with the aerodynamics of small microparticles. Once nano-in-microparticles reach the alveolar surface, the micron skeleton (mainly sugars, e.g., trehalose) easily dissolves in alveolar fluids and the drug nanocrystals are rapidly outwardly exposed126.
Regarding ocular drug delivery, conventional formulations usually result in very low bioavailability (usually <5%) due to rapid blinking reflex and lacrimation. Accordingly, efforts have been made to prolong the retention of drugs. Romero et al.133 prepared a cationic nanocrystal formulation containing dexamethasone acetate nanocrystals and polymyxin B for ophthalmic application, in which the generation of the positive charge was achieved by the addition of cationic excipients cetylpyridinium chloride and benzalkonium chloride. In vitro mucoadhesion test results showed the potential of increased mucoadhesion of such cationic nanocrystals compared to standard eye drop formulations.
Although many cosmetic products exploiting the nanocrystal principle are available on the market, dermal delivery of nanocrystals has not been widely used and there are no nanocrystal pharmaceutical products for dermal application in the market. Nanocrystal products were expected to achieve deep skin penetration of poorly soluble agents in a size-dependent manner134. Recently, Pireddu et al.135 produced diclofenac acid nanocrystals as a novel approach to treat skin inflammation. Ex vivo transdermal delivery experiments demonstrated that nanocrystal formulation could produce a higher accumulation of diclofenac in the skin compared to the commercial formulation (Voltaren). Moreover, the nanosuspension provided an in vivo anti-inflammatory effect similar to that of a commercial formulation, but with a higher inhibition of myeloperoxidase activity in the damaged tissue (86%) vs. the commercial formulation (16%).
Various inventions have been described for the preparation of nanosuspensions136, 137. Generally, these methods require expensive equipment, or use high power consumption processes. Recently, some simple and low-cost approaches have been reported138, 139, 140. Although most commercially available nanocrystal-based products are oral dosage formulations, recent work has reported use for i.v. administration. Unique advantages have also been found for delivery to the lung, eye and skin.
3.4. Prodrug nanomedicines
Prodrugs are bioreversible derivatives of drug molecules that are generally inactive but can be transformed into their active forms via chemical or enzyme-catalyzed reactions141. The prodrug nanomedicines (PNs) based on the molecular self-assembly of amphiphilic prodrugs were developed decades ago142, 143. They were named as self-assembled drug delivery systems (SADDS), prodrug self-assemblies, or nanodrugs. The advantages of PNs over traditional drug carriers have led to increased interest in PNs in the fields of pharmaceutics and nanomedicine (Fig. 4). Anticancer and antiviral drugs are commonly used parent drugs for PN design142, 144, 145, 146, 147. For example, the anti-cancer prodrugs paclitaxel and gemcitabine have been widely studied due to their higher loading efficiency, stimuli-responsive drug release and enhanced therapeutic efficiency144, 148, 149, 150. Clinically used prodrugs occupy about 5%–7% of the drugs approved worldwide151.
Figure 4.
Formation and advantages of prodrug nanomedicines (PNs).
Unlike traditional nanocarriers, both the chemical and physical stability of prodrugs in PNs are important. To enhance accumulation of parent drugs in target tissue, functional linkers are usually embedded in prodrug for tissue targeting145, enzyme-triggered release152, pH-triggered release153, or redox-triggered release150. For instance, hepatocyte targeting of PNs was achieved by coating with d-galactide polyoxyethylene cetyl ether152. Lipid–CPT conjugate (CPT-SS-PA) was synthesized by conjugation of CPT and palmitic acid via a disulfide linker154. The results indicated that CPT-SS-PA SLN maintained chemical structural stability in simulated physiological environments but exhibited quick reduction-response release of CPT in the presence of DTT. In addition, pH-responsive prodrug system based on pH-sensitive amphiphilic diblock copolymer conjugated through acid-labile cis-aconityl moiety (mPEG-b-PAE-cis-DOX) have been fabricated which can self-assemble into DOX-conjugated PMs (DOX-cis-PMs)148. Antitumor experiments in tumor-bearing mice demonstrated that the pH-responsive nano-prodrug system effectively enhanced the therapeutic efficacy in comparison to free drug.
Prodrug nanomedicine self-assembled by amphiphilic prodrug usually achieves high drug loading and stability153, 155, 156. pH-responsive micellar nanoparticles are prepared by self-assembly of an amphiphilic poly(ethylene glycol)-acetal-paclitaxel (PEG-acetal-PTX) prodrug, and free PTX can be encapsulated in the hydrophobic core of the nanoparticles. These nanoparticles exhibit excellent storage stability for over 6 months under normal conditions, and have a high drug loading capacity of 60.3%157. The cRGD-decorated polymersomal mertansine prodrug (cRGD-PS-DM1) has been readily fabricated from cRGD-functionalized poly(ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate) with simultaneous loading of mertansine (DM1) via thiol-disulfide exchange reaction and disulfide cross-linking of polymersomal membrane. The study showed that cRGD-PS-DM1 has high drug loading, superior stability, and fast responsive drug release156.
As discussed above, PNs are continuing to develop as a popular DDS. As an interdisciplinary product, the study of PNs needs the participation of the scientists in multiple disciplines to achieve success of the design and molecular simulation of prodrugs and PNs, the preparation of them (especially large scale production), investigation of their properties and in vitro/in vivo behavior, and clinical studies.
4. Inorganic materials for drug delivery
Inorganic nanomaterials are also widely explored as potential materials for delivery systems158, 159, 160, 161, 162, 163, 164, 165, 166 due to their unique and superior physicochemical properties, such as facile preparation/modification, good biocompatibility and storage stability167. Although many challenges need to be overcome, significant progress has been made in recent years based on various materials, e.g., black phosphorus, mesoporous silica, metal-organic framework (MOF)168, carbon nanomaterials169, magnetic nanomaterials170, as well as their combinations171.
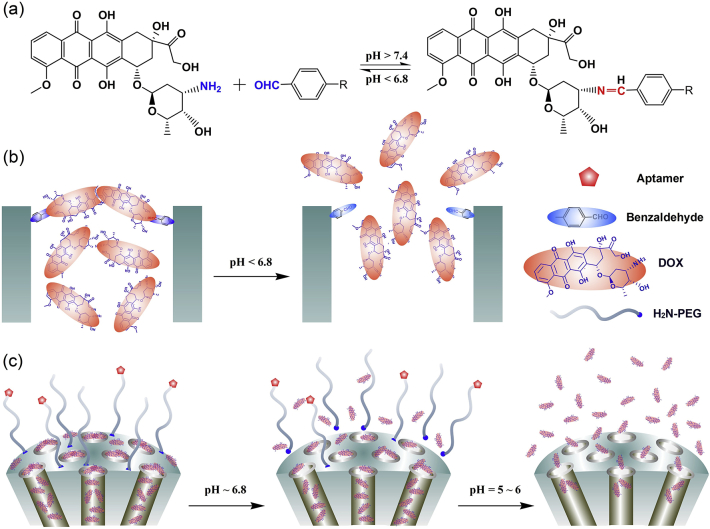
Mesoporous silica nanoparticles (MSNs) with unique properties have attracted increasing interest for drug delivery applications172. Zeng et al.160 demonstrated a novel pH-responsive MSNs that is gated by the delivered DOX itself via a reversible covalent bond. The paradigm shift, caused by bypassing the use of auxiliary capping agents, could simplify inorganic nanomaterials and allow MSNs to function as intelligent drug carriers for therapy of cancer and other diseases. Cheng et al.41 designed a cancer-targeted nanoparticle delivery system based on a DOX-gated MSN encapsulated with permeability glycoprotein (P-gp) small interfering RNA (siRNA) and a polydopamine (PDA) outer layer for DOX loading and folic acid decoration. The multifunctional nanoplatform tactfully integrates chemotherapy agents (DOX), gene (P-gp siRNA), and photothermal (PDA layer) substances in one delivery system. In another work, a nano-delivery system of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS)-functionalized PDA-coated MSNs was designed for sustainable and pH-responsive delivery of DOX for therapy of drug-resistant non-small cell lung cancer173. The MSN-based drug delivery platforms are capable of delivering other hydrophilic and hydrophobic drugs or both (Fig. 5). Quan et al.174 fabricated lactosaminated MSNs targeting the asialoglycoprotein receptor for anticancer drug delivery.
Figure 5.
a) The dynamic interaction between DOX and benzaldehyde via pH-sensitive benzoic–imine bond. b) Schematic illustration of the drug DOX self-gated MSNs with pH-responsive drug release property. c) Dynamically PEGylated and DOX self-gated MSNs with site-specific drug release at weak acidic tumor tissue/cells.
Although MSN is a promising carrier in drug delivery, the slow degradation and metabolism of MSN presently limit its use. In order to solve these problems, some novel mesoporous silica nanoparticles with rapid degradation rate have been synthesized. Zhao's group175 developed uniform monodispersed three-dimensional dendritic mesoporous silica nanospheres with rapid simulated biodegradation, which were completely eliminated within 24 h. Choi and Kim176 promoted the degradation of MSNs in a physiological condition by surface coating of polyethyleneimine (PEI) based on its proton-sponge effect. Efforts have been made to fabricate multi-stimuli responsive surfaces on MSN for environmentally sensitive, site-specific drug delivery with rapid degradation159. Wu's group177 embedded the gelator within the mesopores of silica nanoparticles (MSNs) and formed low molecular weight gel (LMWG) as a gate to fabricate a dual pH and glucose responsive nano drug delivery system. Aiming at easy aggregation in saline buffers and limited circulation lifetime of MSNs, Su et al.178 fabricated laser-responsive MSNs which supported RBC-mimetic nanoparticles with a long circulation time and controlled tumor drug release, to realize efficient anticancer drug delivery. Chai et al.179 developed a red light (660 nm)-responsive drug delivery system based on low-cost cyclodextrin (CD)-gated MSNs containing a photodynamic therapy (PDT) photosensitizer (Chlorin e6, Ce6). The designed CD-gated MSNs are promising to improve the efficacy of PDT and chemotherapy while decreasing the side effects in cancer treatment.
Black phosphorus nanosheets (BPNS) are novel two-dimensional (2D) inorganic nanomaterials which are regarded as a promising candidate for drug delivery platform for synergistic chemo/gene/photothermal therapy158. Tao et al.158 demonstrated the promising application of BPNSs as a robust drug delivery platform, thus opening up an exciting new research point of BP inorganic nanomaterials as a drug delivery carrier. In addition, Zhao et al.180 elucidated the endocytosis pathways and intracellular trafficking of PEGylated BPNSs in cancer cells; this information is essential for understanding how BP and other emerging 2D inorganic nanomaterials can best be used for cancer theranostics. However, BP is very reactive to water and oxygen, leading to compositional and physical changes. The lack of water- and air-stability under ambient conditions impedes BP's progress in drug delivery applications. Zeng et al.181 developed a simple polydopamine modification method to improve the stability and photothermal performance of bare BP nanosheets in order to fabricate a stable multifunctional co-delivery platform for the targeted synergistic chemo/gene/photothermal therapy against multidrug resistant cancer. Furthermore, other single atom nonmetal 2D inorganic nanomaterials have also shown considerable potential in DDS. In recent research, a type of ultrathin boron nanosheets (B NSs) was fabricated through a novel top-down method by coupling thermal oxidation etching and liquid exfoliation technologies. Based on the PEGylated B NSs, a novel photonic drug delivery system was explored, which exhibits multiple promising advantages for cancer therapy and imaging, including excellent biocompatibility, high drug-loading capacity, triggered drug release by NIR light and moderate acidic pH, and multimodal imaging properties (photothermal, photoacoustic, and fluorescence imaging)161.
5. Gene drug delivery
With high selectivity and bioactivity, the gene drugs (such as siRNA, microRNA, pDNA and CRISPR/Cas9) have gained increasing attention, especially the successful marketing authorization application of siRNA-based drug (named Patisiran)182, 183, 184. However, in spite of the outstanding potential and rapid advances, only a few gene drug formulations have been applied to clinical therapy. Poor pharmacokinetic properties are thought to contribute to limited efficacy of these formulations, which include low stability in the circulation, poor tissue-targeting ability and degradation in lysosomes183. To combat these severe challenges, various delivery strategies have been developed to improve clinical application of gene drug.
In order to improve the gene delivery stability in circulation, PEGylation, one of the most common approaches, can enhance colloidal stability and reduce opsonization and clearance by the mononuclear phagocyte system (MPS). Chen's group185 developed pH-triggered PEGylated nanoparticles, in which the negatively charged siRNA was complexed by PEI and PLG to form the siRNA-loaded complex and further tightened by PEG. The PEG corona was stable during circulation but could be detached at tumor sites for facilitating the cellular uptake. In addition, researchers also attempted to encapsulate gene drug in the core of nanoparticles144, 186. Sarett et al.144 reported that siRNA hydrophobization through conjugation to palmitic acid could entrap siRNA in the hydrophobic core to improve stability, in vivo pharmacokinetics, and tumor gene silencing of PEGylated nanopolyplexes (siPA-NPs) compared with unmodified siRNA. Furthermore, Zou et al.186 designed peptide-functionalized reversibly crosslinked chimaeric polymersomes, in which siRNA was completely and tightly loaded into the aqueous lumen. In contrast to common polycationic systems, the siRNA-loaded polymersomes were stable in blood circulation and had a low systemic toxicity due to disulfide crosslinking of the polymersomal membrane and effective stealth by PEG on the outer surface.
For improving cellular uptake of gene drug, some naturally available substances have been widely used in some work due to their natural internalization pathway through receptor-mediated endocytosis. Li et al.187 selected unmodified human apoferritin to encapsulate siRNA and achieve high transfection efficiency in human tumorigenic cells, human primary mesenchymal stem cells (hMSC) and peripheral blood mononuclear cells (PBMCs) at low siRNA concentrations (10 nmol/L). Additionally, cell penetrating peptides are also used to efficiently carry gene drug into cells which are not depend on classical endocytosis. Xu et al.188 developed a NP platform that could achieve TME pH-triggered rapid disassembly of NPs and exposure of tumor cell-targeting and penetrating peptide TCPA/siRNA complexes for enhanced cell uptake. Moreover, surface charge switchable nanoparticle is another common approach to improve cellular uptake. Wang et al.189 developed nanoparticles by conjugating 2,3-dimethylmaleic anhydride (DMMA) molecules to the surface amines of PLGA-PEI micelles and used to delivery siRNAs. The nanoparticles remained negatively charged in physiological condition and positive surface charge was converted to facilitate cellular uptake of siRNAs due to tumor-acidity-activated shedding of DMMA.
After cell internalization, most of siRNA are trapped in the endosomes and further transported to late endosomes/lysosomes190. In order to improve gene silencing efficiency, some functional materials have been widely used to enhance endosomal escape and release therapeutic cargoes into the cytoplasm. He et al.191 constructed lipid-based liquid crystalline nanoparticles that can perform high-efficient endosomal membrane fusion induced by the conformational transition of lipids in the intracellular acidic environment, resulting in direct release of free siRNA into cytoplasm. Wang et al.192 present a novel strategy by using near-infrared (NIR) laser irradiation to activate “bomb-like” nanoparticle to generate heat by the coencapsulated ICG and then to produce CO2 and NH3 gases from the coencapsulated ammonium bicarbonate (NH4HCO3), which helps to efficiently break the endosomes/lysosomes and enhance the cytosolic release of the encapsulated miR-34a. He et al.193 reported a hybrid nanoparticulate system based on a cationic helical polypeptide PPABLG for the efficient delivery of TNF-α siRNA. The pore formation feature of PPABLG was used to facilitate the direct translocation of cell membrane, as well as endosomal escape of TNF-α siRNA.
6. Peptide and protein drug delivery
Peptide and protein drugs, including antibodies and vaccines, increased rapidly among the novel therapeutics in the past three decades194. Compared with chemical drugs, peptides and proteins possess higher specificity and potency, as well as less adverse effects. However, they are significantly different in the physicochemical and biological properties with chemical molecules, primarily involving large molecular weight, poor stability, and low permeability, which lead to the limitation of their formulations.
Parenteral administration is the first choice for clinical application of peptides and proteins due to their intrinsic properties. Therapeutic proteins have increasingly been developed as subcutaneous injection, a route which offers many significant practical benefits195. Peptides and proteins are prone to degradation by enzymes and therefore usually have short half-lives in vivo. Encapsulation into micro- or nanoparticles with biocompatible and absorbable polymers can provide protection and sustained release for these extremely vulnerable macromolecules to achieve prolonged efficacy196. An alternative strategy is loading peptides and proteins into in situ forming depots, which can be injected as viscous liquids and subsequently solidified to hydrogels triggered by the local microenvironment197 or to implants driven by self-assembly198. The common characteristic of these delivery systems is ease of preparation and administration with a standard syringe. Payloads can be released for several weeks.
Great efforts have also been paid for non-invasive delivery of peptides and proteins, among which oral administration is of most interest to improve patient compliance. Oral administration is a preferred method for drug delivery, though achieving effective drug delivery of biomacromolecules is especially challenging due to the multiple absorption barriers presented in the gastrointestinal (GI) tract including the harsh GI environment for biomacromolecules, the trap of mucus and the impermeability of epithelial cells. Proper encapsulation by delivery systems could provide protection for the biomacromolecules, and help to bypass the absorption barriers.
Peppas's group198 recently reviewed the challenges to deliver peptide and protein orally, and discussed the possible approaches. These include the aid of permeation enhancers, protease inhibitors, and polymeric or polysaccharide carriers. Although the oral bioavailability has been significant improved by these approaches, it is often limited to 0.5%–1.0% in clinical trials compared to i.v. or s.c. administration199. A typical example is oral administration of semaglutide in a tablet formulation with the absorption enhancer N-[8-(2-hydroxybenzoyl)amino] caprylate (SNAC). Microenvironment-responsive hydrogel allowed for protection of protein in the stomach but release in the small intestine. Therefore, this seems to be a promising oral peptide and protein carrier200. Self-emulsifying drug delivery system (SEDDS) was employed to deliver hydrophilic peptides and proteins in the presence of hydrophobic ion-pairing agents, and showed an oral bioavailability at least in the single digit percentage range201. The functionalized nanocarriers were also the potential candidates of oral macromolecule delivery systems202.
For overcoming the barriers of mucus, mucus-adhesive NPs (MAPs) and mucus-penetrating NPs (MPPs) have been developed, and results have revealed that MPPs diffused more freely than MAPs, exhibiting improved distribution and access to the absorptive epithelium203, 204. In addition, shape and elasticity are also reported to affect mucus diffusion205, 206. For overcoming the barriers of epithelial cells, exploiting receptor/transporter-mediated pathways, such as neonatal Fc receptor (FcRn), transferrin receptor (TfR), and apical sodium-dependent bile acid transporter (ASBT), have also exhibited enhanced cellular internalization and improved permeability of loaded therapeutics207, 208, 209. It is worth noting that an increasing number of studies realize that the properties required for efficient mucus penetration are typically unsuitable for cellular internalization. Thus, NPs with the capacity to overcome both the mucus and epithelial cell barriers have been extensively explored210. Additionally, with further understanding of the physiology of epithelium, researchers are now developing NPs capable of overcoming intracellular barriers, such as lysosome-escape and exocytosis at the basolateral membranes207, 211.
Oral delivery of proteins has made significant progress, and several oral insulin and glucagon-likepeptide1 (GLP-1) analogue formulations are under clinical trials212, 213. For example, Diasome has developed a liposomal formulation, which has been shown in phase 2 human studies to be very effective in lowering blood glucose levels in Type 1 and Type 2 diabetic patients using very low amounts of oral insulin.
Oral inhalation provides the opportunity for drugs to target the respiratory tract or alternatively be absorbed in the lung for systemic delivery. Inhalation is a particularly attractive route for peptides and proteins. One inhaled dornase alfa (Pulmozyme) and two inhaled insulin (Exubera and Afrezza) have thus far been launched into the clinic. The relative bioavailability of insulin is 33% for Afrezza, almost double the value reported for Exubera214. Although Exubera was subsequently withdrawn from the market, more than a dozen of inhaled protein therapeutics have entered various clinical trials for treatment of respiratory or lung diseases215. Protein nebulization and excipients are important parameters for the inhaled formulations. The former modulates the aerodynamic profiles of aerosols, while the latter offer opportunities to stabilize the protein.
Except for respiratory tract mucosa, buccal202, ocular216, and nasal mucosa217, are all the active targets for peptides and proteins delivery. For example, Oral-Lyn™, an oral insulin spray formulation, has been developed by Generex Biotechnology to deliver insulin through buccal mucosa. When compared to insulin injection administered subcutaneously, Oral-Lyn™ shows a faster onset of action and a shorter duration of action.
Macromolecules cannot penetrate the human skin without assistance. Fortunately, recent advances in polymeric microneedles paved a way for transdermal delivery of proteins and vaccines218. Especially bioresponsive microneedles can be triggered by the physiological signals219, allowing the precise, on-demand release of protein therapeutics220, 221. Nowadays, several clinical trials are ongoing to investigate the effectiveness of microneedles for protein drug delivery, and developers of related products are optimistic for success.
In recent years, immune checkpoint inhibitors (ICI) including peptides and antibodies, have made great progress in cancer immunotherapy by antagonizing immune checkpoint proteins, promoting the activation of T cells, thereby producing anti-tumor immune effects. However, due to the frequently occurring toxic side effects, the ICI delivery has become an important topic in this field. In view of this, Wang et al.222 have recently used the design of novel biomaterials to deliver checkpoint inhibitor antibodies to the TME to improve therapeutic efficacy. For example, a biodegradable MN has been developed to control the delivery of anti-PD-1(aPD1) to melanoma, compared with free-form aPD1, MN enhanced the retention of aPD1 in tumor microenvironment and induced a strong immune response to melanoma in B16F10 mice. In addition, MN patches could also serve as a platform for the combined delivery of other immunocheckpoint inhibitors, such as anti-CTLA4 (aCTLA4)223. In general, the strategy of local melanoma treatment based on biodegradable MN improved the efficacy of immunocheckpoint inhibitors and reduces systemic toxicity. Additionally, checkpoint suppression in combination with other immunomodulators showed synergistically increased antitumor activity. Wang et al.224 demonstrated a novel vector for controlling the release of anti-PD1 antibodies and CpG oligonucleotides (CpG ODNs) in response to postoperative inflammatory conditions. DNCs (DNA nano-cocoons) contained aPD1 and inflammatory reactive triglyceride monostearate (TGMS) nanoparticles caging a restriction enzyme inside, which could specifically divide DNCs into short fragments of CpG ODNs. After injecting TGMS nanoparticles into the tumor resection site, they are digested by inflammation-related proteases and lysed, releasing caged enzymes, which cleaved DNCs into CpG ODN and released aPD1. The CpG DNA-based carrier can not only serve as the treatment loading matrix of aPD1, but also be cut into another drug CpG fragment to the active DC by enzymes, so as to improve the therapeutic effect.
7. Future perspective of drug delivery research
In the past three years, great progress in novel DDS has been achieved in the delivery strategies, construction techniques, and potential materials for improving the bioavailability, biocompatibility and therapeutic index of drugs. In fact, many presently-formulated prescription drugs have unfavorable physicochemical and pharmacokinetic properties, along with various limitations on the dosage regimen and undesirable side effects in the conventional dosage form. The development of new drug delivery systems and new formulations would be potential and promising approaches for increasing these therapeutic indices and reducing side effects. However, it should not be neglected to balance druggability and functional design, as the clinical application should be the final focus of our work. Also, decades of experience in drug research and development has demonstrated that there would be no new pharmaceutical preparations without technological innovation. It is imperative to strengthen the research and development of innovative technologies and drug delivery systems within the pharmaceutical industry. Certainly, a novel technical invention becomes an innovative technology to be applied in drug delivery system, requiring long-term testing, revision and optimization.
Acknowledgments
This review paper was supported by the projects of National Natural Science Foundation of China (Grant Nos. 81773650, 81690264 and 81673376), the Drug Innovation Major Project of China (Grant No. 2018ZX09721003-004).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Park K. Controlled drug delivery systems: past forward and future back. J Control Release. 2014;190:3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenholz Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 3.Kennon S., Tasch E.G., Arm R.N., Wig P.P. The relationship between plaque scores and the development of caries in adult dentition. Clin Prev Dent. 1979;1:26–31. [PubMed] [Google Scholar]
- 4.Li M., Shi K.R., Tang X., Wei J.J., Cun X.L., Chen X.X. pH-sensitive folic acid and dNP2 peptide dual-modified liposome for enhanced targeted chemotherapy of glioma. Eur J Pharm Sci. 2018;124:240–248. doi: 10.1016/j.ejps.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Zhang T.H., Chen X., Xiao C.S., Zhuang X.L., Chen X.S. Synthesis of a phenylboronic ester-linked PEG-lipid conjugate for ROS-responsive drug delivery. Polym Chem. 2017;8:6209–6216. [Google Scholar]
- 6.Chi Y.Y., Yin X.L., Sun K.X., Feng S.S., Liu J.H., Chen D.Q. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Release. 2017;261:113–125. doi: 10.1016/j.jconrel.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Fouladi F., Steffen K.J., Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug Chem. 2017;28:857–868. doi: 10.1021/acs.bioconjchem.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y., Li N.X., Yin H.L., Chen T.Y., Yang Q., Wu M. Thermo- and pH-responsive, lipid-coated, mesoporous silica nanoparticle-based dual drug delivery system to improve the antitumor effect of hydrophobic drugs. Mol Pharm. 2019;16:422–436. doi: 10.1021/acs.molpharmaceut.8b01073. [DOI] [PubMed] [Google Scholar]
- 9.Li Q.P., Li W., Di H.X., Luo L.H., Zhu C.Q., Yang J. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J Control Release. 2018;277:114–125. doi: 10.1016/j.jconrel.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Santos M.A., Goertz D.E., Hynynen K. Focused ultrasound hyperthermia mediated drug delivery using thermosensitive liposomes and visualized with in vivo two-photon microscopy. Theranostics. 2017;7:2718–2731. doi: 10.7150/thno.19662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvatore A., Montis C., Berti D., Baglioni P. Multifunctional magnetoliposomes for sequential controlled release. ACS Nano. 2016;10:7749–7760. doi: 10.1021/acsnano.6b03194. [DOI] [PubMed] [Google Scholar]
- 12.Deng W., Chen W.J., Clement S., Guller A., Zhao Z.J., Engel A. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat Commun. 2018;9:2713. doi: 10.1038/s41467-018-05118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rwei A.Y., Paris J.L., Wang B., Wang W.P., Axon C.D., Vallet-Regí M. Ultrasound-triggered local anaesthesia. Nat Biomed Eng. 2017;1:644–653. doi: 10.1038/s41551-017-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv S.X., Wu Y.C., Cai K.M., He H., Li Y.J., Lan M. High drug loading and sub-quantitative loading efficiency of polymeric micelles driven by donor-receptor coordination interactions. J Am Chem Soc. 2018;140:1235–1238. doi: 10.1021/jacs.7b12776. [DOI] [PubMed] [Google Scholar]
- 15.Wang T.T., Wang D.G., Liu J.P., Feng B., Zhou F.Y., Zhang H.W. Acidity-triggered ligand-presenting nanoparticles to overcome sequential drug delivery barriers to tumors. Nano Lett. 2017;17:5429–5436. doi: 10.1021/acs.nanolett.7b02031. [DOI] [PubMed] [Google Scholar]
- 16.Li S.P., Zhang Y.L., Wang J., Zhao Y., Ji T.J., Zhao X. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat Biomed Eng. 2017;1:667–679. doi: 10.1038/s41551-017-0115-8. [DOI] [PubMed] [Google Scholar]
- 17.Ling X., Chen X., Riddell I.A., Tao W., Wang J.Q., Hollett G. Glutathione-scavenging poly(disulfide amide) nanoparticles for the effective delivery of Pt(IV) prodrugs and reversal of cisplatin resistance. Nano Lett. 2018;18:4618–4625. doi: 10.1021/acs.nanolett.8b01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian C.G., Feng P.J., Yu J.C., Chen Y.L., Hu Q.Y., Sun W.J. Anaerobe-inspired anticancer nanovesicles. Angew Chem Int Ed. 2017;56:2588–2593. doi: 10.1002/anie.201611783. [DOI] [PubMed] [Google Scholar]
- 19.Deng Z.Y., Qian Y.F., Yu Y.Q., Liu G.H., Hu J.M., Zhang G.Y. Engineering intracellular delivery nanocarriers and nanoreactors from oxidation-responsive polymersomes via synchronized bilayer cross-linking and permeabilizing inside live cells. J Am Chem Soc. 2016;138:10452–10466. doi: 10.1021/jacs.6b04115. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z.Q., Zou Y.L., He H., Rao J.M., Ji S.S., Cui X.N. Bifunctional platinated nanoparticles for photoinduced tumor ablation. Adv Mater. 2016;28:10155–10164. doi: 10.1002/adma.201602738. [DOI] [PubMed] [Google Scholar]
- 21.Ye S.Y., Rao J.M., Qiu S.H., Zhao J.L., He H., Yan Z.L. Rational design of conjugated photosensitizers with controllable photoconversion for dually cooperative phototherapy. Adv Mater. 2018;30:1801216. doi: 10.1002/adma.201801216. [DOI] [PubMed] [Google Scholar]
- 22.Gao H.L., He Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin Drug Deliv. 2014;11:409–420. doi: 10.1517/17425247.2014.877442. [DOI] [PubMed] [Google Scholar]
- 23.Guan X.W., Guo Z.P., Wang T.H., Lin L., Chen J., Tian H.Y. A pH-responsive detachable PEG shielding strategy for gene delivery system in cancer therapy. Biomacromolecules. 2017;18:1342–1349. doi: 10.1021/acs.biomac.7b00080. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Y., Zhou Z.X., Fan M.M., Gong T., Zhang Z.R., Sun X. PEGylated cationic vectors containing a protease-sensitive peptide as a miRNA delivery system for treating breast cancer. Mol Pharm. 2017;14:81–92. doi: 10.1021/acs.molpharmaceut.6b00726. [DOI] [PubMed] [Google Scholar]
- 25.Gao H.L., Zhang Q.Y., Yu Z.Q., He Q. Cell-penetrating peptide-based intelligent liposomal systems for enhanced drug delivery. Curr Pharm Biotechnol. 2014;15:210–219. doi: 10.2174/1389201015666140617092552. [DOI] [PubMed] [Google Scholar]
- 26.Chen X.L., Liu L.S., Jiang C. Charge-reversal nanoparticles: novel targeted drug delivery carriers. Acta Pharm Sin B. 2016;6:261–267. doi: 10.1016/j.apsb.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv G.X., Guo W.S., Zhang W., Zhang T.B., Li S.Y., Chen S.Z. Near-infrared emission CuInS/ZnS quantum dots: all-in-one theranostic nanomedicines with intrinsic fluorescence/photoacoustic imaging for tumor phototherapy. ACS Nano. 2016;10:9637–9645. doi: 10.1021/acsnano.6b05419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Huang P., Chen X.Y. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater. 2016;28:7340–7364. doi: 10.1002/adma.201601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R., Xiao W., Hu C., Xie R., Gao H.L. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Hu C., Cun X.L., Ruan S.B., Liu R., Xiao W., Yang X.T. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Ruan S.B., Hu C., Tang X., Cun X.L., Xiao W., Shi K.R. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano. 2016;10:10086–10098. doi: 10.1021/acsnano.6b05070. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P., Xu X.Y., Chen Y.P., Xiao M.Q., Feng B., Tian K.X. Protein corona between nanoparticles and bacterial proteins in activated sludge: characterization and effect on nanoparticle aggregation. Bioresour Technol. 2018;250:10–16. doi: 10.1016/j.biortech.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Yang P.P., Luo Q., Qi G.B., Gao Y.J., Li B.N., Zhang J.P. Host materials transformable in tumor microenvironment for homing theranostics. Adv Mater. 2017;29:1605869. doi: 10.1002/adma.201605869. [DOI] [PubMed] [Google Scholar]
- 34.Subbiah V., Grilley-Olson J.E., Combest A.J., Sharma N., Tran R.H., Bobe I. Phase Ib/II Trial of NC-6004 (Nanoparticle Cisplatin) plus gemcitabine in patients with advanced solid tumors. Clin Cancer Res. 2018;24:43–51. doi: 10.1158/1078-0432.CCR-17-1114. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M., Liu E.G., Cui Y., Huang Y.Z. Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol Med. 2017;14:212–227. doi: 10.20892/j.issn.2095-3941.2017.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng H.G., Chen B.F., Huang W., Tang Y.B., Jiang Y.F., Zhang W.Y. Reprogramming tumor-associated macrophages to reverse EGFRT790M resistance by dual-targeting codelivery of gefitinib/vorinostat. Nano Lett. 2017;17:7684–7690. doi: 10.1021/acs.nanolett.7b03756. [DOI] [PubMed] [Google Scholar]
- 37.Kang X.J., Wang H.Y., Peng H.G., Chen B.F., Zhang W.Y., Wu A.H. Codelivery of dihydroartemisinin and doxorubicin in mannosylated liposomes for drug-resistant colon cancer therapy. Acta Pharmacol Sin. 2017;38:885–896. doi: 10.1038/aps.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang X.J., Zheng Z.N., Liu Z.H., Wang H.Y., Zhao Y.G., Zhang W.Y. Liposomal codelivery of doxorubicin and andrographolide inhibits breast cancer growth and metastasis. Mol Pharm. 2018;15:1618–1626. doi: 10.1021/acs.molpharmaceut.7b01164. [DOI] [PubMed] [Google Scholar]
- 39.Kemp J.A., Shim M.S., Heo C.Y., Kwon Y.J. “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev. 2016;98:3–18. doi: 10.1016/j.addr.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Krauss A.C., Gao X., Li L., Manning M.L., Patel P., Fu W.T. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25:2685–2690. doi: 10.1158/1078-0432.CCR-18-2990. [DOI] [PubMed] [Google Scholar]
- 41.Cheng W., Nie J.P., Gao N.S., Liu G., Tao W., Xiao X.J. A multifunctional nanoplatform against multidrug resistant cancer: merging the best of targeted chemo/gene/photothermal therapy. Adv Funct Mater. 2017;27:1704135. [Google Scholar]
- 42.Zhang F.W., Ni Q.Q., Jacobson O., Cheng S.Y., Liao A., Wang Z.T. Polymeric nanoparticles with a glutathione-sensitive heterodimeric multifunctional prodrug for in vivo drug monitoring and synergistic cancer therapy. Angew Chem Int Ed. 2018;57:7066–7070. doi: 10.1002/anie.201801984. [DOI] [PubMed] [Google Scholar]
- 43.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 44.Qiao H., Cui Z.W., Yang S.B., Ji D.K., Wang Y.G., Yang Y. Targeting osteocytes to attenuate early breast cancer bone metastasis by theranostic upconversion nanoparticles with responsive plumbagin release. ACS Nano. 2017;11:7259–7273. doi: 10.1021/acsnano.7b03197. [DOI] [PubMed] [Google Scholar]
- 45.Arranja A.G., Pathak V., Lammers T., Shi Y. Tumor-targeted nanomedicines for cancer theranostics. Pharmacol Res. 2017;115:87–95. doi: 10.1016/j.phrs.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb B.A., Chimenti M., Jacobson M.P., Barber D.L. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Wang Y.G., Huang G., Gao J.M. Cooperativity principles in self-assembled nanomedicine. Chem Rev. 2018;118:5359–5391. doi: 10.1021/acs.chemrev.8b00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y.G., Zhou K.J., Huang G., Hensley C., Huang X.N., Ma X.P. A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13:204–212. doi: 10.1038/nmat3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao T., Huang G., Li Y., Yang S.C., Ramezani S., Lin Z.Q. A transistor-like pH nanoprobe for tumour detection and image-guided surgery. Nat Biomed Eng. 2016;1 doi: 10.1038/s41551-016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo M., Wang H., Wang Z.H., Cai H.C., Lu Z.G., Li Y. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X.D., Wu J., Liu Y.L., Yu M., Zhao L.L., Zhu X. Ultra-pH-responsive and tumor-penetrating nanoplatform for targeted siRNA delivery with robust anti-cancer efficacy. Angew Chem Int Ed. 2016;55:7091–7094. doi: 10.1002/anie.201601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alkilany A.M., Thompson L.B., Boulos S.P., Sisco P.N., Murphy C.J. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64:190–199. doi: 10.1016/j.addr.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Shanmugam V., Selvakumar S., Yeh C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem Soc Rev. 2014;43:6254–6287. doi: 10.1039/c4cs00011k. [DOI] [PubMed] [Google Scholar]
- 54.Morgan J., Oseroff A.R. Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Deliv Rev. 2001;49:71–86. doi: 10.1016/s0169-409x(01)00126-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X., Yang C.X., Chen L.G., Yan X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat Commun. 2017;8:14998. doi: 10.1038/ncomms14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu S.T., Zhu X.Y., Zhang C., Huang W., Zhou Y.F., Yan D.Y. Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat Commun. 2018;9:2053. doi: 10.1038/s41467-018-04318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X.S., Kwon N., Guo T., Liu Z., Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew Chem Int Ed. 2018;57:11522–11531. doi: 10.1002/anie.201805138. [DOI] [PubMed] [Google Scholar]
- 58.Yue X.L., Zhang Q., Dai Z.F. Near-infrared light-activatable polymeric nanoformulations for combined therapy and imaging of cancer. Adv Drug Deliv Rev. 2017;115:155–170. doi: 10.1016/j.addr.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Chen M., Liang X.L., Gao C., Zhao R.R., Zhang N.S., Wang S.M. Ultrasound triggered conversion of porphyrin/camptothecin-fluoroxyuridine triad microbubbles into nanoparticles overcomes multidrug resistance in colorectal cancer. ACS Nano. 2018;12:7312–7326. doi: 10.1021/acsnano.8b03674. [DOI] [PubMed] [Google Scholar]
- 60.Shi J.J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu M.K., Park J., Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zou L.L., Wang H., He B., Zeng L.J., Tan T., Cao H.Q. Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics. 2016;6:762–772. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang P.F., Liu G., Chen X.Y. Nanobiotechnology: cell membrane-based delivery systems. Nano Today. 2017;13:7–9. doi: 10.1016/j.nantod.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S., Michael I.J., Park J., Granick S., Cho Y.K. Cloaked exosomes: biocompatible, durable, and degradable encapsulation. Small. 2018;14:1802052. doi: 10.1002/smll.201802052. [DOI] [PubMed] [Google Scholar]
- 65.Ingato D., Edson J.A., Zakharian M., Kwon Y.J. Cancer cell-derived, drug-loaded nanovesicles induced by sulfhydryl-blocking for effective and safe cancer therapy. ACS Nano. 2018;12:9568–9577. doi: 10.1021/acsnano.8b05377. [DOI] [PubMed] [Google Scholar]
- 66.Xue J.W., Zhao Z.K., Zhang L., Xue L.J., Shen S.Y., Wen Y.J. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 67.Wu T.T., Zhang D., Qiao Q., Qin X.Y., Yang C.L., Kong M. Biomimetic nanovesicles for enhanced antitumor activity of combinational photothermal and chemotherapy. Mol Pharm. 2018;15:1341–1352. doi: 10.1021/acs.molpharmaceut.7b01142. [DOI] [PubMed] [Google Scholar]
- 68.Molinaro R., Corbo C., Martinez J.O., Taraballi F., Evangelopoulos M., Minardi S. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu C., Yang X.Y., Fu X., Tian R., Jacobson O., Wang Z.T. Converting red blood cells to efficient microreactors for blood detoxification. Adv Mater. 2017;29:1603673. doi: 10.1002/adma.201603673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaneti L., Bronshtein T., Malkah Dayan N., Kovregina I., Letko Khait N., Lupu-Haber Y. Nanoghosts as a novel natural nonviral gene delivery platform safely targeting multiple cancers. Nano Lett. 2016;16:1574–1582. doi: 10.1021/acs.nanolett.5b04237. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song Q.L., Yin Y.J., Shang L.H., Wu T.T., Zhang D., Kong M. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017;17:6366–6375. doi: 10.1021/acs.nanolett.7b03186. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z., Zhao P.F., Luo Z.Y., Zheng M.B., Tian H., Gong P. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 74.Kroll A.V., Fang R.H., Jiang Y., Zhou J.R., Wei X.L., Yu C.L. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29:1703969. doi: 10.1002/adma.201703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang T., Zhu Q.Q., Wei D., Feng J.X., Yao J.H., Jiang T.Z. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11:1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 76.Wu T.T., Qi Y., Zhang D., Song Q.L., Yang C.L., Hu X.M. Bone marrow dendritic cells derived microvesicles for combinational immunochemotherapy against tumor. Adv Funct Mater. 2017;27:1703191. [Google Scholar]
- 77.Usman W.M., Pham T.C., Kwok Y.Y., Vu L.T., Ma V., Peng B.Y. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. 2018;9:2359. doi: 10.1038/s41467-018-04791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lizotte P.H., Wen A.M., Sheen M.R., Fields J., Rojanasopondist P., Steinmetz N.F. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nanotechnol. 2016;11:295–303. doi: 10.1038/nnano.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lebel M.E., Chartrand K., Tarrab E., Savard P., Leclerc D., Lamarre A. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Lett. 2016;16:1826–1832. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- 81.Johnsen K.B., Bak M., Kempen P.J., Melander F., Burkhart A., Thomsen M.S. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics. 2018;8:3416–3436. doi: 10.7150/thno.25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng S., Wang Y.H., Li N., Li C. Enhanced cellular uptake and tumor penetration of nanoparticles by imprinting the “hidden” part of membrane receptors for targeted drug delivery. Chem Commun. 2017;53:11114–11117. doi: 10.1039/c7cc05894b. [DOI] [PubMed] [Google Scholar]
- 83.Kedmi R., Veiga N., Ramishetti S., Goldsmith M., Rosenblum D., Dammes N. A modular platform for targeted RNAi therapeutics. Nat Nanotechnol. 2018;13:214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 84.Zheng Y.Q., Ji X.Y., Yu B.C., Ji K.L., Gallo D., Csizmadia E. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat Chem. 2018;10:787–794. doi: 10.1038/s41557-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott L.J. Brentuximab vedotin: a review in CD30-positive hodgkin lymphoma. Drugs. 2017;77:435–445. doi: 10.1007/s40265-017-0705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Appelbaum F.R., Bernstein I.D. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130:2373–2376. doi: 10.1182/blood-2017-09-797712. [DOI] [PubMed] [Google Scholar]
- 87.Hurvitz S.A., Martin M., Symmans W.F., Jung K.H., Huang C.S., Thompson A.M. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 88.Oller-Salvia B., Sánchez-Navarro M., Ciudad S., Guiu M., Arranz-Gibert P., Garcia C. MiniAp-4: a venom-inspired peptidomimetic for brain delivery. Angew Chem Int Ed. 2016;55:572–575. doi: 10.1002/anie.201508445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guan J., Shen Q., Zhang Z., Jiang Z.X., Yang Y., Lou M.Q. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982. doi: 10.1038/s41467-018-05384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sempkowski M., Zhu C., Menzenski M.Z., Kevrekidis I.G., Bruchertseifer F., Morgenstern A. Sticky patches on lipid nanoparticles enable the selective targeting and killing of untargetable cancer cells. Langmuir. 2016;32:8329–8338. doi: 10.1021/acs.langmuir.6b01464. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Deng C.Y., Liu S., Wu J., Chen Z.B., Li C. Active targeting of tumors through conformational epitope imprinting. Angew Chem Int Ed. 2015;54:5157–5160. doi: 10.1002/anie.201412114. [DOI] [PubMed] [Google Scholar]
- 92.Liu S., Bi Q.Y., Long Y.Y., Li Z.X., Bhattacharyya S., Li C. Inducible epitope imprinting: 'generating' the required binding site in membrane receptors for targeted drug delivery. Nanoscale. 2017;9:5394–5397. doi: 10.1039/c6nr09449j. [DOI] [PubMed] [Google Scholar]
- 93.Norman J., Madurawe R.D., Moore C.M.V., Khan M.A., Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50. doi: 10.1016/j.addr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Giordano R.A., Wu B.M., Borland S.W., Cima L.G., Sachs E.M., Cima M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J Biomater Sci Polym Ed. 1996;8:63–75. doi: 10.1163/156856297x00588. [DOI] [PubMed] [Google Scholar]
- 95.Talebian S., Foroughi J., Wade S.J., Vine K.L., Dolatshahi-Pirouz A., Mehrali M. Biopolymers for antitumor implantable drug delivery systems: recent advances and future outlook. Adv Mater. 2018;30:1706665. doi: 10.1002/adma.201706665. [DOI] [PubMed] [Google Scholar]
- 96.Goyanes A., Buanz A.B.M., Basit A.W., Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476:88–92. doi: 10.1016/j.ijpharm.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 97.Khaled S.A., Burley J.C., Alexander M.R., Yang J., Roberts C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 98.Kyobula M., Adedeji A., Alexander M.R., Saleh E., Wildman R., Ashcroft I. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017;261:207–215. doi: 10.1016/j.jconrel.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 99.Gupta M.K., Meng F.B., Johnson B.N., Kong Y.L., Tian L.M., Yeh Y.W. 3D printed programmable release capsules. Nano Lett. 2015;15:5321–5329. doi: 10.1021/acs.nanolett.5b01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang F.Y., Su Y.C., Zhang J.T., Dinunzio J., Leone A., Huang C.B. Rheology guided rational selection of processing temperature to prepare copovidone/nifedipine amorphous solid dispersions via hot melt extrusion (HME) Mol Pharm. 2016;13:3494–3505. doi: 10.1021/acs.molpharmaceut.6b00516. [DOI] [PubMed] [Google Scholar]
- 101.Bochmann E.S., Neumann D., Gryczke A., Wagner K.G. Micro-scale prediction method for API-solubility in polymeric matrices and process model for forming amorphous solid dispersion by hot-melt extrusion. Eur J Pharm Biopharm. 2016;107:40–48. doi: 10.1016/j.ejpb.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 102.Skowyra J., Pietrzak K., Alhnan M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur J Pharm Sci. 2015;68:11–17. doi: 10.1016/j.ejps.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Holländer J., Genina N., Jukarainen H., Khajeheian M., Rosling A., Mäkilä E. Three-dimensional printed PCL-based implantable prototypes of medical devices for controlled drug delivery. J Pharm Sci. 2016;105:2665–2676. doi: 10.1016/j.xphs.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Goyanes A., Det-Amornrat U., Wang J., Basit A.W., Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–48. doi: 10.1016/j.jconrel.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 105.Li Q.J., Wen H.Y., Jia D.Y., Guan X.Y., Pan H., Yang Y. Preparation and investigation of controlled-release glipizide novel oral device with three-dimensional printing. Int J Pharm. 2017;525:5–11. doi: 10.1016/j.ijpharm.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 106.Boetker J., Water J.J., Aho J., Arnfast L., Bohr A., Rantanen J. Modifying release characteristics from 3D printed drug-eluting products. Eur J Pharm Sci. 2016;90:47–52. doi: 10.1016/j.ejps.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 107.Goole J., Amighi K. 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems. Int J Pharm. 2016;499:376–394. doi: 10.1016/j.ijpharm.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 108.Ma G.J., Wu C.W. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23. doi: 10.1016/j.jconrel.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 109.Kwon K.M., Lim S.M., Choi S., Kim D.H., Jin H.E., Jee G. Microneedles: quick and easy delivery methods of vaccines. Clin Exp Vaccine Res. 2017;6:156–159. doi: 10.7774/cevr.2017.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang H.W., Ye L., Guo X.D., Yang C.L., Compans R.W., Prausnitz M.R. Ebola vaccination using a DNA vaccine coated on PLGA-PLL/γPGA nanoparticles administered using a microneedle patch. Adv Healthc Mater. 2017;6:1600750. doi: 10.1002/adhm.201600750. [DOI] [PubMed] [Google Scholar]
- 111.Johnson K.L., McCann C.M., Wilkinson J.L., Jones M., Tebo B.M., West M. Dissolved Mn(III) in water treatment works: prevalence and significance. Water Res. 2018;140:181–190. doi: 10.1016/j.watres.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 112.Than A., Liu C.H., Chang H., Duong P.K., Cheung C.M.G., Xu C.J. Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nat Commun. 2018;9:4433. doi: 10.1038/s41467-018-06981-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jin X., Zhu D.D., Chen B.Z., Ashfaq M., Guo X.D. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev. 2018;127:119–137. doi: 10.1016/j.addr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 114.Bhatnagar S., Dave K., Venuganti V.V.K. Microneedles in the clinic. J Control Release. 2017;260:164–182. doi: 10.1016/j.jconrel.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 115.Lee H., Choi T.K., Lee Y.B., Cho H.R., Ghaffari R., Wang L. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11:566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 116.Lee H., Song C., Hong Y.S., Kim M.S., Cho H.R., Kang T. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3 doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu J.C., Zhang Y.Q., Ye Y.Q., DiSanto R., Sun W.J., Ranson D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu J.C., Qian C.G., Zhang Y.Q., Cui Z., Zhu Y., Shen Q.D. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17:733–739. doi: 10.1021/acs.nanolett.6b03848. [DOI] [PubMed] [Google Scholar]
- 119.Tong Z., Zhou J., Zhong J., Tang Q., Lei Z., Luo H. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces. 2018;10:20014–20024. doi: 10.1021/acsami.8b04484. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y.Q., Yu J.C., Wang J.Q., Hanne N.J., Cui Z., Qian C.G. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation. Adv Mater. 2017;29:1604043. doi: 10.1002/adma.201604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hirobe S., Azukizawa H., Hanafusa T., Matsuo K., Quan Y.S., Kamiyama F. Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials. 2015;57:50–58. doi: 10.1016/j.biomaterials.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 122.Arya J., Henry S., Kalluri H., McAllister D.V., Pewin W.P., Prausnitz M.R. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7. doi: 10.1016/j.biomaterials.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Malamatari M., Taylor K.M.G., Malamataris S., Douroumis D., Kachrimanis K. Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov Today. 2018;23:534–547. doi: 10.1016/j.drudis.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 124.Sigfridsson K., Skantze P., Skantze U., Svensson L., Löfgren L., Nordell P. Nanocrystal formulations of a poorly soluble drug. 2. Evaluation of nanocrystal liver uptake and distribution after intravenous administration to mice. Int J Pharm. 2017;524:248–256. doi: 10.1016/j.ijpharm.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 125.Bi C., Miao X.Q., Chow S.F., Wu W.J., Yan R., Liao Y.H. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomedicine. 2017;13:943–953. doi: 10.1016/j.nano.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 127.Yang L., Hong J., Di J., Guo Y., Han M., Liu M. 10-Hydroxycamptothecin (HCPT) nanosuspensions stabilized by mPEG1000-HCPT conjugate: high stabilizing efficiency and improved antitumor efficacy. Int J Nanomed. 2017;12:3681–3695. doi: 10.2147/IJN.S134005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li Y.J., Hong J.Y., Li H.W., Qi X.Y., Guo Y.F., Han M.H. Genkwanin nanosuspensions: a novel and potential antitumor drug in breast carcinoma therapy. Drug Deliv. 2017;24:1491–1500. doi: 10.1080/10717544.2017.1384519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hadiwinoto G.D., Lip Kwok P.C., Lakerveld R. A review on recent technologies for the manufacture of pulmonary drugs. Ther Deliv. 2018;9:47–70. doi: 10.4155/tde-2017-0083. [DOI] [PubMed] [Google Scholar]
- 130.Hidalgo A., Cruz A., Pérez-Gil J. Pulmonary surfactant and nanocarriers: toxicity versus combined nanomedical applications. Biochim Biophys Acta. 2017;1859:1740–1748. doi: 10.1016/j.bbamem.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 131.Khurana L.K., Singh R., Singh H., Sharma M. Systematic development and optimization of an in-situ gelling system for moxifloxacin ocular nanosuspension using high-pressure homogenization with an improved encapsulation efficiency. Curr Pharm Des. 2018;24:1434–1445. doi: 10.2174/1381612824666180403115106. [DOI] [PubMed] [Google Scholar]
- 132.Maged A., Mahmoud A.A., Ghorab M.M. Nano spray drying technique as a novel approach to formulate stable econazole nitrate nanosuspension formulations for ocular use. Mol Pharm. 2016;13:2951–2965. doi: 10.1021/acs.molpharmaceut.6b00167. [DOI] [PubMed] [Google Scholar]
- 133.Romero G.B., Keck C.M., Müller R.H., Bou-Chacra N.A. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215–222. doi: 10.1016/j.ejpb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 134.Pelikh O., Stahr P.L., Huang J., Gerst M., Scholz P., Dietrich H. Nanocrystals for improved dermal drug delivery. Eur J Pharm Biopharm. 2018;128:170–178. doi: 10.1016/j.ejpb.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 135.Pireddu R., Caddeo C., Valenti D., Marongiu F., Scano A., Ennas G. Diclofenac acid nanocrystals as an effective strategy to reduce in vivo skin inflammation by improving dermal drug bioavailability. Colloids Surf B Biointerfaces. 2016;143:64–70. doi: 10.1016/j.colsurfb.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 136.Suri G.S., Kaur A., Sen T. A recent trend of drug-nanoparticles in suspension for the application in drug delivery. Nanomedicine. 2016;11:2861–2876. doi: 10.2217/nnm-2016-0238. [DOI] [PubMed] [Google Scholar]
- 137.Chang T.L., Zhan H.L., Liang D.N., Liang J.F. Nanocrystal technology for drug formulation and delivery. Front Chem Sci Eng. 2015;9:1–14. [Google Scholar]
- 138.Romero G.B., Keck C.M., Müller R.H. Simple low-cost miniaturization approach for pharmaceutical nanocrystals production. Int J Pharm. 2016;501:236–244. doi: 10.1016/j.ijpharm.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 139.Han X.F., Wang M.L., Ma Z.H., Xue P., Wang Y.J. A new approach to produce drug nanosuspensions CO2-assisted effervescence to produce drug nanosuspensions. Colloids Surf B Biointerfaces. 2016;143:107–110. doi: 10.1016/j.colsurfb.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y.L., Han X.F., Wang J., Wang Y.J. Preparation, characterization and in vivo evaluation of amorphous tacrolimus nanosuspensions produced using CO2-assisted in situ nanoamorphization method. Int J Pharm. 2016;505:35–41. doi: 10.1016/j.ijpharm.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 141.Amly W., Karaman R. Recent updates in utilizing prodrugs in drug delivery (2013-2015) Exp Opin Drug Deliv. 2016;13:571–591. doi: 10.1517/17425247.2016.1142527. [DOI] [PubMed] [Google Scholar]
- 142.Du L., Jia J.W., Ge P.J., Jin Y.G. Self-assemblies of 5′-cholesteryl-ethyl-phosphoryl zidovudine. Colloid Surf B Biointerfaces. 2016;148:385–391. doi: 10.1016/j.colsurfb.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 143.Gaudin A., Yemisci M., Eroglu H., Lepetre-Mouelhi S., Turkoglu O.F., Dönmez-Demir B. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol. 2014;9:1054–1062. doi: 10.1038/nnano.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarett S.M., Werfel T.A., Chandra I., Jackson M.A., Kavanaugh T.E., Hattaway M.E. Hydrophobic interactions between polymeric carrier and palmitic acid-conjugated siRNA improve PEGylated polyplex stability and enhance in vivo pharmacokinetics and tumor gene silencing. Biomaterials. 2016;97:122–132. doi: 10.1016/j.biomaterials.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Du L., Zhang B.L., Lei Y.J., Wang S., Jin Y.G. Long-circulating and liver-targeted nanoassemblies of cyclic phosphoryl N-dodecanoyl gemcitabine for the treatment of hepatocellular carcinoma. Biomed Pharmacother. 2016;79:208–214. doi: 10.1016/j.biopha.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 146.Cheetham A.G., Zhang P.C., Lin Y.A., Lock L.L., Cui H.G. Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc. 2013;135:2907–2910. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Couvreur P., Stella B., Reddy L.H., Hillaireau H., Dubernet C., Desmaële D. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006;6:2544–2548. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 148.Huang X.X., Liao W.B., Xie Z.H., Chen D.S., Zhang C.Y. A pH-responsive prodrug delivery system self-assembled from acid-labile doxorubicin-conjugated amphiphilic pH-sensitive block copolymers. Mater Sci Eng C. 2018;90:27–37. doi: 10.1016/j.msec.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 149.Zuo J., Tong L., Du L., Yang M., Jin Y.G. Biomimetic nanoassemblies of 1-O-octodecyl-2-conjugated linoleoyl-sn-glycero-3-phosphatidyl gemcitabine with phospholipase A2-triggered degradation for the treatment of cancer. Colloids Surf B Biointerfaces. 2017;152:467–474. doi: 10.1016/j.colsurfb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 150.Luo C., Sun J., Sun B.J., Liu D., Miao L., Goodwin T.J. Facile fabrication of tumor redox-sensitive nanoassemblies of small-molecule oleate prodrug as potent chemotherapeutic nanomedicine. Small. 2016;12:6353–6362. doi: 10.1002/smll.201601597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rautio J., Kumpulainen H., Heimbach T., Oliyai R., Oh D., Järvinen T. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 152.Du L., Wu L.L., Jin Y.G., Jia J.W., Li M., Wang Y. Self-assembled drug delivery systems. Part 7: hepatocyte-targeted nanoassemblies of an adefovir lipid derivative with cytochrome P450-triggered drug release. Int J Pharm. 2014;472:1–9. doi: 10.1016/j.ijpharm.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 153.Zhang J.M., Song H.J., Ji S.L., Wang X.M., Huang P.S., Zhang C.N. NO prodrug-conjugated, self-assembled, pH-responsive and galactose receptor targeted nanoparticles for co-delivery of nitric oxide and doxorubicin. Nanoscale. 2018;10:4179–4188. doi: 10.1039/c7nr08176f. [DOI] [PubMed] [Google Scholar]
- 154.Han L.Q., Wang T.Q., Wu J.L., Yin X.L., Fang H., Zhang N. A facile route to form self-carried redox-responsive vorinostat nanodrug for effective solid tumor therapy. Int J Nanomed. 2016;11:6003–6022. doi: 10.2147/IJN.S118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo C., Sun J., Liu D., Sun B.J., Miao L., Musetti S. Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016;16:5401–5408. doi: 10.1021/acs.nanolett.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meng H., Zou Y., Zhong P., Meng F.H., Zhang J., Cheng R. A smart nano-prodrug platform with reactive drug loading, superb stability, and fast responsive drug release for targeted cancer therapy. Macromol Biosci. 2017;17:1600518. doi: 10.1002/mabi.201600518. [DOI] [PubMed] [Google Scholar]
- 157.Huang D., Zhuang Y.P., Shen H., Yang F., Wang X., Wu D.C. Acetal-linked PEGylated paclitaxel prodrugs forming free-paclitaxel-loaded pH-responsive micelles with high drug loading capacity and improved drug delivery. Mater Sci Eng C. 2018;82:60–68. doi: 10.1016/j.msec.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 158.Tao W., Zhu X.B., Yu X.H., Zeng X.W., Xiao Q.L., Zhang X.D. Black phosphorus nanosheets as a robust delivery platform for cancer theranostics. Adv Mater. 2017;29:1603276. doi: 10.1002/adma.201603276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou Y.X., Quan G.L., Wu Q.L., Zhang X.X., Niu B.Y., Wu B.Y. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm Sin B. 2018;8:165–177. doi: 10.1016/j.apsb.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeng X.W., Liu G., Tao W., Ma Y., Zhang X.D., He F. A drug-self-gated mesoporous antitumor nanoplatform based on pH-sensitive dynamic covalent bond. Adv Funct Mater. 2017;27:1605985. [Google Scholar]
- 161.Ji X.Y., Kong N., Wang J.Q., Li W.L., Xiao Y.L., Gan S.T. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv Mater. 2018;36:1803031. doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim C.K., Ghosh P., Rotello V.M. Multimodal drug delivery using gold nanoparticles. Nanoscale. 2009;1:61–67. doi: 10.1039/b9nr00112c. [DOI] [PubMed] [Google Scholar]
- 163.El-Sherbiny I.M., Elbaz N.M., Sedki M., Elgammal A., Yacoub M.H. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine. 2017;12:387–402. doi: 10.2217/nnm-2016-0341. [DOI] [PubMed] [Google Scholar]
- 164.Yao J., Li P.F., Li L., Yang M. Biochemistry and biomedicine of quantum dots: from biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018;74:36–55. doi: 10.1016/j.actbio.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 165.Liu J.Q., Cui L., Losic D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013;9:9243–9257. doi: 10.1016/j.actbio.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 166.Xu T.T., Xu X.Y., Gu Y., Fang L., Cao F. Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery. Int J Nanomed. 2018;13:917–937. doi: 10.2147/IJN.S148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liang R.Z., Wei M., Evans D.G., Duan X. Inorganic nanomaterials for bioimaging, targeted drug delivery and therapeutics. Chem Commun. 2014;50:14071–14081. doi: 10.1039/c4cc03118k. [DOI] [PubMed] [Google Scholar]
- 168.Chen T.T., Yi J.T., Zhao Y.Y., Chu X. Biomineralized metal-organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J Am Chem Soc. 2018;140:9912–9920. doi: 10.1021/jacs.8b04457. [DOI] [PubMed] [Google Scholar]
- 169.He B., Shi Y.J., Liang Y.Q., Yang A.P., Fan Z.P., Yuan L. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat Commun. 2018;9:2393. doi: 10.1038/s41467-018-04700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schleich N., Po C., Jacobs D., Ucakar B., Gallez B., Danhier F. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J Control Release. 2014;194:82–91. doi: 10.1016/j.jconrel.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 171.Chen F., Zhao E.R., Hableel G., Hu T., Kim T., Li J.T. Increasing the efficacy of stem cell therapy via triple-function inorganic nanoparticles. ACS Nano. 2019;13:6605–6617. doi: 10.1021/acsnano.9b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gao W.X., Hu Y.L., Xu L., Liu M.C., Wu H.Y., He B. Dual pH and glucose sensitive gel gated mesoporous silica nanoparticles for drug delivery. Chin Chem Lett. 2018;29:1795–1798. [Google Scholar]
- 173.Cheng W., Liang C.Y., Xu L., Liu G., Gao N.S., Tao W. TPGS-functionalized polydopamine-modified mesoporous silica as drug nanocarriers for enhanced lung cancer chemotherapy against multidrug resistance. Small. 2017;13:1700623. doi: 10.1002/smll.201700623. [DOI] [PubMed] [Google Scholar]
- 174.Quan G.L., Pan X., Wang Z.H., Wu Q.L., Li G., Dian L.H. Erratum to: lactosaminated mesoporous silica nanoparticles for asialoglycoprotein receptor targeted anticancer drug delivery. J Nanobiotechnol. 2015;13:89. doi: 10.1186/s12951-015-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shen D.K., Yang J.P., Li X.M., Zhou L., Zhang R.Y., Li W. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014;14:923–932. doi: 10.1021/nl404316v. [DOI] [PubMed] [Google Scholar]
- 176.Choi E., Kim S. Surface pH buffering to promote degradation of mesoporous silica nanoparticles under a physiological condition. J Colloid Interface Sci. 2019;533:463–470. doi: 10.1016/j.jcis.2018.08.088. [DOI] [PubMed] [Google Scholar]
- 177.Song Y., Li Y., Xu Q., Liu Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomed. 2016;12:87–110. doi: 10.2147/IJN.S117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Su J.H., Sun H.P., Meng Q.S., Zhang P.C., Yin Q., Li Y.P. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7:523–537. doi: 10.7150/thno.17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chai S.Q., Guo Y., Zhang Z.Y., Chai Z., Ma Y.R., Qi L.M. Cyclodextrin-gated mesoporous silica nanoparticles as drug carriers for red light-induced drug release. Nanotechnology. 2017;28:145101. doi: 10.1088/1361-6528/aa5e74. [DOI] [PubMed] [Google Scholar]
- 180.Zhao Y.T., Wang H.Y., Huang H., Xiao Q.L., Xu Y.H., Guo Z.N. Surface coordination of black phosphorus for robust air and water stability. Angew Chem Int Ed. 2016;55:5003–5007. doi: 10.1002/anie.201512038. [DOI] [PubMed] [Google Scholar]
- 181.Zeng X.W., Luo M.M., Liu G., Wang X.S., Tao W., Lin Y.X. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci. 2018;5:1800510. doi: 10.1002/advs.201800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morrison C. Alnylam prepares to land first RNAi drug approval. Nat Rev Drug Discov. 2018;17:156–157. doi: 10.1038/nrd.2018.20. [DOI] [PubMed] [Google Scholar]
- 183.Khalil I.A., Yamada Y., Harashima H. Optimization of siRNA delivery to target sites: issues and future directions. Expert Opin Drug Deliv. 2018;15:1053–1065. doi: 10.1080/17425247.2018.1520836. [DOI] [PubMed] [Google Scholar]
- 184.Wang H.X., Song Z.Y., Lao Y.H., Xu X., Gong J., Cheng D. Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc Natl Acad Sci U S A. 2018;115:4903–4908. doi: 10.1073/pnas.1712963115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guan X.W., Guo Z.P., Lin L., Chen J., Tian H.Y., Chen X.S. Ultrasensitive pH triggered charge/size dual-rebound gene delivery system. Nano Lett. 2016;16:6823–6831. doi: 10.1021/acs.nanolett.6b02536. [DOI] [PubMed] [Google Scholar]
- 186.Zou Y., Zheng M., Yang W.J., Meng F.H., Miyata K., Kim H.J. Virus-mimicking chimaeric polymersomes boost targeted cancer siRNA therapy in vivo. Adv Mater. 2017;29:1703285. doi: 10.1002/adma.201703285. [DOI] [PubMed] [Google Scholar]
- 187.Li L., Muñozculla M., Carmona U., Lopez M.P., Yang F., Trigueros C. Ferritin-mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials. 2016;98:143–151. doi: 10.1016/j.biomaterials.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 188.Xu X.D., Saw P.E., Tao W., Li Y.J., Ji X.Y., Yu M. Tumor microenvironment-responsive multistaged nanoplatform for systemic RNAi and cancer therapy. Nano Lett. 2017;17:4427–4435. doi: 10.1021/acs.nanolett.7b01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang B., Ding Y.P., Zhao X.Z., Han X.X., Yang N., Zhang Y.L. Delivery of small interfering RNA against Nogo-B receptor via tumor-acidity responsive nanoparticles for tumor vessel normalization and metastasis suppression. Biomaterials. 2018;175:110–122. doi: 10.1016/j.biomaterials.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 190.Chen Y., Gao D.Y., Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi: 10.1016/j.addr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.He S.F., Fan W.W., Wu N., Zhu J.J., Miao Y.Q., Miao X.R. Lipid-based liquid crystalline nanoparticles facilitate cytosolic delivery of siRNA via structural transformation. Nano Lett. 2018;18:2411–2419. doi: 10.1021/acs.nanolett.7b05430. [DOI] [PubMed] [Google Scholar]
- 192.Wang H., Agarwal P., Zhao S.T., Yu J.H., Lu X.B., He X.M. A near-infrared laser-activated “Nanobomb” for breaking the barriers to microRNA delivery. Adv Mater. 2016;28:347–355. doi: 10.1002/adma.201504263. [DOI] [PubMed] [Google Scholar]
- 193.He H., Zheng N., Song Z.Y., Kim K.H., Yao C., Zhang R.J. Suppression of hepatic inflammation via systemic siRNA delivery by membrane-disruptive and endosomolytic helical polypeptide hybrid nanoparticles. ACS Nano. 2016;10:1859–1870. doi: 10.1021/acsnano.5b05470. [DOI] [PubMed] [Google Scholar]
- 194.Morrison C. Fresh from the biotech pipeline-2017. Nat Biotechnol. 2018;36:131–136. doi: 10.1038/nbt.4068. [DOI] [PubMed] [Google Scholar]
- 195.Viola M., Sequeira J., Seiça R., Veiga F., Serra J., Santos A.C. Subcutaneous delivery of monoclonal antibodies: how do we get there?. J Control Release. 2018;286:301–314. doi: 10.1016/j.jconrel.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 196.Ye C., Chi H. A review of recent progress in drug and protein encapsulation: approaches, applications and challenges. Mater Sci Eng C. 2018;83:233–246. doi: 10.1016/j.msec.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 197.Sahoo J.K., VandenBerg M.A., Webber M.J. Injectable network biomaterials via molecular or colloidal self-assembly. Adv Drug Deliv Rev. 2018;127:185–207. doi: 10.1016/j.addr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 198.Wagner A.M., Gran M.P., Peppas N.A. Designing the new generation of intelligent biocompatible carriers for protein and peptide delivery. Acta Pharm Sin B. 2018;8:147–164. doi: 10.1016/j.apsb.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lakkireddy H.R., Urmann M., Besenius M., Werner U., Haack T., Brun P. Oral delivery of diabetes peptides—comparing standard formulations incorporating functional excipients and nanotechnologies in the translational context. Adv Drug Deliv Rev. 2016;106:196–222. doi: 10.1016/j.addr.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 200.Koetting M.C., Guido J.F., Gupta M., Zhang A., Peppas N.A. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J Control Release. 2016;221:18–25. doi: 10.1016/j.jconrel.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mahmood A., Bernkop-Schnürch A. SEDDS: a game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv Drug Deliv Rev. 2018;142:91–101. doi: 10.1016/j.addr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 202.Batista P., Castro P.M., Madureira A.R., Sarmento B., Pintado M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides. 2018;101:112–123. doi: 10.1016/j.peptides.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 203.Maisel K., Ensign L., Reddy M., Cone R., Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release. 2015;197:48–57. doi: 10.1016/j.jconrel.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wu J.W., Zheng Y.X., Liu M., Shan W., Zhang Z.R., Huang Y. Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl Mater Interfaces. 2018;10:9916–9928. doi: 10.1021/acsami.7b16524. [DOI] [PubMed] [Google Scholar]
- 205.Yu M.R., Wang J.L., Yang Y.W., Zhu C.L., Su Q., Guo S.Y. Rotation-facilitated rapid transport of nanorods in mucosal tissues. Nano Lett. 2016;16:7176–7182. doi: 10.1021/acs.nanolett.6b03515. [DOI] [PubMed] [Google Scholar]
- 206.Yu M.R., Xu L., Tian F.L., Su Q., Zheng N., Yang Y.W. Rapid transport of deformation-tuned nanoparticles across biological hydrogels and cellular barriers. Nat Commun. 2018;9:2607. doi: 10.1038/s41467-018-05061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R., Blumberg R.S. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci Transl Med. 2013;5:213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim K.S., Suzuki K., Cho H., Youn Y.S., Bae Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano. 2018;12:8893–8900. doi: 10.1021/acsnano.8b04315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Al-Hilal T.A., Park J., Alam F., Chung S.W., Park J.W., Kim K. Oligomeric bile acid-mediated oral delivery of low molecular weight heparin. J Control Release. 2014;175:17–24. doi: 10.1016/j.jconrel.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 210.Shan W., Zhu X., Liu M., Li L., Zhong J.J., Sun W. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano. 2015;9:2345–2356. doi: 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- 211.Fan W.W., Xia D.N., Zhu Q.L., Li X.Y., He S.F., Zhu C.L. Functional nanoparticles exploit the bile acid pathway to overcome multiple barriers of the intestinal epithelium for oral insulin delivery. Biomaterials. 2018;151:13–23. doi: 10.1016/j.biomaterials.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 212.Moroz E., Matoori S., Leroux J.C. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. doi: 10.1016/j.addr.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 213.Chuang E.Y., Nguyen G.T.H., Su F.Y., Lin K.J., Chen C.T., Mi F.L. Combination therapy via oral co-administration of insulin- and exendin-4-loaded nanoparticles to treat type 2 diabetic rats undergoing OGTT. Biomaterials. 2013;34:7994–8001. doi: 10.1016/j.biomaterials.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 214.de Kruijf W., Ehrhardt C. Inhalation delivery of complex drugs-the next steps. Curr Opin Pharmacol. 2017;36:52–57. doi: 10.1016/j.coph.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 215.Bodier-Montagutelli E., Mayor A., Vecellio L., Respaud R., Heuze-Vourc'h N. Designing inhaled protein therapeutics for topical lung delivery: what are the next steps?. Expert Opin Drug Deliv. 2018;15:729–736. doi: 10.1080/17425247.2018.1503251. [DOI] [PubMed] [Google Scholar]
- 216.Mandal A., Pal D., Agrahari V., Trinh H.M., Joseph M., Mitra A.K. Ocular delivery of proteins and peptides: challenges and novel formulation approaches. Adv Drug Deliv Rev. 2018;126:67–95. doi: 10.1016/j.addr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thwala L.N., Préat V., Csaba N.S. Emerging delivery platforms for mucosal administration of biopharmaceuticals: a critical update on nasal, pulmonary and oral routes. Expert Opin Drug Deliv. 2017;14:23–36. doi: 10.1080/17425247.2016.1206074. [DOI] [PubMed] [Google Scholar]
- 218.Ye Y.Q., Yu J.C., Wen D., Kahkoska A.R., Gu Z. Polymeric microneedles for transdermal protein delivery. Adv Drug Deliv Rev. 2018;127:106–118. doi: 10.1016/j.addr.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yu J.C., Zhang Y.Q., Kahkoska A.R., Gu Z. Bioresponsive transcutaneous patches. Curr Opin Biotechnol. 2017;48:28–32. doi: 10.1016/j.copbio.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hu X.L., Yu J.C., Qian C.G., Lu Y., Kahkoska A.R., Xie Z.G. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11:613–620. doi: 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Qiu C., Wei W., Sun J., Zhang H.T., Ding J.S., Wang J.C. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8:13033–13044. doi: 10.1039/c6nr04034a. [DOI] [PubMed] [Google Scholar]
- 222.Wang C., Ye Y.Q., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of Anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 223.Ehlerding E.B., England C.G., Majewski R.L., Valdovinos H.F., Jiang D.W., Liu G. ImmunoPET imaging of CTLA-4 expression in mouse models of non-small cell lung cancer. Mol Pharm. 2017;14:1782–1789. doi: 10.1021/acs.molpharmaceut.7b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang C., Sun W.J., Wright G., Wang A.Z., Gu Z. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and Anti-PD1 antibody. Adv Mater. 2016;28:8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]