Abstract
Mycophenolic acid (MPA, 1) and its derivatives are first-line immunosuppressants used in organ transplantation and for treating autoimmune diseases. Despite chemical synthetic achievements, the biosynthetic formation of a seven-carbon carboxylic acid pharmacophore side chain of 1, especially the processes involving the cleavage of the prenyl side chain between DHMP (4) and DMMPA (5), remains unknown. In this work, we identified a membrane-bound prenyltransferase, PgMpaA, that transfers FPP to 4 to yield FDHMP (6). Compound 6 undergoes the first cleavage step via a new globin-like enzyme PgMpaB to form a cryptic intermediate 12. Heterologous expression of PgMpa genes in Aspergillus nidulans demonstrates that the second cleavage step (from 12 to 5) of 1 is a PgMpa cluster-independent process in vivo. Our results, especially the discovery of the broad tolerance of substrates recognized by PgMpaB, set up a strategy for the formation of “pseudo-isopentenyl” natural products using fungal globin-like enzymes.
Key words: Biosynthesis, Mycophenolic acid, Prenylation, C‒C bond cleavage, Globin enzyme
Graphical abstract
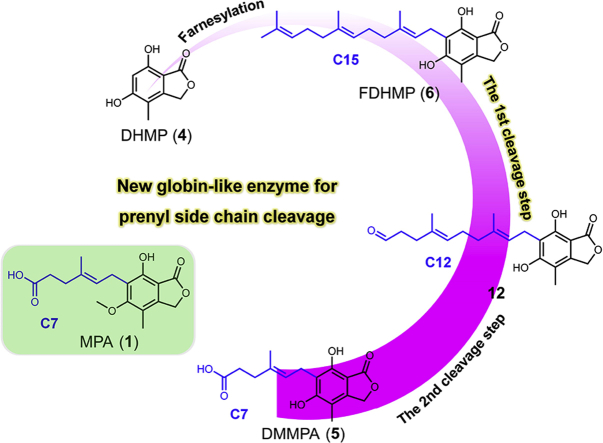
Collaborative biosynthesis—a new globin-like enzyme (PgMpaB) is responsible for the first pivotal C–C bond cleavage step in mycophenolic acid (MPA, 1) biosynthesis. The second cleavage step is a PgMpa cluster-independent process in vivo.
1. Introduction
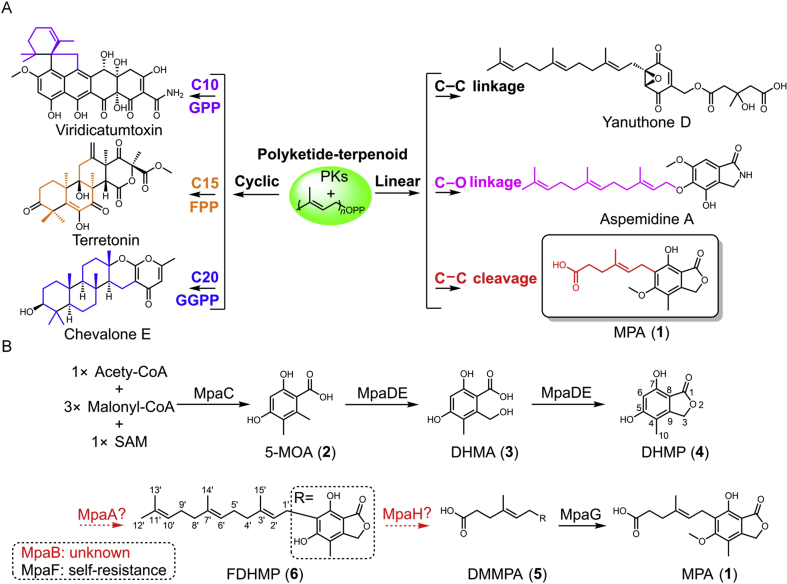
Fungal polyketide-terpenoid hybrid natural products (PK-TPs) belong to the largest class of meroterpenoids exhibiting a wide range of biological activities1. PK-TPs typically consist of a cyclic aromatic backbone featuring a prenyl side chain attached via a C–O or C–C bond linkage2, 3. The prenyl side chains are diverse partially due to different biogenetic origins and include dimethylallyl diphosphate (DMAPP, C5), geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15), and geranylgeranyl diphosphate (GGPP, C20)2, 3. In the development of complex structural scaffolds, after transferring the prenyl chain to the polyketide core, a flavin-dependent oxidase, a membrane-bound terpene cyclase and other redox enzymes usually catalyze tandem epoxidation, cyclization and oxidation reactions4, 5, 6, 7, 8, 9, 10. During the course of these reactions, the linear prenyl side chain can undergo changes in C–C connectivity to form mono- or poly-cyclic chiral centers, whereas the carbons are highly oxidized (Scheme 1A). Mycophenolic acid (MPA, 1) featuring a seven-carbon carboxylic acid pharmacophore, is putatively derived from an intact C15 prenyl moiety by a cleavage reaction rather than through classical epoxidation and cyclization reactions and represents a unique example of fungal PK-TPs (Scheme 1A)2, 3.
Scheme 1.
Biosynthetic diversity of fungal polyketide-terpenoid hybrid natural products. (A) The various modifications of prenyl side chain. (B) Previously proposed biosynthetic pathway of MPA (1).
As a highly potential inosine-5′-monophosphate dehydrogenase (IMPDH) inhibitor, MPA (1) was isolated from Penicillium brevicompactum in 1893. MPA and its derivatives are first-line immunosuppressants used in organ transplantations and treating autoimmune diseases11, 12, 13, 14. However, the genetic and enzymatic mechanisms for 1 have lagged far behind its discovery, synthesis, and medicinal applications, especially with respect to the installation and cleavage of prenyl side chain and associated responsible enzymes. Previous biosynthetic studies have suggested several mechanisms and enzymes (Scheme 1B): (i) a nonreducing polyketide synthase (NRPKS) MpaC catalyzes the formation of 5-methylorsellinic acid (5-MOA, 2) from one acetyl-CoA unit, three malonyl-CoA units and one S-adenosyl-l-methionine (SAM) unit15, 16; (ii) an unusual cytochrome P450 and hydrolase fused enzyme MpaDE catalyzes the hydroxylation of 2 to form 4,6-dihydroxy-2-(hydroxymethyl)-3-methylbenzoic acid (DHMB, 3) and subsequently catalyzes intramolecular dehydration to produce 5,7-dihydroxy-4-methylphthalide (DHMP, 4)17; (iii) MpaG catalyzes the last step of a C5 O-methylation of demethylmycophenolic acid (DMMPA, 5) to yield the final product 118; and (iv) MpaF, an IMPDH-like protein, plays a critical role in self-resistance toward 119. Although gene silencing studies have indicated that three additional genes (MpaA, MpaB and MpaH) are involved in 1 production20, the actual functions and roles of these genes and conversion details between 4 and 5 remain unresolved.
Here we identified a membrane-bound prenyltransferase PgMpaA catalyzing the regioselective C6 farnesylation of 4 to yield FDHMP (6), the resulting C15 prenyl side chain of which was undergone the first pivotal cleavage step by a new globin-like enzyme PgMpaB. Subsequent heterologous expression of PgMpa genes in Aspergillus nidulans demonstrated that the second cleavage step of 1 was a PgMpa cluster-independent process in vivo.
2. Results and discussions
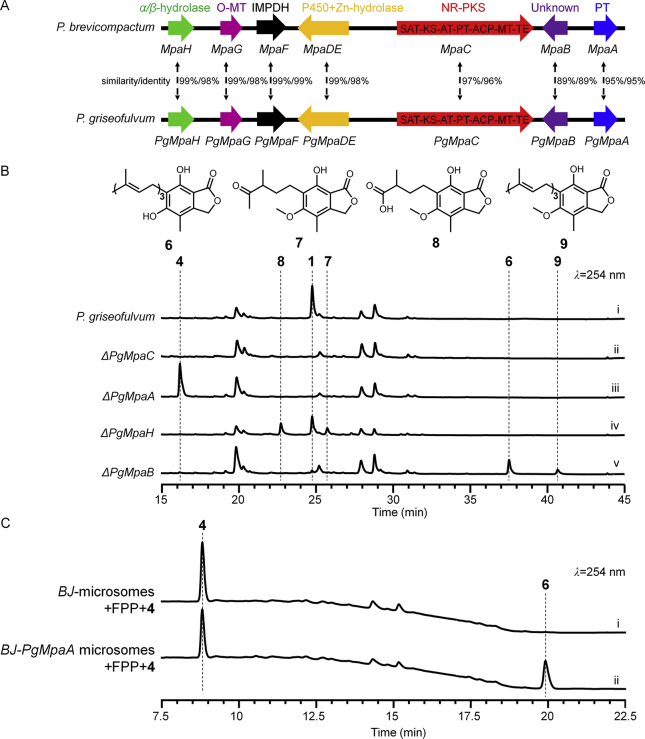
In our continuous studies on discovering enzymes for the cleavage of prenyl chains in natural product biosyntheses21, 22, we have focused on 1 using an MPA high-producing strain Penicillium griseofulvum (Supporting Information Fig. S1)23, 24. The strain provides a convenient platform for analyzing accumulated intermediates produced by gene mutants. One PgMpa gene cluster was computationally identified; this cluster showed high similarity/identity and conserved gene organization to the Pbmpa gene cluster from P. brevicompactum (Fig. 1A). Deletion of PgMpaC (SI) led to the complete abolishment of 1 production (Fig. 1B, i and ii; Supporting Information Fig. S2B), confirming a clear relationship of the PgMpa cluster with 1 biosynthesis in P. griseofulvum. To identify the three genes PgMpaA, PgMpaB and PgMpaH functionally responsible for the seven-carbon carboxylic acid side-chain formation, we first examined PgMpaA. PgMpaA catalyzes Friedel–Crafts farnesylation to yield 6-farnesyl-5,7-dihydroxy-4-methylphtalide (FDHMP, 6) using 4 as the substrate. The ΔPgMpaA mutant strain was unable to produce 1 and instead, accumulated 4 (Fig. 1B iii), which was purified and confirmed by NMR characterization (Supporting Information Table S3, and Figs. S17 and S18) and used as a substrate for biochemical assays.
Figure 1.
Confirmation of the PgMpa gene cluster. (A) Organization and proposed function of PgMpa A‒H. (B) LC‒MS analyses of culture extracts from P. griseofulvum wild type and mutants. (C) In vitro biochemical assays of BJ-PgMpaA or BJ control microsome with FPP.
PgMpaA is a fungal membrane-bound prenyltransferase containing six transmembrane regions (Supporting Information Fig. S3). Phylogenetic tree analyses (Supporting Information Fig. S4) showed that PgMpaA was closest to Pyr6, the farnesyltransferase from the pyripyropene A biosynthetic gene cluster25. Compound 4 was incubated with 0.2 mmol/L FPP, 10 mmol/L Mg2+, and microsome fractions of S. cerevisiaeBJ5464-NpgA∷PgMpaA (BJ-PgMpaA, SI). Expectedly, there was a nearly ∼25% conversion of 4 into a new compound in 3 h with mass (m/z 383 [M‒H]‒). This was consistent with farnesyl-4, whereas the BJ control remained inactive (Fig. 1C, i and ii). When DMAPP, GPP or GGPP were used, one new compound consistent with the mass of geranyl-4 (m/z 315 [M‒H]‒) was detected by LC‒MS, albeit with a lower turnover (Supporting Information Fig. S5). A competition and time-course assay of BJ-PgMpaA microsome in the presence of equimolar amounts of GPP and FPP (SI) confirmed that PgMpaA preferentially used FPP as the donor for the prenylation of 4 (Supporting Information Fig. S6). Indeed, under a sustainable supply of isoprene intermediate metabolic pools in vivo, the whole-cell biotransformation using BJ-PgMpaA with 4 only produced farnesyl-4 (Supporting Information Fig. S7). After product extraction and purification, NMR characterization confirmed the compound as 6 (Supporting Information Table S4 and Figs. S19–S22). Feeding 6 into the ΔPgMpaC mutant restored the production of 1, which demonstrated the role of 6 as a key precursor (Supporting Information Fig. S8). The in vivo and in vitro results indicated (i) PgMpaA exhibited broad substrate tolerance toward FPP and GPP. However, PgMpaA specifically catalyzed the regioselective C6 farnesylation of 4 to 6. (ii) The seven-carbon carboxylic acid side chain of 1 comes from an intact FPP (C15) moiety of 6. The unusual cleavage mechanism existed downstream in the pathway.
We next identified enzymes responsible for this cleavage step. The ideal candidate was PgMpaH based on bioinformatic analyses, which showed that PgMpaH contained an α/β-hydrolase fold and had very weak amino acid sequence similarity/identity with 1-H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase (HOD) and 1-H-3-hydroxy-4-oxoquinoline 2,4-dioxygenase (QDO)26. These two enzymes are cofactor-independent dioxygenases involved in the cleavage of the C–C double bond in N-heteroaromatic compounds26. To clarify the function of PgMpaH, it was inactivated in P. griseofulvum. Unexpectedly, the ΔPgMpaH mutant retained ∼50% ability to produce 1 and two new compounds with m/z 291 [M‒H]‒ (7) and m/z 293 [M‒H]‒ (8) (Fig. 1B iv). Purification and structural characterization (Supporting Information Tables S5 and 6 and Figs. S23–S28) indicated that 7 and 8 were the C5- and C6-chain analogues of 5 (C7-chain), respectively (Fig. 1B). They are probable abnormal cleavage products of 6 in vivo but not the precursors of 1. Indeed, feeding 7 and 8 into the ΔPgMpaC mutant did not restore 1 production (Fig. S8). These results excluded the direct role of PgMpaH taking part in the breakdown of the C15-chain of 6 in vivo. However, the data suggested PgMpaH may be functional as an assistant enzyme to ensure a correct and efficient whole cleavage process.
Gene PgMpaH was excluded from the candidate genes for C–C bond cleavage prompting the investigation of the function of PgMpaB. NCBI blast or Pfam domain analyses showed that PgMpaB was an unknown function protein. A potential conserved three-dimensional structure using Phyre2 analysis showed that PgMpaB possibly belonged to the globin superfamily protein (Supporting Information Fig. S9). PgMpaB showed 41%/22% amino acid similarity/identity with the bacterial globin-like enzyme latex-clearing protein (Lcpk30) from Streptomyces sp.27, 28. Phylogenetic tree analyses of PgMpaB with other globin-like enzymes, such as bacterial Lcpk30, rubber oxygenase (RoxA29, from Xanthomonas sp.) and fungal flavohemoglobin (FHb30, from Aspergillus sp.), showed that PgMpaB was clustered into an independent clade (Supporting Information Fig. S10). Lcpk30 and RoxA catalyzed the oxidative cleavage of poly (cis-1,4-isoprene) during rubber degradation. This reaction was similar to the cleavage of the prenyl side chain in 1 biosynthesis.
To uncover its function, gene PgMpaB was inactivated in P. griseofulvum, and the metabolites of the ΔPgMpaB mutant were analyzed by LC‒MS. This revealed that the production of 1 dramatically decreased (∼98%) in the ΔPgMpaB mutant, indicating that PgMpaB played an important role for 1 biosynthesis (Fig. 1B v). The trace yield of 1 retained in the ΔPgMpaB mutant suggested the existence of minor complementary activity for PgMpaB in P. griseofulvum. Indeed, two other PgMpaB homologues genes (PgMpaB-scaffold3 and PgMpaB-scaffold17, Supporting Information Fig. S11) existed in the genome of P. griseofulvum, whereas the PgMpaB-scaffold3 was actively transcribed (Fig. S11). In addition to the 6, a minor new compound 9 with m/z 397 [M‒H]‒ was also detected in the ΔPgMpaB mutant, whose structure was determined to be 5-O-methyl-6 (Fig. 1B) by NMR analyses (Supporting Information Table S7 and Figs. S29–S33). Compound 9 was the shunt methyl-product of 6, possibly catalyzed by PgMpaG, because its broad substrate tolerance has been previously demonstrated18. The successful bioconversion of 6 to 9 in the BJ-PgMpaG strain (Supporting Information Fig. S12) also confirmed that PgMpaG was responsible for this step.
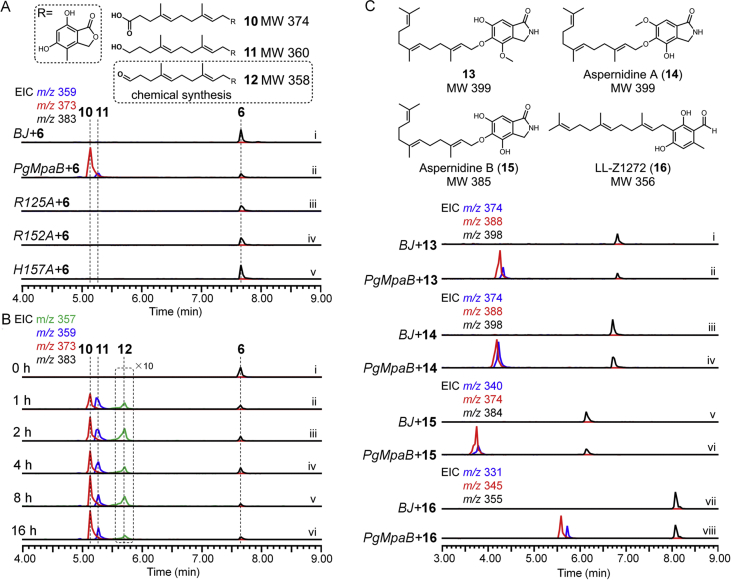
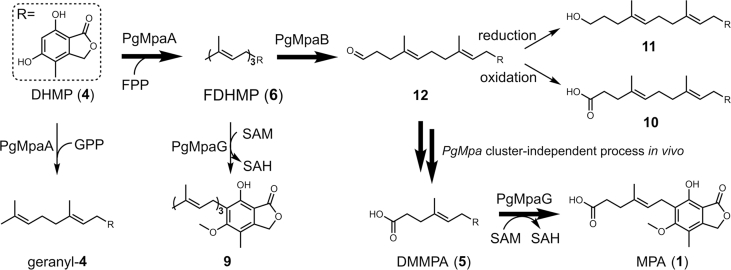
The accumulation of 6 in the ΔPgMpaB mutant suggested PgMpaB may be the real cleavage enzyme. Due to the insoluble expression of PgMpaB in Escherichia coli, the intron-free PgMpaB was introduced into BJ (BJ-PgMpaB) under control of the ADH2 promoter. When 6 was incubated with the cell-free extract of BJ-PgMpaB, two new compounds with m/z 373 [M‒H]‒ (10) and m/z 359 [M‒H]‒ (11) were detected (Fig. 2A). Purified 10 and 11 from large-scale conversion assays, followed by structural NMR characterization, indicated that 10 and 11 were the twelve-carbon carboxylic acid and hydroxylated side-chain analogues of 6, respectively (Fig. 2A, Supporting Information Tables S8 and S9, and Figs. S34–43). Compounds 10 and 11 were likely the spontaneous oxidized and reduced products of the corresponding aldehyde precursor 12 during the formation of complex cell-free extracts, respectively (Fig. 2A). We proposed that compound 12 was the real product from the PgMpaB-catalyzed cleavage of the terminal olefin (Δ10’) of 6, which was detected in the time-course analyses (Fig. 2B). We chemically semisynthesized 12 using 10 as the starting material (SI) and confirmed 12 indeed as the precursor of 1 due to its ability to restore 1 production in the ΔPgMpaC mutant (Fig. S8). Bioinformatic analyses of PgMpaB and its homologues from the NCBI database showed that these fungal globin-like proteins were rich in arginine, glutamic acid, lysine and histidine (Supporting Information Fig. S13). Mutation results confirmed that (i) Arg125, Arg152 and His157 were essential for the activity of PgMpaB (Fig. 2A); (ii) Arg62, Arg99, Glu135, Arg145 and Glu245 mutants significantly decreased the activity of PgMpaB (Supporting Information Fig. S14); (iii) Lys254 and His275 had no effect on the activity of PgMpaB (Fig. S14). Further investigations of the substrate tolerance showed that PgMpaB had the ability to catalyze the cleavage of various O- and C-farnesyl side-chain analogues of 1 (Fig. 2C), which indicated future applications of PgMpaB as a biocatalyst for C–C bond cleavage.
Figure 2.
In vitro biochemical assays of the PgMpaB cell-free extracts with different substrates. (A) Identification of the key amino acid residues for PgMpaB activity. (B) Time-course analyses of the PgMpaB-catalyzed cleavage reaction. (C) The substrate tolerance of PgMpaB. The extracted ion chromatograms (EIC) under negative ionization are shown.
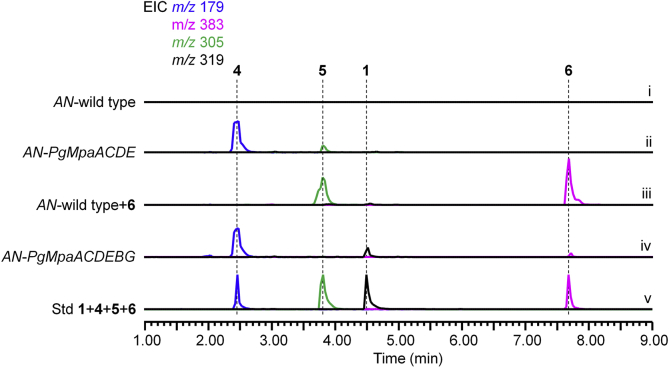
The production of 12 but not 5 in a PgMpaB-catalyzed reaction as well as the feeding of 12 to restore the production of 1 indicated (i) PgMpaB exclusively catalyzed the regioselective double bond (Δ10’) cleavage and that (ii) the mature process of C7 side chain of 1 should be performed by two cleavage steps (from 6 to 12, and from 12 to 5). The latter step (from 12 to 5) was likely an independent process in vivo not related with the PgMpa cluster. To prove this hypothesis, we introduced the PgMpa genes into the heterologous host A. nidulans, the strain that could not produce 131. Bioinformatic analyses showed that two PgMpaB homologues (AN10293.4 and AN1187.2) existed in the genome of A. nidulans, showing 73%/54% and 73%/57% (similarity/identity) to PgMpaB, respectively (Supporting Information Fig. S15). Compared with PgMpaB, these two proteins had the same identical active amino acid residues (Fig. S15), indicating that they had the ability to complement the activity of PgMpaB. Indeed, biochemical assays showed that these two proteins (AN10293.4 and AN1187.2) could catalyze the C–C bond cleavage of 6 to form 12 (Supporting Information Fig. S16). Upon the coexpression of PgMpaACDE in A. nidulans (AN-PgMpaACDE, SI), besides 4 and 6, one compound with m/z 305 [M‒H]- was detected (Fig. 3, i and ii), which was further isolated and identified as 5 by NMR analyses (Supporting Information Table S10 and Figs. S44–S47). Further feeding of 6 to A. nidulans showed that 5 was detected (Fig. 3 iii). These results unambiguously confirmed that the latter cleavage step of the farnesyl side chain in 1 biosynthesis was a PgMpa cluster-independent process (Scheme 2). We finally reconstructed the whole pathway of 1 in A. nidulans by the coexpression of AN-PgMpaABCDEG (Fig. 3 iv). Overlapping in time frame with the work presented here, a preprint manuscript reported that the β-oxidation of primary metabolism was proposedly involved in the biosynthesis of 132. However, the in vitro biochemical details required further investigation.
Figure 3.
Reconstruction of 1 pathway in A. nidulans confirms two cleavage stages of the C15 prenyl side chain in 1 biosynthesis. The extracted ion chromatograms (EIC) under negative ionization are shown.
Scheme 2.
The updated biosynthetic pathway and confirmed key intermediates of 1. Bold and plain arrows indicate the on-pathway and shunt pathway of 1, respectively.
3. Conclusions
In this study, the missing step of the biosynthetic pathway of the immunosuppressive drug mycophenolic acid (1) was identified from P. griseofulvum. A membrane-bound prenyltransferase PgMpaA transferred an isopentenyl unit to the polyketide backbone 4 to yield 6 containing the C15 farnesyl moiety, which subsequently underwent the first cleavage step by a new globin-like enzyme (PgMpaB) to form cryptic intermediate 12. Heterologous expression of the PgMpa gene cluster in A. nidulans indicated that the second cleavage step of 1 was a PgMpa cluster-independent process in vivo. Our results, especially the discovery of the broad tolerance of substrate recognized by PgMpaB, set up a strategy for the formation of “pseudo-isopentenyl” natural products using fungal globin-like enzymes.
Acknowledgments
We thank Prof. Blaine Pfeifer from the University at Buffalo (UB, USA), the State University of New York (USA) for a critical reading of the manuscript. This work is supported by the National Natural Science Foundation of China (31870022), the Fundamental Research Funds for the Central Universities (XDJK2018B035 and 201941001, China), and the Scientific Research Starting Foundation of Southwest University (SWU117034, China), the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao, China) (No. 2018SDKJ0401-2), and Taishan Scholar Youth Expert Program in Shandong Province (tsqn201812021, China). Yi Zou is supported by the Venture & Innovation Support Program for Chongqing Overseas Returnees and the Thousand Young Talents Program of China.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.06.009.
Contributor Information
Dehai Li, Email: dehaili@ouc.edu.cn.
Yi Zou, Email: zouyi31@swu.edu.cn.
Appendix A. Supporting information
The following is the Supplementary data to this article:
References
- 1.Geris R., Simpson T.J. Meroterpenoids produced by fungi. Nat Prod Rep. 2009;26:1063–1094. doi: 10.1039/b820413f. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda Y., Abe I. Biosynthesis of fungal meroterpenoids. Nat Prod Rep. 2016;33:26–53. doi: 10.1039/c5np00090d. [DOI] [PubMed] [Google Scholar]
- 3.Schor R., Cox R. Classic fungal natural products in the genomic age: the molecular legacy of Harold Raistrick. Nat Prod Rep. 2018;35:230–256. doi: 10.1039/c8np00021b. [DOI] [PubMed] [Google Scholar]
- 4.Mori T., Iwabuchi T., Hoshino S., Wang H., Matsuda Y., Abe I. Molecular basis for the unusual ring reconstruction in fungal meroterpenoid biogenesis. Nat Chem Biol. 2017;13:1066–1073. doi: 10.1038/nchembio.2443. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda Y., Iwabuchi T., Wakimoto T., Awakawa T., Abe I. Uncovering the unusual D-ring construction in terretonin biosynthesis by collaboration of a multifunctional cytochrome P450 and a unique isomerase. J Am Chem Soc. 2015;137:3393–3401. doi: 10.1021/jacs.5b00570. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima Y., Mitsuhashi T., Matsuda Y., Senda M., Sato H., Yamazaki M. Structural and computational bases for dramatic skeletal rearrangement in anditomin biosynthesis. J Am Chem Soc. 2018;140:9743–9750. doi: 10.1021/jacs.8b06084. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda Y., Iwabuchi T., Fujimoto T., Awakawa T., Nakashima Y., Mori T. Discovery of key dioxygenases that diverged the paraherquonin and acetoxydehydroaustin pathways in Penicillium brasilianum. J Am Chem Soc. 2016;138:12671–12677. doi: 10.1021/jacs.6b08424. [DOI] [PubMed] [Google Scholar]
- 8.Tang M.C., Cui X., He X., Ding Z., Zhu T., Tang Y. Late-stage terpene cyclization by an integral membrane cyclase in the biosynthesis of isoprenoid epoxycyclohexenone natural products. Org Lett. 2017;19:5376–5379. doi: 10.1021/acs.orglett.7b02653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chooi Y.H., Hong Y.J., Cacho R.A., Tantillo D.J., Tang Y. A cytochrome P450 serves as an unexpected terpene cyclase during fungal meroterpenoid biosynthesis. J Am Chem Soc. 2013;135:16805–16808. doi: 10.1021/ja408966t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W.G., Du L.Q., Sheng S.L., Li A., Li Y.P., Cheng G.G. Genome mining for fungal polyketide-diterpenoid hybrids: discovery of key terpene cyclases and multifunctional P450s for structural diversification. Org Chem Front. 2019;6:571–578. [Google Scholar]
- 11.Bentley R. Mycophenolic acid: a one hundred year odyssey from antibiotic to immunosuppressant. Chem Rev. 2000;100:3801–3826. doi: 10.1021/cr990097b. [DOI] [PubMed] [Google Scholar]
- 12.Marzano A.V., Dassoni F., Caputo R. Treatment of refractory blistering autoimmune diseases with mycophenolic acid. J Dermatol Treat. 2006;17:370–376. doi: 10.1080/09546630600964999. [DOI] [PubMed] [Google Scholar]
- 13.de Winter B.C., van Gelder T. Therapeutic drug monitoring for mycophenolic acid in patients with autoimmune diseases. Nephrol Dial Transplant. 2008;23:3386–3388. doi: 10.1093/ndt/gfn497. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson C.A., Carlsten H. Mycophenolic acid inhibits inosine 5′-monophosphate dehydrogenase and suppresses immunoglobulin and cytokine production of B cells. Int Immunopharmacol. 2003;3:31–37. doi: 10.1016/s1567-5769(02)00210-2. [DOI] [PubMed] [Google Scholar]
- 15.Regueira T.B., Kildegaard K.R., Hansen B.G., Mortensen U.H., Hertweck C., Nielsen J. Molecular basis for mycophenolic acid biosynthesis in Penicillium brevicompactum. Appl Environ Microbiol. 2011;77:3035–3043. doi: 10.1128/AEM.03015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen B.G., Salomonsen B., Nielsen M.T., Nielsen J.B., Hansen N.B., Nielsen K.F. Versatile enzyme expression and characterization system for Aspergillus nidulans, with the Penicillium brevicompactum polyketide synthase gene from the mycophenolic acid gene cluster as a test case. Appl Environ Microbiol. 2011;77:3044–3051. doi: 10.1128/AEM.01768-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen B.G., Mnich E., Nielsen K.F., Nielsen J.B., Nielsen M.T., Mortensen U.H. Involvement of a natural fusion of a cytochrome P450 and a hydrolase in mycophenolic acid biosynthesis. Appl Environ Microbiol. 2012;78:4908–4913. doi: 10.1128/AEM.07955-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Cao S., Qiu L., Qi F., Li Z., Yang Y. Functional characterization of MpaG', the O-methyltransferase involved in the biosynthesis of mycophenolic acid. Chembiochem. 2015;16:565–569. doi: 10.1002/cbic.201402600. [DOI] [PubMed] [Google Scholar]
- 19.Hansen B.G., Genee H.J., Kaas C.S., Nielsen J.B., Regueira T.B., Mortensen U.H. A new class of IMP dehydrogenase with a role in self-resistance of mycophenolic acid producing fungi. BMC Microbiol. 2011;11:202. doi: 10.1186/1471-2180-11-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del-Cid A., Gil-Durán C., Vaca I., Rojas-Aedo J.F., García-Rico R.O., Levicán G. Identification and functional analysis of the mycophenolic acid gene cluster of Penicillium roqueforti. PLoS One. 2016;11:e0147047. doi: 10.1371/journal.pone.0147047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Y., Garcia-Borràs M., Tang M.C., Hirayama Y., Li D.H., Li L. Enzyme-catalyzed cationic epoxide rearrangements in quinolone alkaloid biosynthesis. Nat Chem Biol. 2017;13:325–332. doi: 10.1038/nchembio.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y., Zhan Z., Li D., Tang M., Cacho R.A., Watanabe K. Tandem prenyltransferases catalyze isoprenoid elongation and complexity generation in biosynthesis of quinolone alkaloids. J Am Chem Soc. 2015;137:4980–4983. doi: 10.1021/jacs.5b03022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng H., Yin G., Wei Q., Li D., Wang Y., Hu Y. Unprecedented [5.5.5.6]dioxafenestrane ring construction in fungal insecticidal sesquiterpene biosynthesis. Angew Chem Int Ed. 2019;58:6569–6573. doi: 10.1002/anie.201813722. [DOI] [PubMed] [Google Scholar]
- 24.Shim S.H., Swenson D.C., Gloer J.B., Dowd P.F., Wicklow D.T. Penifulvin A: a sesquiterpenoid-derived metabolite containing a novel dioxa[5,5,5,6]fenestrane ring system from a fungicolous isolate of Penicillium griseofulvum. Org Lett. 2006;8:1225–1228. doi: 10.1021/ol060107c. [DOI] [PubMed] [Google Scholar]
- 25.Itoh T., Tokunaga K., Matsuda Y., Fujii I., Abe I., Ebizuka Y. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat Chem. 2010;2:858–864. doi: 10.1038/nchem.764. [DOI] [PubMed] [Google Scholar]
- 26.Steiner R.A., Janssen H.J., Roversi P., Oakley A.J., Fetzner S. Structural basis for cofactor-independent dioxygenation of N-heteroaromatic compounds at the α/β-hydrolase fold. Proc Natl Acad Sci U S A. 2010;107:657–662. doi: 10.1073/pnas.0909033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Röther W., Austen S., Birke J., Jendrossek D. Cleavage of rubber by the latex clearing protein (Lcp) of Streptomyces sp. strain K30: molecular insights. Appl Environ Microbiol. 2016;82:6593–6602. doi: 10.1128/AEM.02176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilcu L., Röther W., Birke J., Brausemann A., Einsle O., Jendrossek D. Structural and functional analysis of latex clearing protein (Lcp) provides insight into the enzymatic cleavage of rubber. Sci Rep. 2017;7:6179. doi: 10.1038/s41598-017-05268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birke J., Jendrossek D. Rubber oxygenase and latex clearing protein cleave rubber to different products and use different cleavage mechanisms. Appl Environ Microbiol. 2014;80:5012–5020. doi: 10.1128/AEM.01271-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogewijs D., Dewilde S., Vierstraete A., Moens L., Vinogradov S.N. A phylogenetic analysis of the globins in fungi. PLoS One. 2012;7:e31856. doi: 10.1371/journal.pone.0031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez J.F., Somoza A.D., Keller N.P., Wang C.C. Advances in Aspergillus secondary metabolite research in the post-genomic era. Nat Prod Rep. 2012;29:351–371. doi: 10.1039/c2np00084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W., Du L., Qu Z., Zhang X., Li F., Li Z. Compartmentalized biosynthesis of mycophenolic acid. BioRxiv. 2019 doi: 10.1101/524025. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.