Abstract
The sigma-1 receptor (σ1R) is a unique intracellular protein. σ1R plays a major role in various pathological conditions in the central nervous system (CNS), implicated in several neuropsychiatric disorders. Imaging of σ1R in the brain using positron emission tomography (PET) could serve as a noninvasively tool for enhancing the understanding of the disease's pathophysiology. Moreover, σ1R PET tracers can be used for target validation and quantification in diagnosis. Herein, we describe the radiosynthesis, in vivo PET/CT imaging of novel σ1R 11C-labeled radioligands based on 6-hydroxypyridazinone, [11C]HCC0923 and [11C]HCC0929. Two radioligands have high affinities to σ1R, with good selectivity. In mice PET/CT imaging, both radioligands showed appropriate kinetics and distributions. Additionally, the specific interactions of two radioligands were reduced by compounds 13 and 15 (self-blocking). Of the two, [11C]HCC0929 was further investigated in positive ligands blocking studies, using classic σ1R agonist SA 4503 and σ1R antagonist PD 144418. Both σ1R ligands could extensively decreased the uptake of [11C]HCC0929 in mice brain. Besides, the biodistribution of major brain regions and organs of mice were determined in vivo. These studies demonstrated that two radioligands, especially [11C]HCC0929, possessed ideal imaging properties and might be valuable tools for non-invasive quantification of σ1R in brain.
KEY WORDS: σ1R, PET, Brain imaging, 6-Hydroxypyridazinone, 11C-labeled radioligand
Abbreviations: 3D, three-dimensional; σ1R, sigma-1 receptor; σ2R, sigma-2 receptor; AF, ammonium formate; BBB, brain blood barrier; BP, binding potential; CNS, center nervous systems; CRPS, complex regional pain syndrome; DMF, dimethyl formamide; DMSO, dimethylsulfoxide; ER, endoplasmic reticulum; LCP, lipidic cubic phase; MAM, mitochondria-associated ER membrane; PCP, phencyclidine; PET, positron emission tomography; TFA, trifluoroacetic acid
Graphical abstract
Of the two novel 11C-labeled sigma-1 receptor radioligands, [11C]HCC0929 has possessed better kinetic property and specificity, which could potentially accelerate preclinical research and medical development in sigma-1 receptor-related center nervous systems disease.
1. Introduction
As an enigmatic intracellular protein, the history of sigma (σ) receptor was originally categorized as an opioid receptor subtype1, and later confused with the phencyclidine (PCP) receptor due to the lack of selective ligands2. Subsequent pharmacological studies and molecular biology have finally identified that the σ receptor is a non-opioid and non-PCP protein, which was at least two known subtypes, classified as sigma-1 (σ1) and sigma-2 (σ2) receptor. These two subtypes are pharmacologically similar but genetically unrelated, with different body distribution, biological function and pharmacological profiles3, 4, 5, 6.
At present, sigma-1 receptor (σ1R) is known as a unique protein that shares no sequence homology with opioids or any other human proteins but is highly conserved across mammalian species. σ1R is a 23.5 kDa that is 223 amino acids in length5. σ1R is widely expressed in the central nervous system (CNS) and peripheral tissues and organs7, 8.
Mainly residing in the mitochondria-associated endoplasmic reticulum (ER) membrane (MAM) of cell, σ1R has been reported to interacted with numerous neurotransmitter receptors and ion channels, involved in diverse basic biochemical processes and pathological conditions related to neurodegeneration, pain sensitization, psychiatric disorders, and drug addiction7, 9, 10. σ1R is also found overexpressed in many known human cancers in lung, breast, prostate, and glioma cells11, 12.
The first crystal structure of human σ1R was recently solved using lipidic cubic phase (LCP) crystallography. The three-dimensional (3D) protein structure of human σ1R receptor showed a membrane-bound trimeric assembly with one transmembrane region, modifying the previous hypothesis that the receptor had two transmembrane domains13, 14. Despite the fact that structural information has only recently become available, and no endogenous ligand has been established for the receptor, numerous small molecule ligands for σ1R have been reported over the past few decades. Among these compounds, some have been developed as radiolabeled imaging tracers (Fig. 1) for positron emission tomography (PET) applications15, 16, 17.
Figure 1.
Selected σ1 receptor (σ1R) ligand and radioligands.
As a translational noninvasive imaging method, PET imaging of σ1R is a promising modality to evaluate distribution and expression in inaccessible regions and tissues. Additionally, with the specificity and selectivity radioligands, examining σ1R through PET could facilitate the investigation of in vivo role in pathology and progression of σ1R in different diseases directly18.
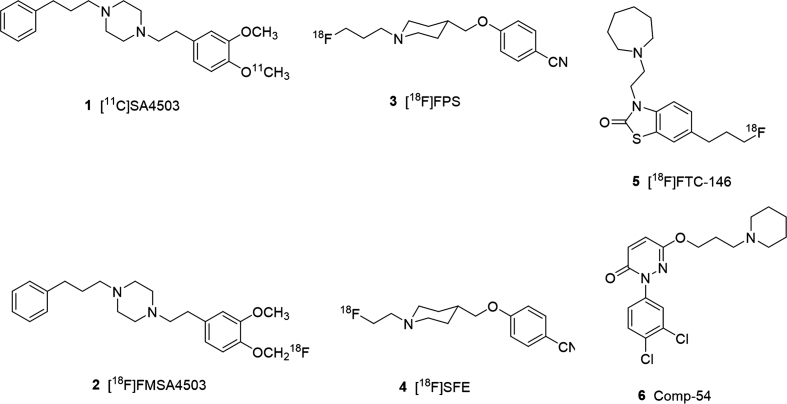
Several radiolabeled σ1R ligands have been studied in PET imaging of human investigation, but only a few of them have been used clinically. [11C]SA 4503 (1) is the first σ1R radioligand in human studies18. The ex vivo binding assays of SA 4503 was initially reported as nanomolar affinity of σ1R (Ki=4.6 nmol/L) and highly selectivity to σ1R (σ2/σ1=103)19, later the selectivity to σ2R was reinvestigated as 13.3–55.018, 20. Besides, the low selectivity either to the emopamil binding protein (EBP) or to the vesicular acetylcholine transporter (VAChT) limited its broad use in clinic studies19, 20. There are also fluorine-18-labeled σ1R radioligands, including but not limited to [18F]FMSA450321 (2), [18F]FPS22 (3), [18F]SFE23, 24 (4), and [18F]FTC-14625 (5). Although most of them have been studied in human researches, and [18F]FTC-146 have completed early phase I trial of PET/MRI in healthy volunteers, and in complex regional pain syndrome (CRPS) and sciatica26, 27, still each of them have unmet requirements and needs further investigation for practical clinic translation.
In our previous work, we identified 6-hydroxypyridazinone class of compounds with high σ1R affinity and high selectivity over σ2R28. Ex vivo tests suggested comp-54 (6) of 6-hydroxypyridazinone derivatives were reported as the most promising candidate of high binding affinity (σ1R Ki=1.4 nmol/L) and apparent good selectivity (σ2/σ1=1365.7). The pharmacological test of in vivo evaluation in rodent showed it was a σ1R antagonist and could possibly penetrate the blood‒brain barrier (BBB), and get into the σ1R-expressed region in mice brain, which exerted a highly potency of modified into σ1R radioligand for brain PET imaging. Aiming to preserve this high affinity and selectivity, we devised a strategy to modify 6 in ways that would incorporate a carbon-11 radiolabel without greatly altering the original framework of 6-hydroxypyridazinone in the target molecules.
To the best of our knowledge, no similar compounds with 6-hydroxypyridazinone scaffold have been developed as σ1R PET radiotracer. Since the core structure is distinct from existing PET radioligands for σ1R imaging, it could expand the diversity of available probes and facilitate future advances in a σ1R imaging. Here, we report the radiosynthesis of two novel carbon-11-labeled σ1R radioligands, [11C]HCC0923 and [11C]HCC0929, and demonstrate the in vivo pharmacokinetic properties, biodistribution of brain regions and major organs through PET/CT imaging for σ1R in the mice.
2. Results and discussion
2.1. Chemical synthesis
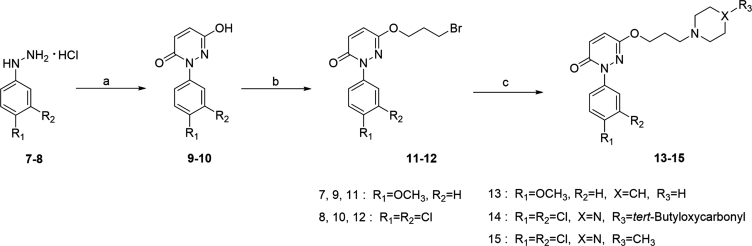
The chemical synthesis of the compounds 13–15 was illustrated in Scheme 1. The 6-hydroxypyridazinone derivatives were prepared following our previously reported work with minor changes28, 29.
Scheme 1.
Synthesis of 6-hydroxypyridazinone derivatives. Reagents and conditions: (a) maleic anhydride, H2O, conc. HCl, 100 °C, 8 h; (b) Br(CH2)3Br, K2CO3, acetone, 58 °C, 4 h; (c) Cs2CO3, acetonitrile, 62 °C, 2 h.
Briefly, through a one-step cyclization reaction of substitute phenylhydrazine hydrochloride and maleic anhydride, the intermediates 9 and 10 were prepared as white solid; Next, standard alkylation with 1,3-dibromopropane in acetone using potassium carbonate (K2CO3) was conducted to produce 11 and 12, and then reacted with the piperidine, tert-butyl piperazine-1-carboxylate or 1-methylpiperazine respectively in the presence of cesium carbonate (Cs2CO3) in acetonitrile to afford the compound 13–15 in moderate yields.
2.2. Preparation of the precursors and radiosynthesis of [11C]HCC0923 and [11C]HCC0929
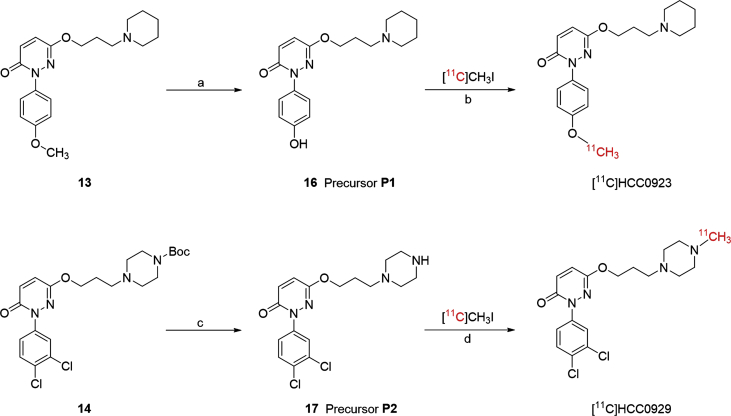
The preparation of the precursors P1 and P2 were using straightforward methods, as presented in Scheme 2. For precursor P1, the methoxy group in compound 13 was turned to hydroxy group by demethylation of BBr3 in ‒78 °C30. For precursor P2, the tert-butyl ester in compound 14 was hydrolyzed in the presence of HCl solution in diethyl ether (2 mol/L) to form the designed precursor.
Scheme 2.
Synthesis of radiolabeling precursor P1 (16), P2 (17) and radiosynthesis of [11C]HCC0923 and [11C]HCC0929. Reagents and conditions: (a) BBr3, dichloromethane, ‒78 °C to room temperature, overnight; (b) NaOH, DMF, 120 °C, 3 min; (c) 2 mol/L HCl in diethyl ether, dichloromethane, room temperature, overnight; (d) K2CO3, DMF, 120 °C, 3 min.
Two carbon-11-labeled radioligands were prepared through a standard methylation method, under the catalyzation of different bases, respectively. [11C]HCC0923 was prepared through precursor P1 with [11C]CH3I. [11C]CH3I was produced from cyclotron and then trapped in a sealed vial with the precursor in DMF solution in the presence of sodium hydroxide (NaOH) followed by heating at 120 °C for 3 min. The reaction was consequently quenched with water and purified by semipreparative HPLC. Including formulation, [11C]HCC0923 was prepared in 35–40 min after the end of bombardment with adequate radiochemical yields (6%‒15%, uncorrected for decay and based on trapped [11C]CH3I) and high radiochemical purity (>95%).
The procedure for synthesis of [11C]HCC0929 was almost the same as [11C]HCC0923, except using precursor P2 with K2CO3 as the base catalyst instead of NaOH. The entire preparation including formulation takes approximately 35–40 min, with the radiochemical yields of 3%‒8% (uncorrected for decay and based on trapped [11C]CH3I) and radiochemical purity over 95%.
2.3. Ex vivo σ1R binding affinity, selectivity and logD of compounds 13 and 15
An essential property of developing binding-based radiotracer is the binding potential (BP), which is usually represented as the ratio of receptor's density (Bmax) to binding affinity. A radiotracer which is suitable for quantitative comparisons with PET imaging has at least a value of BP over 5, especially in clinical investigation; in a non-research clinical setting, BP should typically be greater than 1031.
The Bmax of σ1R in human brain was measured to be approximately 30‒600 fmol/mg (3‒60 nmol/L)32, 33. Thus, the radioligand with affinity of 0.6‒12 nmol/L could be used for σ1R imaging, and range of 0.3‒6 nmol/L will be more suitable. The ex vivo σ1R binding affinity of unlabeled HCC0923 (compound 13) and HCC0929 (compound 15) were measured through previously demonstrated methods (as described in Supporting Information)28, 29. The σ1R binding affinities of 13 and 15 were 10.3 and 5.6 nmol/L, with the selectivities of 111.3- and 272.8-fold to σ2R, respectively (Table 113, 27). Compared to the most promising compound 6, both 13 and 15 showed a slight decrease of σ1R binding affinity and selectivity, due to structure modifications necessary for radiolabeling; however, based on the criteria mentioned above, 13 and 15 still possessed suitable affinities for in vivo PET imaging of σ1R.
Table 1.
Ex vivo binding affinities for σ1R and σ2R of PD 144418, compounds 6, 13 and 15.
| Compd. |
σ1R Kd or Ki (nmol/L) |
σ2R Ki (nmol/L) |
Selectivity (σ2R/σ1R) |
|---|---|---|---|
| PD 14441813 | 4.3 ± 0.1 | 1377 ± 179 | – |
| 627 | 1.4 ± 0.1 | 1912 ± 210 | 1365.7 |
| 13 | 10.3 ± 1.1 | 1146 ± 116 | 111.3 |
| 15 | 5.6 ± 0.7 | 1528 ± 120 | 272.8 |
−Not applicable.
Besides the binding affinity and selectivity to the target receptor, the logD is also another important parameter, especially for the radiotracer for brain imaging. The experimental logDPBS,pH7.4±SD of compounds 13 and 15 were measured34 to be 0.89±0.06 and 1.73±0.08, respectively.
2.4. Molecular docking studies of 6-hydroxypyridazinone derivatives
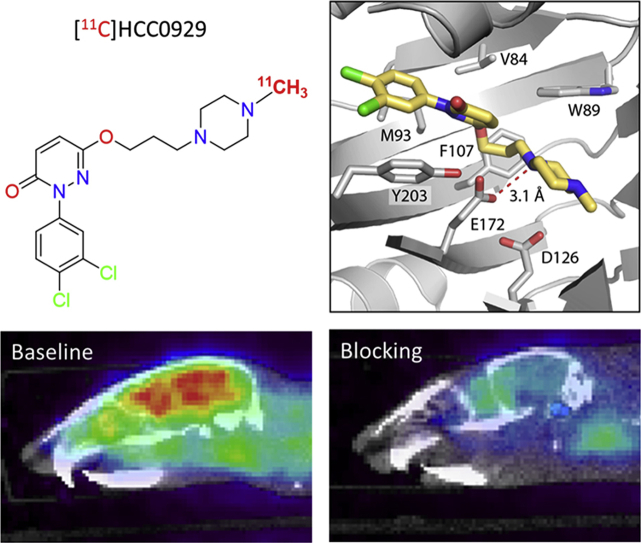
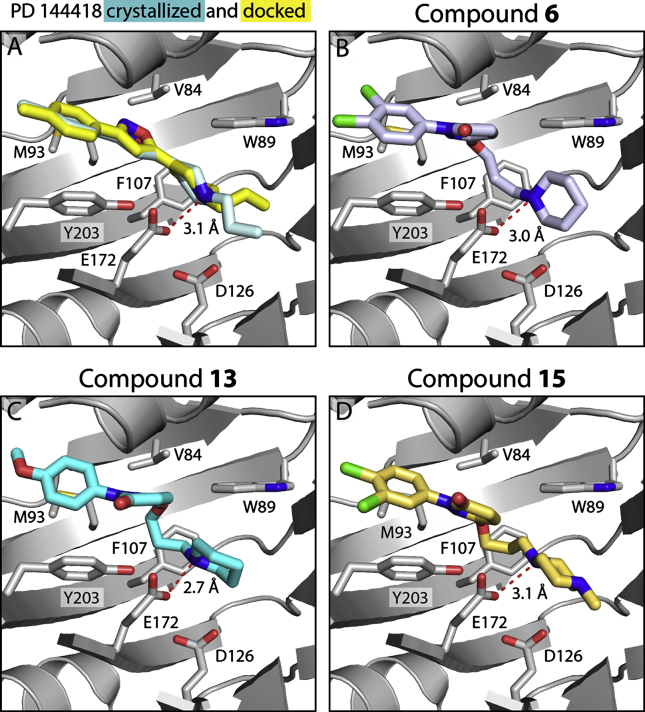
To predict the possible binding mode of the two radioligands, we performed molecular docking with Schrödinger Glide software (Schrödinger, LLC, New York, NY, USA) using the 2.5 Å resolution structure of the σ1R bound to PD 144418, in a similar manner as reported previously13, 14, 35.
Encouragingly, the top-ranked docked pose of PD 144418 in Fig. 2A (yellow) was nearly identical to that seen in the crystal structure (Fig. 2A, cyan), and the Glide score was comparable to what has been reported previously for high-affinity σ1R ligands13, 14, 35.
Figure 2.
Glide docking of compounds into the σ1R (PDB 5HK1). (A) Pose of co-crystallized PD 144418 (pale cyan) and top-ranked pose from Glide docking (yellow). Best docked poses for (B) compound 6 (periwinkle), (C) compound 13 (cyan), (D) compound 15 (gold). In all panels, the receptor is shown in gray.
The reference compound 6, along with 13 and 15, was docked in the same way. All three compounds adopted similar conformations in the ligand-binding site as PD 144418, with good Glide Scores (Table 2). Like all ligands co-crystallized with the receptor to date, the poses for compounds 6, 13, and 15 featured an electrostatic interaction between the positively charged nitrogen in the ligand and E172 (Table 2, Fig. 2B‒D). Additionally, as reported for all currently co-crystallized σ1R antagonists13, 14, the primary hydrophobic regions of these compounds were positioned near Y203, pointing towards the membrane, and the secondary hydrophobic regions were pointing towards the bottom of the ligand binding site past D126. These results suggest that these compounds likely bind the receptor similarly to other high-affinity σ1R ligands.
Table 2.
Distance of the electrostatic interaction between ligand's basic amine and E172, with Glide scores of PD 144418, HCC0923 and HCC0929 in molecular docking.
| Compd. | Distance to E172 (Å) | Glide Score (kcal/mol) |
|---|---|---|
| PD 144418 (co-crystallized) | 3.1 | – |
| PD 144418 (docking) | 3.1 | ‒10.460 |
| 6 | 3.0 | ‒10.251 |
| 13 | 2.7 | ‒10.578 |
| 15 | 3.1 | ‒10.577 |
−Not applicable.
2.5. In vivo PET-CT imaging with [11C]HCC0923 in mice36, 37
Following the encouraging data in ex vivo σ1R binding assays and molecular docking, two radioligands, [11C]HCC0923 and [11C]HCC0929, were a step forward to further in vivo investigation.
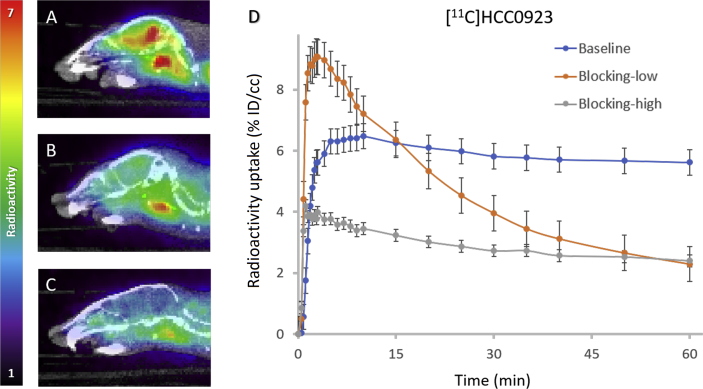
We firstly assessed [11C]HCC0923 in vivo conducting dynamic PET imaging focused on mice brains. In PET-CT studies, [11C]HCC0923 exerted high BBB penetration and fast uptake when administered by intravenous bolus injection (100‒150 μCi per animal), as shown in Fig. 3. Based on a whole-brain analysis, the concentration of [11C]HCC0923 in the mice brain reached a maximum uptake of 6.48% ID/cc within the first few minutes after injection, and sustained binding over the scanning time (60 min).
Figure 3.
Mice brain PET/CT images 25‒60 min after intravenous administration (i.v.) of radioligand [11C]HCC0923 and time‒activity curve. (A) Baseline PET/CT image. (B) and (C) PET/CT image from blocking study, involving i.v. pretreatment with unlabeled HCC0923 (self-blocking, B: 1.25 mg/kg, C: 12.5 mg/kg) 5.0 min before radioligand injection. (D) Time‒activity curve demonstrating uptake of radiolingand for baseline and blocking studies (low & high dose).
To investigate the specificity of [11C]HCC0923, we performed PET imaging studies in mice with a 5-min pretreatment of compound 13 (unlabeled HCC0923) at different doses (1.25 and 12.5 mg/kg). Compared with the non-pretreat control (baseline, Fig. 3A), we found that the [11C]HCC0923 binding in mice brain was blocked in a dose-dependent manner with a stepwise reduction in the percent tracer uptake after administration of 13 (Fig. 3B and C). At 1.25 mg/kg, we found an approximate 38% reduction in binding, estimated as the percent change in whole-brain radioactivity between peak uptake at 10 min and the lowest uptake at 60 min. Increasing the dose of 13 to 12.5 mg/kg resulted in a ∼54% reduction in [11C]HCC0923 brain uptake, a dose-dependent response to self-blockade and ∼45% of uptake attributed to non-specific binding. We observed a similar blockade level at the last 10 min, indicating saturation at 1.25 mg/kg. This finding demonstrates a high specific binding of [11C]HCC0923 for σ1R, with a dose-dependent response to self-blockade.
2.6. In vivo PET-CT imaging with [11C]HCC0929 in mice36, 37
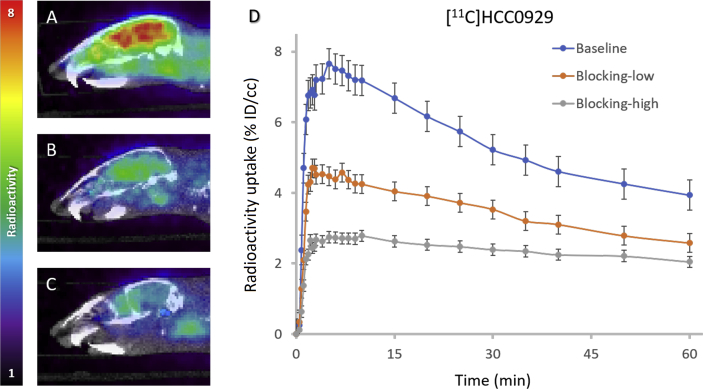
[11C]HCC0929 was also studied in mice PET-CT imaging due to its better σ1R affinity. To test [11C]HCC0929 as a radiotracer in vivo, we conducted PET imaging focused on the mice brains. Compared to [11C]HCC0923, we determined that [11C]HCC0929 exhibited more potent properties in PET imaging studies: higher BBB penetration and faster signal decrease over the 60-min scan when administered by intravenous bolus injection (100‒150 μCi per animal), as shown in Fig. 4.
Figure 4.
Mice brain PET/CT images 25‒60 min after intravenous administration (i.v.) of radioligand [11C]HCC0929 and time‒activity curve. (A) Baseline PET/CT image. (B) and (C) PET/CT image from blocking study, involving i.v. pretreatment with unlabeled HCC0929 (self-blocking, B: 0.288 mg/kg, C: 2.88 mg/kg) 5.0 min before radioligand injection. (D) Time‒activity curve demonstrating uptake of radioligand for baseline and blocking studies (low & high dose).
Unlike the slow gradient of baseline curve of [11C]HCC0923, the whole-brain analysis that exerted the concentration of [11C]HCC0929 in the mice brain reached a maximum uptake of 7.66% ID/cc at ∼5 min after injection with moderate wash-out rate during the scanning period (60 min), indicating a faster brain clearance kinetic property compared to [11C]HCC0923. The specificity of [11C]HCC0929 was investigated in mice PET imaging studies with a 5-min i.v. pretreatment of compound 15 (unlabeled HCC0929) at doses of 0.288 and 2.88 mg/kg.
We found that administration of 0.288 mg/kg unlabeled HCC0929 (15) blocked [11C]HCC0929 binding in the mice brain by approximately 36%, measured as the percent change in whole radioactivity between peak uptake at ∼5 min and the lowest uptake at 60 min. Increasing the dose of 15 to 2.88 mg/kg resulted in a ∼58% reduction in [11C]HCC0929 mice brain uptake (Fig. 4D), which represents a dose-dependent response to self-blockade and ∼40% of uptake attributed to non-specific binding. The mice in vivo PET-CT studies demonstrated a high uptake and good mice brain clearance kinetic of [11C]HCC0929 for σ1R imaging in brain, with a dose-dependent response to self-blockade.
2.7. Positive ligands blocking study of in vivo PET-CT imaging using [11C]HCC0929 in mice
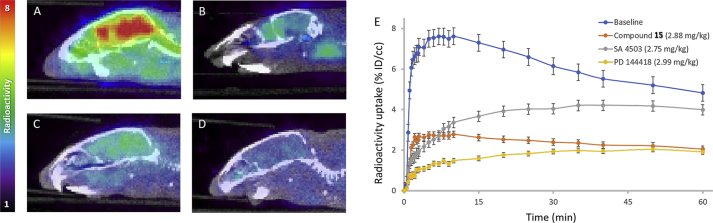
To further validate the selectivity of σ1R of the candidate radioligand [11C]HCC0929, two highly σ1R selective ligands, SA 4503 (σ1R agonist) and PD 144418 (σ1R antagonist) were adopted for positive ligands blocking study36, 37.
Through the in vivo PET-CT imaging in mice brain (Fig. 5), we found that administration of SA 4503 (2.75 mg/kg) or PD 144418 (2.99 mg/kg) could remarkably reduce the [11C]HCC0929 binding in the mice brain by approximately 41% and 67%, respectively, measured as the percent change in whole radioactivity between peak uptake at ∼5 min and the lowest uptake at 60 min. The different blocking effects of two positive ligands might due to their entirely opposite functional profiles. The shape of time‒active curve of self-blocking was close to the curve of PD 144418, since the compound 15 acted as the same as antagonist, but the binding affinity to σ1R was a little higher than PD 144418.
Figure 5.
Mice brain PET/CT images 25‒60 min after intravenous administration (i.v.) of radioligand [11C]HCC0929 and time‒activity curve. (A) Baseline PET/CT image (n=2); PET/CT image from blocking study, involving i.v. pretreatment with positive compounds 5.0 min before radioligand injection: (B) unlabeled HCC0929 (self-blocking, 2.88 mg/kg); (C) SA 4503 (σ1R agonist, 2.75 mg/kg); (D) PD 144418 (σ1R agonist, 2.99 mg/kg); (E) Time‒activity curve demonstrating uptake of radioligand for baseline and blocking studies (low & high dose).
2.8. In vivo biodistribution studies of [11C]HCC0929 in mice
The biodistribution of radioligand [11C]HCC0929 in mice was investigated by in vivo PET-CT imaging, and the data were acquired by using the FUSION module in PMOD (PMOD 4.003, PMOD Technologies Ltd., Zurich, Switzerland).
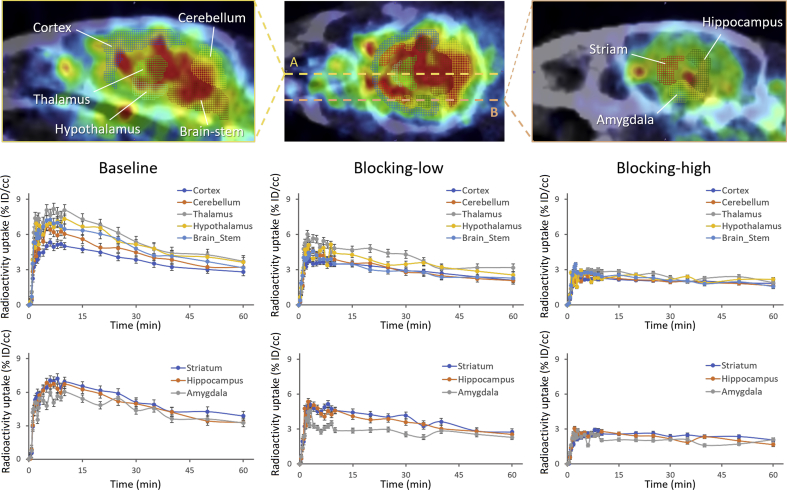
The analysis of detailed distribution of different brain regions of mice was obtained through the mouse (Ma-Benveniste-Mirrione) VOI atlas38, 39. Eight important functional regions of mice brain were selected: cortex, cerebellum, brain stem, thalamus, hypothalamus, striatum, hippocampus and amygdala. The radioligand [11C]HCC0929 distributed in the selected brain regions were investigated and showed quite similar distribution patterns40 without significant regional differences (Fig. 6). In blocking studies, the uptake of [11C]HCC0929 in different regions of mice brain was significantly decreased by co-injection of HCC0929. In high dose injection (2.88 mg/kg), all the selected brain regions of mice were decreased significantly as the same; while pretreated a low dose of HCC0929 (0.288 mg/kg), the cortex, striatum and hippocampus showed a moderate decrease compared to other mice brain regions, which were mainly because of the different express of σ1R in these regions.
Figure 6.
Time-activity curve demonstrating uptake of radioligand [11C]HCC0929 for baseline and blocking studies (self-blocking, low blocking dose: 0.288 mg/kg, high blocking dose: 2.88 mg/kg) of different brain regions of mice brain, including cortex, cerebellum, brain stem, thalamus, hypothalamus, striatum, hippocampus, amygdala.
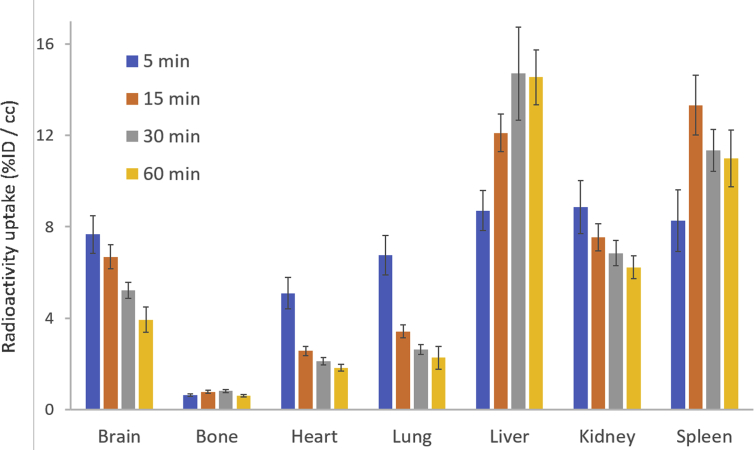
The distribution of major organs in mice was analyzed using the PBAS module in PMOD 4.003. The mean radioactive uptake in brain and major organs at each time point is showed in Fig. 7. The highest uptake occurred at 5 min in brain (7.66±0.82% ID/cc), heart (5.10±0.68% ID/cc), lung (6.75±0.86% ID/cc) and kidney (8.87±1.16% ID/cc), and then the radioligand was gradually washed out from these organs. Whereas in liver and spleen, due to the accumulation of [11C]HCC0929, the time point of maximum uptake was behind other organs. The uptake in liver peaked at 30 min at 14.70±2.03% ID/cc and slightly decreased in 60 min; in spleen, the maximum radioactivity uptake reached in 15 min at 13.32±2.03% ID/cc and then was slowly washed out during study.
Figure 7.
Biodistribution of radioligand [11C]HCC0929 in rats at 5, 15, 30, and 60 min after injection of radioligand (n=3 for each time point). Error bars represent SEM.
3. Conclusions
In summary, two novel carbon-11 labeled σ1R radioligands with a 6-hydroxypyridazinone scaffold, [11C]HCC0923 and [11C]HCC0929, were successfully prepared and evaluated in mice. Both two radioligands can highly bind to σ1R in the mice brain, with good selectivity and specificity. Of the two novel 11C-labeled sigma-1 receptor radioligands, [11C]HCC0929 possessed better kinetic property and specificity which was further investigated in positive ligands blocking studies in mice PET-CT brain imaging, using classic σ1R agonist SA 4503 and σ1R antagonist PD 144418. Both σ1R ligands could extensively decreased the uptake of [11C]HCC0929 in mice brain, with different kinetic uptake and washout properties. Besides, the biodistribution of major brain regions and organs of mice were determined in vivo. The radioligand [11C]HCC0929 distributed in the selected brain regions showed quite similar distribution patterns as reported, and the distribution in major organs extent the good pharmacokinetic properties in vivo. These results demonstrated its promise as preclinical tools for visualizing and quantitating σ1R density in the mice brain in vivo.
Application [11C]HCC0929 as PET probes could be used to quantify σ1R expression in various neurological disorders, and would also be valuable for evaluation of potential drugs in living subjects. The approach is also valuable for expanding the variety and diversity of PET radiotracers for σ1R imaging to meet the requirements for practical clinic application and therefore warrants further investigation.
4. Experimental
4.1. General methods and materials
All commercially available chemical reagents and solvents were of ACS-grade purity or higher, and used without further purification.
1H NMR and 13C NMR spectra data were recorded on a JEOL JNM-ECZ500R Spectrometer (JEOL Ltd, Tokyo, Japan) at 500 MHz (1H) and 126 MHz (13C) using chloroform-d. Chemical shifts were given in δ values (ppm), using tetramethylsilane (TMS) as the internal standard; coupling constants (J) were given in Hz. Signal multiplicities were characterized as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad signal).
Analytical thin layer chromatography (TLC) was performed on silica gel GF254. Column chromatographic purification was carried out using silica gel. Analytical separation was conducted on an Agilent 1100 series HPLC (Agilent Technologies, Inc., Santa Clara, CA, USA) fitted with a diode-array detector, quaternary pump, vacuum degasser, and autosampler. Mass spectrometry data were recorded on an Agilent 6310 ion trap mass spectrometer (ESI source, Agilent Technologies, Inc., Santa Clara, CA, USA) connected to an Agilent 1200 series HPLC with quaternary pump, vacuum degasser, diode-array detector, and autosampler.
[11C]CO2 (1.2 Ci) was obtained via the 14N (p, α) 11C reaction on nitrogen with 2.5% oxygen, with 11 MeV protons (Siemens Eclipse cyclotron, Siemens Healthcare GmbH, Erlangen, Germany), and trapped on molecular sieves in a TRACERlab FX-MeI synthesizer (General Electric, GE Healthcare, Boston, MA, USA). [11C]CH4 was obtained by the reduction of [11C]CO2 in the presence of Ni/hydrogen at 350 °C and recirculated through an oven containing I2 to produce [11C]CH3I via a radical reaction.
All animal studies were carried out at Massachusetts General Hospital (MGH, PHS Assurance of Compliance No. A3596-01). The Subcommittee on Research Animal Care (SRAC) serves as the Institutional Animal Care and Use Committee (IACUC) for the Massachusetts General Hospital. SRAC reviewed and approved all procedures detailed in this paper.
Micro PET-CT imaging was performed in anesthetized (isoflurane) mice (BALB/c) to minimize discomfort. Highly trained animal technicians monitored animal safety throughout all procedures, and veterinary staff were responsible for daily care. All mice were socially housed in cages appropriate for the physical and behavioral health of the individual animal and were given unlimited access to food and water, with additional nutritional supplements provided as prescribed by the attending veterinary staff.
4.2. Chemical synthesis
4.2.1. General procedure for the preparation of intermediates 9 and 10
6-Hydroxy-2-(4-methoxyphenyl)pyridazin-3(2H)-one (9). To a mixture of (4-methoxyphenyl)hydrazine hydrochloride (7, 55 mmol) and maleic anhydride (50 mmol) in H2O (400 mL), concentrated hydrochloric acid solution (40 mL) was added slowly with stirring. The mixture was heated at 120 °C for 8 h. The progress of the reaction was monitored by TLC. After cooling to room temperature and filtrating, the resulting solid was washed with ice‒water and dissolved in a saturated sodium bicarbonate solution. After another filtration, the resulting filtrate was neutralized with 1 mol/L hydrochloric acid and formed a precipitate. The precipitate was then filtered and washed with water to yield 6-hydroxy-2-(4-methoxyphenyl)pyridazin-3(2H)-one, 9, as a white solid (7.22 g, 66.2%). 1H NMR (500 MHz, chloroform-d) δ 7.49 (d, J=8.7 Hz, 2H), 7.10–7.02 (m, 2H), 7.00–6.94 (m, 2H), 3.84 (s, 3H). A signal for the OH-proton is not visible. LC–MS Calcd. for C11H10N2O3 expected [M+H]+: 219.1; Found [M+H]+: 219.1.
2-(3,4-Dichlorophenyl)-6-hydroxypyridazin-3(2H)-one (10). The procedure described for the synthesis of 9 was applied to the initial compound 8 (55 mmol), maleic anhydride (50 mmol), and H2O (400 mL) with concentrated HCl solution (40 mL) to afford 2-(3,4-dichlorophenyl)-6-hydroxypyridazin-3(2H)-one, 10 as a white solid (9.01 g, 70.1%). 1H NMR (500 MHz, chloroform-d) δ 7.89 (d, J=2.3 Hz, 1H), 7.65 (dd, J=8.7, 2.5 Hz, 1H), 7.52 (d, J=8.8 Hz, 1H), 7.01 (s, 2H). A signal for the OH-proton is not visible. LC–MS Calcd. for C10H6Cl2N2O2 expected [M+H]+: 258.1; Found [M+H]+: 258.1.
4.2.2. General procedure for the preparation of intermediates 11 and 12
6-(3-Bromopropoxy)-2-(4-methoxyphenyl)pyridazin-3(2H)-one (11). To a solution of 9 (10 mmol) and 1,3-dibromopropane (20 mmol) in acetone (100 mL), potassium carbonate (20 mmol) was added and the mixture was refluxed for 4 h. The progress of the reaction was monitored by TLC. After cooled to room temperature, the mixture was filtered and the solvent was evaporated under reduced pressure. The crude product was purified by means of flash chromatography (hexane/ethyl acetate=50/1) to yield 6-(3-bromopropoxy)-2-(4-methoxyphenyl)pyridazin-3(2H)-one, 11, as a pale-yellow oil (1.89 g, 55.6%). 1H NMR (500 MHz, chloroform-d) δ 7.57 (d, J=8.8 Hz, 2H), 7.06–7.01 (m, 2H), 7.00–6.95 (m, 2H), 4.31 (t, J=5.9 Hz, 2H), 3.84 (s, 3H), 3.55 (t, J=6.4 Hz, 2H), 2.30 (p, J=6.2 Hz, 2H). 13C NMR (126 MHz, chloroform-d) δ 158.89, 152.50, 134.68, 134.08, 126.56, 126.36, 113.96, 64.80, 55.62, 31.75, 29.61. LC–MS Calcd. for C14H15BrN2O3 expected [M+H]+: 340.2; Found [M+H]+: 340.1.
6-(3-Bromopropoxy)-2-(3,4-dichlorophenyl)pyridazin-3(2H)-one (12). The procedure described for the synthesis of 11 was applied to intermediate 10 (10 mmol), 1,3-dibromopropane (20 mmol), and potassium carbonate (20 mmol) in acetone (100 mL) to afford 6-(3-bromopropoxy)-2-(3,4-dichlorophenyl)pyridazin-3(2H)-one, 12 as a light yellow oil (1.97 g, 52.0%). 1H NMR (500 MHz, chloroform-d) δ 7.88 (d, J=2.3 Hz, 1H), 7.64 (dd, J=8.7, 2.5 Hz, 1H), 7.51 (d, J=8.8 Hz, 1H), 7.00 (s, 2H), 4.33 (t, J=5.9 Hz, 2H), 3.56 (t, J=6.5 Hz, 2H), 2.32 (p, J=6.2 Hz, 2H). 13C NMR (126 MHz, chloroform-d) δ 158.55, 152.77, 140.63, 134.34, 132.58, 131.48, 130.23, 127.23, 126.70, 124.09, 65.01, 31.62, 29.47. LC–MS Calcd. for C13H11BrCl2N2O2 expected [M+H]+: 379.1; Found [M+H]+: 379.1.
4.2.3. General procedure for the preparation of compounds 13 to 15
2-(4-Methoxyphenyl)-6-(3-(piperidin-1-yl)propoxy)pyridazin-3(2H)-one (13). A mixture of intermediate 11 (5 mmol) and piperidine (5.5 mmol) in acetonitrile (50 mL) and cesium carbonate (10 mmol) was heated and refluxed for 2 h. After filtering, the resulting filtrate was evaporated to dryness under reduced pressure. The residue was suspended in water (50 mL) and extracted with dichloromethane (3 × 25 mL). The combined organic layers were dried with anhydrous magnesium sulfate, the filtrate was evaporated under reduced pressure, and the crude product was purified by means of flash chromatography (CH2Cl2/CH3OH=10/1) to yield 2-(4-methoxyphenyl)-6-(3-(piperidin-1-yl)propoxy)pyridazin-3(2H)-one, 13, as a yellow oil (1.46 g, 84.8%). 1H NMR (500 MHz, chloroform-d) δ 7.56 (d, J=8.7 Hz, 2H), 7.03–6.93 (m, 4H), 4.19 (t, J=6.4 Hz, 2H), 3.84 (s, 3H), 2.53–2.30 (m, 6H), 1.96 (p, J=6.7 Hz, 2H), 1.60 (p, J=5.6 Hz, 4H), 1.51–1.40 (m, 2H). 13C NMR (126 MHz, chloroform-d) δ 158.91, 158.81, 152.79, 134.81, 133.89, 126.79, 126.38, 113.93, 65.86, 55.98, 55.61, 54.67, 26.23, 25.90, 24.40. LC–MS Calcd. for C19H25N3O3 expected [M+H]+: 344.2; Found [M+H]+: 344.2, HR-MS Calcd. for C19H25N3O3 expected [M+H]+: 344.1964; Found [M+H]+: 344.1969.
tert-Butyl 4-(3-((1-(3,4-dichlorophenyl)-6-oxo-1,6-dihydropyridazin-3-yl)oxy)-propyl)piperazine-1-carboxylate (14). The procedure described for the synthesis of 13 was applied to intermediate 12 (5 mmol), tert-butyl piperazine-1-carboxylate (5.5 mmol), and cesium carbonate (10 mmol) in acetonitrile (50 mL) to afford 4-(3-((1-(3,4-dichlorophenyl)-6-oxo-1,6-dihydropyridazin-3-yl)oxy)-propyl)piperazine-1-carboxylate, 14 as a light yellow oil (1.78 g, 73.5%). 1H NMR (500 MHz, chloroform-d) δ 7.87 (d, J=2.5 Hz, 1H), 7.64 (dd, J=8.8, 2.4 Hz, 1H), 7.51 (d, J=8.8 Hz, 1H), 6.99 (s, 2H), 4.23 (t, J=6.4 Hz, 2H), 3.44–3.30 (m, 4H), 2.50 (t, J=7.3 Hz, 2H), 2.45–2.34 (m, 4H), 1.96 (p, J=6.7 Hz, 2H), 1.46 (s, 9H). 13C NMR (126 MHz, chloroform-d) δ 158.59, 153.05, 140.74, 134.13, 132.53, 131.38, 130.18, 127.49, 126.72, 124.13, 65.88, 55.11, 55.04, 53.18, 46.05, 26.16. LC–MS Calcd. for C22H28Cl2N4O4 expected [M+H]+: 484.4; Found [M+H]+: 484.3.
2-(3,4-Dichlorophenyl)-6-(3-(4-methylpiperazin-1-yl)propoxy)pyridazin-3(2H)-one (15). The procedure described for the synthesis of 13 was applied to intermediate 12 (5 mmol), 1-methylpiperazine (5.5 mmol), and cesium carbonate (10 mmol) in acetonitrile (50 mL) to afford 2-(3,4-dichlorophenyl)-6-(3-(4-methylpiperazin-1-yl)propoxy) pyridazin-3(2H)-one, 15 as a light yellow oil (1.60 g, 80.6%). 1H NMR (500 MHz, chloroform-d) δ 7.87 (d, J=2.4 Hz, 1H), 7.64 (dd, J=8.7, 2.4 Hz, 1H), 7.50 (d, J=8.7 Hz, 1H), 6.99 (s, 2H), 4.22 (t, J=6.4 Hz, 2H), 2.62–2.40 (m, 10H), 2.31 (s, 3H), 1.96 (p, J=6.7 Hz, 2H). 13C NMR (126 MHz, chloroform-d) δ 158.57, 154.80, 153.03, 140.72, 134.16, 132.53, 131.39, 130.18, 127.45, 126.70, 124.11, 79.76, 65.76, 55.11, 55.04, 53.10, 28.51, 26.07. LC–MS Calcd. for C18H22Cl2N4O2 expected [M+H]+: 396.1; Found [M+H]+: 397.2, HR-MS Calcd. for C18H22Cl2N4O2 expected [M+H]+: 397.1193; Found [M+H]+: 397.1190.
2-(4-Hydroxyphenyl)-6-(3-(piperidin-1-yl)propoxy)pyridazin-3(2H)-one (16, precursor P1) Under N2, a solution of 13 (1 mmol) in dichloromethane (14 mL) was kept in an acetone-dry ice bath at ‒78 °C. 6 mL of Boron tribromide solution (1.0 mol/L in dichloromethane) was added carefully to the stirring solution and kept at ‒78 °C for 2 h. As the solution of boron tribromide was added, a pale yellow precipitate formed. The reaction mixture was gradually warmed to room temperature and kept stirring overnight. The reaction mixture was then hydrolyzed by careful shaking with 40 mL of H2O, thus precipitating a white solid, which was dissolved by the addition of 30 mL of dichloromethane. The organic layer was separated and extracted with 20 mL of 2 mol/L sodium hydroxide; the alkaline extract was neutralized with dilute hydrochloric acid and extracted with dichloromethane (3 × 10 mL). The combined organic layers were dried with anhydrous magnesium sulfate, the filtrate was evaporated under reduced pressure, and the crude product was purified by means of flash chromatography (CH2Cl2/CH3OH=10/1) to yield 2-(4-hydroxyphenyl)-6-(3-(piperidin-1-yl)propoxy)pyridazin-3(2H)-one (16, precursor P1) as a pale yellow oil (0.142 g, 43.2%). 1H NMR (500 MHz, chloroform-d) δ 7.32 (d, J=8.5 Hz, 2H), 7.00–6.91 (m, 2H), 6.84–6.73 (m, 2H), 4.17 (t, J=6.2 Hz, 2H), 3.00–2.44 (m, 6H), 2.08 (t, J=7.5 Hz, 2H), 1.82–1.66 (m, 4H), 1.59–1.41 (m, 2H). A weak signal for the Ar–OH–proton is in δ 8.04, hardly visible. 13C NMR (126 MHz, chloroform-d) δ 159.17, 156.92, 152.77, 133.59, 133.39, 126.89, 126.53, 115.74, 65.32, 55.57, 54.22, 25.02, 24.61, 23.52. LC–MS Calcd. for C18H23N3O3 expected [M+H]+: 330.2; Found [M+H]+: 330.2, HR-MS Calcd. for C18H23N3O3 expected [M+H]+: 330.1812; Found [M+H]+: 330.1813.
2-(3,4-Dichlorophenyl)-6-(3-(piperazin-1-yl)propoxy)pyridazin-3(2H)-one (17, precursor P2) Under N2, a solution of 14 (1 mmol) in dichloromethane (18 mL) was kept in an ice bath at 0 °C. 2 mL of hydrogen chloride solution (1.0 mol/L in diethyl ether) was added to the stirring solution. As the solution of hydrogen chloride was added, a pale yellow precipitate was formed. The reaction mixture was gradually warmed to room temperature and kept stirring overnight. After that, the reaction mixture was neutralized by 30 mL of saturated sodium bicarbonate solution and extracted with dichloromethane (3 × 10 mL). The combined organic layers were dried with anhydrous magnesium sulfate, the filtrate was evaporated under reduced pressure, and the crude product was purified by means of flash chromatography (CH2Cl2/CH3OH=10/1) to yield 2-(3,4-dichlorophenyl)-6-(3-(piperazin-1-yl)propoxy)pyridazin-3(2H)-one (17, precursor P2) as a yellow oil (0.353 g, 92.1%). 1H NMR (500 MHz, chloroform-d) δ 7.86 (d, J=2.4 Hz, 1H), 7.63 (dd, J=8.8, 2.4 Hz, 1H), 7.51 (d, J=8.7 Hz, 1H), 7.03–6.95 (m, 2H), 4.22 (t, J=6.4 Hz, 2H), 3.25–3.16 (m, 2H), 3.09–2.40 (m, 9H), 2.09–1.89 (m, 2H). 13C NMR (126 MHz, chloroform-d) δ 158.54, 152.93, 134.27, 134.19, 130.24, 130.20, 127.42, 127.34, 126.72, 124.14, 65.31, 54.58, 50.08, 43.80, 25.88. LC–MS Calcd. for C17H20Cl2N4O2 expected [M+H]+: 383.1; Found [M+H]+: 383.1, HR-MS Calcd. for C17H20Cl2N4O2 expected [M+H]+: 383.1036; Found [M+H]+: 383.1032.
4.3. Radiosynthesis
[11C]HCC0923. [11C]methyl iodide ([11C]CH3I) was trapped in a TRACERlab FX-M synthesizer reactor (General Electric) preloaded with a solution of precursor P1 in anhydrous DMF (2.0 mg/mL, 0.3 mL) and NaOH (8 mg). The solution was stirred at 120 °C for 3 min, and 0.1% trifluoroacetic acid (TFA) in water (1.2 mL) was added. The reaction mixture was purified by reverse phase semipreparative HPLC (Agilent Eclipse XDB-C18, 5 μm, 250 mm × 9.4 mm, flow rate=5.0 mL/min, mobile phase=0.1% TFA in water/0.1% TFA in acetonitrile, 82/18, v/v), and the desired fraction was collected. The final product was reformulated by loading onto a solid-phase exchange (SPE) C-18 cartridge, rinsing with H20 (5 mL), eluting with DMSO (1 mL), and diluting with saline solution (0.9%, 9 mL).
The average time required for the synthesis from end of cyclotron bombardment to end of synthesis was approximate 40‒50 min. The average radiochemical yield was 6%−15% (nondecay corrected to trapped [11C]CH3I). Chemical and radiochemical purities were ≥95% with a specific activity 1.29±0.2 Ci/μmol (EOB).
[11C]HCC0929. [11C]methyl iodide ([11C]CH3I) was trapped in a TRACERlab FX-M synthesizer reactor (General Electric) preloaded with a solution of precursor P2 in anhydrous DMF (2.0 mg/mL, 0.3 mL) and K2CO3 (8 mg). The solution was stirred at 120 °C for 3 min, and 0.1 mol/L ammonium formate (AF) in water (1.2 mL) was added. The reaction mixture was purified by reverse phase semipreparative HPLC (Phenomenex Luna 5u C8(2), 250 mm × 10 mm, 5 μm, flow rate=5.0 mL/min, mobile phase=0.1 mol/L AF in water/acetonitrile, 65/35, v/v), and the desired fraction was collected. The final product was reformulated by loading onto a solid-phase exchange (SPE) C-18 cartridge, rinsing with H20 (5 mL), eluting with EtOH (1 mL), and diluting with saline solution (0.9%, 9 mL).
The average time required for the synthesis from end of cyclotron bombardment to end of synthesis was approximate 40‒50 min. The average radiochemical yield was 3%−8% (nondecay corrected to trapped [11C]CH3I). Chemical and radiochemical purities were ≥95% with a specific activity 1.62±0.2 Ci/μmol (EOB).
4.4. Molecular docking
Molecular docking into the σ1R was performed in the manner of previous work13, 14, 35 with Glide 5.5 extra precision (XP) Maestro 11 Schrodinger software (Schrödinger, LLC, New York, NY, USA) release 2016–341.
The 2.5 Å resolution structure of σ1R in complex with PD 144418 (PDB 5KH1) was used for docking, compared with the ex vivo binding affinity42. Because the structure has three protomers in the asymmetric unit, only chain C was used for docking studies. Lipids, ions, and waters were removed before protein preparation, thus leaving only the protein and ligand. Hydrogen atoms were added, and the protein was further refined by assignment of hydrogen bonds and minimization of energy for the OPLS3 force field. The grid used for docking was centered on the location of the co-crystallized ligand PD 144418, and was 20 Å in the x, y, and z dimensions. Poses were ranked according to glide score and inspected visually. Only poses in which the ligand's basic amine made an electrostatic interaction with E172 were considered plausible, as this has been observed in all five existing σ1R crystal structures currently available13, 14.
4.5. Mice PET-CT acquisition and post processing
Male Balb/c mice were tested in groups, each group contained 4 mice, anesthetized with inhalational isoflurane (Patterson Vet Supply, Inc., Greeley, CO, USA) at 2% in a carrier of 2 L/min medial oxygen, and maintained at 1% isoflurane for the duration of the scan.
The mice were arranged side-by-side in two layers in a Triumph Trimodality PET/CT/SPECT scanner (Gamma Medica, Northridge, CA, USA). Mice were injected standard references or vehicle via a lateral tail vein catheterization at the start of PET acquisition. Dynamic PET acquisition lasted for 60 min followed by computed tomography (CT) for anatomic coregistration. PET data were reconstructed using a 3D-MLEM method resulting in a full width at half-maximum resolution of 1 mm. Reconstructed images were exported from the scanner in DICOM format along with an anatomic CT for rodent studies. These files were imported and analyzed using AMIDE (a medical imaging data examiner) software (an open-source software, Los Angeles, CA, USA)43 and PMOD (PMOD4.003, PMOD Technologies Ltd., Zurich, Switzerland).
4.6. Mice PET-CT image analysis
Volumes of interest (VOIs) were generated manually in forms of spheres under the guide of high-resolution CT structural images and summed PET data in mice brain regions, with a radius no less than 1 mm to minimize partial volume effects. Time-activity curves (TACs) were exported as decay-corrected activity per unit volume. The TACs were expressed as percent injected dose per unit volume for analysis.
Acknowledgments
This work was supported by a pilot funding from the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital (Changning Wang, USA), National Natural Science Foundation of China (Grant No.81602946, Yu Lan) and Natural Science Foundation of Hubei Province of China (Grant No. 2016CFB258, Yu Lan). The authors are grateful to Prof. Andrew C. Kruse in Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School for the constructive discussion and enthusiastic help in molecular docking, the Athinoula A. Martinos Center Radiopharmacy Lab staff for assistant in radiochemistry and Prof. Xudong Cao in Xuzhou Medical School for the discussion in chemistry and structure identification.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.07.002.
Appendix A. Supporting information
The following is the Supporting data to this article:
References
- 1.Martin W.R., Eades C.G., Thompson J.A., Huppler R.E., Gilbert P.E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 2.Zukin S.R., Brady K.T., Slifer B.L., Balster R.L. Behavioral and biochemical stereoselectivity of sigma opiate/PCP receptors. Brain Res. 1984;294:174–177. doi: 10.1016/0006-8993(84)91326-x. [DOI] [PubMed] [Google Scholar]
- 3.Hellewell S.B., Bowen W.D. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of Guinea pig brain. Brain Res. 1990;527:244–253. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 4.Su T.P., Su T.C., Nakamura Y., Tsai S.Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol Sci. 2016;37:262–278. doi: 10.1016/j.tips.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanner M., Moebius F.F., Flandorfer A., Knaus H.G., Striessnig J., Kempner E. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alon A., Schmidt H.R., Wood M.D., Sahn J.J., Martin S.F., Kruse A.C. Identification of the gene that codes for the σ2 receptor. Proc Natl Acad Sci U S A. 2017;114:7160–7165. doi: 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabba M. The essential roles of protein‒protein interaction in sigma-1 receptor functions. Front Cell Neurosci. 2003;7:50. doi: 10.3389/fncel.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T., Su T.P. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci U S A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER‒mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Maurice T., Su T.P. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Waarde A., Rybczynska A.A., Ramakrishnan N., Ishiwata K., Elsinga P.H., Dierckx R.A. Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands. Curr Pharmaceut Des. 2010;16:3519–3537. doi: 10.2174/138161210793563365. [DOI] [PubMed] [Google Scholar]
- 12.Tesei A., Cortesi M., Zamagni A., Arienti C., Pignatta S., Zanoni M. Sigma receptors as endoplasmic reticulum stress “gatekeepers” and their modulators as emerging new weapons in the fight against cancer. Front Pharmacol. 2018;9:711. doi: 10.3389/fphar.2018.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt H.R., Zheng S., Gurpinar E., Koehl A., Manglik A., Kruse A.C. Crystal structure of the human σ1 receptor. Nature. 2016;532:527–530. doi: 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt H.R., Betz R.M., Dror R.O., Kruse A.C. Structural basis for σ1 receptor ligand recognition. Nat Struct Mol Biol. 2018;25:981–987. doi: 10.1038/s41594-018-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brust P., Deuther-Conrad W., Lehmkuhl K., Jia H., Wünsch B. Molecular imaging of σ1 receptors in vivo: current status and perspectives. Curr Med Chem. 2014;21:35–69. doi: 10.2174/09298673113209990214. [DOI] [PubMed] [Google Scholar]
- 16.Weber F., Brust P., Laurini E., Pricl S., Wünsch B. Fluorinated PET tracers for molecular imaging of σ1 receptors in the central nervous system. In: Smith S.B., Su T.P., editors. Sigma receptors: their role in disease and as therapeutic targets. Springer; Cham: 2017. pp. 31–48. [DOI] [PubMed] [Google Scholar]
- 17.Jia H., Zhang Y., Huang Y. Imaging sigma receptors in the brain: new opportunities for diagnosis of Alzheimer's disease and therapeutic development. Neurosci Lett. 2019;691:3–10. doi: 10.1016/j.neulet.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Toyohara J., Sakata M., Ishiwata K. PET imaging of sigma1 receptors. In: Dierckx R.A., Otte A., De Vries E.F., Van Waarde A., Luiten P.G., editors. PET and SPECT of neurobiological systems. Springer; Berlin, Heidelberg: 2014. pp. 741–763. [Google Scholar]
- 19.Kawamura K., Ishiwata K., Tajima H., Ishii S.I., Matsuno K., Homma Y. In vivo evaluation of [11C]SA4503 as a PET ligand for mapping CNS sigma1 receptors. Nucl Med Biol. 2000;27:255–261. doi: 10.1016/s0969-8051(00)00081-0. [DOI] [PubMed] [Google Scholar]
- 20.Shen B., James M.L., Andrews L., Lau C., Chen S., Palner M. Further validation to support clinical translation of [18F]FTC-146 for imaging sigma-1 receptors. EJNMMI Res. 2015;5:49. doi: 10.1186/s13550-015-0122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura K., Tsukada H., Shiba K., Tsuji C., Harada N., Kimura Y. Synthesis and evaluation of fluorine-18-labeled SA4503 as a selective sigma1 receptor ligand for positron emission tomography. Nucl Med Biol. 2007;34:571–577. doi: 10.1016/j.nucmedbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Waterhouse R.N., Collier T.L. In vivo evaluation of [18F]1-(3-fluoropropyl)-4-(4-cyanophenoxymethyl)piperidine: a selective sigma-1 receptor radioligand for PET. Nucl Med Biol. 1997;24:127–134. doi: 10.1016/s0969-8051(96)00184-9. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse R.N., Zhao J., Stabin M.G., Ng H., Schindler-Horvat J., Chang R.C. Preclinical acute toxicity studies and dosimetry estimates of the novel sigma-1 receptor radiotracer, [18F]SFE. Mol Imaging Biol. 2006;8:284–291. doi: 10.1007/s11307-006-0056-1. [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse R.N., Chang R.C., Zhao J., Carambot P.E. In vivo evaluation in rats of [18F]1-(2-fluoroethyl)-4-[(4-cyanophenoxy)methyl]piperidine as a potential radiotracer for PET assessment of CNS sigma-1 receptors. Nucl Med Biol. 2006;33:211–215. doi: 10.1016/j.nucmedbio.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25.James M.L., Shen B., Zavaleta C.L., Nielsen C.H., Mesangeau C., Vuppala P.K. New positron emission tomography (PET) radioligand for imaging σ-1 receptors in living subjects. J Med Chem. 2012;55:8272–8282. doi: 10.1021/jm300371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjørnevik T., Cipriano P.W., Shen B., Park J.H., Gulaka P., Holley D. Biodistribution and radiation dosimetry of 18F-FTC-146 in humans. J Nucl Med. 2017;58:2004–2009. doi: 10.2967/jnumed.117.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NIH-U.S. National Library of Medicine, ClinicalTrals. gov, Identifier: NTC02753101. Available from: https://clinicaltrials.gov/ct2/show/NCT02753101?term=FTC-146&rank=1#wrapper.
- 28.Cao X., Chen Y., Zhang Y., Lan Y., Zhang J., Xu X. Synthesis and biological evaluation of novel σ1 receptor ligands for treating neuropathic pain: 6-hydroxypyridazinones. J Med Chem. 2016;59:2942–2961. doi: 10.1021/acs.jmedchem.5b01416. [DOI] [PubMed] [Google Scholar]
- 29.Lan Y., Chen Y., Cao X., Zhang J., Wang J., Xu X. Synthesis and biological evaluation of novel sigma-1 receptor antagonists based on pyrimidine scaffold as agents for treating neuropathic pain. J Med Chem. 2014;57:10404–10423. doi: 10.1021/jm501207r. [DOI] [PubMed] [Google Scholar]
- 30.McOmie J.F., West D.E. 3,3'-dihydroxybiphenyl. Org Synth. 1969;49:50–52. [Google Scholar]
- 31.Van De Bittner G.C., Ricq E.L., Hooker J.M. A philosophy for CNS radiotracer design. Acc Chem Res. 2014;47:3127–3134. doi: 10.1021/ar500233s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissman A.D., Su T.P., Hedreen J.C., London E.D. Sigma receptors in post-mortem human brains. J Pharmacol Exp Ther. 1988;247:29–33. [PubMed] [Google Scholar]
- 33.Kornhuber J., Schoppmeyer K., Bendig C., Riederer P. Characterization of [3H]pentazocine binding sites in post-mortem human frontal cortex. J Neural Transm (Vienna) 1996;103:45–53. doi: 10.1007/BF01292615. [DOI] [PubMed] [Google Scholar]
- 34.Andrés A., Rosés M., Ràfols C., Bosch E., Espinosa S., Segarra V. Setup and validation of shake-flask procedures for the determination of partition coefficients (log D) from low drug amounts. Eur J Pharm Sci. 2015;76:181–191. doi: 10.1016/j.ejps.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Linkens K., Schmidt H.R., Sahn J.J., Kruse A.C., Martin S.F. Investigating isoindoline, tetrahydroisoquinoline, and tetrahydrobenzazepine scaffolds for their sigma receptor binding properties. Eur J Med Chem. 2018;151:557–567. doi: 10.1016/j.ejmech.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Schroeder F.A., Hooker J.M. Development of new positron emission tomography radiotracer for BET imaging. ACS Chem Neurosci. 2017;8:17–21. doi: 10.1021/acschemneuro.6b00288. [DOI] [PubMed] [Google Scholar]
- 37.Wang C., Schroeder F.A., Wey H.Y., Borra R., Wagner F.F., Reis S. In vivo imaging of histone deacetylases (HDACs) in the central nervous system and major peripheral organs. J Med Chem. 2014;57:7999–8009. doi: 10.1021/jm500872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma Y., Hof P.R., Grant S.C., Blackband S.J., Bennett R., Slatest L. A three-dimensional digital atlas database of the adult C57BL/6J mouse brain by magnetic resonance microscopy. Neuroscience. 2005;135:1203–1215. doi: 10.1016/j.neuroscience.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Mirrione M.M., Schiffer W.K., Fowler J.S., Alexoff D.L., Dewey S.L., Tsirka S.E. A novel approach for imaging brain-behavior relationships in mice reveals unexpected metabolic patterns during seizures in the absence of tissue plasminogen activator. Neuroimage. 2007;38:34–42. doi: 10.1016/j.neuroimage.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamura K., Kobayashi T., Matsuno K., Ishiwata K. Different brain kinetics of two sigma1 receptor ligands, [3H](+)-pentazocine and [11C]SA4503, by P-glycoprotein modulation. Synapse. 2003;48:80–86. doi: 10.1002/syn.10190. [DOI] [PubMed] [Google Scholar]
- 41.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 42.Akunne H.C., Whetzel S.Z., Wiley J.N., Corbin A.E., Ninteman F.W., Tecle H. The pharmacology of the novel and selective sigma ligand, PD 144418. Neuropharmacology. 1997;36:51–62. doi: 10.1016/s0028-3908(96)00161-x. [DOI] [PubMed] [Google Scholar]
- 43.Loening A.M., Gambhir S.S. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging. 2003;2:131–137. doi: 10.1162/15353500200303133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.