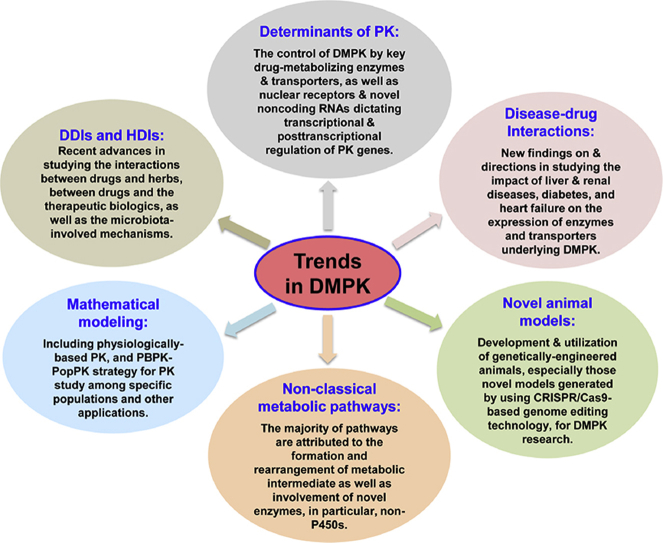
Abstract
Pharmacokinetics (PK) is the study of the absorption, distribution, metabolism, and excretion (ADME) processes of a drug. Understanding PK properties is essential for drug development and precision medication. In this review we provided an overview of recent research on PK with focus on the following aspects: (1) an update on drug-metabolizing enzymes and transporters in the determination of PK, as well as advances in xenobiotic receptors and noncoding RNAs (ncRNAs) in the modulation of PK, providing new understanding of the transcriptional and posttranscriptional regulatory mechanisms that result in inter-individual variations in pharmacotherapy; (2) current status and trends in assessing drug–drug interactions, especially interactions between drugs and herbs, between drugs and therapeutic biologics, and microbiota-mediated interactions; (3) advances in understanding the effects of diseases on PK, particularly changes in metabolizing enzymes and transporters with disease progression; (4) trends in mathematical modeling including physiologically-based PK modeling and novel animal models such as CRISPR/Cas9-based animal models for DMPK studies; (5) emerging non-classical xenobiotic metabolic pathways and the involvement of novel metabolic enzymes, especially non-P450s. Existing challenges and perspectives on future directions are discussed, and may stimulate the development of new research models, technologies, and strategies towards the development of better drugs and improved clinical practice.
KEY WORDS: Pharmacokinetics, Drug metabolism, Drug–drug interactions, Modeling, Metabolizing enzymes, Transporters, Nuclear receptors, Noncoding RNAs
Graphical abstract
Understanding of DMPK properties is essential for drug development and precision medication. In this article, we provided an overview of recent research on DMPK with focuses on the regulatory mechanisms of pharmacokinetics, drug–drug interaction, mathematical modeling, non-classical metabolism and so on. Existing challenges and perspectives on future directions are also discussed.
1. Introduction
Pharmacokinetics (PK) is defined as the quantitative study of drug absorption, distribution, metabolism, and excretion (ADME)—i.e., the ways the body processes a drug1 while the drug exerts its actions in the body. The scope of PK not only covers studies on healthy subjects but also includes broad research on variations under a variety of physiologic or pathologic conditions and the underlying mechanisms, potential drug–drug interactions (DDI), and possible strategies such as dose adjustment to achieve precision medication. Collectively, these aspects of PK allow customization of drug dosage regimens to enhance therapeutic outcomes1. Therefore, PK study is a prerequisite to establish the relations and the underlying mechanisms of a drug to its activities and clinical benefits. The information obtained is crucial for lead identification and optimization in drug discovery, as well as dosage regimen design and adjustment in clinical practice2. The complexity of PK has evolved, largely in relation to the rapid developments in analytical chemistry, computer science, molecular biology and biochemistry. Although much is known with regard to the PK of many drugs, and many technologies have been established for PK research, recent studies are revealing the existence of new mechanisms by which how drugs are metabolized and how PK is regulated. New experimental models and computational modeling algorithms are arising for an improved understanding of the significance of PK in a whole-body system; nonetheless, many challenges remain.
This review will provide a comprehensive overview of recent developments in the areas of PK research. First, we will provide an update of findings on drug-metabolizing enzymes and transporters in the control of PK, as well as advances in nuclear receptors and noncoding RNAs (ncRNAs) in the modulation of PK, which will provide new insights into understanding the transcriptional and posttranscriptional regulatory mechanisms behind inter-individual variations in pharmacotherapy. Second, we will review the current status and trends in assessing DDIs, especially the interactions between drugs and herbs, between drugs and therapeutic biologics, and microbiota-mediated DDIs and HDIs. Third, we will summarize recent advances in disease–drug interactions, in particular, regulation of metabolizing enzymes and transporters and alteration of PK by different diseases or physiological states. Fourth, we will summarize the trends in mathematical modeling including physiologically-based PK, which could be applied to support clinical investigations. In addition, we will discuss novel animal models such as CRISPR/Cas9-based animal models for DMPK research and overview some interesting non-classical biotransformation pathways including those utilizing novel drug-metabolizing enzymes. Existing challenges and future perspectives are also discussed. It is expected that this review will provide an update on recent advances in PK fields and may stimulate the establishment of new research models, technologies, and strategies towards the development of better drugs and improvements in clinical practice.
2. Determinants of PK
Drug-metabolizing enzymes and transporters play a very important role in the control of PK. Furthermore, transcriptional and posttranscriptional factors such as nuclear receptors and noncoding RNAs (ncRNAs) are critical in the modulation of PK and provide in-depth insight into understanding regulatory mechanisms to solve problems in PK. These mechanism-driven PK studies can improve the success of drug development related to its efficacy and safety and improve the rational use of medication in clinical practice.
2.1. Drug-metabolizing enzymes in the control of PK
Drug-metabolizing enzymes mediate the metabolism of exogenous and endogenous substances. Most drugs lose their pharmacological activities mainly through metabolic transformation, yielding metabolites with high water solubility that are readily excreted. Hence, metabolizing enzymes play an extremely important role in the control of drug PK. The biotransformation of xenobiotics by xenobiotic-metabolizing enzymes (XMEs) may be classified into Phase I and Phase II reactions. Advanced characterizations of enzymes involved in human drug metabolism are urgently needed, which help to avoid severe adverse drug reactions. Advances are being made in understanding the role of drug-metabolizing enzymes in the control of PK, including individual isoforms of many enzymes such as cytochrome P450s (CYPs) and UGTs, and their selective substrates, inducers and inhibitors. Other non-P450 oxidative enzymes and conjugative enzymes are also discussed in this section since an increasing number of drugs are metabolized via these enzymes3.
2.1.1. CYPs critical for PK
CYPs can oxidize foreign substances, enhance the water solubility and make drugs easier to be eliminated from the body. Most drugs are metabolized by CYPs, which mainly are located in the inner membrane of mitochondria or the endoplasmic reticulum of cells4. There are a total of 57 human CYP genes in 18 families. The members of CYP1 to CYP4 families oxidize thousands of exogenous and endogenous substrates (Table 1); whereas all members of CYP5 family and higher principally metabolize endogenous substrates in a highly substrate-specific manner5.
Table 1.
Endogenous and exogenous substrates of CYPs and ligands of transcription factors.
| Family | Enzyme | Endogenous substrate | Xenobiotic substrate | Transcription factor |
|---|---|---|---|---|
| CYP1 | CYP1A1 | Steroid (especially estrogen), aromatic amines, polycyclic aromatic hydrocarbons | Benzo[a]pyrene | AhR CAR |
| CYP1A2 | Phenacetin11 | AhR, CAR | ||
| CYP1B1 | Steroid (especially estrogen), melatonin6 | Aromatic amines, polycyclic aromatic hydrocarbons | AhR | |
| CYP2 | CYP2A6 | Steroid | Nicotine, cotinine, coumarin12,13 | PXR, NFE2L2, ER, GR, PXR, HNF4α |
| CYP2A13 | Unknown | Nicotine, coumarin, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)14, naphthalene15 | FOXA2 | |
| CYP2B6 | Synthesis of cholesterol, steroids and other lipids. | Bupropion11, efavirenz | CAR, PXR, HNF4α | |
| CYP2C8 | Arachidonic acid16 | Paclitaxela, repaglinide, AZD9496, Taxol | CAR, PXR, ROR, VDR | |
| CYP2C9 | Serotonin, polyunsaturated fatty acids, arachidonic acid. | Warfarin, phenytoin, tolbutamide | PXR, CAR, VDR, HNF4α | |
| CYP2C18 | Arachidonic acid, linoleic acid, docosahexaenoic acid (DHA), Eicosapentaenoic acid (EPA). | Tolbutamide, cyclophosphamide, ifosfamide | Unknown | |
| CYP2C19 | Arachidonic acid | S-Mephenytoin | PXR, CAR, FOXA3 | |
| CYP2D6 | Hydroxytryptamines, neurosteroids, m-tyramine, p-tyramine8 | Tamoxifen, gefitinib, cyclophosphamide, bufuralol | HNF4α | |
| CYP2E1 | Arachidonic acid | Chlorzoxazone (CHZ), acetaminophen | LXR, HNF1α, NRF2 | |
| CYP2F1 | 3-Methylindole (3MI) | Naphthalene, benzene, 1,1-dichloroethylene | Unknown | |
| CYP2J2 | Arachidonic acid, vitamin D3 | Astemizole | Unknown | |
| CYP2R1 | Vitamin D3 | Unknown | Unknown | |
| CYP2S1 | Prostaglandin G(2)/H(2), thromboxane A(2), oxygenated eicosanoids | Benzo[a]pyrene-7,8-diol | Unknown | |
| CYP2U1 | Arachidonic acid, docosahexaenoic acid (DHA) | Debrisoquin sulfate | Unknown | |
| CYP2W1 | Fatty acids, lysophospholipids,retinoic acid | Canduocarmycin | Unknown | |
| CYP3 | CYP3A4 | Steroid (including testosterone), vitamin D3 | Midazolam, rivaroxaban, 3-acetyl-11-keto-β-boswellic acid (AKBA) | CAR, PXR, FXR, HNF4α, LXR, VDR |
| CYP3A5 | Steroid (including testosterone), progesterone, Rostenedione | Diltiazem, cyclosporine, 3-acetyl-11-keto-β-boswellic acid (AKBA) | PXR, LXR, HNF4α | |
| CYP3A7 | Steroid (including testosterone) | 3-acetyl-11-keto-β-boswellic acid (AKBA) | Glucocorticoid receptor (GR), PXR | |
| CYP3A43 | Androgen | Alprazolam | Unknown | |
| CYP4 | CYP4A11 CYP4A22 |
Arachidonic acid, fatty acid, lauric acid | Unknown | PPARα |
| CYP4B1 | Furan pro-toxin 4-ipomeanol | Pneumotoxin, 4-ipomeanol, aromatic amines, 2-aminofluorene | Unknown | |
| CYP4F2 | Arachidonic acid, vitamin K menaquinone, leukotrienes, prostaglandins | Pafuramidine, fingolimod | Unknown | |
| CYP4F3 | Arachidonic acid, prostaglandins, leukotriene-B4 | Pafuramidine | Unknown | |
| CYP4F8 | Arachidonic acid, prostaglandins, eicosanoids, dihomo-γ-linolenic acid, leukotrienes, 19-hydroxylase of prostaglandin endoperoxides (PGEs) |
Unknown | Unknown | |
| CYP4F11 | Arachidonic acid, vitamin K menaquinone9, prostaglandins, leukotrienes | Benzphetamine, ethylmorphine, chlorpromazine, imipramine, erythromycin | RXR | |
| CYP4F12 | Arachidonic acid, docosahexaenoic and eicosapentaenoic acids, prostaglandins, leukotrienes | Ebastine, terfenadine | PXR | |
| CYP4F22 | Arachidonic acid, eicosanoids, prostaglandins, leukotrienes | Unknown | Unknown | |
| CYP4V2 | Medium chain fatty acids | Unknown | PPARγ | |
| CYP4X1 | Arachidonic acid, anandamide | Unknown | PPARα | |
| CYP4Z1 | Lauric acid, myristic acid | Unknown | Unknown |
Most known chemical carcinogens, including aromatic amines and polycyclic aromatic hydrocarbons (PAHs), are substrates of CYP1 family, and their metabolism often results in the formation of active carcinogenic metabolites. In 2018, CYP1B1 was found in the mitochondria of cancer cells, where it reportedly metabolizes melatonin to form the metabolite N-acetylserotonin (NAS), which has antitumor effects6. CYP2D6, another important metabolic enzyme, is involved in the metabolism of many anti-cancer drugs, such as cyclophosphamide, tamoxifen, and gefitinib7. Recent research has found that in brain, CYP2D6 can metabolize both m-tyramine and p-tyramine into dopamine8. The CYP4 family has gained increasing attention for its potential to generate interesting metabolites and dispose of endogenous substrates in recent years. CYP4F11, together with CYP4F2, plays an important role in the synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE) from arachidonic acid, and participates in the metabolism of vitamin K9. Cyp2a5, the mouse correlate of human CYP2A6, encodes an enzyme that exhibited circadian regulation10. The other CYP1 to CYP4 subfamilies are involved in metabolism of different endogenous and exogenous substrates, as listed in Table 1.
Understanding variation in mechanism-based enzyme activity is crucial for improving the clinical use of drugs. Highly selective inducers and inhibitors of CYPs have been cited in Guidance for Industry by FDA (https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers). Recent studies have revealed new chemicals and herb products as inducers or inhibitors of CYPs. For example, CYP7A1 is upregulated by an intestinal HIF-2α inhibitor called PT238517. The ketene intermediate of erlotinib can inactivate CYP3A4 and CYP3A5, which can result in liver injury18. Due to the complexity of components in the extract of herbs it is common that herb products exhibit different effects on the regulation of multiple enzymes. Sophora flavescens can inhibit CYP2B6, CYP2C8, CYP2C9, and CYP3A activities, while catalpol can inhibit the activity of CYP3A4, CYP2E1 and CYP2C919,20. Other regulatory factors can also alter the expression of CYPs. For example, tumor suppressor p53 can regulate Cyp2b10 directly and thereby attenuate APAP-induced hepatotoxicity21.
Herbs may be used singly or in combination in the treatment of diseases22. It is very important to understand how drug exposure alters molecular mechanisms underlying many complex drug interactions. For example, data show that ellagic acid from pomegranate peel guava leaf extract can significantly increase the AUC of warfarin with concomitant use. A significant reduction in CYP2C8, 2C9, and 3A4 activity was the main reason for this interaction23.
Based on recently available data, new information on the relative content of individual isoforms of P450 has been generated. Total CYP concentrations are significantly different between Chinese and Caucasian populations and the metabolic capabilities of CYPs in Chinese liver microsomes was significantly lower (<50%) in the CLint for substrates of CYP1A2, CYP2C9, CYP2C19 and CYP2E1 than those of Caucasian populations24. Large variations in protein content, mRNA levels, and intrinsic activities of ten P450s (CYP3A4, 1A2, etc) have been revealed and some single nucleotide polymorphisms had significant impact on P450 expression; for example, CYP2C19 activity varied more than 600-fold25. A recent human PK study further evaluated CYP1A2 content in Chinese compared with Caucasian populations, enhancing the confidence in pharmacokinetic prediction of CYP1A2 content using two substrates (caffeine and theophylline)26.
Other organs like kidney and intestine also have significant metabolic capacity. There is definitive evidence for CYP2B6 and 3A5 expression in human kidney, while multiple CYPs are expressed in intestine27,28. The role of renal and intestinal enzymes in herbal product metabolism has been uncovered. Aminoglycoside antibiotics are leading causes for nephrotoxicity; combination with herbs or dietary supplements at reduced dosage is possible to reduce the risk of drug-mediated renal toxicity. A recent study revealed that moringa oleifera seed oil could limit gentamicin-induced oxidative nephrotoxicity29. Additional herbs have been identified as having effects on intestinal metabolism, such as the extracts of Yin-Chen-Hao Tang (YCHT), a very popular hepatoprotective three-herb formula in China and Japan30. These findings contribute to the understanding of the metabolic characteristics of renal and intestinal metabolism.
2.1.2. Non-P450 oxidative enzymes
The contribution of non-P450 enzymes to drug metabolism can be significant and affect the overall development of drugs. Non-CYP enzymes can be divided into four general categories: namely oxidative, reductive, conjugative, and hydrolytic. Non-CYP oxidative enzymes include flavin-containing monooxygenases (FMOs), monoamine oxidases (MAOs), peroxidases, xanthine oxidases (XO), aldehyde oxidase (AO), alcohol dehydrogenase (ADHs) and aldehyde dehydrogenase (ALDHs)31.
Very little is known about the regulation of content and activity of non-P450 oxidative enzymes. Recently, some selective substrates and inhibitors of non-P450 enzymes have been identified in natural products and other sources. FMOs are involved in the metabolism of a wide array of xenobiotics. Well-known inhibitors of FMOs include indole-3-carbinol and methimazole, and 2-mercaptobenzimidazole32. Classified into two different isoforms (MAO-A, MAO-B), MAOs are enzymes involved in the catabolism of monoamines. Benextramine and its derivatives were identified as novel human monoamine oxidases inhibitors, which could be considered as candidate drugs for the treatment of neurodegenerative diseases33. In addition, 3-(3-(dimethylamino)propanoyl)-7-hydroxy-5-methyl-2H-chromen-2-one hydrochloride has been found to function as a novel selective hMAO-B inhibitor, which is expected to be a promising multifunctional Parkinson's disease treatment agent34. XO and AO are involved in the oxidation of aldehydes and heterocycles, and carbazeran was used as a selective probe substrate of AO in hepatocytes35. Allopurinol and S-allyl cysteine (SAC) are XO inhibitors used in the treatment of gout and hyperuricemia36. A single-nucleotide polymorphism of human cytochrome P450 oxidoreductase (POR) in the Chinese population can regulate the content of POR and P450 isoforms37. Identifying specific inhibitor compounds will greatly facilitate investigation of enzyme-mediated drug disposition and drug interactions.
2.1.3. Importance of UDP-glucuronyltransferases (UGTs) in PK
UDP-glucuronyltransferases (UGTs) are a family of endoplasmic reticulum-bound enzymes which are responsible for the process of glucuronidation, a major part of phase II metabolism38. Human UGTs include 22 different functional enzymes and are classified into four gene families, UGT1, UGT2, UGT3 and UGT839. The UGT1 and UGT2 families are primarily enzymes involved in drug glucuronidation, while the contribution of the UGT3 and UGT8 families to drug metabolism is minimal40.
Recently, UGT1A3 was found to be involved in the glucuronidation of alpinetin41. UGT1A4 is involved in the glucuronidation of metizolam42. Other UGT isoforms involved endogenous and exogenous substrates are listed in Table 223,43, 44, 45, 46.
Table 2.
Endogenous and exogenous substrates of UGTs and ligands of transcription factors.
| Family | Enzyme | Endogenous substrate | Xenobiotic substrate | Transcription factor |
|---|---|---|---|---|
| UGT1A | UGT1A1 | Bilirubin, estradiol, fatty acids | SN-38, leonurine, bergenin, axitinib | CAR, PXR, PPARα, AhR43, NRF2 |
| UGT1A3 | Bile acid, arachidonic | Polyaromatic amines, non-steroidal anti-inflammatory drugs, statins, ahydroxygenkwanin, genkwanin, ursolic acid44, fimasartan45, alpinetin23 | PPARα, HNF1, AhR, LXR, PXR | |
| UGT1A4 | Eicosanoids | Imipramine, lamotrigine, clonazolam, deschloroetizolam, etizolam, flubromazolammetizolam | HNF1, PPARα, PXR, CAR, AhR, HNF1α | |
| UGT1A6 | Serotonin | 1-Naphthol 4-nitrophenol | AhR, CAR, PXR, PPARα | |
| UGT1A7 | Unknown | Icaritin, carcinogens | AhR, HNF1, HNF4α, NRF2 | |
| UGT1A8 | Fatty acids | Retinoids, catechol estrogens, opioids, coumarins, flavonoids, anthraquinones, phenols, raloxifene | HNF1, HNF4α, AhR, NRF2 | |
| UGT1A9 | Steroids, fatty acids | Bulky phenols, propofol, mycophenolic acid, niflumic acid, psoralidin | CAR, HNF1, HNF4α, PPARα, AhR, NRF2 | |
| UGT1A10 | Estrogens | Nitrosamine, flavonoids, polycyclic aromatic hydrocarbons, raloxifene, dopamine | HNF1α, HNF4α, AhR, NRF2 | |
| UGT2A | UGT2A2/3 | Hyodeoxycholic acid | Tobacco carcinogen | HNF1, LXR |
| UGT2B | UGT2B4 | Arachidonic acid | Naftopidil, deoxynivalenol | PPARα, AhR, FXR |
| UGT2B7 | Sex-steroid hormones, glucocorticoids, mineralocorticoid, bile acids | Naftopidil, deoxynivalenol, mirabegron, efavirenz, zidovudine, codeine, morphine | HNF1α46, CAR, PXR, FXR, PPAR, NRF2 | |
| UGT2B10 | Eicosanoids | Amitriptyline, imipramine, clomipramine, trimipramine | CAR, FXR, AR | |
| UGT2B11 | Unknown | 3a-Hydroxyandrogens, 3a-pregnanes, Hydroxylestrogens | ER, AR | |
| UGT2B15 | Sex-steroid hormones | Oxazepam, lorazepam, sipoglitazar, bisphenol-A | AR, ER, HNF3α, FXR | |
| UGT2B17 | Sex-steroid hormones | Coumarins, anthraquinones flavonoids, chlorantraniliprole | HNF1α, HNF4α, HNF3α, AR, ER | |
| UGT2B28 | Sex-steroid hormones | Unknown | ER, AR | |
| UGT3 | UGT3 | Unknown | N-Acetylglucosamine | Unknown |
| UGT8 | UGT8A1 | Bile acids | Unknown | LXR |
Highly selective substrates and selective inhibitors of UGTs have been found in natural products and from other sources. Resveratrol can activate UGT1A8 expression, and is used for breast cancer treatment47. Different doses of emodin can inhibit the activity of UGT2B748.
In some cases, herbal products are metabolized by multiple UGTs. Linoleic acid and glutaric acid can inhibit the glucuronidation of berberrubine, a lipid-lowing metabolite of berberine, as well as the activities of UGT isoforms, such as UGT1A7, 1A8, 1A949. Glucuronidation of catalposide, an active component of Veronica species, was catalyzed by gastro-intestine-specific UGTs 1A8 and 1A1050.
Gene polymorphisms are a key factor in the regulation of the content and activity of UGTs. UGT1A and UGT2B genetic variation can alter nicotine and nitrosamine glucuronidation in European and African American smokers51. In addition, the UGT1A4*3 genetic polymorphism is associated with low posaconazole plasma concentrations in patients with hematological malignancies52. UGT1A1*6 polymorphisms are correlated with irinotecan-induced neutropenia in cancer patients53.
2.1.4. Other conjugative enzymes important for PK studies
In addition to UGTs, sulfonyl transferases (SULTs) and glutathione S-transferases (GSTs) are also important conjugative enzymes mediating phase II reaction.
SULTs catalyze the transfer of the water-soluble sulfonate group from 3′-phosphoadenosine 5′-phosphosulfate to drugs or endogenous molecules that contain hydroxy or amine group(s)54. At present, four families of human SULTs have been discovered, namely SULT1, SULT2, SULT4 and SULT6. SULT1E1 plays an important role in the metabolism and detoxification of estrogens and flavonoids55. SULT2 enzymes, mainly SULT2A and SULT2B, are primarily responsible for catalyzing the sulfation of hydroxysteroids56. A recent study found that tumor suppressor p53 could regulate the expression of SULTs57.
GSTs are a group of phase II drug-metabolizing enzymes that catalyze the binding of glutathione to various electrophilic compounds. In humans, cytosolic GST isoenzymes of the alpha, zeta, theta, mu, pi, sigma and omega classes have been found. GSTA1 plays a significant role in the metabolism of acetaminophen58. GSTA4 metabolizes electrophilic and carcinogenic substances such as endogenous carcinogen 4-hydroxy-2-nonenal59. The detailed substrates of SULTs and GSTs are listed in Table 3.
Table 3.
Endogenous and xenobiotic substrates for GSTs and SULTs that are also ligands of particular transcription factors.
| Enzyme | Endogenous substrate | Xenobiotic substrate | Transcription factor | |
|---|---|---|---|---|
| GSTs | Steroids, bilirubin, heme, fatty acids | 1-Chloro-2,4-dinitrobenzene (CDNB), 1,2-dichloro-4-nitrobenzene (DCNB), 4-nitrobenzyl chloride (pNBC), ethacrynic acid (ETHA), aflatoxin B1 (AFB1), 4-hydroxynonenal (4HNE), acrolein, N-acetyl-p-benzoquinone imine (NAPQI), cisplatin, busulfan, dichloroacetate, cyclophosphamide, azathioprine (AZA) | PXR, CAR, steroidogenic factor 1 (SF-1), RXR | |
| SULT1 | SULT1A1 | 4-Methylphenol, iodothyronines | 4-Nitrophenol | PXR, CAR |
| SULT1A2 | Dopamine, estrogens, catechol estrogens | 4-Nitrophenol, 2-naphthol, naloxone, minoxidil | PXR, CAR, FXR, HNF4α | |
| SULT1A3 | Dopamine, norepinephrine, iodothyronines | 6-Hydroxydopamine, hydromorphone | ||
| SULT1B1 | Thyroxine | 3-OHB[a]P,1-naphthol | ||
| SULT1C1 | Thyroxine | N-Hydroxyarylamines | ||
| SULT1E1 | Iodine thyroxine, pregnenolone | 1-Naphthol, naringenin, genistein, 4-hydroxytamoxifen |
||
| SULT2 | SULT2A | Dehydroepiandrosterone (DHEA), bile acid, cholesterol, estrone | Tibolone, budesonide | |
| SULT2B | Dehydroepiandrosterone (DHEA), bile acid, cholesterol, estrone | 3β-Hydroxysteroids | ||
2.1.5. Updates on the nuclear receptor-mediated regulation of xenobiotic-metabolizing enzymes
The human nuclear receptors comprise a family of 48 ligand-regulated transcription factors that in turn regulate target genes involved in metabolism and other physiological functions. Some of these receptors (e.g., peroxisome proliferators-activated receptor (PPAR), liver X receptor (LXR), hepatocyte nuclear factor (HNF)) are of particular interest in regard to drug metabolism and disposition as they have been found to regulate many XMEs in recent years.
PPARα induces the expression of CYP4A in response to a heterogeneous group of peroxisome proliferators. PPARγ also regulates the expression of CYP4V2, a fatty acid metabolizing enzyme, in human tetrahydropyranyl 1 (THP1) macrophages60. LXR controls the transcription of Cyp7a1 and Cyp27a1, Cyp3a11 and Cyp2e161, 62, 63.
Traditional transcriptional factors can bind directly to specific DNA sequences and thus control the gene expression. However, epigenetic regulation like histone modification and DNA methylation modulates transcription of UGTs or CYPs mainly by changing chromatin architecture. For example, the UGT1A gene can be repressed by the recruitment of histones in females64. Several studies determined that microRNAs (miRNAs), could down-regulate the expression of metabolizing enzymes, which will be further reviewed in Section 2.3.
In summary, the expression and activity of metabolizing enzymes can be regulated by multiple factors, including drugs, nuclear receptors, gene polymorphisms, and even ethnic categories. Non-P450 enzymes and other conjugative metabolizing enzymes have gained attention in drug metabolism in recent years. It is desirable to illustrate the key factors responsible for variable expression and activity of drug metabolizing enzymes, as it may be beneficial in the prediction of potential therapeutics, drug–drug interactions, and in modifying the PK of drugs.
2.2. Transporters in the control of PK
2.2.1. Introduction of transporters
Transporters are membrane-bound proteins expressed on the cell membrane in most tissues with varying abundance. They can transport a variety of endogenous or exogenous substrates (such as drugs and their metabolites) in and out of cells. For drugs, transporters are the gatekeepers for cells and control the uptake and efflux of drugs. Transporters are involved in the ADME process of drugs. Therefore, transporters play critical roles in the pharmacokinetics, efficacy and toxicity of drugs. Alteration of transporter function or expression may significantly change the blood and/or tissue exposure of drugs, leading to significant changes in pharmacokinetics. Furthermore, the induction or inhibition of transporters by co-administered drugs can change PK and pharmacodynamics of therapeutic drugs and produce DDI.
There are more than 400 membrane transporters belonging to two major superfamilies: adenosine triphosphate (ATP)-binding cassette (ABC) and solute carrier (SLC) transporters. They utilize the energy that is released by ATP hydrolysis or an electrochemical ion gradient to translocate drugs across the membrane.
2.2.1.1. The ABC family of drug transporters
ABC transporters mainly act as exporters and pump drug molecules out of cells by utilizing the energy released by the hydrolysis of ATP. According to the organization and sequence of ATP-binding domains, 49 ABC transporters are classified into seven subfamilies: ABC1/ABCA, multidrug resistance (MDR)/TAP/ABCB, MRP/ABCC, ALD/ABCD, OABP/ABCE, GCN20/ABCF and White/ABCG65. Among them, P-glycoprotein (P-gp, MDR1, ABCB1), MRPs/ABCCs, breast cancer resistance protein (BCRP/ABCG2) and bile salt export pump (BSEP/ABCB11) are recognized for their importance in drug disposition66. P-gp, which is expressed at a high level in the intestine, liver, kidney, brain and placenta, is the most studied ABC transporter. Many substrates of P-gp including antibiotics, statins, immunosuppressants, anticancer drugs and a broad spectrum of drugs overlap with the substrates of CYPs. The expression of P-gp is regulated by several transcription factors including PXR, CAR, vitamin D receptor (VDR) and CCAAT/enhancer binding protein (C/EBP) and some microRNA such as miR-451, miR-27a and miR-14567,68. Furthermore, P-gp is usually overexpressed in cancer cells and plays a critical role in MDR. For example, during chemotherapy, P-gp may be an obstacle for drug exposure if the therapeutic drugs are P-gp substrates69. Besides its role in MDR induction, P-gp plays a critical role in pharmacokinetics, pharmacology and toxicology. Through pumping multiple drugs out of cells, P-gp decreases the bioavailability of oral drugs and increases drug efflux into urine or bile. Furthermore, P-gp also plays a vital role in the maintenance of the blood–brain barrier by pumping drugs or toxins out of the CNS70. Another important ABC transporter group is the MRP family that consists of 9 MRP proteins (MRP1–MRP9). Among them, MRP2 is important in drug pharmacokinetics. MRP2, once known as the canalicular multispecific organic anion transporter, is highly expressed in liver, intestine and kidney. Chemotherapeutics such as methotrexate, melphalan, and statins are the classical substrates of MRP2. Since co-expressed in the liver, many liver-enriched transcription factors such as LXR, farnesoid X receptor (FXR), HNF and C/EBP regulate the transcription of MRP2. Another efflux transporter BCRP is a half transporter and is expressed at a high level in a wide variety of tissues such as intestine, kidney, liver, testis and brain. BCRP is modulated by the progesterone receptor B (PRB) and estrogen receptor (ER). Another ABC family drug transporter, BSEP, is primarily expressed in the liver and pumps bile acids and non-bile acid drugs such as pravastatin into bile.
2.2.1.2. The SLC family of drug transporters
The SLC family consists of 55 gene subfamilies and more than 360 family members. SLC transporters mainly utilize the energy stored in the ion gradients across membranes, but do not depend directly on ATP hydrolysis71. Several SLC family transporters play important roles in drug disposition including organic anion-transporting proteins (OATPs/SLC21/SLCO), organic anion and cation transporters (OATs and OCTs/SLC22), peptide transporters (PEPTs/SLC15) and sodium-dependent bile acid transporters (NTCP/SLC10A1). The OATP family consists of 11 members. Among them, four transporters including OATP1A2 (SLCO1A2), OATP1B1 (SLCO1B1), OATP1B3 (SLCO1B3) and OATP2B1 (SLCO2B1) are involved in drug transport72. OATP1A2 is expressed in the intestinal epithelium, renal epithelium and brain capillary endothelial cells, while OATP1B1, OATP1B3 and OATP2B1 are expressed predominantly in hepatocytes. Statins and anti-cancer drugs like paclitaxel, sorafenib and methotrexate are known as the substrates of OATPs. The SLC22 family consists of 23 members, including OCTs, zwitterion/cation transporters (OCTNs) and OATs. Among OCTs, OCT1 (SLC22A1) is mainly expressed in the liver, OCT2 (SLC22A2) is located at a high level in proximal tubular cells, and OCT3 (SLC22A3) has a broader expression range. Several drugs have been identified as OCT substrates including anesthetic drugs, the anti-diabetic drug metformin, antidepressants, β-blockers and anti-cancer chemotherapeutics. Among OATs, OAT1 (SLC22A6) and OAT3 (SLC22A8) have a broader expression range with the highest expression in kidney, while OAT2 (SLC22A7) is primarily expressed in the liver. OAT1 substrates include antiviral drugs, antibiotics, diuretics and angiotensin-converting enzyme (ACE) inhibitors. For the SLC15 subfamily, PEPT1 and PEPT2 are the most studied transporters. Both mediate oligopeptide uptake. PEPT1 is highly expressed in the intestine and mediates drug absorption, while PEPT2 is mainly expressed in kidney and affects renal reabsorption.
2.2.2. Transporters are critical for PK
The ADME process determines the blood and tissue concentration of drugs, as well as subsequent pharmacological or toxicological effects. The intestine and liver, both of which tightly regulate the entry of drugs into the blood circulation, are important organs in determining the bioavailability of oral drugs. Elimination of drugs or their active metabolites occurs either by metabolism to inactive metabolites that are excreted, or by direct excretion of drugs or active metabolites in the kidney. The transporters expressed in intestine, liver and kidney are involved in the absorption, distribution and excretion processes of drugs, and are the major determinant in blood and tissue concentration of drugs.
2.2.2.1. Transporter-mediated oral drug absorption
Oral drug absorption primarily occurs in the intestine, which is the major determinant of drug bioavailability, together with the first-pass extraction in the liver. Drug molecules pass through the membranes in the intestine through two pathways: passive diffusion and transporter-mediated absorption.
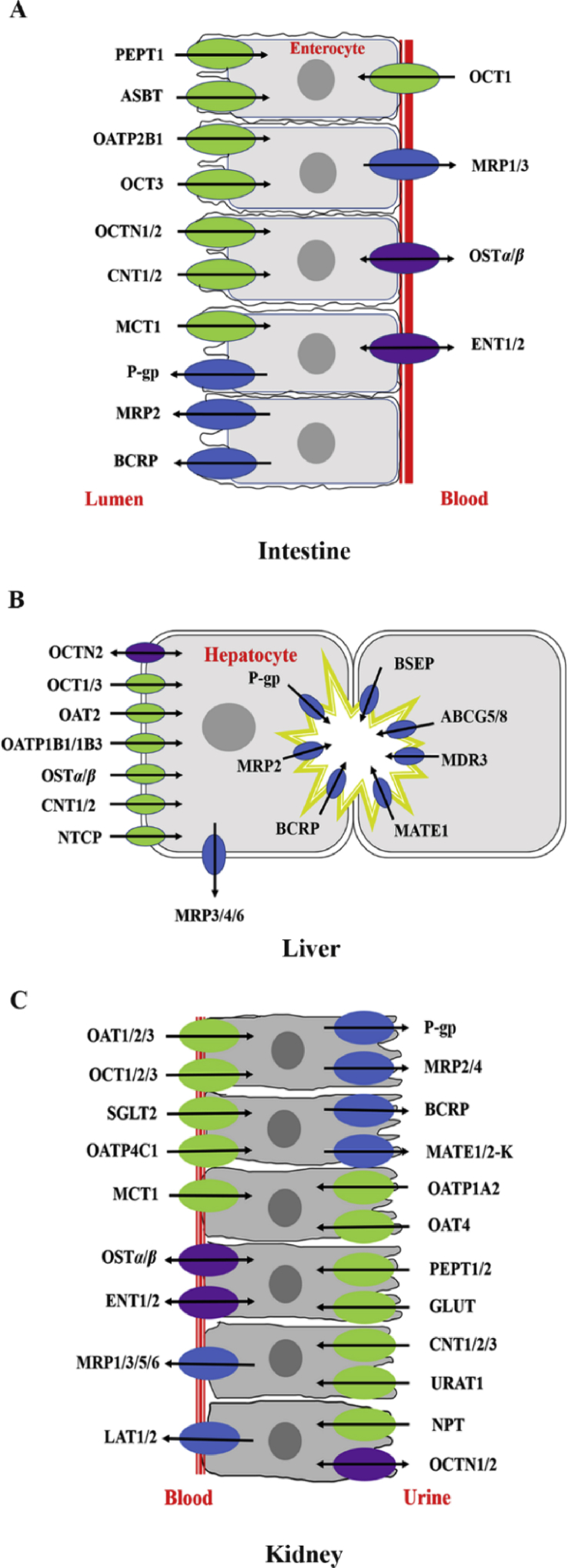
The process of transporter-mediated oral drug absorption consists of two parallel transport processes including transporter-mediated uptake and transporter-mediated efflux73,74 (Fig. 1A). In general, net drug absorption depends on multiple uptake and efflux transporters in the intestine. Uptake transporters such as OATP2B1, PEPT1 and sodium-dependent bile acid transporter (ASBT/SLC10A2) are involved in the intestinal uptake of drugs across the brush border membrane75. For example, PEPT1 transports di/tripeptides-like anticancer drugs such as bestatin and β-lactam antibiotics into enterocytes76, 77, 78. Efflux transporters expressed on the brush border membrane of the intestine, are considered as the barriers for intestinal drug absorption. P-gp, MRP2 and BCRP are three major efflux transporters in the intestine. P-gp, the most studied efflux transporter, has broad substrate specificity and significantly limits the bioavailability of many oral drugs79. For example, co-treatment with verapamil, a P-gp inhibitor, increases the intestinal absorption of afatinib or bestatin due to P-gp inhibition in the intestine80,81. On the contrary, rifampin, a P-gp inducer, decreases the oral absorption of cyclosporine and tacrolimus through the induction of P-gp in the intestine82. BCRP is another efflux transporter expressed in the intestine and suppresses the intestinal absorption of drugs83. Due to only one ATP binding site and six putative transmembrane helices, BCRP is considered a “half-transporter”. The substrates of BCRP include statins (pitavastatin, rosuvastatin), antiviral drugs (lamivudine, zidovudine, abacavir), anticancer drugs (methotrexate, SN-38, irinotecan, gefitinib, imatinib, erlotinib) and antibiotics (nitrofurantoin, ciprofloxacin)84. The efflux transporter MRP2 is also expressed on the brush border membrane of the intestine and transports a variety of substrates conjugated with sulfate, glutathione and glucuronide, as well as various unmodified drugs. Previous studies showed that resveratrol inhibited MRP2 and thereby increased the intestinal absorption of methotrexate85.
Figure 1.
Drug transporter expression in tissues. Drug transporter expression in the intestine (A), liver (B) and kidney (C). The arrows indicate the general directions in which the substrates are transported.
2.2.2.2. Transporter-mediated drug distribution
Transporters also affect the tissue distribution and contribute to the selective distribution of drugs to specific tissues. For example, OATP1B1 and OATP1B3 are the major uptake transporters in the liver for cilostazol, and MRP2, BCRP, P-gp pump cilostazol out of the liver into bile86. These transporters assist the liver-specific distribution of cilostazol. Another example is pravastatin, which enters into the liver through OATP1B1 and OATP1B3. After being excreted into the bile, pravastatin is reabsorbed in the intestine to the portal vein and taken up by the liver, and effectively undergoes enterohepatic circulation87. Therefore, the liver concentration should be higher than that in the circulating blood, leading to a high pharmacological effect at a relatively low plasma concentration. Transporters are also expressed on the blood–brain barrier and play critical roles in restricting the distribution of drugs into the brain. Increasing evidence has demonstrated that P-gp on the blood–brain barrier can suppress the distribution of drugs into the CNS88,89. Also, BCRP is recognized as an efflux transporter on the blood–brain barrier suppressing drug entry into the brain. Except for efflux transporters, uptake transporters are also expressed on the blood–brain barrier and play key roles in the uptake of neuroactive drugs. OAT3 is highly expressed on the basolateral membrane of brain capillaries90, and OCT2 is expressed in neurons and the choroid plexus. OCT2 is involved in the reabsorption of many drugs such as serotonin, norepinephrine, dopamine, choline and histamine from the cerebrospinal fluid.
2.2.2.3. Transporter-mediated drug excretion
Drug elimination primarily occurs in the liver and kidney. Hepatobiliary elimination processes can be summarized as follows: (1) the uptake of drugs into hepatocytes via uptake transporters or passive diffusion; (2) drug metabolism in hepatocytes including CYP metabolism (phase I metabolism) and conjugation (phase II metabolism); (3) excretion from hepatocytes into bile or portal blood via efflux transporters. Hepatobiliary transport of drugs is attributable to transporters located on the basolateral (sinusoidal) or canalicular (apical) membrane of hepatocytes (Fig. 1B). SLC superfamily transporters are responsible for drug uptake from the portal blood into hepatocytes. Among them, OAT2, OCT1, OATPs and NTCP are major uptake transporters. Efflux transporters such as P-gp, BCRP, MRP2 and BSEP are responsible for the hepatobiliary excretion of drugs and their metabolites. In addition, the efflux transporter MRP3, 4 and 6 expressed on the basolateral membrane are responsible for the basolateral efflux of drugs from the liver into the blood circulation. The hepatic transporters OAT2, OATP1B1/1B3 and OCT1 are highly expressed in the liver and are considered to be of particular importance for hepatic drug elimination, PK and efficacy. Much like the interplay of transport and metabolic enzymes at the intestinal barrier, these transporters also have a “gatekeeper” function in the drug movement from the blood into hepatocytes; they regulate both the number of drugs available for metabolism by liver enzymes and the subsequent biliary excretion. Efflux transporters including P-gp, BCRP, MRP2 and BSEP are responsible for the biliary excretion of endogenous and exogenous molecules. Many studies have shown that P-gp transports amphiphilic cationic drugs such as doxorubicin, digoxin and vinblastine into bile91. BCRP is involved in the biliary excretion of sulfated conjugates of steroids and drugs such as doxorubicin, mitoxantrone and daunorubicin, while BSEP transports drugs including vinblastine and taxol, et al. Due to their important roles in hepatobiliary efflux, the inhibition of BSEP, BCRP and MRP2 may lead to cholestasis. Therefore, the effects of chemicals on transporter-mediated hepatobiliary excretion must be determined in drug discovery92.
The kidney is the major organ of drug excretion. Renal clearance of drugs consists of glomerular filtration, tubular secretion and reabsorption. The proximal tubule region is responsible for the active secretion and reabsorption of drugs. Many transporters are located at the renal tubular epithelial cells and are involved in the proximal tubular secretion and reabsorption (Fig. 1C). These transporters include OCTs, OATs, multidrug and toxin extrusion proteins (MATE1 and MATE2-K), sodium-phosphate transporter (NPT/SLC17A1), OATPs and PEPTs, as well as equilibrium and concentration nucleoside transporters (ENTs and CNTs/SLC28A). Among them, OCTs, OATs and MATEs play critical roles in the active secretion of renal proximal tubule. These transporters work in concert with efflux transporters to transfer drugs into urine. OATs mainly transport anionic drugs such as beta-lactam antibiotics and anti-inflammatory drugs. The competitive inhibition of OATs may lead to a decrease in renal tubular secretion and an increase in the systemic concentration of drugs. For example, co-administration of probenecid, one OAT inhibitor, decreases renal secretion, leading to an increase in the plasma concentration of bestatin93. JBP485, a dipeptide with potential protective activity against kidney, liver and intestinal injury, has been demonstrated to be a substrate of OATs. Co-administration of JBP485 and cephalexin decreased the accumulative renal excretion and renal clearance of both compounds77. When JBP485 and lisinopril were co-administered, the competitive inhibition of OAT1 and OAT3 were also observed in OAT1/3-HEK293 cells94. In addition, acyclovir, an antiviral drug, was also a substrate of OAT1/3 and JBP485 can inhibit its renal excretion95. Furthermore, the DDIs between JBP485 and entecavir through the competitive inhibition of OAT1 and OAT3 significantly decreased the renal excretion of both compounds96. On the other hand, OATs are involved in drug-related nephrotoxicity. Probenecid, by inhibiting OAT1 and OAT3, reduced the accumulation of cephaloridine and subsequently nephrotoxicity97,98.
Three OCT isoforms including OCT1, OCT2 and OCT3 have been found in the kidney. Among the three OCTs, OCT2 is the major transporter for renal secretion of a variety of drugs such as memantine, metformin and amantadine. DDIs may also occur through the competitive inhibition of OCTs. For example, through inhibiting OCT2, cimetidine decreases the renal excretion of metformin and increases its plasma concentration99. On the other hand, OCT2, by modulating the exposure of drugs to renal proximal tubule cells regulates the nephrotoxicity of anticancer drug cisplatin and its analogs100. Substrates taken up from the systemic circulation may subsequently undergo efflux across the brush border membrane of proximal tubule cells by various ABC efflux transporters such as P-gp and BCRP. For example, a probe P-gp substrate, methotrexate, is actively secreted into urine. Co-treatment with bestatin, another P-gp substrate, increases plasma concentrations and decreases the renal clearance of methotrexate101. MATE1 and MATE2-K are expressed on the brush border membrane of proximal tubular cells. MATE1 mediated the renal secretion of fluoroquinolones including gatifloxacin, ciprofloxacin, levofloxacin, enoxacin, pazufloxacin, norfloxacin and tosufloxacin.
In summary, the expression and activity of transporters can be regulated by drugs and competitive inhibition may occur after co-administration of more than one drug. Furthermore, species differences in transporters complicate pharmacokinetic scaling from preclinical species to humans. Additionally, the expression of transporters may also be regulated by disease progression102. Modulation of transporter expression by disease states can potentially modify the PK of drugs.
2.3. ncRNAs in the regulation of drug metabolism and pharmacokinetics
ncRNAs are genome-derived RNA molecules that are not translated into proteins. Indeed, the human genome is comprised of over 95% of noncoding sequences103 that are transcribed into various forms of functional ncRNAs including miRs, transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small nucleolar RNAs (snoRNAs), and long noncoding RNAs (lncRNAs). Among them, miRNAs usually lead to translation inhibition or enhance mRNA degradation in cells through complementary base pairing with target transcripts. Many miRNAs have been shown to modulate the expression of drug-metabolizing enzymes or transporters, and consequently alter cellular drug metabolism and transport capacity, as well as drug responses (see recent reviews104, 105, 106). For instance, miR-27b reduces CYP1B1 protein expression in human carcinoma cells and thus suppresses CYP1B1 enzymatic activity, as indicated by a P450-Glo™ luminescent assay107. Meanwhile, miR-27b modulates CYP3A4 expression through direct targeting of CYP3A4 3ʹ-untranslated region (3ʹUTR) and “indirect” targeting of transcriptional factors such as NR1I1/VDR108, which may significantly alter CYP3A4-mediated drug metabolism109,110. Furthermore, miR-27a/b regulates the expression of a number of transporters such as ABCB1/P-gp111, 112, 113, and thus influences intracellular drug accumulation and chemosensitivity. In addition, a number of Phase 2 drug-metabolizing enzymes such as the UGTs are regulated by miRNAs at the posttranscriptional level114, 115, 116, 117, 118, 119. Findings on miRNA-controlled regulation of DMPK provide new insights into mechanisms behind inter-individual variations in pharmacotherapy.
Recent studies on miRNA regulation in DMPK also led to the development of novel research approaches and technologies. For example, while the luciferase reporter assay, gene mutagenesis and correlation analysis are helpful methods for the assessment of the interactions between miRNAs and target transcripts, a more direct approach has been established which is based on the change of RNA mobility after binding to miRNA, namely RNA electrophoretic mobility shift assay (EMSA)120,121. Using this RNA EMSA and other methods, a number of CYP genes (e.g., CYP2C19, CYP2E1 and CYP2D6) and regulators have been shown to be regulated post-transcriptionally by particular miRNAs121, 122, 123, 124, 125. It is also noteworthy that miRNA research is limited to the use of miRNA-expressing plasmids or viruses, or chemically-synthesized or chemo-engineered miRNA mimics126, 127, 128. To better capture the properties of biologic RNA molecules and cellular miRNA machinery, a novel RNA bioengineering technology has been established for the production of biologic miRNA agents in living cells109,129, 130, 131, 132, 133. With such novel bioengineered miRNA agents produced cost effectively and on a large scale, extensive functional studies have been conducted and the results showed rather a modest change in the PK of major CYP probe drugs in mouse models134. Further studies have demonstrated the utility of miRNAs as therapeutics or sensitizing agents for the treatment of human diseases in various animal models133,135, 136, 137, 138, 139.
There is also growing evidence that lncRNAs may regulate the expression of drug-metabolizing enzymes and transporters. For example, expression of HNF1α antisense RNA 1 (HNF1α-AS1) and HNF4α antisense RNA 1 (HNF4α-AS1) shows a significant influence on the basal and drug-induced expression of drug-metabolizing enzymes in human cells140. H19, an lncRNA highly expressed in liver tissues, induces the expression of efflux transporter P-gp/MDR1/ABCB1 in drug-resistant HepG2 cells141. The lncRNA MRUL confers the overexpression of ABCB1 in drug-resistant gastric cancer cells142. Furthermore, some studies have demonstrated that a lncRNA modulates drug sensitivity through its action on miRNA-transporter axis143,144. In addition, as RNA editing and posttranscriptional modifications are critical for RNA stability and biological function, very recent studies have also demonstrated the alteration of DMPK gene expression following RNA editing145, 146, 147. Future studies in these areas will undoubtedly advance our understanding of RNA-based regulation in DMPK.
In summary, research on miRNA-controlled regulation of DMPK provides new insights into understanding the posttranscriptional regulatory mechanisms behind inter-individual variations. Novel technologies and research approaches are also established during the investigation of ncRNA regulation of DMPK gene expression, which should have broad impact on biomedical research. Evidence is accumulating that some lncRNAs may be involved in the regulation of DMPK, which represents a new area of research.
3. Drug–drug interactions
3.1. Current status of research on drug–drug interactions
DDIs may result in favorable or toxic effects. Patients frequently use more than one medication at a time. Depending on the clinical settings and the number of drugs prescribed, the incidence of potential DDIs ranges between 15% and 80%148. DDIs can be classified mechanistically into 3 major types: physio-chemical incompatibility, PK interactions, and pharmacodynamic interactions149. Physio-chemical interactions usually occur when positively and negatively charged compounds are mixed before they are administrated or absorbed. Pharmacokinetics-based DDIs, characterized by altered concentration of unbound drugs that exert pharmacological effects, can be caused by several mechanisms, including: 1) alteration of drug metabolizing enzymes (e.g., CYPs)150, 2) alteration of transporters involved in the absorption, distribution and excretion of drugs (e.g., MDR1, OAT, OCT, etc)150, 3) influence on plasma protein binding affinity149, and 4) changes in the function of organs (e.g., gut motility or stomach content pH)149. Pharmacodynamics-based DDIs are characterized by a shift of the unbound drug concentration versus response curve149. New responses that are not present when either of the drugs is given alone may also be observed when drugs are used in combination.
In vitro, in vivo and clinical studies are usually conducted to identify any potential DDIs. The in vitro studies are usually simple systems that can be used for high throughput screening and provide mechanistic information for potential DDIs. In vivo animal studies are often conducted using clinically relevant dosages and pharmacodynamic endpoints to confirm the in vitro observations. If evidence obtained from in vitro and in vivo animal models suggests strong DDIs potential further clinical trials are recommended150,151. Recently, mathematical modeling, particularly physiologically-based pharmacokinetic (PBPK) modeling has also been applied to investigate potential pharmacokinetic-based DDIs. A recent review by Min et al.152 depicted how pharmacokinetic modeling improves and simplifies the investigation on DDIs. In addition, systematic reviews and databases summarize all the experimental and predicted data on DDIs, which are useful for providing warning and proper advice to patients in clinical practice153.
Although DDIs between small molecule drugs have been well investigated and documented, knowledges on interactions between drugs and herbs, interactions between therapeutic biologics, and interactions mediated by the gut microbiome are currently not well understood. The cutting-edge investigations on these aspects are briefly introduced in the following sections.
3.2. Current status of research on herb–drug interactions
Herbal plants and herbal products are commonly used as remedies and dietary supplements. When herbs are concurrently administered with drugs unrecognized herb–drug interactions (HDIs) may lead to side effects and toxicity. HDIs basically share the same mechanisms as DDIs. To avoid physio-chemical interactions between herbal components and drugs, it is usually recommended that herbs should be taken at two hours before or after the drugs. Moreover, herbs may sometimes alter the PK and/or pharmacodynamics of the concurrently administered drugs. PK and pharmacodynamic interactions have been reported between herbs and drugs with narrow therapeutic indexes, especially drugs for CNS and cardiovascular diseases154. For example, St John's wort (Hypericum perforatum) was reported to decrease warfarin plasma concentrations via inducing the activity of CYPs, leading to the loss of anticoagulant activity155. A traditional Chinese herb Danshen (Salvia miltiorrhiza) was reported to interact with warfarin on both its PK profiles and pharmacodynamic effects, resulting in over-anticoagulation and increased risk of bleeding155.
Investigation of HDIs is often more complicated than those of DDIs, due to the complex herbal components and the batch-to-batch variation of herbal products. As demonstrated in Table 4, compared with DDIs, research on HDIs is still insufficient. In vitro screening assays, which are efficient ways for detecting potential DDIs, may not be applicable for testing crude herbs or herb extracts, due to the fact that some of the herbal components may not be bioavailable, and adding such herbal components to the in vitro cell/microsome systems may alter results. By using LC–MS/MS, several multi-compound pharmacokinetic studies allowed the simultaneous detection of the plasma/tissue concentrations of multiple components after ingestion of the studied herb, facilitating the discovery of the bioavailable active components and subsequent in vitro and in vivo mechanistic studies on potential HDIs156,157. Most of the reported HDIs are based on in vitro and in vivo animal models, providing evidence with low clinical relevance. Moreover, many clinical studies were conducted among healthy populations, where the impact of the herbs on the pharmacodynamics effects of the concurrent drug may not be determined. On the other hand, the wide variation between different batches of herbal products also leads to poor reproducibility of the tests. Although not true in all countries, herbal products in China are generally regulated and used as medicine with standardization of the content of the major active components, and the herbal products are sometimes investigated not only as the effector but also as the affected agent of HDIs. In addition to experimental approaches based on the pre-clinical and clinical data, mathematical models have been established to predict HDIs, demonstrating the feasibility of using PBPK modeling for the prediction of HDIs152. For example, PBPK modeling of two major active components from Wuzhi capsule (Schisandra sphenanthera extract) predicts its interaction with tacrolimus metabolism by CYP3A4 inhibition158. However, the application of modeling and simulation on the investigation of HDIs is still restricted by the limited human pharmacokinetic data of herbal components152. More sophisticated designs of clinical studies are warranted to evaluate the safety and efficacy of the concomitant use of herbs and drugs.
Table 4.
| Type of investigation | DDIs | HDIs |
|---|---|---|
| In vitro studies |
|
|
| In vivo animal studies |
|
|
| Clinical studies |
|
|
| Simulation and modeling |
|
|
| Systematic reviews and databases |
|
|
3.3. Trends in drug–drug interactions of therapeutic biologics
Therapeutic biologics include therapeutic proteins, monoclonal antibodies (mAbs), vaccines, and peptide and nucleic acid derivatives that are manufactured for pharmaceutical uses159. Development of therapeutic biologics is growing fast, and in clinical practice the risk of DDIs with biologics is increasing.
3.3.1. PK of therapeutic biologics
The PK of biologics is different from those of small molecules. Since most therapeutic biologics undergo rapid degradation in the gastrointestinal tract after oral administration, alternative routes, such as intravenous, intramuscular, and subcutaneous injection are often used for drug delivery159, 160, 161. The distribution of therapeutic biologics is mainly mediated by interstitial penetration, lymphatic drainage, transcytosis, and receptor-mediated cell uptake159, 160, 161. Therapeutic proteins usually have a limited volume of distribution and do not bind to plasma proteins, and their biliary and renal excretion is generally negligible162. Catabolism via proteolytic degradation is the predominant clearance pathway for most therapeutic proteins159, 160, 161, while target antigen-mediated disposition also plays a role161. Moreover, fragment crystallizable receptor (FcR)-mediated antibody recycling by monocytes, macrophages, and dendritic cells is a salvage pathway that prolongs the half-lives of many mAbs159, 160, 161. Immune responses participate in both the catabolism and the antibody recycling process, and therefore immunogenicity can significantly influence the clearance of therapeutic proteins159. A recent review by Ferri et al.162 has summarized the pharmacokinetic DDIs of therapeutic antibodies. Unlike therapeutic proteins, nucleic acid and peptide drugs160 are rapidly eliminated by peptidases and nucleases159,163, and may also undergo slow renal excretion161. Plasma binding of these oligomers can sometimes be very high and has been reported to affect their distribution and clearance160.
3.3.2. Pharmacokinetics-based interactions of therapeutic biologics
Direct competition between therapeutic biologics and small molecules in PK is not common due to their distinct pharmacokinetic pathways163. However, certain indirect pharmacokinetic DDI may occur. Immunosuppressive agents may decrease the immunogenicity of the therapeutic protein so as to hinder its clearance163. For example, concomitant treatment with the immunosuppressant methotrexate can decrease the clearance of mAbs including golimumab164, adalimumab162, and infliximab165. Another indirect pharmacokinetic DDI mechanism is cytokine–CYP modulation. Several biologics with immunomodulatory effects may alter CYP activities via modulating the cytokine levels leading to the altered PK of co-administered small molecules that are substrates of the affected CYPs159,163,166. For instance, tocilizumab, which can induce CYP3A4 activity by decreasing interleukin 6 levels, was found to reduce simvastatin systemic exposure167. Similarly, by triggering inflammation, influenza vaccination has been reported to decrease CYP activity and thus influence the systemic exposure of CYP substrates such as clozapine168. PBPK modeling is a powerful tool for the investigation of pharmacokinetic-based interactions between therapeutic biologics and small molecules, and has been successfully applied to quantitatively predict DDIs of CYP-modulating protein drugs (such as blinatumomab and sirukumab) and small molecule CYP substrates in patients169,170. On the other hand, pharmacokinetic interaction between two therapeutic biologics has seldom been reported. However, such pharmacokinetic DDIs may occur due to specific binding between two biologics. For example, palifermin is a truncated form of the endogenous fibroblast growth factor which contains the heparin-binding domains. Co-administration of palifermin with heparin was found to increase the systemic exposure to palifermin up to 5-fold171.
3.3.3. Pharmacodynamics-based interactions of therapeutic biologics
Comparing to the pharmacokinetics-based DDIs of therapeutics biologics, their pharmacodynamics-based DDIs are more commonly reported. A large volume of cases has demonstrated pharmacodynamic interactions among various hormones owing to their complex signaling networks159. For instance, insulin can interact with numerous drugs including hormones, antidiabetics, antibiotics, antipsychotics, etc172. Recombinant growth hormones interact with small molecule hormones such as glucocorticoids, estrogens, thyroxin, etc.159. Although co-administration of biologics indicated for the same disease usually results in additive or synergistic efficacy, co-administration may also induce toxicity. Both anakinra and etanercept are approved for the treatment of rheumatoid arthritis. However, combined use of the two biologics led to severe adverse effects including increased risk of infection and increased neutropenia without significant improvement in therapeutic efficacy173.
3.3.4. Risk assessment for DDIs of therapeutic biologics
Due to the distinct pharmacokinetic and pharmacodynamic properties of therapeutic biologics, the classic approach for DDIs prediction for small molecules may not applicable for therapeutic biologics. With the increase in therapeutic biologics in the market, it is critical to call for building strategies and regulations on the potential DDIs involving biologics. Based on the current findings on the major mechanisms for the pharmacokinetic-based DDIs of therapeutic biologics, assessment of the modulation of CYP activities and immunogenicity are recommended. In terms of pharmacodynamics-based DDIs, identification and monitoring of clinical endpoints relevant to both the efficacy as well the adverse effects of therapeutic biologics is highly recommended.
3.4. Trends in microbiota mediated drug–drug interactions
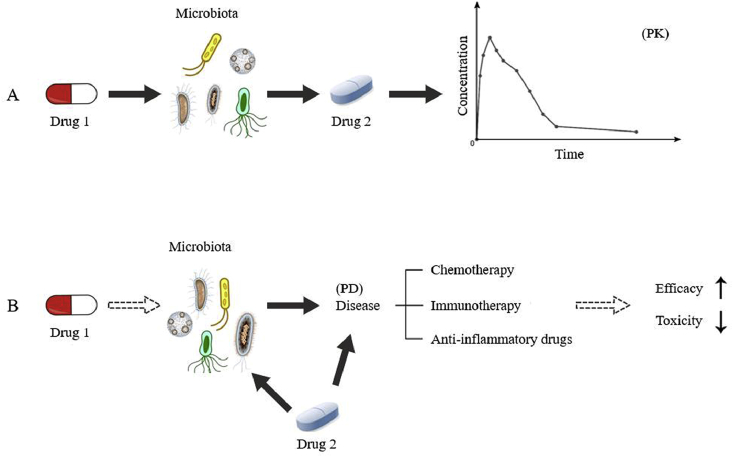
Recent studies have indicated that the microbiota is a vital drug target in many disease treatments. Many therapeutics have great effects on altering the composition of the microbiota. As indicated in Fig. 2A, changes in microbiota in the gastrointestinal tract may influence the metabolism of co-administered drugs, leading to altered pharmacokinetics. Findings have shown that gut microbiota can mediate drug metabolism including reduction174, oxidation175, dehydroxylation, decarboxylation176, etc. DDIs between antibiotics and drugs that are metabolized by gut microbiota are commonly reported. Many antibiotics can disturb the PK of a co-administered drug by affecting the enzymatic activities and composition of gut microbiota177, leading to an altered therapeutic effect. For example, the coagulant drug sulfinpyrazone can be metabolized to sulfinpyrazone sulfide in the gut contents. It was found that the plasma pharmacokinetic profile of sulfinpyrazone and sulfinpyrazone sulfide was changed in patients treated with the antibiotic metronidazole178. After reduction via azoreductases in gut microbiota, prontosil was metabolized to sulfanilamide, which exhibits potent antibacterial activities. In addition, it was noted in rats that the conversion of prontosil to sulfanilamide can be suppressed by antibiotics, leading to the reduced antibacterial effects174,179. Most recently, gnotobiotic mouse models and PBPK models have been established to untangle host and microbial contributions to the pharmacokinetic profile180. These novel experimental and computational strategies can be incorporated in future investigations on microbiota-mediated DDIs.
Figure 2.
Microbiota-mediated pharmacokinetic and pharmacodynamic interactions between different drugs. (The solid arrow indicates an effect supported by obtained evidence, and the dotted arrow indicates potential effects.)
In addition to effects on pharmacokinetics, altered microbiota composition may also lead to pharmacodynamics changes in the concomitant drugs (Fig. 2B). It was noted that the presence of a certain type of bacteria may have an impact on chemotherapy and immunotherapy181,182. Clinical trials are currently conducted on microbiota interventions, such as probiotics and fecal microbiota transplant (FMT), to explore their influence on the efficacy and toxicity of co-administrated chemotherapeutic agents, immunotherapeutic agents and anti-inflammatory drugs183. The potential benefits of probiotics and FMT to increase the efficacy of pembrolizumab in the treatment of PD-1 resistance patients184 and to reduce the adverse effects of aspirin185 and irinotecan186 are currently under clinical investigation.
Besides well-known influences on the microbiota from antibiotics and probiotics, influences from other types of drugs or natural products are very limited. Although evidence of gut microbiota-mediated DDIs remain limited, the growing interest in microbiota will definitely provide a better understanding on their influence on the PK and pharmacodynamics of drugs. Nevertheless, the impact of herbal medicine on the gut microbiome is unavoidable, and such research is expected to provide more in-depth understanding on herb–drug interactions. In summary, in addition to consideration of classical PK and pharmacodynamic interactions, microbiota-mediated drug–drug/herb–drug interactions are expected to bring additional insight into their therapeutic effects.
3.5. Summary
Investigation of herb–drug interactions (HDIs) is often more complicated than that on DDIs, due to the complex herbal components and the batch-to-batch variation of herbal products. More pharmacokinetic and pharmacodynamic data on the bioavailable herbal components from clinical studies using standardized herbal products are warranted for better understanding of HDIs. With the increasing number of therapeutic biologics in the market, it is critical to build strategies and regulations on the potential DDIs involved biologics. Based on the current findings on pharmacokinetic- and pharmacodynamic-based DDIs of therapeutic biologics, assessments on the modulation of CYP activity and immunogenicity, and identification and monitoring of clinical endpoints of the therapeutic biologics is recommended. In addition to consideration of classical PK and pharmacodynamics interactions, microbiota-mediated HDIs/DDIs are expected to bring additional insight into their interactions. Novel experimental and computational strategies, such as gnotobiotic animal models and physiologically-based pharmacokinetic modeling can be incorporated in future investigations on microbiota-mediated HDIs/DDIs.
In summary, the incidence of interactions between various therapeutics is high in patients taking multiple drugs and dietary supplements. Although DDIs between small molecule drugs are relatively well-characterized, other potential interactions are not fully explored. It is essential to develop efficient strategies for the investigation of the interactions between drugs and herbs, and between therapeutic biologics. Furthermore, the growing knowledge on the microbiota as therapeutic targets and as a site of drug metabolism leads us to pay more attention to microbiota-mediated interactions when examining potential DDIs and HDIs.
4. Disease–drug interactions
Understanding disease–drug interactions is clinically important due to the risk of treatment failure and the incidence of adverse reactions. An accumulation of strong research evidence indicates that disease–drug and drug–disease interactions can have a profound effect on the response to a medication, yet most of the existing results are only from animal models. Moreover, there are differences between animal disease models and human diseases187. Differences between different species should be also taken into account. In recent years PBPK modeling has gradually been applied to the prediction of disease–drug interactions188,57. However, further clinical study or real-life experience is needed to justify results from PBPK modeling. Additionally, the potential mechanism of disease–drug interactions remains poorly characterized. Therefore, further studies are also needed to reveal the in-depth and comprehensive mechanism involved in disease–drug interactions.
In recent years, apart from the DDI, disease–drug interactions have attracted lots of attention due to their potential impact on efficacy and safety of clinical therapy. Disease–drug interactions mainly refer to the disease itself can lead to changes in PK and pharmacodynamics of drugs, and also include the influence of alteration of endogenous substrates related to metabolism on disease status. Both effects of disease on drug metabolism and effects of metabolism regulation on diseases have the potential to increase the risk of treatment failure and the incidence of adverse reactions189. Although there have been some reports published on disease–drug interactions, there are still many unknown issues to be characterized. This review provides an update on the research on disease–drug interactions and offers an in-depth perspective on new strategies for the elucidation of disease–drug interactions.
4.1. Effects of diseases on drug metabolism
Disease is a vital factor affecting clinical medication. Disease changes the PK of a drug by altering the ADME process; on the other hand, disease can also change the sensitivity of the body to drugs by altering the number of receptors and their function in organs. Clinical practice should take into account the effects of a disease on a drug for the best therapeutic outcome and to avoid serious adverse reactions by adjusting the dose, the interval of administration, and the route of administration, etc. Current progress on disease effects on drug metabolism are listed in Table 5.
Table 5.
Summary of the effects of specific diseases on drug metabolism.
| Type of diseases | Affected drugs | Related mechanisms |
|---|---|---|
| Diabetes mellitus |
|
|
| Liver disease |
|
|
| Heart failure |
|
|
| Renal disease |
|
|
| Sepsis |
|
4.1.1. Effects of diabetes on drug metabolism
Diabetes mellitus, commonly referred to as diabetes, is a group of metabolic disorders in which there are high blood sugar levels over a prolonged period. Diabetes mellitus is also a well-known risk factor for cardiovascular disease and atherosclerotic complications, especially coronary heart disease209. In recent years there have been many reports of the effect of diabetes on drug metabolism. Alterations in function and expression of ABC transporters at the blood–brain barrier in diabetes have been observed210; for instance, it was found that the uptake of vincristine by cultured rat brain microvessel endothelial cells incubated in diabetic rat serum were higher than uptake in nondiabetic rat serum, which was related to the impairment of P-gp function and expression at the blood–brain barrier of diabetic rats190. Moreover, in brain cortex, STZ-induced diabetes mellitus may induce an impairment of function and expression of BCRP. The uptake of prazosin and cimetidine, two typical substrates of BCRP, was significantly increased in diabetic rats compared to uptake in non-diabetic rats191. However, different from the impairment of function and expression of P-gp and BCRP, diabetes may enhance MRP2 function and expression in liver, kidney and intestine, which then leads to increased excretion of sulfobromophthalein (a substrate of MRP2) via the bile, urine and intestinal perfusate192. Atorvastatin is a substrate of OATP1B1, an influx transporter expressed on the sinusoidal membrane of hepatocytes. Recent studies found that diabetes mellitus could enhance the hepatotoxicity and decrease exposure to atorvastatin in rats partly through upregulating hepatic Oatp2193,194.
In addition to Oatp2, upregulation of hepatic Cyp3a also contributes to the decreased exposure to atorvastatin, simvastatin and simvastatin acid in diabetic rats195, 194, 193. Accumulated evidence shows that diabetes mellitus apparently alters the expression and activity of cytochrome P450 (CYP450) enzymes196,211. In diabetic rats, the AUC of theophylline was significantly smaller than that of normal rats because of significantly faster time-averaged total body clearance in diabetic rats, which was attributed to upregulated hepatic CYP1A2 and CYP2E1. Furthermore, diabetes mellitus could significantly increase exposure (area under the curve and peak concentration) to glibenclamide after oral administration. Data with hepatic microsomes suggested the impairment of glibenclamide metabolism and efflux in diabetic rats197. Accumulating evidence also has shown that diabetes increased the metabolism of CYP3A4 substrates by upregulating the function and expression of CYP3A4 in hepatic cells198. Interestingly, diabetes mellitus showed a tissue-specific effect on CYP3A expression and activity (induced in liver and inhibited in intestine), resulting in opposite pharmacokinetic behavior for verapamil after oral and intravenous administration to diabetic rats212. UGTs, the major phase II conjugation enzymes, can also be affected by diabetes mellitus. It was reported that the UGT1 family is adaptively upregulated in the diabetic gastrointestinal tract199. Given the essential regulatory role of the gastrointestinal site in drug disposition, such changes in UGTs may have an impact on the metabolism of therapeutic drugs and endogenous substrates.
4.1.2. Effects of liver disease on drug metabolism
There is growing evidence to suggest that many hepatic diseases can affect drug metabolism. The effect of liver disease on drug metabolism is mainly due to the alteration of liver hemodynamics and activity of liver microsomal enzymes. Local and systemic liver injuries have a major effect on the expression and activity of DMEs in the liver213. For example, compared to control rats, there were significant changes in pharmacokinetic profiles after administrations of rhubarb anthraquinone-extracts in CCl4-induced liver-injury rats. The plasma concentrations of the four pharmacokinetic markers (Rhein, emodin, aloe-emodin, chrysophanol) of rhubarb anthraquinone extract increased, which indicated that their metabolism and excretion changed after liver injury200. Liver failure is often associated with hepatic encephalopathy, due to dyshomeostasis of the central nervous system (CNS). One study showed that the function and expression of P-gp and BCRP decreased, while the function and expression of MRP2 increased in the brain of acute liver failure (ALF) mice214. The attenuated function and expression of P-gp at the BBB might enhance phenobarbital distribution in the brain and increase phenobarbital efficacy on the CNS of ALF mice201. In addition, ALF could enhance oral plasma exposure of zidovudine in rats by downregulation of hepatic UGT2B7 and intestinal P-gp202.
Fatty liver disease, also known as hepatic steatosis, is a condition where excess fat builds up in the liver. Previous research showed that valproic acid with a high-fat diet-induced fatty liver could upregulate UGTs and was accompanied by the increased expression of CAR and PPARα215. Further analysis revealed that liver disease in warfarin users was associated with a significant increase in the likelihood of hemorrhage216.
4.1.3. Effects of heart failure on drug metabolism
Heart failure (HF) is considered an epidemic disease in the modern world affecting approximately 1%–2% of the adult population. Many CYP enzymes have been identified in the heart and their levels have been reported to be altered during HF. There is a great deal of discrepancy between various reports on CYP alterations during HF, likely due to differences in disease severity, the species in question and other underlying conditions. A recent review by Aspromonte et al.217 has summarized a comprehensive modulation of cardiac CYP in patients with HF. In general, cardiac CYP1B and CYP2A, CYP2B, CYP2J, CYP4A and CYP11 mRNA levels and related enzyme activities are usually increased in HF217,218. On the other hand, HF plays an important role in the down-regulation of hepatic CYP involved in drug metabolism through several mechanisms which include hepatocellular damage, hypoxia, elevated levels of pro-inflammatory cytokines, and increased production of heme oxygenase-1219. For example, the plasma concentrations of caffeine (CYP1A2 probe), mephenytoin (CYP2C19 probe), dextromethorphan (CYP2D6 probe) and chlorzoxazone (CYP2E1 probe) were significantly elevated in patients with congestive HF203. It was suggested that the doses of these CYP enzymes substrates should be decreased when used in patients with congestive HF.
4.1.4. Effects of renal disease on drug metabolism
Evaluation of drug metabolism in patients with end-stage renal disease is important because these patients use a large number of medications and are at risk of adverse reactions and DDI. Previous studies found that end-stage renal disease patients had a 50% increase in the plasma warfarin S/R ratio relative to control subjects. This may be reflective of a selective decrease in hepatic CYP3A and CYP2C9 activity in renal failure204,205. Furthermore, results from a “cocktail” approach showed that the enzyme activities of CYP3A4 and CYP2C9 of patients with renal failure were selectively inhibited220. Therefore, if CYP34A and CYP2C9 substrates are used in patients with renal failure, the dose needs to be lowered. Although chronic renal failure (CRF) has been found to be associated with a decrease in liver CYP, the mechanism remains poorly understood. The N-demethylation of erythromycin was decreased by more than 35% (P < 0.001) in hepatocytes incubated with serum from rats with CRF221. It is speculated that the mediator(s) of uremic serum may down-regulate the CYP of normal hepatocytes. In addition, a recent study investigated the effects of adenine-induced chronic kidney disease (CKD) in rats on the activities of some XMEs in liver and kidneys. It was found that the plasma theophylline concentration was significantly increased in rats with CKD206. Moreover, a reduced metabolism of midazolam could be observed in rats with acute kidney injury (AKI)207.
4.1.5. Effects of sepsis on drug metabolism
Sepsis is the systemic inflammatory response syndrome caused by infection, which is a common complication following surgery, especially abdominal surgery, with higher mortality. It has been well documented that hepatocellular dysfunction occurs early in sepsis and contributes to multiple organ failure and ultimately death222. Among them, the effects of polymicrobial sepsis on the activity and gene expression of hepatic microsomal CYP450 have attracted considerable attention due to their potential disease–drug interactions in clinical therapy. It has been reported that the major hepatic CYP isoforms CYP1A1, 1A2, 2B1, 2E1 were down-regulated during polymicrobial sepsis208,203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224. Moreover, results from mechanistic studies show that nitric oxide (NO) and the AhR play key potential roles in down-regulation of hepatic CYP during sepsis225,226. Therefore, treatment with pharmaceutical agents that regulate or are metabolized by CYP enzymes might be approached cautiously in the septic patient.
On the other hand, early and appropriate antimicrobial treatment is the predominant intervening measure to decrease patient mortality227. However, the pathophysiologic changes during sepsis such as systemic capillary leak syndrome, altered shift of body fluid and hypoalbuminemia can lead to changes in pharmacokinetics/pharmacodynamics (PK/PD) parameters such as apparent volume of distribution (Vd)228 and clearance (CL)229 that affect the achievement of PK targets and increase the risk of treatment failure with routine dosing. In addition, it is likely to cause low blood protein symptoms in sepsis due to the increased capillary permeability, decreased hepatic albumin synthesis and a large number of infusions230. Therefore, the effect of hypoalbuminemia on antibiotic PK also cannot be ignored. It is crucial to reduce patient mortality by adjusting antimicrobial doses and improving drug infusion to optimize antimicrobial therapy according to the characteristics of PK/PD during sepsis.
4.2. Effects of endogenous metabolism mediated by nuclear receptors on diseases
In recent years the regulation of endogenous metabolism mediated by nuclear receptors on diseases has received increasing attention with improvements in bioanalytical technology, especially the intervention of the various “omics”. Among them, PXR and CAR are two closely related and liver-enriched nuclear hormone receptors originally defined as xenobiotic receptors. However, an increasing body of evidence suggests that PXR and CAR also have endobiotic functions that impact glucose and lipid metabolism, as well as the pathogenesis of metabolic diseases. PXR and CAR not only regulate the transcription of drug-metabolizing enzymes and transporters, but also orchestrate energy metabolism and immune responses231. The cutting-edge investigations on these aspects are briefly shown in Table 6.
Table 6.
Summary of endogenous substances related to diseases and nuclear receptors.
| Endogenous substance | Diseases involved | Related nuclear receptor or protein |
|---|---|---|
| Glucose and lipid |
|
|
| Biliary bile acid |
|
|
| Corticosterone and aldosterone |
|
|
| Fatty acids |
|
|
| Sterol |
|
|
| Oleic acid |
|
|
| Hexokinase II |
|
A recent study revealed that PXR ablation inhibited high-fat diet-induced obesity, hepatic steatosis, and insulin resistance232. These results may help to establish PXR as a novel therapeutic target, and PXR antagonists may be used for the prevention and treatment of obesity and type 2 diabetes. PXR was also reported to play a vital role in maintaining biliary bile acid homeostasis by regulating the biosynthesis and transport of bile salts233. Activation of the PXR pathway was associated with decreased lithocholic acid-induced cholestasis in mice241. PXR may be developed as a therapeutic target for cholesterol gallstone disease. Interestingly, study has revealed a function of PXR in enlarging liver size and changing liver cell fate by activation of the yes-associated protein (YAP) signaling pathway. This has implications for understanding the physiological functions of PXR242. In addition, PXR plays an important endobiotic role in adrenal steroid homeostasis. Activation of PXR markedly increased plasma concentrations of corticosterone and aldosterone234. These results suggest that PXR is a potential endocrine disrupting factor that may have broad implications in steroid homeostasis and drug–hormone interactions.
CAR has also been increasingly appreciated for its endobiotic functions in influencing glucose and lipid metabolism, with dysregulation implicated in two of the most prevalent metabolic disorders, obesity and type 2 diabetes243. Further study found that CAR suppresses hepatic gluconeogenesis by facilitating the ubiquitination and degradation of PGC1α244. Given the metabolic benefits of CAR activation, CAR may represent an attractive therapeutic target to manage obesity and type 2 diabetes.
Nonalcoholic steatohepatitis (NASH) is common and medically significant because it is closely related to metabolic syndrome and has the potential to progress into the more harmful cirrhosis. Emerging evidence points to an important function of AhR in the uptake of fatty acids in the liver and the pathogenesis of fatty liver disease236. Activation of the AhR sensitizes mice to NASH by deactivating mitochondrial sirtuin deacetylase Sirt3245. These results suggest that the use of AhR antagonists might be a viable approach to prevent and treat NASH.
LXRs are known as sterol sensors that impact cholesterol and lipid homeostasis, as well as inflammation. The hepatic functions of LXRs are well documented and the pathophysiological role of LXRs was uncovered progressively in recent years. Activation of LXR prevents lipopolysaccharide-induced lung injury by regulating antioxidant enzymes and the implication of this regulation is pulmonary tissue protection237. Moreover, a recent study demonstrated that activation of LXR attenuates OA-induced acute respiratory distress syndrome by attenuating the inflammatory response and enhancing antioxidant capacity238.
FXR, a nuclear receptor mainly expressed in enterohepatic tissues, is a master regulator for bile acid, lipid and glucose homeostasis246. Emerging evidence indicates that restoration of FXR protein levels may represent a new strategy for enterohepatic and metabolic diseases. Hepatitis B virus X protein (HBx) is a hepatitis B virus protein that has multiple cellular functions, but its role in the pathogenesis of hepatocellular carcinoma (HCC) has been controversial. It was reported that transactivation of FXR by full-length HBx may represent a protective mechanism to inhibit HCC247. Additionally, FXR antagonism was also reported to be pivotal in attenuating obstructive cholestasis in bile duct-ligated mice235. These results suggest that FXR may be developed as a therapeutic target for cholesterol gallstone disease.
The tumor suppressor p53 is traditionally recognized as a surveillance molecule to preserve genome integrity. Recent studies have demonstrated that it contributes to metabolic diseases. It was found that the activation of p53 participated in promoting bile acid disposition and alleviating cholestatic syndrome by up-regulating the expression of Cyp2b10, Sult2a1 and Abcc2/3/4, which provides a potential therapeutic target for cholestasis235. In addition, p53 could attenuate acetaminophen-induced hepatotoxicity by regulating the CYPs, SULTs and MRPs, which provides a potential new therapeutic target for APAP-induced liver injury248.
Metabolism regulation mediated by downstream targets of the above transcriptional factors may also play an important role in diseases. For example, NAD(P)H: quinone oxidoreductase 1 (NQO1) has been reported to be a prognostic biomarker and a promising therapeutic target for patients with NSCLC due to its frequent overexpression and significantly increased activity in NSCLC. It was found that depleting tumor-NQO1 potentiates anoikis and inhibits the growth of NSCLC239. Furthermore, recent results from a metabolomics analysis have revealed that inhibition of cell proliferation upon NQO1 depletion was accompanied by suppressed glycometabolism in NQO1 high-expression human NSCLC A549 cells. Also, NQO1 depletion significantly decreased the gene expression of hexokinase II240.
4.3. Summary
Understanding disease–drug interactions is clinically important due to the risk of treatment failure and the incidence of adverse reactions. An accumulation of strong research evidence indicates that disease–drug and drug–disease interactions can have a profound effect on the response to a medication, but most of the existing results are only from animal models. In recent years, PBPK modeling has also gradually been applied to the prediction of disease–drug interactions57,188. However, further clinical study or real-life experience is certainly needed to justify the results from PBPK modeling. Additionally, the potential mechanisms of disease–drug interactions are not well-characterized. Therefore, further studies are needed to reveal the in-depth and comprehensive mechanism involved in disease–drug interactions.
5. Mathematical modeling
The application of mathematical modeling to problems in PK has a rich history in the form of pharmacokinetic modeling to explore how simulation can be used to improve our understanding of common issues not readily addressed in human pharmacology249. Animal models are mainly used in experimental physiology, experimental pathology and experimental therapeutics, especially in the study of new drugs. In the earliest stage of drug discovery/development, various cell-based models and animal models were used for the prediction of human PK and toxicokinetics250. In this section, the current status and future challenges on PBPK modeling and animal models are summarized.
5.1. The current status and challenges of PBPK modeling
As early as 1937251 physiological parameters were introduced into pharmacokinetic parameter estimation. The term PBPK model appeared in 1977252. Although the PBPK method was proposed a long time ago, it was applied to support new drug development in the last decade since the mechanism of drug metabolism and transport gained clarity. Two known milestones of extensive application for industry are: 1) A PBPK review team was set up in the office of clinical pharmacology (CDER/FDA, US) because of increasing numbers of PBPK submissions in 2013253; 2) PBPK guidance was released by FDA254 and EMA255 respectively during 2016–2018. A total of 217 PBPK submissions were reviewed by the FDA in 2016256. As one of the four major pharmacometric research methods257, the strategy of waiving clinical trials through PBPK study has been extensively accepted in western society and is gradually being accepted in China.
5.1.1. Basic concepts of PBPK
PBPK can be utilized to mechanistically understand and predict in vivo pharmacokinetic characteristics from a whole body perspective by integrating system-specific parameters (such as physiological parameters), drug-specific parameters (such as physical–chemical and mechanistic pharmacokinetic data), and specific PBPK model structure258. It can quantitatively describe drug concentration kinetics in the blood and each tissue through a series of mathematical differential equations, which allows it to accurately predict target tissue drug concentration as well as to understand drug absorption, distribution, metabolism, elimination, and transportation (ADMET) processes. Because it incorporates system-specific parameters into equations of each tissue, it can also be used to predict drug concentration in tissues under different scenarios, such as co-administration of enzyme inhibitor or in a specific population (hepatic- or renal-impaired patients, pediatrics, or elders), which could support new drug development strategy, clinical trial design, and improved clinical development efficiency.
5.1.2. PBPK in drug development
5.1.2.1. PK drug–drug interaction study
As of August 2016, 60% of PBPK study cases submitted to FDA were related to drug–drug interactions (DDI)256. Among the three predominant DDI mechanisms, enzyme-259, transporter-260, and disease-mediated DDI261,262, enzyme-mediated DDI cases showed the best predictive performance in PBPK. Hsueh et al.259 summarized 104 publications with DDI predictions, a total of 126 and 360 cases were reported for drug as metabolic “victim” and “perpetrator” respectively. The predictive performance of CYP3A- and CYP2D6-mediated DDI was found to be the best for new drugs as victim using the PBPK method. Two enzymes are involved in metabolism of large proportion of marketed drugs and well-established probe perpetrators are available256,263. The predictive performance was poorer for new drugs as perpetrator259. In order to accurately predict the quantitative effects of an enzyme inhibitor264 or inducer265 on a substrate, the FDA suggested the following study strategy256,266: a) Develop an initial PBPK model of enzyme–substrate based on in vitro data followed by verification using human single-dose PK data; b) develop a PBPK model of inducer or inhibitor and validate its enzyme modulation effect using in vivo (or literature data) data; c) predict the effect of inhibitor/inducer on substrate PK characteristics in humans using the PBPK model, which will support DDI study strategy or clinical trial design, especially for the dose selection; d) if a dedicated DDI was required and conducted, then the initial PBPK model will be verified and modified based on observed DDI data; e) predict other untested scenarios and validate dose selection. Following this strategy, predictive performance was summarized in report published by Hsueh et al.259. As stated in submitted cases to the FDA, AUCR or CmaxR (ratio of AUC or Cmax) was estimated within the range of (0.80, 1.25) and (0.50, 2.00) for higher than 73% and 77% cases respectively. Although overall DDI of CYP-mediated interactions could be estimated well, prediction of time-dependent DDI and intestinal enzyme-mediated DDI was still challenging256.
Because tissue concentration can indicate efficacy or safety better than plasma concentration, it is more important to be predicted, especially for the drugs with a “disconnected” concentration in tissues compared to plasma concentration, which may be caused by significantly different distribution through transporters267. Unfortunately, the best prediction method theoretically, the PBPK method, showed worse predictive performance for transporter-mediated DDI compared to that of enzyme-mediated DDI, which was due to ubiquitous tissue distribution, unique cellular localization, and competing active and passive processes268. Furthermore, the lack of knowledge pertaining to disease- or population-specific factors makes PBPK more challenging for transporter-mediated DDI prediction. In order to accurately predict unbound and intra/subcellular drug concentrations while considering the role of a transporter, selecting appropriate in vitro (such as imaging) and in vivo experimental methods to determine tissue concentration followed by verification of PBPK model in animals may be helpful267. Recently, disease-mediated DDI received greater attention, especially for renal impairment affecting liver enzymes262. However, research on disease-mediated DDI are limited, and few PBPK cases to predict this kind of DDI have been reported269,270, and so further research to uncover the rationales behind of disease and physiological parameters is needed.
5.1.2.2. Specific population study
One of the most known characteristics of the PBPK model is that it can integrate drug-specific and system-specific parameters, which includes age, gender, disease status, and specific physiological status. This characteristic allows us to predict PK exposure changes by mechanistically changing specific parameters according to the different populations, such as pediatric, elderly, and in patients with hepatic or renal impairment. However, accurate prediction for these specific populations is still quite challenging because changes in system-specific parameters generally are not available or quantified accurately271. The FDA and other scientists summarized PBPK prediction strategy in patients with renal impairment272 or hepatic impairment273, in the elderly274, pediatric275, fetal276, and pregnant patients277 but, because of the above limits, these predictions could be utilized only to aid in clinical trial design in these specific populations rather than to waive these dedicated clinical trials without any verification in these specific populations.
5.1.2.3. Generic drug development
In comparison to the in vitro–in vivo correlation (IVIVC) method the PBPK method was advantageous because it could identify the contribution of penetration, intestinal metabolism and transport to the absorption–drug concentration–time curve. Therefore, PBPK analysis can estimate in vivo dissolution characteristics more accurately, which will be useful to guide drug development278,279. Therefore, the US FDA continuously held modeling and simulation workshops to make PBPK methods more useful in generic drug development280, 281, 282 as well as suggested a research strategy for industry283,284. Additionally, physiologically-based oral absorption modeling can be utilized to guide Quality by Design (QbD) and predict food effects, effect of acid-reducing agents, SUPAC activities, and to influence label language. Although it was potentially powerful, its application is still limited because of physiological information missing in PBPK system models. The European OrBiTo (Oral Biopharmaceutical Tool) Project results showed that less than 50% of drugs could receive 2-fold error prediction performance using the PBPK modeling method285. Modified in vitro experiment data with more similarities to in vivo status and accurate physiological parameters affecting the rate-limiting absorption process may be able to improve its predictive performance.
5.1.2.4. Other applications and trends of PBPK modeling methods
In addition to the above applications, the PBPK modeling method also could be used to predict first-in-human PK profiles286. It may be helpful for those drugs with nonlinear metabolism characteristics. Recently, a semi-PBPK model (or minimal PBPK model)287,288 was reported to extensively survey human biologics PK profiles to assess the predominant clearance site and dynamically describe system plasma concentrations and two other virtual compartments, lumped tissues with continuous and fenestrated vascular endothelium. This semi-PBPK model structure could allow investigators quickly estimate PBPK parameters using system drug concentrations considering drug-receptor binding in systems as well as in tissues, as described in two recent reviews289,290.
The PBPK modeling method is not an independent modeling method, and sometimes it is better to be integrated with other modeling methods for better results. In order to understand PK characteristics in mechanism, allometric scaling291 and in vitro–in vivo extrapolation methods292 can also be used to analyze preclinical data and compare the results with human data, which can provide more key information from different angles to develop a PBPK model more accurately indicating the real disposition process in humans293. Taking advantage of the PBPK ability to predict drug tissue concentration, a PBPK-PD model could be developed to capture pharmacodynamic characteristics in a more accurate way with more understanding of the mechanism294,295, which is helpful for those drugs with significantly inconsistent exposure between system and targeted tissues. For a new moiety entity clinical development, verification of an established PBPK model based on human data with the specific ADMET mechanism is required, which may need an additional clinical trial. Recently, global development is going to become routine strategy, and ethnic differences in PK characteristics will be important. Therefore, PBPK could support evaluation of ethnic differences by its unique contribution to the mechanistic understanding296. Because population PK (PopPK) is routinely used to identify the key factors affecting PK profiles followed by quantifying these key factors, a PopPK study could verify PBPK simulations under some extreme scenarios, which may allow sponsors to waive some dedicated clinical trials (PBPK-PopPK strategy)297. Under many scenarios, we only pay attention to drug concentration in tissues related to PK, PD, or safety characteristics, so we don't need to accurately capture drug kinetics in other tissues. Therefore, in order to increase parameter reliability without a decrease in PBPK power, we could shrink the typical PBPK model integrating each tissue in humans to a semi-PBPK model integrating necessary target tissues and replace other tissues with one or two compartments.
Along with the coadministration of herbal or natural products, the potential herb–drug interaction is gaining increasing attention, and can be predicted using a PBPK modeling method. But accurate prediction of herb–drug interactions is still a challenging mission because of the complex composition and relatively limited knowledge of individual constituents that produce the interactions. A feasible procedure is to firstly identify the major constituents followed by compound–compound interaction prediction as previous introduced158,298. The major concern with this procedure is to prove that the interaction of major constituents is similar to that of the whole herb.
5.2. Summary
In summary, PBPK can be utilized to mechanistically understand and predict a priori in vivo pharmacokinetic characteristics from a whole body perspective by integrating system-specific and drug-specific parameters. PBPK modeling has been routinely conducted for new entities to illustrate pharmacokinetic characteristics when drug–drug interactions happen or when dosing in specific populations needs optimization. The predictive performance of CYP3A- and CYP2D6-mediated DDI was found to be best for new drugs as victim using PBPK method, which could be applied to waive part of clinical trial. Due to unclear changes in transporter-mediated mechanism and system-specific parameters in specific populations, PBPK modeling power is limited to supporting clinical trial design.
6. Novel animal models for DMPK studies
Animal models are mainly used in experimental physiology, experimental pathology and experimental therapeutics, especially in the study of new drugs. In the earliest stage of drug discovery/development, various cell-based models and animal models were used for the prediction of human PK and toxicokinetics250. The common laboratory animals for DMPK include rats, rabbits, dogs, monkeys, etc. However, with the development of gene editing technology, animal models of special ADME genes are needed to better study the mechanisms of DMPK, including the metabolic pathway and its regulatory mechanism.
6.1. Conventional transgenic animal models for DMPK research
Traditional animal models are constructed by homologous recombination in embryonic stem cells. This method implements foreign gene knock-in, but the recombination efficiency is very low, and the recombinant site has certain randomness299. In 2009, the discovery and application of nucleic acid engineering enzymes greatly advanced gene knock-in technology300. Zinc-finger nucleases (ZFNs) are the first nucleic acid engineering enzymes to be discovered300. They cleave DNA at specific sites to form double-strand breaks (DSBs), which are then repaired by cell homology and used as templates by exogenous donor DNA. The repair of DSBs result in knocking out the foreign gene300. Another engineering nuclease that was subsequently discovered for gene editing is transcription activator-like effector nucleases (TALENs)300,301. Since the 1990s, Cyp knockout (KO) mice have been successfully constructed using gene KO techniques, such as Cyp1a2, Cyp2e1, Cyp2c9, Cyp3a4 and Cyp2d6302, 303, 304, 305. In recent years some of mouse models have been used to study the DMPK of drugs under specific Cyp knockout conditions. To overcome the differences in subtype composition, protein expression, catalytic activity and substrate specificity between mouse and human CYP enzymes, scientists have built humanized animal models to better evaluate drug metabolism characteristics of human CYPs. For example, in 2007 humanized Cyp1a1/2 mice were constructed for a toxicology study306. Humanized Cyp2c19 mice for drug metabolism307, humanized Cyp3a4 mice for drug interactions308, and humanized Cyp2d6 mice for drug interactions309 were reported in 2008, 2011 and 2012, respectively. In 2012, Cyp2c knockout mice and Cyp2c9 humanized mice were generated for drug metabolism and drug interaction studies304. In 2015, humanized Cyp2b6 mice were also constructed for drug metabolism310.
Both Cyp gene KO and humanized mouse models have been constructed by traditional knockout techniques, i.e. homologous recombination of foreign DNA fragments with genes of the same or similar sequence in the host genome, thus replacing the corresponding gene sequences in the genome of the recipient cells and integrating them into the host. In the cell genome, the key technologies of this method include the acquisition of embryonic stem cells, the design of target, and the screening of embryonic stem cells. Homologous recombination is time-consuming, costly, as well as inefficient in gene editing, and may lead to adverse mutations. As it is difficult to obtain and culture embryonic stem cells in rats, the construction and application of knockout or knock-in rat models have lagged behind the mouse models. Rats are a rodent model animal widely used in DMPK and have many advantages over mice, such as larger size, easy manipulation, high tolerance to blood volume loss and large sample size. Moreover, rats in certain physiological and pathological states such as diabetes and breast cancer, are closer to humans than mice311,312. Therefore, it is particularly urgent to construct novel rat models of DMPK-related genes through KO and humanization.
6.2. Novel CRISPR/Cas9-based animal models for DMPK research
The clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated 9 (Cas9) system, as the third generation of artificial nuclease technology, provides a promising tool for genetic engineering. It offers an efficient approach to develop genetically modified animal models and a potential strategy for targeted gene therapies. The CRISPR/Cas9 system allows simultaneous digestion of multiple targets at multiple sites in the same cell, making it possible to knock out or knock in multiple genes. CRISPR/Cas9 as a new gene editing technology has many characteristics and advantages, including high targeting accuracy, simultaneous knockout of multiple sites of target genes, simplicity of operation and no species restriction. In recent years, CRISPR/Cas9 has been applied to the study of drug absorption, disposition, metabolism and excretion, as well as the preparation of ADME animal models.
Today CRISPR/Cas9 technology enables DMPK scientists to develop better and more predictable ADME models in vitro and in vivo, especially to study ADME genes that have not been fully explored previously. Most published papers of CRISPR/Cas9-mediated ADME describe CYP drug metabolic enzymes and ABC drug transporters. For example, in 2016, the rat Cyp2d gene locus (containing Cyp2d1-5) was knocked out and replaced with human CYP2D6 in Wistar rats, but a functional characterization was not reported313. In the same year the rat Cyp2e1 gene was knocked out in Sprague Dawley rats, and the KO rats were physiological normal and lost the expression and function of the CYP2E1 enzyme314. In 2017, Cyp3a1 and Cyp3a2 double KO rats were generated by CRISPR/Cas9 technology315, and Cyp2c (Cyp2c6, Cyp2c11 and Cyp2c12) genes were also knocked out in rats316. Finally, Cyp2c11 gene was knocked out in Sprague Dawley rats317. In vitro and in vivo metabolic studies of the CYP substrates indicated that the target CYP isoform was functionally inactive in all KO rats314,315. It should be noted that KO models resulted in the compensatory regulation of other CYP isoforms involved in drug metabolism314,315. However, the potential mechanisms of these compensatory changes remain unclear. In addition, these KO models showed some differences, such as changes in serum testosterone concentrations315 or alkaline phosphatase314. Some of these differences can be attributed to the deficiency of CYP functions, such as CYP3A-mediated testosterone metabolism. Therefore, these physiological changes in KO rats should be considered when comparing ADME data from KO models with data from wild-type rats. In addition to the rat KO models, a Cyp2b9/10/13 KO mouse model was also generated via CRISPR/Cas9 technology318. It is interesting that there were no significant compensatory changes in other CYP isoforms in Cyp2b KO mice, which may be due to low CYP2B hepatic expression, especially in male mice318. In 2019, a novel MDR1 (Mdr1a/b) double-knockout rat model was generated in Sprague Dawley rats by the CRISPR/Cas9 technology319. The loss of MDR1 function significantly increased digoxin uptake in Mdr1a/b−/− rats. The MDR1 KO rat model is of great significance to study the function of MDR1 in drug transport, toxicity and drug resistance.
6.3. Summary
In summary, genome editing based on CRISPR/Cas9 has been identified as a breakthrough technology in constructing animal models. Novel animal models are not only conducive to the basic research of human diseases, but also can be used to study the molecular mechanisms of drug pharmacodynamics, toxicity and clinical use. Furthermore, DMPK animal models will promote the study of DMPK mechanisms and strengthen the relationship between drug metabolism and pharmacology/toxicology. For example, the potentials and mechanisms of DDI between erlotinib and docetaxel was studies by using Cyp3a1/2 KO rats320. Docetaxel significantly increased the maximum concentration and systemic exposure of erlotinib in wild type (WT) rats, but the DDI was significantly attenuated in Cyp3a1/2 KO rats, suggesting that the CYP3A plays the perpetrating role of docetaxel on erlotinib.
7. Non-classical xenobiotic metabolic pathways
Drug metabolism or drug biotransformation is the process by which xenobiotics are enzymatically modified to make them more readily excretable and eliminate pharmacological activity. Drug metabolism is the prominent process in drug disposition. Understanding the metabolic fate and the corresponding enzymes are important with regard to metabolite toxicity and drug–drug interaction risks. Detailed data from drug metabolism studies aid in the drug clinical practice and drug design and modification. Over the past decades the basic mechanism and rules of drug metabolism, especially mediated by CYP, have been clarified. The strategies and approaches used for drug metabolism investigations have come to maturity and industrialization. Recently, with the rapid development of the separation technology and qualitative techniques, such as IMS-TOF/MS or novel 2D NMR technology, and the considerable amount of attention directed at non-CYP enzymes, several undesirable drug metabolites have been identified, and novel metabolic reactions were discovered. Some outwardly rational reactions are newly described based on the novel understandings of the mechanism underlying common biotransformations. This section briefly reviews a series of cases of novel metabolic reactions and pathways to provide readers new insights into investigations on drug metabolism.
7.1. Oxidative pathways
Oxidative pathways, including sp3-hybridized C-hydroxylation, unsaturated C-oxidation, N-dealkylation/deamination, O-dealkylation, S-dealkylation, N-oxidation, S-oxidation, and oxidative cleavage of esters and amides classified by functional groups are the most common biotransformations. In recent years some unexpected oxidative reactions or pathways have been reported.
Aromatic ring-containing drugs are most common and generally metabolized by P450-mediated π-electron oxidations to form an arene-epoxidized intermediate. The latter undergoes a hydride shift spontaneously to produce stable phenol metabolite(s). However, for some specific structures, unstable epoxides are preferentially attacked by nucleophilic substances, thereby leading to reactive intermediate-related covalent attachments. For example, for cocaine, the covalent adducts of biological thiols are first characterized321. In vitro investigations revealed that CYP1A2, 2C9, and 2D6 catalyze the formation of a reactive epoxide intermediate from the oxidation of the cocaine phenyl moiety (Fig. 3A). Although an aryl moiety is generally considered a stable functional group, epoxide ring opening is attacked by nucleophilic thiolates, such as N-acetylcysteine or glutathione, for cocaine.
Figure 3.
Cases of some unusual metabolic pathways of oxidation, including: (A) proposed mechanism for cocaine metabolism to thiol-related adduction, and (B) Baeyer–Villiger oxidation mediated by FMO5.
Carbon–carbon cleavage and formation reactions are rare in xenobiotic metabolism. Recent studies have focused on the roles of flavin-containing monooxygenases (FMOs) to catalyze unexpected Baeyer–Villiger oxidations, which is a kind of carbon–carbon bond cleavage reaction. E7016, a potential anticancer agent with a 4-hydroxypiperidine moiety was confirmed to be a substrate of FMO5322. The generation of the major ring-opened hydroxyl-carboxylate metabolite was proposed by a three-step reaction, as follows: dehydrogenation of the secondary alcohol on the parent drug to form piperidine-4-one, followed by insertion of an oxygen atom to form a lactone via the Baeyer–Villiger oxidation, and further CEs-mediated hydrolysis. Recently, the 2,3-dihydropyridin-4-one (DHPO) ring in MRX-I (an analog of the antibiotic linezolid) was also reported to undergo a similar carbon–carbon cleavage reaction in humans323. However, different from piperidine-4-one, Baeyer–Villiger oxidation of the DHPO ring forms an enol lactone and is further hydrolyzed to an enol, which can be transformed to an aldehyde intermediate by enol–aldehyde tautomerism. The aldehyde intermediate underwent either oxidation catalyzed by short-chain dehydrogenase, aldehyde ketone reductase, and aldehyde dehydrogenase (ALDH) or reduction mediated by ALDH to generate the observed directed DHPO ring-opening metabolites MRX459 or MRX455-1, respectively (Fig. 3B). H218O experiments were conducted to elucidate the mechanism underlying the formation of the two metabolites.
7.2. Reductive metabolic pathways
The majority of in vivo biotransformations are oxidation, while reductive reactions preferentially occur in anaerobic or low-O conditions. A considerable number of the same enzymes that catalyze oxidative metabolism, such as P450s, aldo-keto reductase, carbonyl reductase, xanthine oxidase, aldehyde oxidase, and quinone oxidoreductase, can also be involved in reductions. Under the catalysis of some specific enzymes or the involvement of reducing agents, some uncommon reductive metabolic pathways are observed.
NADPH-cytochrome P450 reductase (POR) and cytochrome-b5 is crucial for P450 electron transporter chain integrity because they donate electrons to P450s from NADPH. Thus, most marketed recombinant P450 enzymes generally contain cytochrome-b5 and POR to enhance their oxidative efficiencies. In some cases, POR alone can also catalyze one-electron reduction, such as with aristolochic acid324. Another reported substrate of POR is an aldehyde intermediate (M-CHO) that is formed during the metabolism of imrecoxib, which is a moderate COX-2 inhibitor325. POR expresses dual effects on further M-CHO metabolism, namely oxidation to form carboxylic acid metabolite (M2) and unexpected reduction to form a hydroxymethyl metabolite (M1), by donating electrons to P450s or competitively to the substrate, respectively (Fig. 4A). The two opposite metabolic pathways, especially M-CHO reduction, led to an underestimation of the amount of M2 in static in vitro incubations.
Figure 4.
Instances of some unusual metabolic pathways for reduction, hydrolysis, and conjugation, including: (A) formation mechanism for M1 and M2, the major imrecoxib metabolites in humans, (B) hydrolysis pathways of vicagrel in humans, and (C) N+-glucuronidation of morinidazole.
7.3. Hydrolysis pathways
Many drugs with specific functional moieties, including esters, amides, thioesters, epoxides, sulfates, and glucuronides can be metabolized by adding water. Hydrolysis is generally carried out by the corresponding enzymes, such as esterase or amidases. Prodrug design has received increasing interest, thereby leading to considerable attention to the important roles of hydrolytic metabolism. Some novel hydrolytic enzymes also catalyze undesirable reactions.
For example, arylacetamide deacetylase (AADAC) is a serine hydrolase expressed in human liver and intestine that is rarely reported compared with other hydrolytic enzymes (CEs and paraxonase). Only one AADAC isoform is present in humans. AADAC is identified as a lipase that is capable of hydrolyzing endogenous cholesterol ester326; however, it has been recently found to be responsible for some clinical drugs, such as prasugrel327 and vicagrel328. Different from clopidogrel, the thiolactone metabolite of vicagrel is formed via a rapid hydrolysis before intestinal absorption329. The first activation step for vicagrel was initially believed to be mediated only by human intestine CES-2 (CES2) until a recent finding showed that AADAC also contributed to vicagrel hydrolysis (Fig. 4B). The activation of the parent drug before entering the systemic circulation guarantees short onset time and avoidance of “clopidogrel resistance” attributed to CYP2C9 gene polymorphisms.
Another case of hydrolytic enzymes newly identified is dipeptidyl peptidases (DPPs), which can catalyze the hydrolysis of cyanopyrrolidine DDP-4 inhibitors. Generally, a nitrile group in the drug structures prevents metabolism because of its well-known inertness, and as a result, a nitrile moiety is increasing introduced as a block on metabolically labile sites in drug design330. However, for vildagliptin, anagliptin, and besigliptin (not saxagliptin), the biotransformation of the nitrile group into carboxylic acid is the major metabolic pathway in vivo by the DPP family such as DPP-4, DPP-2, DPP-8, DPP-9, and fibroblast activation protein-α331. Among them, DPP-2 has the highest hydrolytic capacity after DPP-4. However, other substrates containing a nitrile group, such as lacosamide and flutamide, cannot be hydrolyzed by DPPs probably because the nitrile moiety in these structures cannot be positioned in the catalytic triad of Asp-His-Ser of DPPs.
7.4. Conjugation pathways
Generally, conjugation pathways involve the addition of an endogenous hydrophilic group to a drug or its metabolite(s), including glucuronidation, sulfation, glutathione conjugation, amino acid conjugation, acetylation, and methylation. Conjugation generally introduces polar groups to facilitate drug excretion, except for methylation and acetylation. Although this finding is true for many cases, several unusual conjugative reactions were reported in recent years.
The substrates for glucuronidation generally have an OH (i.e., alcohols, phenol, and carboxylic acids), amino (i.e., aliphatic tertiary amine, aromatic primary amine, and sulfonamide) or thiophenol group. In general, for drugs possessing both tertiary amine and hydroxyl groups, O-glucuronidation is always formed preferentially over N-glucuronidation. However, a reversible regioselectivity is observed in the conjugative metabolism of morinidazole in humans, where glucuronidation prefers the tertiary nitrogen of the morpholine ring to the aliphatic hydroxyl group at the side chain of morinidazole (Fig. 4C)332. Additionally, molecular modeling studies indicated that the regioselectivity for morinidazole glucuronidation is unrelated to steric hindrance. UGT1A4, as well as UGT1A3 and 2B10, are often considered enzymes that play important roles in N+-glucuronidation333, 334, 335. However, according to the morinidazole experience, UGT1A9 can be identified as a new UGT isoform specializing in tertiary aliphatic amine N-glucuronidation.
In addition, several novel conjugates, including carnitine conjugation to cyclopropanecarboxylic acid336 creatinine conjugation to andrographolide337, and phosphoethanolamine conjugation to pimasertib338, recently have been discovered. The combination or further modification of the common conjugation process has been also reported339,340.
7.5. Summary
In recent years there has been an increased effort to better understand the role of enzymes beyond P450, UDP-glucuronosyltransferase, and aldehyde oxidase in drug metabolism. Recently, several biological enzymes responsible for endogenous substrate catalysis, such as dipeptidyl peptidases and arylacetamide deacetylase, are newly proven to have additional capabilities in drug transformation. Drugs that rely on these non-P450 enzymes for their in vivo clearance, however, usually undergo non-classical metabolic pathways. The basic mechanism and rules of drug metabolism cannot be characterized based on the structures of the drugs alone, because the presence of metabolic intermediates that would allow for the intra-molecular rearrangement are likely factors in unusual metabolite formation. This subtle but potentially significant hypothesis suggests that the electron or radical-mediated modulation of biotransformation characteristics may represent uncommon underlying mechanisms for undesirable metabolic pathways, with relevant toxicological consequences.
Several novel and unusual reactions and pathways have been reviewed. Most of these reactions are attributed to (I) metabolic intermediate formation and rearrangement and (II) the involvement of novel metabolic enzymes, especially non-P450s. Considering the availability of sophisticated and sensitive analytical instrumentation, as well as the introduction of modern approaches in drug metabolism investigation, new metabolic reactions continue to be discovered. Accurately predicting drug metabolism in an empirical manner and clarifying the metabolism mechanisms responsible for drug adverse reactions and drug–drug interactions will increase in the future. Additionally, valuable inspiration may be provided for rational drug design and modification with the expansion of metabolic enzymes, many of which are recognized as new therapeutic targets.
8. Conclusions and perspectives
DMPK research is essential for understanding the efficacy and safety of medications. Integrated studies on drug-metabolizing enzymes and transporters underlying the ADME processes as well as their transcriptional and posttranscriptional regulation mechanisms provide a comprehensive understanding of interindividual variations in pharmacotherapy. Future studies in these areas will undoubtedly advance our understanding to achieve better prediction of PK properties. Understanding the DDIs and disease–drug interactions is clinically important as such interactions may increase the risk of adverse reactions or lead to treatment failure. Although DDIs between small molecule drugs are relatively well-characterized, other potential interactions are not fully explored, including interactions with herbal biologics and other new forms of therapeutics. Furthermore, more attention should be paid to the microbiota-mediated drug interactions when examining potential DDIs and HDIs. There is emerging evidence indicating that disease–drug interactions can have a profound impact on the therapeutic outcomes. Further studies are needed to reveal the critical mechanisms by which disease–drug interactions are produced. While the benefits of PBPK are obvious for clinical trials, it is better to integrate PBPK with other modeling methods and consult experimental findings to design clinical trials in support of new drug development. Novel animal models such as those created through CRISPR-Cas9-based gene editing techniques should be an invaluable addition to current tools for PK studies. With the application of sensitive and accurate analytical instruments and technologies, many new metabolic reactions and biotransformation pathways have been and will be discovered. Predicting drug metabolism more accurately and clarifying the metabolic mechanisms responsible for adverse drug reactions and DDIs will become possible in the future. Collectively, DMPK research awaits further innovation and mechanistic studies while DMPK remains a critical component in drug development, and is essential for practicing precision medication.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grants: 81573489, 81522047, 81730103, 81320108027, 81660618, and 81773808), the National Key Research and Development Program (grant: 2017YFE0109900 and 2017YFC0909303, China), the 111 project (grant: B16047, China), the Key Laboratory Foundation of Guangdong Province (grant: 2017B030314030, China), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y093, China), National Engineering and Technology Research Center for New drug Druggability Evaluation (Seed Program of Guangdong Province, 2017B090903004, China), Natural Science Foundation of Guangdong (grant: 2017A030311018 and 2015A030313124, China), and National Institutes of Health (grants No. R01CA225958 and R01GM113888 to Ai-Ming Yu, USA).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.10.001.
Contributor Information
Xiaoyan Chen, Email: xychen@simm.ac.cn.
Kexin Liu, Email: liukexin89@163.com.
Ai-Ming Yu, Email: aimyu@ucdavis.edu.
Zhong Zuo, Email: joanzuo@cuhk.edu.hk.
Huichang Bi, Email: bihchang@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Currie G.M. Pharmacology, part 2: introduction to pharmacokinetics. J Nucl Med Technol. 2018;46:221–230. doi: 10.2967/jnmt.117.199638. [DOI] [PubMed] [Google Scholar]
- 2.Yan R., Yang Y., Chen Y. Pharmacokinetics of Chinese medicines: strategies and perspectives. Chin Med. 2018;13:24. doi: 10.1186/s13020-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan J., Ma S., Zhang D. Non-cytochrome P450-mediated bioactivation and its toxicological relevance. Drug Metab Rev. 2016;48:473–501. doi: 10.1080/03602532.2016.1225756. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya S., Sinha K., Sil P.C. Cytochrome P450s: mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr Drug Metab. 2014;15:719–742. doi: 10.2174/1389200215666141125121659. [DOI] [PubMed] [Google Scholar]
- 5.Nebert D.W., Dalton T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- 6.Yu Z., Tian X., Peng Y., Sun Z., Wang C., Tang N. Mitochondrial cytochrome P450 (CYP) 1B1 is responsible for melatonin-induced apoptosis in neural cancer cells. J Pineal Res. 2018;65 doi: 10.1111/jpi.12478. [DOI] [PubMed] [Google Scholar]
- 7.Ruwali M., Dhawan A., Pant M.C., Rahman Q., Khurana S.M., Parmar D. Clinical management of head and neck cancer cases: role of pharmacogenetics of CYP2 and GSTs. Oncol Res Treat. 2016;39:221–226. doi: 10.1159/000444608. [DOI] [PubMed] [Google Scholar]
- 8.Wang X., Li J., Dong G., Yue J. The endogenous substrates of brain CYP2D. Eur J Pharmacol. 2014;724:211–218. doi: 10.1016/j.ejphar.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Yi M., Cho S.A., Min J., Kim D.H., Shin J.G., Lee S.J. Functional characterization of a common CYP4F11 genetic variant and identification of functionally defective CYP4F11 variants in erythromycin metabolism and 20-HETE synthesis. Arch Biochem Biophys. 2017;620:43–51. doi: 10.1016/j.abb.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Deng J., Guo L., Wu B. Circadian regulation of hepatic cytochrome P450 2a5 by peroxisome proliferator-activated receptor γ. Drug Metab Dispos. 2018;46:1538–1545. doi: 10.1124/dmd.118.083071. [DOI] [PubMed] [Google Scholar]
- 11.Cannady E.A., Suico J.G., Wang M.D., Friedrich S., Rehmel J.R., Nicholls S.J. CYP-mediated drug–drug interactions with evacetrapib, an investigational CETP inhibitor: in vitro prediction and clinical outcome. Br J Clin Pharmacol. 2015;80:1388–1398. doi: 10.1111/bcp.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siu E.C., Tyndale R.F. Selegiline is a mechanism-based inactivator of CYP2A6 inhibiting nicotine metabolism in humans and mice. J Pharmacol Exp Ther. 2008;324:992–999. doi: 10.1124/jpet.107.133900. [DOI] [PubMed] [Google Scholar]
- 13.Pelkonen O., Rautio A., Raunio H., Pasanen M. CYP2A6: a human coumarin 7-hydroxylase. Toxicology. 2000;144:139–147. doi: 10.1016/s0300-483x(99)00200-0. [DOI] [PubMed] [Google Scholar]
- 14.He X.Y., Shen J., Ding X., Lu A.Y., Hong J.Y. Identification of critical amino acid residues of human CYP2A13 for the metabolic activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco-specific carcinogen. Drug Metab Dispos. 2004;32:1516–1521. doi: 10.1124/dmd.104.001370. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Carratt S., Hartog M., Kovalchuk N., Jia K., Wang Y. Human CYP2A13 and CYP2F1 mediate naphthalene toxicity in the lung and nasal mucosa of CYP2A13/2F1-humanized mice. Environ Health Perspect. 2017;125 doi: 10.1289/EHP844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M., Hao H., Jiang L., Long F., Wei Y., Ji H. In vitro inhibitory effects of ethanol extract of Danshen (Salvia miltiorrhiza) and its components on the catalytic activity of soluble epoxide hydrolase. Phytomedicine. 2015;22:444–451. doi: 10.1016/j.phymed.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Xie C., Gao X., Sun D., Zhang Y., Krausz K.W., Qin X. Metabolic profiling of the novel hypoxia-inducible factor 2α inhibitor PT2385 in vivo and in vitro. Drug Metab Dispos. 2018;46:336–345. doi: 10.1124/dmd.117.079723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Li S., Yang Z., Peng Y., Chen X., Zheng J. Identification of ketene-reactive intermediate of erlotinib possibly responsible for inactivation of P450 enzymes. Drug Metab Dispos. 2018;46:442–450. doi: 10.1124/dmd.117.079327. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Cao X., Li T., Li X. Effects of catalpol on the activity of human liver cytochrome P450 enzymes. Xenobiotica. 2019;49:1289–1295. doi: 10.1080/00498254.2018.1558309. [DOI] [PubMed] [Google Scholar]
- 20.Yim D., Kim M.J., Shin Y., Lee S.J., Shin J.G., Kim D.H. Inhibition of cytochrome P450 activities by Sophora flavescens extract and its prenylated flavonoids in human liver microsomes. Evid Based Complement Alternat Med. 2019;2019:2673769. doi: 10.1155/2019/2673769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen P., Li D., Chen Y., Sun J., Fu K., Guan L. p53-mediated regulation of bile acid disposition attenuates cholic acid-induced cholestasis in mice. Br J Pharmacol. 2017;174:4345–4361. doi: 10.1111/bph.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Showande S.J., Fakeye T.O., Kajula M., Hokkanen J., Tolonen A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal herbs-Implication for herb–drug interactions. Food Sci Nutr. 2019;7:44–55. doi: 10.1002/fsn3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alnaqeeb M., Mansor K.A., Mallah E.M., Ghanim B.Y., Idkaidek N., Qinna N.A. Critical pharmacokinetic and pharmacodynamic drug-herb interactions in rats between warfarin and pomegranate peel or guava leaves extracts. BMC Complement Altern Med. 2019;19:29. doi: 10.1186/s12906-019-2436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., He M.M., Niu W., Wrighton S.A., Li L., Liu Y. Metabolic capabilities of cytochrome P450 enzymes in Chinese liver microsomes compared with those in Caucasian liver microsomes. Br J Clin Pharmacol. 2012;73:268–284. doi: 10.1111/j.1365-2125.2011.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao N., Tian X., Fang Y., Zhou J., Zhang H., Wen Q. Gene polymorphisms and contents of cytochrome P450s have only limited effects on metabolic activities in human liver microsomes. Eur J Pharm Sci. 2016;92:86–97. doi: 10.1016/j.ejps.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Li G.F., Zheng Q.S., Yu Y., Zhong W., Zhou H.H., Qiu F. Impact of ethnicity-specific hepatic microsomal scaling factor, liver weight, and cytochrome P450 (CYP) 1A2 content on physiologically based prediction of CYP1A2-mediated pharmacokinetics in young and elderly Chinese adults. Clin Pharmacokinet. 2019;58:927–941. doi: 10.1007/s40262-019-00737-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaminsky L.S., Zhang Q.Y. The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos. 2003;31:1520–1525. doi: 10.1124/dmd.31.12.1520. [DOI] [PubMed] [Google Scholar]
- 28.Knights K.M., Rowland A., Miners J.Q. Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT) Br J Clin Pharmacol. 2013;76:587–602. doi: 10.1111/bcp.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edeogu C.O., Kalu M.E., Famurewa A.C., Asogwa N.T., Onyeji G.N., Ikpemo K.O. Nephroprotective effect of Moringa oleifera seed oil on gentamicin-induced nephrotoxicity in rats: biochemical evaluation of antioxidant, anti-inflammatory, and antiapoptotic pathways. J Am Coll Nutr. 2019;12:1–9. doi: 10.1080/07315724.2019.1649218. [DOI] [PubMed] [Google Scholar]
- 30.Liu H., Chen M., Yin H., Hu P., Wang Y., Liu F. Exploration of the hepatoprotective chemical base of an orally administered herbal formulation (YCHT) in normal and CCl4-intoxicated liver injury rats. Part 1: metabolic profiles from the liver-centric perspective. J Ethnopharmacol. 2019;237:81–91. doi: 10.1016/j.jep.2019.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Marchitti S.A., Brocker C., Stagos D., Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyajima A., Sakemi-Hoshikawa K., Usami M., Mitsunaga K., Irie T., Ohno Y. Thyrotoxic rubber antioxidants, 2-mercaptobenzimidazole and its methyl derivatives, cause both inhibition and induction of drug-metabolizing activity in rat liver microsomes after repeated oral administration. Biochem Biophys Res Commun. 2017;492:116–120. doi: 10.1016/j.bbrc.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Di Paolo M.L., Cozza G., Milelli A., Zonta F., Sarno S., Minniti E. Benextramine and derivatives as novel human monoamine oxidases inhibitors: an integrated approach. FEBS J. 2019. Available from doi: 10.1111/febs.14994. [DOI] [PubMed] [Google Scholar]
- 34.Tao D., Wang Y., Bao X.Q., Yang B.B., Gao F., Wang L. Discovery of coumarin Mannich base derivatives as multifunctional agents against monoamine oxidase B and neuroinflammation for the treatment of Parkinson's disease. Eur J Med Chem. 2019;173:203–212. doi: 10.1016/j.ejmech.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Foti R.S., Dalvie D.K. Cytochrome P450 and non-cytochrome P450 oxidative metabolism: contributions to the pharmacokinetics, safety, and efficacy of xenobiotics. Drug Metab Dispos. 2016;44:1229–1245. doi: 10.1124/dmd.116.071753. [DOI] [PubMed] [Google Scholar]
- 36.Johnson P., Loganathan C., Iruthayaraj A., Poomani K., Thayumanavan P. S-allyl cysteine as potent anti-gout drug: insight into the xanthine oxidase inhibition and anti-inflammatory activity. Biochimie. 2018;154:1–9. doi: 10.1016/j.biochi.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H.F., Li Z.H., Liu J.Y., Liu T.T., Wang P., Fang Y. Correlation of cytochrome P450 oxidoreductase expression with the expression of 10 isoforms of cytochrome P450 in human liver. Drug Metab Dispos. 2016;44:1193–1200. doi: 10.1124/dmd.116.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mano E.C., Scott A.L., Honorio K.M. UDP-glucuronosyltransferases: structure, function and drug design studies. Curr Med Chem. 2018;25:3247–3255. doi: 10.2174/0929867325666180226111311. [DOI] [PubMed] [Google Scholar]
- 39.Mazerska Z., Mróz A., Pawlowska M., Augustin E. The role of glucuronidation in drug resistance. Pharmacol Ther. 2016;159:35–55. doi: 10.1016/j.pharmthera.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Nair P.C., Meech R., Mackenzie P.I., McKinnon R.A., Miners J.O. Insights into the UDP-sugar selectivities of human UDP-glycosyltransferases (UGT): a molecular modeling perspective. Drug Metab Rev. 2015;47:335–345. doi: 10.3109/03602532.2015.1071835. [DOI] [PubMed] [Google Scholar]
- 41.Qi C., Fu J., Zhao H., Xing H., Dong D., Wu B. Identification of UGTs and BCRP as potential pharmacokinetic determinants of the natural flavonoid alpinetin. Xenobiotica. 2019;49:276–283. doi: 10.1080/00498254.2018.1440657. [DOI] [PubMed] [Google Scholar]
- 42.Pettersson Bergstrand M., Richter L.H., Maurer H.H., Wagmann L., Meyer M.R. In vitro glucuronidation of designer benzodiazepines by human UDP-glucuronyltransferases. Drug Test Anal. 2019;11:45–50. doi: 10.1002/dta.2463. [DOI] [PubMed] [Google Scholar]
- 43.Hu D.G., Meech R., McKinnon R.A., Mackenzie P.I. Transcriptional regulation of human UDP-glucuronosyltransferase genes. Drug Metab Rev. 2014;46:421–458. doi: 10.3109/03602532.2014.973037. [DOI] [PubMed] [Google Scholar]
- 44.Gao R., Liu M., Chen Y., Xia C., Zhang H., Xiong Y. Identification and characterization of human UDP-glucuronosyltransferases responsible for the in vitro glucuronidation of ursolic acid. Drug Metab Pharmacokinet. 2016;31:261–268. doi: 10.1016/j.dmpk.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Jeong E.S., Kim Y.W., Kim H.J., Shin H.J., Shin J.G., Kim K.H. Glucuronidation of fimasartan, a new angiotensin receptor antagonist, is mainly mediated by UGT1A3. Xenobiotica. 2015;45:10–18. doi: 10.3109/00498254.2014.942810. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Cao G., Wang G., Hao H. Regulation of mammalian UDP-glucuronosyltransferases. Curr Drug Metab. 2018;19:490–501. doi: 10.2174/1389200219666180307122945. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X., Zhao Y., Wang J., Wang X., Chen C., Yin D. Resveratrol represses estrogen-induced mammary carcinogenesis through NRF2-UGT1A8-estrogen metabolic axis activation. Biochem Pharmacol. 2018;155:252–263. doi: 10.1016/j.bcp.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Wu L., Chen Y., Liu H., Zhan Z., Liang Z., Zhang T. Emodin-induced hepatotoxicity was exacerbated by probenecid through inhibiting UGTs and MRP2. Toxicol Appl Pharmacol. 2018;359:91–101. doi: 10.1016/j.taap.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y., Cao S., Wang Y., Xu P., Yan J., Bin W. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia. 2014;92:230–237. doi: 10.1016/j.fitote.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Hwang D.K., Kim J.H., Shin Y., Choi W.G., Kim S., Cho Y.Y. Identification of catalposide metabolites in human liver and intestinal preparations and characterization of the relevant sulfotransferase, UDP-glucuronosyltransferase, and carboxylesterase enzymes. Pharmaceutics. 2019;11:355. doi: 10.3390/pharmaceutics11070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wassenaar C.A., Conti D.V., Das S., Chen P., Cook E.H., Ratain M.J. UGT1A and UGT2B genetic variation alters nicotine and nitrosamine glucuronidation in european and african american smokers. Cancer Epidemiol Biomarkers Prev. 2015;24:94–104. doi: 10.1158/1055-9965.EPI-14-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suh H.J., Yoon S.H., Yu K.S., Cho J.Y., Park S.I., Lee E. The genetic polymorphism UGT1A4*3 is associated with low posaconazole plasma concentrations in hematological malignancy patients receiving the oral suspension. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.02230-17. e02230-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Yin J.F., Zhang J., Kong S.J., Zhang H.Y., Chen X.M. UGT1A1*6 polymorphisms are correlated with irinotecan-induced neutropenia: a systematic review and meta-analysis. Cancer Chemother Pharmacol. 2017;80:135–149. doi: 10.1007/s00280-017-3344-3. [DOI] [PubMed] [Google Scholar]
- 54.Suiko M., Kurogi K., Hashiguchi T., Sakakibara Y., Liu M.C. Updated perspectives on the cytosolic sulfotransferases (SULTs) and SULT-mediated sulfation. Biosci Biotechnol Biochem. 2016;81:63–72. doi: 10.1080/09168451.2016.1222266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S., Yuan X., Lu D., Guo L., Wu B. Farnesoid X receptor regulates SULT1E1 expression through inhibition of PGC1α binding to HNF4α. Biochem Pharmacol. 2017;145:202–209. doi: 10.1016/j.bcp.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 56.Falany C.N., Rohn-Glowacki K.J. SULT2B1: unique properties and characteristics of a hydroxysteroid sulfotransferase family. Drug Metab Rev. 2013;45:388–400. doi: 10.3109/03602532.2013.835609. [DOI] [PubMed] [Google Scholar]
- 57.Sun J., Wen Y., Zhou Y., Jiang Y., Chen Y., Zhang H. p53 attenuates acetaminophen-induced hepatotoxicity by regulating drug-metabolizing enzymes and transporter expression. Cell Death Dis. 2018;9:536. doi: 10.1038/s41419-018-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R., Liu F., Chang Y., Ma X., Li M., Li C. Glutathione S-transferase A1 (GSTA1) as a marker of acetaminophen-induced hepatocyte injury in vitro. Toxicol Mech Methods. 2017;27:401–407. doi: 10.1080/15376516.2017.1320457. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y., Huycke M.M., Herman T.S., Wang X. Glutathione S-transferase alpha 4 induction by activator protein 1 in colorectal cancer. Oncogene. 2016;35:5795–5806. doi: 10.1038/onc.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yi M., Shin J.G., Lee S.J. Expression of CYP4V2 in human THP1 macrophages and its transcriptional regulation by peroxisome proliferator-activated receptor gamma. Toxicol Appl Pharmacol. 2017;330:100–106. doi: 10.1016/j.taap.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Jeong S.J., Park J.G., Kim S., Kweon H.Y., Seo S., Na D.S. Extract of Rhus verniciflua stokes protects the diet-induced hyperlipidemia in mice. Arch Pharm Res. 2015;38:2049–2058. doi: 10.1007/s12272-015-0579-6. [DOI] [PubMed] [Google Scholar]
- 62.Graham A. Mitochondrial regulation of macrophage cholesterol homeostasis. Free Radic Biol Med. 2015;89:982–992. doi: 10.1016/j.freeradbiomed.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Saini S.P., Zhang B., Niu Y., Jiang M., Gao J., Zhai Y. Activation of liver X receptor increases acetaminophen clearance and prevents its toxicity in mice. Hepatology. 2011;54:2208–2217. doi: 10.1002/hep.24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalthoff S., Winkler A., Freiberg N., Manns M.P., Strassburg C.P. Gender matters: estrogen receptor alpha (ERα) and histone deacetylase (HDAC) 1 and 2 control the gender-specific transcriptional regulation of human uridine diphosphate glucuronosyltransferases genes (UGT1A) J Hepatol. 2013;59:797–804. doi: 10.1016/j.jhep.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 65.Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 66.Shugarts S., Benet L.Z. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm Res. 2009;26:2039–2054. doi: 10.1007/s11095-009-9924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kota B.P., Tran V.H., Allen J., Bebawy M., Roufogalis B.D. Characterization of PXR mediated P-glycoprotein regulation in intestinal LS174T cells. Pharmacol Res. 2010;62:426–431. doi: 10.1016/j.phrs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Lopes-Rodrigues V., Seca H., Sousa D., Sousa E., Lima R.T., Vasconcelos M.H. The network of P-glycoprotein and microRNAs interactions. Int J Cancer. 2014;135:253–263. doi: 10.1002/ijc.28500. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y., Wang C., Meng Q., Liu Z., Huo X., Sun P. Targeting P-glycoprotein and SORCIN: dihydromyricetin strengthens anti-proliferative efficiency of adriamycin via MAPK/ERK and Ca2+-mediated apoptosis pathways in MCF-7/ADR and K562/ADR. J Cell Physiol. 2018;233:3066–3079. doi: 10.1002/jcp.26087. [DOI] [PubMed] [Google Scholar]
- 70.Robey R.W., Pluchino K.M., Hall M.D., Fojo A.T., Bates S.E., Gottesman M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rives M.L., Javitch J.A., Wickenden A.D. Potentiating SLC transporter activity: emerging drug discovery opportunities. Biochem Pharmacol. 2017;135:1–11. doi: 10.1016/j.bcp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 72.Rocha K.C., Pereira B.M., Rodrigues A.C. An update on efflux and uptake transporters as determinants of statin response. Expert Opin Drug Metab Toxicol. 2018;14:613–624. doi: 10.1080/17425255.2018.1482276. [DOI] [PubMed] [Google Scholar]
- 73.Zakeri-Milani P., Valizadeh H. Intestinal transporters: enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin Drug Metab Toxicol. 2014;10:859–871. doi: 10.1517/17425255.2014.905543. [DOI] [PubMed] [Google Scholar]
- 74.Yu J., Zhou Z., Tay-Sontheimer J., Levy R.H., Ragueneau-Majlessi I. Intestinal drug interactions mediated by OATPs: a systematic review of preclinical and clinical findings. J Pharm Sci. 2017;106:2312–2325. doi: 10.1016/j.xphs.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Oostendorp R.L., Beijnen J.H., Schellens J.H. The biological and clinical role of drug transporters at the intestinal barrier. Cancer Treat Rev. 2009;35:137–147. doi: 10.1016/j.ctrv.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Cheng Z., VanPelt J., Bergstrom A., Bethel C., Katko A., Miller C. A noncanonical metal center drives the activity of the Sediminispirochaeta smaragdinae metallo-β-lactamase SPS-1. Biochemistry. 2018;57:5218–5229. doi: 10.1021/acs.biochem.8b00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J., Wang C., Liu Q., Meng Q., Cang J., Sun H. Pharmacokinetic interaction between JBP485 and cephalexin in rats. Drug Metab Dispos. 2010;38:930–938. doi: 10.1124/dmd.110.032060. [DOI] [PubMed] [Google Scholar]
- 78.Cang J., Zhang J., Wang C., Liu Q., Meng Q., Wang D. Pharmacokinetics and mechanism of intestinal absorption of JBP485 in rats. Drug Metab Pharmacokinet. 2010;25:500–507. doi: 10.2133/dmpk.dmpk-10-rg-045. [DOI] [PubMed] [Google Scholar]
- 79.Nieto Montesinos R., Béduneau A., Pellequer Y., Lamprecht A. Delivery of P-glycoprotein substrates using chemosensitizers and nanotechnology for selective and efficient therapeutic outcomes. J Control Release. 2012;161:50–61. doi: 10.1016/j.jconrel.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 80.Pedersen K.E., Christiansen B.D., Klitgaard N.A., Nielsen-Kudsk F. Effect of quinidine on digoxin bioavailability. Eur J Clin Pharmacol. 1983;24:41–47. doi: 10.1007/BF00613925. [DOI] [PubMed] [Google Scholar]
- 81.Huo X., Liu Q., Wang C., Meng Q., Sun H., Peng J. Enhancement effect of P-gp inhibitors on the intestinal absorption and antiproliferative activity of bestatin. Eur J Pharm Sci. 2013;50:420–428. doi: 10.1016/j.ejps.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Yigitaslan S., Erol K., Cengelli C. The effect of P-glycoprotein inhibition and activation on the absorption and serum levels of cyclosporine and tacrolimus in rats. Adv Clin Exp Med. 2016;25:237–242. doi: 10.17219/acem/35254. [DOI] [PubMed] [Google Scholar]
- 83.Karibe T., Imaoka T., Abe K., Ando O. Curcumin as an in vivo selective intestinal breast cancer resistance protein inhibitor in cynomolgus monkeys. Drug Metab Dispos. 2018;46:667–679. doi: 10.1124/dmd.117.078931. [DOI] [PubMed] [Google Scholar]
- 84.Yamagata T., Kusuhara H., Morishita M., Takayama K., Benameur H., Sugiyama Y. Effect of excipients on breast cancer resistance protein substrate uptake activity. J Control Release. 2007;124:1–5. doi: 10.1016/j.jconrel.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Jia Y., Liu Z., Wang C., Meng Q., Huo X., Liu Q. P-gp, MRP2 and OAT1/OAT3 mediate the drug–drug interaction between resveratrol and methotrexate. Toxicol Appl Pharmacol. 2016;306:27–35. doi: 10.1016/j.taap.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 86.Wang C., Huo X., Wang C., Meng Q., Liu Z., Sun P. Organic anion-transporting polypeptide and efflux transporter-mediated hepatic uptake and biliary excretion of cilostazol and its metabolites in Rats and humans. J Pharm Sci. 2017;106:2515–2523. doi: 10.1016/j.xphs.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Watanabe T., Kusuhara H., Maeda K., Shitara Y., Sugiyama Y. Physiologically based pharmacokinetic modeling to predict transporter-mediated clearance and distribution of pravastatin in humans. J Pharmacol Exp Ther. 2009;328:652–662. doi: 10.1124/jpet.108.146647. [DOI] [PubMed] [Google Scholar]
- 88.De Lange E.C., Vd Berg D.J., Bellanti F., Voskuyl R., Syvänen S. P-glycoprotein protein expression versus functionality at the blood–brain barrier using immunohistochemistry, microdialysis and mathematical modeling. Eur J Pharm Sci. 2018;124:61–70. doi: 10.1016/j.ejps.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 89.Hartz A.M., Zhong Y., Shen A.N., Abner E.L., Bauer B. Preventing P-gp ubiquitination lowers Aβ brain levels in an Alzheimer's disease mouse model. Front Aging Neurosci. 2018;10:186. doi: 10.3389/fnagi.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohtsuki S., Asaba H., Takanaga H., Deguchi T., Hosoya K.I., Otagiri M. Role of blood–brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83:57–66. doi: 10.1046/j.1471-4159.2002.01108.x. [DOI] [PubMed] [Google Scholar]
- 91.Matsson E.M., Eriksson U.G., Palm J.E., Artursson P., Karlgren M., Lazorova L. Combined in vitro–in vivo approach to assess the hepatobiliary disposition of a novel oral thrombin inhibitor. Mol Pharm. 2013;10:4252–4262. doi: 10.1021/mp400341t. [DOI] [PubMed] [Google Scholar]
- 92.Notenboom S., Weigand K.M., Proost J.H., van Lipzig M.M., van de Steeg E., van den Broek P.H. Development of a mechanistic biokinetic model for hepatic bile acid handling to predict possible cholestatic effects of drugs. Eur J Pharm Sci. 2018;115:175–184. doi: 10.1016/j.ejps.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Y., Meng Q., Wang C., Liu Q., Sun H., Kaku T. Organic anion transporters involved in the excretion of bestatin in the kidney. Peptides. 2012;33:265–271. doi: 10.1016/j.peptides.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 94.Guo X., Meng Q., Liu Q., Wang C., Mao Q., Sun H. Peptide cotransporter 1 in intestine and organic anion transporters in kidney are targets of interaction between JBP485 and lisinopril in rats. Drug Metab Pharmacokinet. 2012;27:232–241. doi: 10.2133/dmpk.dmpk-11-rg-089. [DOI] [PubMed] [Google Scholar]
- 95.Ye J., Liu Q., Wang C., Meng Q., Peng J., Sun H. Inhibitory effect of JBP485 on renal excretion of acyclovir by the inhibition of OAT1 and OAT3. Eur J Pharm Sci. 2012;47:341–346. doi: 10.1016/j.ejps.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Xu Q., Wang C., Meng Q., Liu Q., Sun H., Peng J. OAT1 and OAT3: targets of drug–drug interaction between entecavir and JBP485. Eur J Pharm Sci. 2013;48:650–657. doi: 10.1016/j.ejps.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 97.Takeda M., Tojo A., Sekine T., Hosoyamada M., Kanai Y., Endou H. Role of organic anion transporter 1 (OAT1) in cephaloridine (CER)-induced nephrotoxicity. Kidney Int. 1999;56:2128–2136. doi: 10.1046/j.1523-1755.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 98.Jung K.Y., Takeda M., Shimoda M., Narikawa S., Tojo A., Kim D.K. Involvement of rat organic anion transporter 3 (rOAT3) in cephaloridine-induced nephrotoxicity: in comparison with rOAT1. Life Sci. 2002;70:1861–1874. doi: 10.1016/s0024-3205(02)01500-x. [DOI] [PubMed] [Google Scholar]
- 99.Müller F., Weitz D., Mertsch K., König J., Fromm M.F. Importance of OCT2 and MATE1 for the cimetidine-metformin interaction: insights from investigations of polarized transport in single- and double-transfected MDCK cells with a focus on perpetrator disposition. Mol Pharm. 2018;15:3425–3433. doi: 10.1021/acs.molpharmaceut.8b00416. [DOI] [PubMed] [Google Scholar]
- 100.El-Arabey A.A. Dual function of OCT2 and MATE1 in cisplatin induced nephrotoxicity. Pharmacol Res. 2017;119:493. doi: 10.1016/j.phrs.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 101.Zhu Y., Meng Q., Wang C., Liu Q., Huo X., Zhang A. Methotrexate-bestatin interaction: involvement of P-glycoprotein and organic anion transporters in rats. Int J Pharm. 2014;465:368–377. doi: 10.1016/j.ijpharm.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 102.Zhang R., Yang X., Li J., Wu J., Peng W.X., Dong X.Q. Upregulation of rat renal cortical organic anion transporter (OAT1 and OAT3) expression in response to ischemia/reperfusion injury. Am J Nephrol. 2008;28:772–783. doi: 10.1159/000129073. [DOI] [PubMed] [Google Scholar]
- 103.Mattick J.S. RNA regulation: a new genetics?. Nat Rev Genet. 2004;5:316–323. doi: 10.1038/nrg1321. [DOI] [PubMed] [Google Scholar]
- 104.Yu A.M., Tian Y., Tu M.J., Ho P.Y., Jilek J.L. MicroRNA pharmacoepigenetics: posttranscriptional regulation mechanisms behind variable drug disposition and strategy to develop more effective therapy. Drug Metab Dispos. 2016;44:308–319. doi: 10.1124/dmd.115.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu A.M., Ingelman-Sundberg M., Cherrington N.J., Aleksunes L.M., Zanger U.M., Xie W. Regulation of drug metabolism and toxicity by multiple factors of genetics, epigenetics, lncRNAs, gut microbiota, and diseases: a meeting report of the 21st International Symposium on Microsomes and Drug Oxidations (MDO) Acta Pharm Sin B. 2017;7:241–248. doi: 10.1016/j.apsb.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakano M., Nakajima M. Current knowledge of microRNA-mediated regulation of drug metabolism in humans. Expert Opin Drug Metab Toxicol. 2018;14:493–504. doi: 10.1080/17425255.2018.1472237. [DOI] [PubMed] [Google Scholar]
- 107.Tsuchiya Y., Nakajima M., Takagi S., Taniya T., Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 108.Pan Y.Z., Gao W., Yu A.M. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009;37:2112–2117. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M.M., Wang W.P., Wu W.J., Huang M., Yu A.M. Rapid production of novel pre-microRNA agent hsa-mir-27b in Escherichia coli using recombinant RNA technology for functional studies in mammalian cells. Drug Metab Dispos. 2014;42:1791–1795. doi: 10.1124/dmd.114.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li X., Tian Y., Tu M.J., Ho P.Y., Batra N., Yu A.M. Bioengineered miR-27b-3p and miR-328-3p modulate drug metabolism and disposition via the regulation of target ADME gene expression. Acta Pharm Sin B. 2019;9:639–647. doi: 10.1016/j.apsb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu H., Wu H., Liu X., Evans B.R., Medina D.J., Liu C.G. Role of microRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Z., Hu S., Wang J., Cai J., Xiao L., Yu L. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119:125–130. doi: 10.1016/j.ygyno.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Feng D.D., Zhang H., Zhang P., Zheng Y.S., Zhang X.J., Han B.W. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15:2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Papageorgiou I., Court M.H. Identification and validation of the microRNA response elements in the 3ʹ-untranslated region of the UDP glucuronosyltransferase (UGT) 2B7 and 2B15 genes by a functional genomics approach. Biochem Pharmacol. 2017;146:199–213. doi: 10.1016/j.bcp.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Papageorgiou I., Court M.H. Identification and validation of microRNAs directly regulating the UDP-glucuronosyltransferase 1A subfamily enzymes by a functional genomics approach. Biochem Pharmacol. 2017;137:93–106. doi: 10.1016/j.bcp.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dluzen D.F., Sun D., Salzberg A.C., Jones N., Bushey R.T., Robertson G.P. Regulation of UDP-glucuronosyltransferase 1A1 expression and activity by microRNA 491-3p. J Pharmacol Exp Ther. 2014;348:465–477. doi: 10.1124/jpet.113.210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wijayakumara D.D., Hu D.G., Meech R., McKinnon R.A., Mackenzie P.I. Regulation of human UGT2B15 and UGT2B17 by miR-376c in prostate cancer cell lines. J Pharmacol Exp Ther. 2015;354:417–425. doi: 10.1124/jpet.115.226118. [DOI] [PubMed] [Google Scholar]
- 118.Tatsumi N., Tokumitsu S., Nakano M., Fukami T., Nakajima M. miR-141-3p commonly regulates human UGT1A isoforms via different mechanisms. Drug Metab Pharmacokinet. 2018;33:203–210. doi: 10.1016/j.dmpk.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 119.Sutliff A.K., Watson C.J., Chen G., Lazarus P. Regulation of UGT2A1 by miR-196a-5p and miR-196b-5p. J Pharmacol Exp Ther. 2019;369:234–243. doi: 10.1124/jpet.118.255935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu D., Green B., Tolleson W.H., Jin Y., Mei N., Guo Y. MicroRNA hsa-miR-29a-3p modulates CYP2C19 in human liver cells. Biochem Pharmacol. 2015;98:215–223. doi: 10.1016/j.bcp.2015.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mencia N., Selga E., Noé V., Ciudad C.J. Underexpression of miR-224 in methotrexate resistant human colon cancer cells. Biochem Pharmacol. 2011;82:1572–1582. doi: 10.1016/j.bcp.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y., Yu D., Tolleson W.H., Yu L.R., Green B., Zeng L. A systematic evaluation of microRNAs in regulating human hepatic CYP2E1. Biochem Pharmacol. 2017;138:174–184. doi: 10.1016/j.bcp.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y., Zeng L., Wang Y., Tolleson W.H., Knox B., Chen S. The expression, induction and pharmacological activity of CYP1A2 are post-transcriptionally regulated by microRNA hsa-miR-132-5p. Biochem Pharmacol. 2017;145:178–191. doi: 10.1016/j.bcp.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zeng L., Chen Y., Wang Y., Yu L.R., Knox B., Chen J. MicroRNA hsa-miR-370-3p suppresses the expression and induction of CYP2D6 by facilitating mRNA degradation. Biochem Pharmacol. 2017;140:139–149. doi: 10.1016/j.bcp.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu D., Wu L., Gill P., Tolleson W.H., Chen S., Sun J. Multiple microRNAs function as self-protective modules in acetaminophen-induced hepatotoxicity in humans. Arch Toxicol. 2018;92:845–858. doi: 10.1007/s00204-017-2090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duan Z., Yu A.M. Bioengineered non-coding RNA agent (BERA) in action. Bioengineered. 2016;7:411–417. doi: 10.1080/21655979.2016.1207011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho P.Y., Yu A.M. Bioengineering of noncoding RNAs for research agents and therapeutics. Wiley Interdiscip Rev RNA. 2016;7:186–197. doi: 10.1002/wrna.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu A.M., Jian C., Yu A.H., Tu M.J. RNA therapy: are we using the right molecules?. Pharmacol Ther. 2019;196:91–104. doi: 10.1016/j.pharmthera.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Q.X., Wang W.P., Zeng S., Urayama S., Yu A.M. A general approach to high-yield biosynthesis of chimeric RNAs bearing various types of functional small RNAs for broad applications. Nucleic Acids Res. 2015;43:3857–3869. doi: 10.1093/nar/gkv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li M.M., Addepalli B., Tu M.J., Chen Q.X., Wang W.P., Limbach P.A. Chimeric microRNA-1291 biosynthesized efficiently in Escherichia coli is effective to reduce target gene expression in human carcinoma cells and improve chemosensitivity. Drug Metab Dispos. 2015;43:1129–1136. doi: 10.1124/dmd.115.064493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ho P.Y., Duan Z., Batra N., Jilek J.L., Tu M.J., Qiu J.X. Bioengineered noncoding RNAs selectively change cellular miRNome profiles for cancer therapy. J Pharmacol Exp Ther. 2018;365:494–506. doi: 10.1124/jpet.118.247775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li P.C., Tu M.J., Ho P.Y., Jilek J.L., Duan Z., Zhang Q.Y. Bioengineered NRF2-siRNA is effective to interfere with NRF2 pathways and improve chemosensitivity of human cancer cells. Drug Metab Dispos. 2018;46:2–10. doi: 10.1124/dmd.117.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang W.P., Ho P.Y., Chen Q.X., Addepalli B., Limbach P.A., Li M.M. Bioengineering novel chimeric microRNA-34a for prodrug cancer therapy: high-yield expression and purification, and structural and functional characterization. J Pharmacol Exp Ther. 2015;354:131–141. doi: 10.1124/jpet.115.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jilek J.L., Tian Y., Yu A.M. Effects of microRNA-34a on the pharmacokinetics of cytochrome P450 probe drugs in mice. Drug Metab Dispos. 2017;45:512–522. doi: 10.1124/dmd.116.074344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao Y., Tu M.J., Yu Y.F., Wang W.P., Chen Q.X., Qiu J.X. Combination therapy with bioengineered miR-34a prodrug and doxorubicin synergistically suppresses osteosarcoma growth. Biochem Pharmacol. 2015;98:602–613. doi: 10.1016/j.bcp.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jian C., Tu M.J., Ho P.Y., Duan Z., Zhang Q., Qiu J.X. Co-targeting of DNA, RNA, and protein molecules provides optimal outcomes for treating osteosarcoma and pulmonary metastasis in spontaneous and experimental metastasis mouse models. Oncotarget. 2017;8:30742–30755. doi: 10.18632/oncotarget.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jilek J.L., Zhang Q.Y., Tu M.J., Ho P.Y., Duan Z., Qiu J.X. Bioengineered Let-7c inhibits orthotopic hepatocellular carcinoma and improves overall survival with minimal immunogenicity. Mol Ther Nucleic Acids. 2019;14:498–508. doi: 10.1016/j.omtn.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tu M.J., Ho P.Y., Zhang Q.Y., Jian C., Qiu J.X., Kim E.J. Bioengineered miRNA-1291 prodrug therapy in pancreatic cancer cells and patient-derived xenograft mouse models. Cancer Lett. 2019;442:82–90. doi: 10.1016/j.canlet.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alegre F., Ormonde A.R., Snider K.M., Woolard K., Yu A.M., Wittenburg L.A. A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen L., Bao Y., Piekos S.C., Zhu K., Zhang L., Zhong X.B. A transcriptional regulatory network containing nuclear receptors and long noncoding RNAs controls basal and drug-Induced expression of cytochrome P450s in hepaRG cells. Mol Pharmacol. 2018;94:749–759. doi: 10.1124/mol.118.112235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsang W.P., Kwok T.T. Riboregulator H19 induction of MDR1-associated drug resistance in human hepatocellular carcinoma cells. Oncogene. 2007;26:4877–4881. doi: 10.1038/sj.onc.1210266. [DOI] [PubMed] [Google Scholar]
- 142.Wang Y., Zhang D., Wu K., Zhao Q., Nie Y., Fan D. Long noncoding RNA MRUL promotes ABCB1 expression in multidrug-resistant gastric cancer cell sublines. Mol Cell Biol. 2014;34:3182–3193. doi: 10.1128/MCB.01580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang J., Ye C., Liu J., Hu Y. UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem Biophys Res Commun. 2018;501:1034–1040. doi: 10.1016/j.bbrc.2018.05.104. [DOI] [PubMed] [Google Scholar]
- 144.Han Z., Shi L. Long non-coding RNA LUCAT1 modulates methotrexate resistance in osteosarcoma via miR-200c/ABCB1 axis. Biochem Biophys Res Commun. 2018;495:947–953. doi: 10.1016/j.bbrc.2017.11.121. [DOI] [PubMed] [Google Scholar]
- 145.Nakano M., Fukami T., Gotoh S., Nakajima M. A-to-I RNA editing up-regulates human dihydrofolate reductase in breast cancer. J Biol Chem. 2017;292:4873–4884. doi: 10.1074/jbc.M117.775684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakano M., Fukami T., Gotoh S., Takamiya M., Aoki Y., Nakajima M. RNA editing modulates human hepatic aryl hydrocarbon receptor expression by creating microRNA recognition sequence. J Biol Chem. 2016;291:894–903. doi: 10.1074/jbc.M115.699363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nozaki K., Nakano M., Iwakami C., Fukami T., Nakajima M. RNA editing enzymes modulate the expression of hepatic CYP2B6, CYP2C8, and other cytochrome P450 isoforms. Drug Metab Dispos. 2019;47:639–647. doi: 10.1124/dmd.119.086702. [DOI] [PubMed] [Google Scholar]
- 148.Kannan B., Nagella A.B., Sathia Prabhu A., Sasidharan G.M., Ramesh A.S., Madhugiri V. Incidence of potential drug–drug interactions in a limited and stereotyped prescription setting-comparison of two free online pharmacopoeias. Cureus. 2016;8:e886. doi: 10.7759/cureus.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Freedman M.D. Drug interactions: classification and systematic approach. Am J Ther. 1995;2:433–443. [PubMed] [Google Scholar]
- 150.U.S. Food and Drug Administration . 2017. In vitro metabolism- and transporter-mediated drug–drug interaction studies guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-metabolism-and-transporter-mediated-drug-drug-interaction-studies-guidance-industry Available from: [Google Scholar]
- 151.U.S. Food and Drug Administration . 2017. Clinical drug interaction studies—study design, data analysis, and clinical implications guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-study-design-data-analysis-and-clinical-implications-guidance Available from: [Google Scholar]
- 152.Min J.S., Bae S.K. Prediction of drug–drug interaction potential using physiologically based pharmacokinetic modeling. Arch Pharm Res. 2017;40:1356–1379. doi: 10.1007/s12272-017-0976-0. [DOI] [PubMed] [Google Scholar]
- 153.Chen X.W., Sneed K.B., Pan S.Y., Cao C., Kanwar J.R., Chew H. Herb–drug interactions and mechanistic and clinical considerations. Curr Drug Metab. 2012;13:640–651. doi: 10.2174/1389200211209050640. [DOI] [PubMed] [Google Scholar]
- 154.Tsai H.H., Lin H.W., Simon Pickard A., Tsai H.Y., Mahady G.B. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: a systematic literature review. Int J Clin Pract. 2012;66:1056–1078. doi: 10.1111/j.1742-1241.2012.03008.x. [DOI] [PubMed] [Google Scholar]
- 155.Ge B., Zhang Z., Zuo Z. Updates on the clinical evidenced herb-warfarin interactions. Evid Based Complement Alternat Med. 2014;2014:957362. doi: 10.1155/2014/957362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lan K., Xie G., Jia W. Towards polypharmacokinetics: pharmacokinetics of multicomponent drugs and herbal medicines using a metabolomics approach. Evid Based Complement Alternat Med. 2013;2013:819147. doi: 10.1155/2013/819147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li C. Multi-compound pharmacokinetic research on Chinese herbal medicines: approach and methodology. China J Chin Mater Med. 2017;42:607–617. doi: 10.19540/j.cnki.cjcmm.2017.0016. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H., Bu F., Li L., Jiao Z., Ma G., Cai W. Prediction of drug–drug interaction between tacrolimus and principal ingredients of wuzhi capsule in Chinese healthy volunteers using physiologically-based pharmacokinetic modelling. Basic Clin Pharmacol. 2018;122:331–340. doi: 10.1111/bcpt.12914. [DOI] [PubMed] [Google Scholar]
- 159.Zhou H., Meibohm B. John Wiley & Sons; New York: 2013. Drug–drug interactions for therapeutic biologics. [Google Scholar]
- 160.Vugmeyster Y. Pharmacokinetics and toxicology of therapeutic proteins: advances and challenges. World J Biol Chem. 2012;3:73–92. doi: 10.4331/wjbc.v3.i4.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dietrich U., Dürr R., Koch J. Peptides as drugs: from screening to application. Curr Pharm Biotechnol. 2013;14:501–512. doi: 10.2174/13892010113149990205. [DOI] [PubMed] [Google Scholar]
- 162.Ferri N., Bellosta S., Baldessin L., Boccia D., Racagni G., Corsini A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol Res. 2016;111:592–599. doi: 10.1016/j.phrs.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 163.Seitz K., Zhou H. Pharmacokinetic drug–drug interaction potentials for therapeutic monoclonal antibodies: reality check. J Clin Pharmacol. 2007;47:1104–1118. doi: 10.1177/0091270007306958. [DOI] [PubMed] [Google Scholar]
- 164.Zhuang Y., Xu Z., Frederick B., de Vries D.E., Ford J.A., Keen M. Golimumab pharmacokinetics after repeated subcutaneous and intravenous administrations in patients with rheumatoid arthritis and the effect of concomitant methotrexate: an open-label, randomized study. Clin Ther. 2012;34:77–90. doi: 10.1016/j.clinthera.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 165.Baert F., Noman M., Vermeire S., Van Assche G., D'Haens G., Carbonez A. Influence of immunogenicity on the long-term efficacy of infliximab in crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 166.Pellegrino P., Perrotta C., Clementi E., Radice S. Vaccine–drug interactions: cytokines, cytochromes, and molecular mechanisms. Drug Saf. 2015;38:781–787. doi: 10.1007/s40264-015-0330-8. [DOI] [PubMed] [Google Scholar]
- 167.Schmitt C., Kuhn B., Zhang X., Kivitz A.J., Grange S. Disease–drug–drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735–740. doi: 10.1038/clpt.2011.35. [DOI] [PubMed] [Google Scholar]
- 168.Raaska K., Raitasuo V., Neuvonen P. Effect of influenza vaccination on serum clozapine and its main metabolite concentrations in patients with schizophrenia. Eur J Clin Pharmacol. 2001;57:705–708. doi: 10.1007/s002280100375. [DOI] [PubMed] [Google Scholar]
- 169.Xu Y., Hijazi Y., Wolf A., Wu B., Sun Y.N., Zhu M. Physiologically based pharmacokinetic model to assess the influence of blinatumomab-mediated cytokine elevations on cytochrome P450 enzyme activity. CPT Pharmacometrics Syst Pharmacol. 2015;4:507–515. doi: 10.1002/psp4.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jiang X., Zhuang Y., Xu Z., Wang W., Zhou H. Development of a physiologically based pharmacokinetic model to predict disease-mediated therapeutic protein–drug interactions: modulation of multiple cytochrome P450 enzymes by interleukin-6. AAPS J. 2016;18:767–776. doi: 10.1208/s12248-016-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yang B.B., Gillespie B., Smith B., Smith W., Lissmats A., Rudebeck M. Pharmacokinetic and pharmacodynamic interactions between palifermin and heparin. J Clin Pharmacol. 2015;55:1109–1118. doi: 10.1002/jcph.516. [DOI] [PubMed] [Google Scholar]
- 172.U.S. Food & Drug Administration. Drugs@FDA: FDA Approved Drug Products. [accessed 2018 Aug 27]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
- 173.Genovese M.C., Cohen S., Moreland L., Lium D., Robbins S., Newmark R. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 2004;50:1412–1419. doi: 10.1002/art.20221. [DOI] [PubMed] [Google Scholar]
- 174.Gingell R., Bridges J.W., Williams R.T. The role of the gut flora in the metabolism of prontosil and neoprontosil in the rat. Xenobiotica. 1971;1:143–156. doi: 10.3109/00498257109044386. [DOI] [PubMed] [Google Scholar]
- 175.Shu Y.Z., Kingston D.G., Van Tassell R.L., Wilkins T.D. Metabolism of levamisole, an anti-colon cancer drug, by human intestinal bacteria. Xenobiotica. 1991;21:737–750. doi: 10.3109/00498259109039513. [DOI] [PubMed] [Google Scholar]
- 176.Bakke O.M. Degradation of dopa by intestinal microorganisms in vitro. Acta Pharmacol Toxicol. 1971;30:115–121. doi: 10.1111/j.1600-0773.1971.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 177.Kim D.H. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab Dispos. 2015;43:1581–1589. doi: 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- 178.Strong H.A., Renwick A.G., George C.F., Liu Y.F., Hill M.J. The reduction of sulphinpyrazone and sulindac by intestinal bacteria. Xenobiotica. 1987;17:685–696. doi: 10.3109/00498258709043976. [DOI] [PubMed] [Google Scholar]
- 179.Gingell R., Bridges J.W. Intestinal azo-reduction and glucuronide conjugation of prontosil. Xenobiotica. 1973;3:599–604. doi: 10.3109/00498257309151548. [DOI] [PubMed] [Google Scholar]
- 180.Zimmermann M., Zimmermann-Kogadeeva M., Wegmann R., Goodman A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science. 2019;363 doi: 10.1126/science.aat9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lehouritis P., Cummins J., Stanton M., Murphy C.T., McCarthy F.O., Reid G. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5:14554. doi: 10.1038/srep14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Routy B., Le Chatelier E., Derosa L., Duong C.P., Alou M.T., Daillère R. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 183.Wilkinson E.M., Ilhan Z.E., Herbst-Kralovetz M.M. Microbiota-drug interactions: impact on metabolism and efficacy of therapeutics. Maturitas. 2018;112:53–63. doi: 10.1016/j.maturitas.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 184.Fecal Microbiota Transplant (FMT) in melanoma patients. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03341143 [accessed 2018 Sep 27].
- 185.The effect of a probiotic strain on aspirin-induced GI damage. (PIP-D). Available from: https://www.clinicaltrials.gov/ct2/show/NCT03228589 [accessed 2018 Sep 27].
- 186.Prevention of irinotecan induced diarrhea by probiotics. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02819960 [accessed 2018 Sep 27].
- 187.Zhang K., Chen D., Ma K., Wu X., Hao H., Jiang S. NAD(P)H: quinone oxidoreductase 1 (NQO1) as a therapeutic and diagnostic target in cancer. J Med Chem. 2018;61:6983–7003. doi: 10.1021/acs.jmedchem.8b00124. [DOI] [PubMed] [Google Scholar]
- 188.Cheng X., Liu F., Liu H., Wang G., Hao H. Enhanced glycometabolism as a mechanism of NQO1 potentiated growth of NSCLC revealed by metabolomic profiling. Biochem Biophys Res Commun. 2018;496:31–36. doi: 10.1016/j.bbrc.2017.12.160. [DOI] [PubMed] [Google Scholar]
- 189.Staudinger J.L. Disease, drug metabolism, and transporter interactions. Pharm Res. 2013;30:2171–2173. doi: 10.1007/s11095-013-1129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu H., Xu X., Yang Z., Deng Y., Liu X., Xie L. Impaired function and expression of P-glycoprotein in blood–brain barrier of streptozotocin-induced diabetic rats. Brain Res. 2006;1123:245–252. doi: 10.1016/j.brainres.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 191.Liu Y.C., Liu H.Y., Yang H.W., Wen T., Shang Y., Liu X.D. Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem Pharmacol. 2007;74:1766–1772. doi: 10.1016/j.bcp.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 192.Mei D., Li J., Liu H., Liu L., Wang X., Guo H. Induction of multidrug resistance-associated protein 2 in liver, intestine and kidney of streptozotocin-induced diabetic rats. Xenobiotica. 2012;42:709–718. doi: 10.3109/00498254.2011.654363. [DOI] [PubMed] [Google Scholar]
- 193.Shu N., Hu M., Ling Z., Liu P., Wang F., Xu P. The enhanced atorvastatin hepatotoxicity in diabetic rats was partly attributed to the upregulated hepatic Cyp3a and SLCO1B1. Sci Rep. 2016;6:33072. doi: 10.1038/srep33072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shu N., Hu M., Liu C., Zhang M., Ling Z., Zhang J. Decreased exposure of atorvastatin in diabetic rats partly due to induction of hepatic Cyp3a and Oatp2. Xenobiotica. 2016;46:875–881. doi: 10.3109/00498254.2016.1141437. [DOI] [PubMed] [Google Scholar]
- 195.Xu D., Li F., Zhang M., Zhang J., Liu C., Hu M.Y. Decreased exposure of simvastatin and simvastatin acid in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2014;35:1215–1225. doi: 10.1038/aps.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kim Y.C., Lee A.K., Lee J.H., Lee I., Lee D.C., Kim S.H. Pharmacokinetics of theophylline in diabetes mellitus rats: induction of CYP1A2 and CYP2E1 on 1, 3-dimethyluric acid formation. Eur J Pharm Sci. 2005;26:114–123. doi: 10.1016/j.ejps.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 197.Liu H., Liu L., Li J., Mei D., Duan R., Hu N. Combined contributions of impaired hepatic CYP2C11 and intestinal breast cancer resistance protein activities and expression to increased oral glibenclamide exposure in rats with streptozotocin-induced diabetes mellitus. Drug Metab Dispos. 2012;40:1104–1112. doi: 10.1124/dmd.111.043513. [DOI] [PubMed] [Google Scholar]
- 198.Hu N., Hu M., Duan R., Liu C., Guo H., Zhang M. Increased levels of fatty acids contributed to induction of hepatic CYP3A4 activity induced by diabetes—in vitro evidence from HepG2 cell and Fa2N-4 cell lines. J Pharmacol Sci. 2014;124:433–444. doi: 10.1254/jphs.13212fp. [DOI] [PubMed] [Google Scholar]
- 199.Xie H., Sun S., Cheng X., Yan T., Zheng X., Li F. Dysregulations of intestinal and colonic UDP-glucuronosyltransferases in rats with type 2 diabetes. Drug Metab Pharmacokinet. 2013;28:427–434. doi: 10.2133/dmpk.dmpk-13-rg-020. [DOI] [PubMed] [Google Scholar]
- 200.Li P., Lu Q., Jiang W., Pei X., Sun Y., Hao H. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed Pharmacother. 2017;91:425–435. doi: 10.1016/j.biopha.2017.04.109. [DOI] [PubMed] [Google Scholar]
- 201.Liu L., Miao M., Chen Y., Wang Z., Sun B., Liu X. Altered function and expression of ABC transporters at the blood–brain barrier and increased brain distribution of phenobarbital in acute liver failure mice. Front Pharmacol. 2018;9:190. doi: 10.3389/fphar.2018.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang F., Miao M.X., Sun B.B., Wang Z.J., Tang X.G., Chen Y. Acute liver failure enhances oral plasma exposure of zidovudine in rats by downregulation of hepatic UGT2B7 and intestinal P-gp. Acta Pharmacol Sin. 2017;38:1554–1565. doi: 10.1038/aps.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Frye R.F., Schneider V.M., Frye C.S., Feldman A.M. Plasma levels of TNF-α and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8:315–319. doi: 10.1054/jcaf.2002.127773. [DOI] [PubMed] [Google Scholar]
- 204.Dowling T.C., Briglia A.E., Fink J.C., Hanes D.S., Light P.D., Stackiewicz L. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–434. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 205.Dreisbach A.W., Japa S., Gebrekal A.B., Mowry S.E., Lertora J.J., Kamath B.L. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003;73:475–477. doi: 10.1016/s0009-9236(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 206.Al Za'abi M., Shalaby A., Manoj P., Ali B.H. The in vivo effects of adenine-induced chronic kidney disease on some renal and hepatic function and CYP450 metabolizing enzymes. Physiol Res. 2017;66:263–271. doi: 10.33549/physiolres.933374. [DOI] [PubMed] [Google Scholar]
- 207.Sukkummee W., Jittisak P., Wonganan P., Wittayalertpanya S., Chariyavilaskul P., Leelahavanichkul A. The prominent impairment of liver/intestinal cytochrome P450 and intestinal drug transporters in sepsis-induced acute kidney injury over acute and chronic renal ischemia, a mouse model comparison. Ren Fail. 2019;41:314–325. doi: 10.1080/0886022X.2019.1602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lee S.H., Lee S.M. Suppression of hepatic cytochrome p450-mediated drug metabolism during the late stage of sepsis in rats. Shock. 2005;23:144–149. doi: 10.1097/01.shk.0000150778.39484.54. [DOI] [PubMed] [Google Scholar]
- 209.Fox C.S., Golden S.H., Anderson C., Bray G.A., Burke L.E., de Boer I.H. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2015;38:1777–1803. doi: 10.2337/dci15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu L., Liu X.D. Alterations in function and expression of ABC transporters at blood–brain barrier under diabetes and the clinical significances. Front Pharmacol. 2014;5:273. doi: 10.3389/fphar.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Barnett C.R., Gibson G.G., Wolf C.R., Flatt P.R., Ioannides C. Induction of cytochrome P450III and P450IV family proteins in streptozotocin–induced diabetes. Biochem J. 1990;268:765–769. doi: 10.1042/bj2680765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hu N., Xie S., Liu L., Wang X., Pan X., Chen G. Opposite effect of diabetes mellitus induced by streptozotocin on oral and intravenous pharmacokinetics of verapamil in rats. Drug Metab Dispos. 2011;39:419–425. doi: 10.1124/dmd.110.035642. [DOI] [PubMed] [Google Scholar]
- 213.Guo Y., Hu B., Xie Y., Billiar T.R., Sperry J.L., Huang M. Regulation of drug-metabolizing enzymes by local and systemic liver injuries. Expert Opin Drug Metab Toxicol. 2016;12:245–251. doi: 10.1517/17425255.2016.1139574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Y., Zhang J., Xu P., Sun B., Zhong Z., Liu C. Acute liver failure impairs function and expression of breast cancer-resistant protein (BCRP) at rat blood–brain barrier partly via ammonia-ROS-ERK1/2 activation. J Neurochem. 2016;138:282–294. doi: 10.1111/jnc.13666. [DOI] [PubMed] [Google Scholar]
- 215.Zhang L., Chu X., Wang H., Xie H., Guo C., Cao L. Dysregulations of UDP-glucuronosyltransferases in rats with valproic acid and high fat diet induced fatty liver. Eur J Pharmacol. 2013;721:277–285. doi: 10.1016/j.ejphar.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 216.Zhang K., Young C., Berger J. Administrative claims analysis of the relationship between warfarin use and risk of hemorrhage including drug–drug and drug–disease interactions. J Manag Care Pharm. 2006;12:640–648. doi: 10.18553/jmcp.2006.12.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Aspromonte N., Monitillo F., Puzzovivo A., Valle R., Caldarola P., Iacoviello M. Modulation of cardiac cytochrome P450 in patients with heart failure. Expert Opin Drug Metab Toxicol. 2014;10:327–339. doi: 10.1517/17425255.2014.872240. [DOI] [PubMed] [Google Scholar]
- 218.Tan F.L., Moravec C.S., Li J., Apperson-Hansen C., McCarthy P.M., Young J.B. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.El-Kadi A.O., Zordoky B.N. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metab. 2008;9:122–128. doi: 10.2174/138920008783571792. [DOI] [PubMed] [Google Scholar]
- 220.Lanchote V.L., Ping W.C., Santos S.R. Influence of renal failure on cytochrome P450 activity in hypertensive patients using a “cocktail” of antipyrine and nifedipine. Eur J Clin Pharmacol. 1996;50:83–89. doi: 10.1007/s002280050073. [DOI] [PubMed] [Google Scholar]
- 221.Guévin C., Michaud J., Naud J., Leblond F.A., Pichette V. Down-regulation of hepatic cytochrome p450 in chronic renal failure: role of uremic mediators. Br J Pharmacol. 2002;137:1039–1046. doi: 10.1038/sj.bjp.0704951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jacob A., Zhou M., Wu R., Wang P. The role of hepatic cytochrome P-450 in sepsis. Int J Clin Exp Med. 2009;2:203–211. [PMC free article] [PubMed] [Google Scholar]
- 223.Park S.W., Lee S.M. The beneficial effect of Trolox on sepsis-induced hepatic drug metabolizing dysfunction. Arch Pharm Res. 2004;27:232–238. doi: 10.1007/BF02980111. [DOI] [PubMed] [Google Scholar]
- 224.Crawford J.H., Yang S., Zhou M., Simms H.H., Wang P. Down-regulation of hepatic CYP1A2 plays an important role in inflammatory responses in sepsis. Crit Care Med. 2004;32:502–508. doi: 10.1097/01.CCM.0000109453.57709.E2. [DOI] [PubMed] [Google Scholar]
- 225.Eum H.A., Yeom D.H., Lee S.M. Role of nitric oxide in the inhibition of liver cytochrome P450 during sepsis. Nitric Oxide. 2006;15:423–431. doi: 10.1016/j.niox.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 226.Zhou M., Maitra S.R., Wang P. The potential role of transcription factor aryl hydrocarbon receptor in downregulation of hepatic cytochrome P-450 during sepsis. Int J Mol Med. 2008;21:423–428. [PMC free article] [PubMed] [Google Scholar]
- 227.Martin G.S. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10:701–706. doi: 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pea F. Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr Clin Pharmacol. 2013;8:5–12. [PubMed] [Google Scholar]
- 229.Roberts J.A., Abdul-Aziz M.H., Lipman J., Mouton J.W., Vinks A.A., Felton T.W. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ulldemolins M., Roberts J.A., Rello J., Paterson D.L., Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet. 2011;50:99–110. doi: 10.2165/11539220-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 231.Ito R., Takahashi T., Katano I., Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ono C., Hsyu P.H., Abbas R., Loi C.M., Yamazaki S. Application of physiologically based pharmacokinetic modeling to the understanding of bosutinib pharmacokinetics: prediction of drug–drug and drug–disease interactions. Drug Metab Dispos. 2017;45:390–398. doi: 10.1124/dmd.116.074450. [DOI] [PubMed] [Google Scholar]
- 233.Xu R., Ge W., Jiang Q. Application of physiologically based pharmacokinetic modeling to the prediction of drug–drug and drug–disease interactions for rivaroxaban. Eur J Clin Pharmacol. 2018;74:755–765. doi: 10.1007/s00228-018-2430-8. [DOI] [PubMed] [Google Scholar]
- 234.Gao J., Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He J., Gao J., Xu M., Ren S., Stefanovic-Racic M., O'Doherty R.M. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 2013;62:1876–1887. doi: 10.2337/db12-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.He J., Nishida S., Xu M., Makishima M., Xie W. PXR prevents cholesterol gallstone disease by regulating biosynthesis and transport of bile salts. Gastroenterology. 2011;140:2095–2106. doi: 10.1053/j.gastro.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zeng H., Li D., Qin X., Chen P., Tan H., Zeng X. Hepatoprotective effects of Schisandra sphenanthera extract against lithocholic acid-induced cholestasis in male mice are associated with activation of the pregnane X receptor pathway and promotion of liver regeneration. Drug Metab Dispos. 2016;44:337–342. doi: 10.1124/dmd.115.066969. [DOI] [PubMed] [Google Scholar]
- 238.Jiang Y., Feng D., Ma X., Fan S., Gao Y., Fu K. Pregnane X receptor regulates liver size and liver cell fate by yes-associated protein activation in mice. Hepatology. 2019;69:343–358. doi: 10.1002/hep.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhai Y., Pai H.V., Zhou J., Amico J.A., Vollmer R.R., Xie W. Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol Endocrinol. 2007;21:138–147. doi: 10.1210/me.2006-0291. [DOI] [PubMed] [Google Scholar]
- 240.Jiang M., Xie W. Role of the constitutive androstane receptor in obesity and type 2 diabetes: a case study of the endobiotic function of a xenobiotic receptor. Drug Metab Rev. 2013;45:156–163. doi: 10.3109/03602532.2012.743561. [DOI] [PubMed] [Google Scholar]
- 241.Gao J., Yan J., Xu M., Ren S., Xie W. CAR Suppresses hepatic gluconeogenesis by facilitating the ubiquitination and degradation of PGC1α. Mol Endocrinol. 2015;29:1558–1570. doi: 10.1210/me.2015-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.He J., Lee J.H., Febbraio M., Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood) 2011;236:1116–1121. doi: 10.1258/ebm.2011.011128. [DOI] [PubMed] [Google Scholar]
- 243.He J., Hu B., Shi X., Weidert E.R., Lu P., Xu M. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol. 2013;33:2047–2055. doi: 10.1128/MCB.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gong H., He J., Lee J.H., Mallick E., Gao X., Li S. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J Biol Chem. 2009;284:30113–30121. doi: 10.1074/jbc.M109.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhao Z., Xu D., Li S., He B., Huang Y., Xu M. Activation of liver X receptor attenuates oleic acid-induced acute respiratory distress syndrome. Am J Pathol. 2016;186:2614–2622. doi: 10.1016/j.ajpath.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang H., He Q., Wang G., Xu X., Hao H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018;28:765–782. doi: 10.1080/13543776.2018.1527906. [DOI] [PubMed] [Google Scholar]
- 247.Niu Y., Xu M., Slagle B.L., Huang H., Li S., Guo G.L. Farnesoid X receptor ablation sensitizes mice to hepatitis b virus X protein-induced hepatocarcinogenesis. Hepatology. 2017;65:893–906. doi: 10.1002/hep.28924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen P., Li J., Fan X., Zeng H., Deng R., Li D. Oleanolic acid attenuates obstructive cholestasis in bile duct-ligated mice, possibly via activation of NRF2-MRPs and FXR antagonism. Eur J Pharmacol. 2015;765:131–139. doi: 10.1016/j.ejphar.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 249.Riviere J.E., Gabrielsson J., Fink M., Mochel J. Mathematical modeling and simulation in animal health. Part I: moving beyond pharmacokinetics. J Vet Pharmacol Ther. 2016;39:213–223. doi: 10.1111/jvp.12278. [DOI] [PubMed] [Google Scholar]
- 250.Satoh D., Abe S., Kobayashi K., Nakajima Y., Oshimura M., Kazuki Y. Human and mouse artificial chromosome technologies for studies of pharmacokinetics and toxicokinetics. Drug Metab Pharmacokinet. 2018;33:17–30. doi: 10.1016/j.dmpk.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 251.Teorell T. Kinetics of distribution of substances administered to the body. I. The extra-vascular modes of administration. Arch Int Pharmacodyn Ther. 1937;57:205–225. [Google Scholar]
- 252.Harrison L.I., Gibaldi M. Physiologically based pharmacokinetic model for digoxin disposition in dogs and its preliminary application to humans. J Pharm Sci. 1977;66:1679–1683. doi: 10.1002/jps.2600661206. [DOI] [PubMed] [Google Scholar]
- 253.Meeting of the Pharmaceutical Science and Clinical Pharmacology Advisory Committee. 2012.
- 254.FDA . 2018. Physiologically based pharmacokinetic analyses-format and content guidance for industry.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/physiologically-based-pharmacokinetic-analyses-format-and-content-guidance-industry Available from: [Google Scholar]
- 255.EMA . 2016. Guideline on the qualification and reporting of physiologically based pharmacokinetic (PBPK) modelling and simulation.https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-physiologically-based-pharmacokinetic-pbpk-modelling-simulation_en.pdf Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhao P. FDA; Washington, DC: 2017. Towards consistent regulatory assessment of physiologically-based pharmacokinetic modeling to support dosing recommendations. [Google Scholar]
- 257.Liu D., Wang K., Ma G., Xiang X., Liu J., Zhao P. The value and general consideration of pharmacometric study in new drug development. Chin J Clin Pharmacol Ther. 2018;23:961–973. [Google Scholar]
- 258.Rowland M., Peck C., Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. doi: 10.1146/annurev-pharmtox-010510-100540. [DOI] [PubMed] [Google Scholar]
- 259.Hsueh C.H., Hsu V., Pan Y., Zhao P. Predictive performance of physiologically-based pharmacokinetic models in predicting drug–drug interactions involving enzyme modulation. Clin Pharmacokinet. 2018;57:1337–1346. doi: 10.1007/s40262-018-0635-8. [DOI] [PubMed] [Google Scholar]
- 260.Zhang L., Huang S.M., Lesko L.J. Transporter-mediated drug–drug interactions. Clin Pharmacol Ther. 2011;89:481–484. doi: 10.1038/clpt.2010.359. [DOI] [PubMed] [Google Scholar]
- 261.Aghazadeh-Habashi A., Asghar W., Jamali F. Drug–disease interaction: effect of inflammation and nonsteroidal anti-inflammatory drugs on cytochrome p450 metabolites of arachidonic acid. J Pharm Sci. 2018;107:756–763. doi: 10.1016/j.xphs.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 262.Tan M.L., Yoshida K., Zhao P., Zhang L., Nolin T.D., Piquette-Miller M. Effect of chronic kidney disease on nonrenal elimination pathways: a systematic assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP. Clin Pharmacol Ther. 2018;103:854–867. doi: 10.1002/cpt.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yoshida K., Budha N., Jin J.Y. Impact of physiologically based pharmacokinetic models on regulatory reviews and product labels: frequent utilization in the field of oncology. Clin Pharmacol Ther. 2017;101:597–602. doi: 10.1002/cpt.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wagner C., Pan Y., Hsu V., Grillo J.A., Zhang L., Reynolds K.S. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin Pharmacokinet. 2015;54:117–127. doi: 10.1007/s40262-014-0188-4. [DOI] [PubMed] [Google Scholar]
- 265.Wagner C., Pan Y., Hsu V., Sinha V., Zhao P. Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin Pharmacokinet. 2016;55:475–483. doi: 10.1007/s40262-015-0330-y. [DOI] [PubMed] [Google Scholar]
- 266.Wagner C., Zhao P., Pan Y., Hsu V., Grillo J., Huang S.M. Application of physiologically based pharmacokinetic (PBPK) modeling to support dose selection: report of an FDA public workshop on PBPK. CPT Pharmacometrics Syst Pharmacol. 2015;4:226–230. doi: 10.1002/psp4.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Guo Y., Chu X., Parrott N.J., Brouwer K.L., Hsu V., Nagar S. Advancing predictions of tissue and intracellular drug concentrations using in vitro, imaging and physiologically based pharmacokinetic modeling approaches. Clin Pharmacol Ther. 2018;104:865–889. doi: 10.1002/cpt.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pan Y., Hsu V., Grimstein M., Zhang L., Arya V., Sinha V. The application of physiologically based pharmacokinetic modeling to predict the role of drug transporters: scientific and regulatory perspectives. J Clin Pharmacol. 2016;56(Suppl 7):S122–S131. doi: 10.1002/jcph.740. [DOI] [PubMed] [Google Scholar]
- 269.Nguyen T.V., Ukairo O., Khetani S.R., McVay M., Kanchagar C., Seghezzi W. Establishment of a hepatocyte-kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters. Drug Metab Dispos. 2015;43:774–785. doi: 10.1124/dmd.114.061317. [DOI] [PubMed] [Google Scholar]
- 270.Yoshida K., Sun B., Zhang L., Zhao P., Abernethy D.R., Nolin T.D. Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5. Clin Pharmacol Ther. 2016;100:75–87. doi: 10.1002/cpt.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Feng B., Varma M.V. Evaluation and quantitative prediction of renal transporter-mediated drug–drug interactions. J Clin Pharmacol. 2016;56(Suppl 7):S110–S121. doi: 10.1002/jcph.702. [DOI] [PubMed] [Google Scholar]
- 272.Hsu V., de L T Vieira M., Zhao P., Zhang L., Zheng J.H., Nordmark A. Towards quantitation of the effects of renal impairment and probenecid inhibition on kidney uptake and efflux transporters, using physiologically based pharmacokinetic modelling and simulations. Clin Pharmacokinet. 2014;53:283–293. doi: 10.1007/s40262-013-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Edginton A.N., Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47:743–752. doi: 10.2165/00003088-200847110-00005. [DOI] [PubMed] [Google Scholar]
- 274.Schlender J.F., Meyer M., Thelen K., Krauss M., Willmann S., Eissing T. Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet. 2016;55:1573–1589. doi: 10.1007/s40262-016-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yellepeddi V., Rower J., Liu X., Kumar S., Rashid J., Sherwin C.M. State-of-the-art review on physiologically based pharmacokinetic modeling in pediatric drug development. Clin Pharmacokinet. 2019;58:1–13. doi: 10.1007/s40262-018-0677-y. [DOI] [PubMed] [Google Scholar]
- 276.Abduljalil K., Jamei M., Johnson T.N. Fetal physiologically based pharmacokinetic models: systems information on the growth and composition of fetal organs. Clin Pharmacokinet. 2019;58:235–262. doi: 10.1007/s40262-018-0685-y. [DOI] [PubMed] [Google Scholar]
- 277.Xia B., Heimbach T., Gollen R., Nanavati C., He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15:1012–1024. doi: 10.1208/s12248-013-9505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lin L., Wong H. Predicting Oral Drug Absorption: mini review on physiologically-based pharmacokinetic models. Pharmaceutics. 2017;9:41. doi: 10.3390/pharmaceutics9040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mitra A. Maximizing the role of physiologically based oral absorption modeling in generic drug development. Clin Pharmacol Ther. 2019;105:307–309. doi: 10.1002/cpt.1242. [DOI] [PubMed] [Google Scholar]
- 280.Suarez-Sharp S., Cohen M., Kesisoglou F., Abend A., Marroum P., Delvadia P. Applications of clinically relevant dissolution testing: workshop summary report. AAPS J. 2018;20:93. doi: 10.1208/s12248-018-0252-3. [DOI] [PubMed] [Google Scholar]
- 281.Tsume Y., Patel S., Fotaki N., Bergstrӧm C., Amidon G.L., Brasseur J.G. In vivo predictive dissolution and simulation workshop report: facilitating the development of oral drug formulation and the prediction of oral bioperformance. AAPS J. 2018;20:100. doi: 10.1208/s12248-018-0260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Fang L., Kim M.J., Li Z., Wang Y., Diliberti C.E., Au J. Model-informed drug development and review for generic products: summary of FDA public workshop. Clin Pharmacol Ther. 2018;104:27–30. doi: 10.1002/cpt.1065. [DOI] [PubMed] [Google Scholar]
- 283.Kesisoglou F., Mitra A. Application of absorption modeling in rational design of drug product under quality-by-design paradigm. AAPS J. 2015;17:1224–1236. doi: 10.1208/s12248-015-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Babiskin A.H., Zhang X. Application of physiologically based absorption modeling for amphetamine salts drug products in generic drug evaluation. J Pharm Sci. 2015;104:3170–3182. doi: 10.1002/jps.24474. [DOI] [PubMed] [Google Scholar]
- 285.Margolskee A., Darwich A.S., Pepin X., Aarons L., Galetin A., Rostami-Hodjegan A. IMI – oral biopharmaceutics tools project-evaluation of bottom-up PBPK prediction success part 2: an introduction to the simulation exercise and overview of results. Eur J Pharm Sci. 2017;96:610–625. doi: 10.1016/j.ejps.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 286.Vuppugalla R., Marathe P., He H., Jones R.D., Yates J.W., Jones H.M. PhRMA CPCDC initiative on predictive models of human pharmacokinetics, part 4: prediction of plasma concentration-time profiles in human from in vivo preclinical data by using the Wajima approach. J Pharm Sci. 2011;100:4111–4126. doi: 10.1002/jps.22551. [DOI] [PubMed] [Google Scholar]
- 287.Cao Y., Jusko W.J. Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2012;39:711–723. doi: 10.1007/s10928-012-9280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Cao Y., Jusko W.J. Survey of monoclonal antibody disposition in man utilizing a minimal physiologically-based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2014;41:571–580. doi: 10.1007/s10928-014-9374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Wong H., Chow T.W. Physiologically based pharmacokinetic modeling of therapeutic proteins. J Pharm Sci. 2017;106:2270–2275. doi: 10.1016/j.xphs.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 290.Niederalt C., Kuepfer L., Solodenko J., Eissing T., Siegmund H.U., Block M. A generic whole body physiologically based pharmacokinetic model for therapeutic proteins in PK-Sim. J Pharmacokinet Pharmacodyn. 2018;45:235–257. doi: 10.1007/s10928-017-9559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Liu D., Song H., Song L., Liu Y., Cao Y., Jiang J. A unified strategy in selection of the best allometric scaling methods to predict human clearance based on drug disposition pathway. Xenobiotica. 2016;46:1105–1111. doi: 10.1080/00498254.2016.1205761. [DOI] [PubMed] [Google Scholar]
- 292.Liu D., Ma X., Liu Y., Zhou H., Shi C., Wu F. Quantitative prediction of human pharmacokinetics and pharmacodynamics of imigliptin, a novel DPP-4 inhibitor, using allometric scaling, IVIVE and PK/PD modeling methods. Eur J Pharm Sci. 2016;89:73–82. doi: 10.1016/j.ejps.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 293.Song L., Zhang Y., Jiang J., Ren S., Chen L., Liu D. Development of a Physiologically based pharmacokinetic model for sinogliatin, a first-in-class glucokinase activator, by integrating allometric scaling, in vitro to in vivo exploration and steady-state concentration-mean residence time methods: mechanistic understanding of its pharmacokinetics. Clin Pharmacokinet. 2018;57:1307–1323. doi: 10.1007/s40262-018-0631-z. [DOI] [PubMed] [Google Scholar]
- 294.Rose R.H., Neuhoff S., Abduljalil K., Chetty M., Rostami-Hodjegan A., Jamei M. Application of a physiologically based pharmacokinetic model to predict OATP1B1-related variability in pharmacodynamics of rosuvastatin. CPT Pharmacometrics Syst Pharmacol. 2014;3:e124. doi: 10.1038/psp.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Chen Y., Zhao K., Liu F., Li Y., Zhong Z., Hong S. Predicting antitumor effect of deoxypodophyllotoxin in NCI-H460 tumor-bearing mice on the basis of in vitro pharmacodynamics and a physiologically based pharmacokinetic-pharmacodynamic model. Drug Metab Dispos. 2018;46:897–907. doi: 10.1124/dmd.117.079830. [DOI] [PubMed] [Google Scholar]
- 296.Feng S., Shi J., Parrott N., Hu P., Weber C., Martin-Facklam M. Combining ‘bottom-up’ and ‘top-down’ methods to assess ethnic difference in clearance: bitopertin as an example. Clin Pharmacokinet. 2016;55:823–832. doi: 10.1007/s40262-015-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jorga K., Chavanne C., Frey N., Lave T., Lukacova V., Parrott N. Bottom-up meets top-down: complementary physiologically based pharmacokinetic and population pharmacokinetic modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. Clin Pharmacol Ther. 2016;100:761–769. doi: 10.1002/cpt.449. [DOI] [PubMed] [Google Scholar]
- 298.Gufford B.T., Barr J.T., González-Pérez V., Layton M.E., White J.R., Jr., Oberlies N.H. Quantitative prediction and clinical evaluation of an unexplored herb–drug interaction mechanism in healthy volunteers. CPT Pharmacometrics Syst Pharmacol. 2015;4:701–710. doi: 10.1002/psp4.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Capecchi M.R. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- 300.Gaj T., Gersbach C.A., Barbas C.F., III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Qiu Z., Liu M., Chen Z., Shao Y., Pan H., Wei G. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic Acids Res. 2013;41 doi: 10.1093/nar/gkt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Diliberto J.J., Burgin D., Birnbaum L.S. Role of CYP1A2 in hepatic sequestration of dioxin: studies using CYP1A2 knock-out mice. Biochem Biophys Res Commun. 1997;236:431–433. doi: 10.1006/bbrc.1997.6973. [DOI] [PubMed] [Google Scholar]
- 303.Lu Y., Wu D., Wang X., Ward S.C., Cederbaum A.I. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med. 2010;49:1406–1416. doi: 10.1016/j.freeradbiomed.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Scheer N., Kapelyukh Y., Chatham L., Rode A., Buechel S., Wolf C.R. Generation and characterization of novel cytochrome P450 Cyp2c gene cluster knockout and CYP2C9 humanized mouse lines. Mol Pharmacol. 2012;82:1022–1029. doi: 10.1124/mol.112.080036. [DOI] [PubMed] [Google Scholar]
- 305.van Waterschoot R.A., van Herwaarden A.E., Lagas J.S., Sparidans R.W., Wagenaar E., van der Kruijssen C.M. Midazolam metabolism in cytochrome P450 3A knockout mice can be attributed to up-regulated CYP2C enzymes. Mol Pharmacol. 2008;73:1029–1036. doi: 10.1124/mol.107.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Dragin N., Uno S., Wang B., Dalton T.P., Nebert D.W. Generation of ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2–/– mouse line. Biochem Biophys Res Commun. 2007;359:635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Löfgren S., Baldwin R.M., Hiratsuka M., Lindqvist A., Carlberg A., Sim S.C. Generation of mice transgenic for human CYP2C18 and CYP2C19: characterization of the sexually dimorphic gene and enzyme expression. Drug Metab Dispos. 2008;36:955–962. doi: 10.1124/dmd.107.019349. [DOI] [PubMed] [Google Scholar]
- 308.Hasegawa M., Kapelyukh Y., Tahara H., Seibler J., Rode A., Krueger S. Quantitative prediction of human pregnane X receptor and cytochrome P450 3A4 mediated drug–drug interaction in a novel multiple humanized mouse line. Mol Pharmacol. 2011;80:518–528. doi: 10.1124/mol.111.071845. [DOI] [PubMed] [Google Scholar]
- 309.Scheer N., Kapelyukh Y., McEwan J., Beuger V., Stanley L.A., Rode A. Modeling human cytochrome P450 2D6 metabolism and drug–drug interaction by a novel panel of knockout and humanized mouse lines. Mol Pharmacol. 2012;81:63–72. doi: 10.1124/mol.111.075192. [DOI] [PubMed] [Google Scholar]
- 310.Liu Z., Li L., Wu H., Hu J., Ma J., Zhang Q.Y. Characterization of CYP2B6 in a CYP2B6-humanized mouse model: inducibility in the liver by phenobarbital and dexamethasone and role in nicotine metabolism in vivo. Drug Metab Dispos. 2015;43:208–216. doi: 10.1124/dmd.114.061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Zanger U.M., Klein K., Thomas M., Rieger J.K., Tremmel R., Kandel B.A. Genetics, epigenetics, and regulation of drug-metabolizing cytochrome p450 enzymes. Clin Pharmacol Ther. 2014;95:258–261. doi: 10.1038/clpt.2013.220. [DOI] [PubMed] [Google Scholar]
- 312.Aitman T.J., Critser J.K., Cuppen E., Dominiczak A., Fernandez-Suarez X.M., Flint J. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40:516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- 313.Yoshimi K., Kunihiro Y., Kaneko T., Nagahora H., Voigt B., Mashimo T. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat Commun. 2016;7:10431. doi: 10.1038/ncomms10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wang X., Tang Y., Lu J., Shao Y., Qin X., Li Y. Characterization of novel cytochrome P450 2E1 knockout rat model generated by CRISPR/Cas9. Biochem Pharmacol. 2016;105:80–90. doi: 10.1016/j.bcp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 315.Lu J., Shao Y., Qin X., Liu D., Chen A., Li D. CRISPR knockout rat cytochrome P450 3A1/2 model for advancing drug metabolism and pharmacokinetics research. Sci Rep. 2017;7:42922. doi: 10.1038/srep42922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lu J., Liu M., Wang X. Gene targeting on Cyp2c locus in rats using the CRISPR/Cas9 system. In: Wang X., editor. CRISPR: advances in research and applications. NOVA Science Publishers, Inc; New York: 2017. pp. 95–111. [Google Scholar]
- 317.Wei Y., Yang L., Zhang X., Sui D., Wang C., Wang K. Generation and characterization of a CYP2C11-null rat model by using the CRISPR/Cas9 method. Drug Metab Dispos. 2018;46:525–531. doi: 10.1124/dmd.117.078444. [DOI] [PubMed] [Google Scholar]
- 318.Kumar R., Mota L.C., Litoff E.J., Rooney J.P., Boswell W.T., Courter E. Compensatory changes in CYP expression in three different toxicology mouse models: cAR-null, Cyp3a-null, and Cyp2b9/10/13-null mice. PLoS One. 2017;12 doi: 10.1371/journal.pone.0174355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Liang C., Zhao J., Lu J., Zhang Y., Ma X., Shang X. Development and characterization of MDR1 (Mdr1a/b) CRISPR/Cas9 knockout rat model. Drug Metab Dispos. 2019;47:71–79. doi: 10.1124/dmd.118.084277. [DOI] [PubMed] [Google Scholar]
- 320.Qin X., Lu J., Wang P., Xu P., Liu M., Wang X. Cytochrome P450 3A selectively affects the pharmacokinetic interaction between erlotinib and docetaxel in rats. Biochem Pharmacol. 2017;143:129–139. doi: 10.1016/j.bcp.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 321.Schneider K.J., DeCaprio A.P. Covalent thiol adducts arising from reactive intermediates of cocaine biotransformation. Chem Res Toxicol. 2013;26:1755–1764. doi: 10.1021/tx4003116. [DOI] [PubMed] [Google Scholar]
- 322.Lai W.G., Farah N., Moniz G.A., Wong Y.N. A Baeyer-Villiger oxidation specifically catalyzed by human flavin-containing monooxygenase 5. Drug Metab Dispos. 2011;39:61–70. doi: 10.1124/dmd.110.035360. [DOI] [PubMed] [Google Scholar]
- 323.Meng J., Zhong D., Li L., Yuan Z., Yuan H., Xie C. Metabolism of MRX-I, a novel antibacterial oxazolidinone, in humans: the oxidative ring opening of 2, 3-dihydropyridin-4-one catalyzed by non-P450 enzymes. Drug Metab Dispos. 2015;43:646–659. doi: 10.1124/dmd.114.061747. [DOI] [PubMed] [Google Scholar]
- 324.Aigrain L., Pompon D., Truan G. Role of the interface between the FMN and FAD domains in the control of redox potential and electronic transfer of NADPH-cytochrome P450 reductase. Biochem J. 2011;435:197–206. doi: 10.1042/BJ20101984. [DOI] [PubMed] [Google Scholar]
- 325.Hou X., Zhou J., Yu S., Zhou L., Zhang Y., Zhong D. Differences in the in vivo and in vitro metabolism of imrecoxib in humans: formation of the rate-limiting aldehyde intermediate. Drug Metab Dispos. 2018;46:1320–1328. doi: 10.1124/dmd.118.081182. [DOI] [PubMed] [Google Scholar]
- 326.Fukami T., Yokoi T. The emerging role of human esterases. Drug Metab Pharmacokinet. 2012;27:466–477. doi: 10.2133/dmpk.dmpk-12-rv-042. [DOI] [PubMed] [Google Scholar]
- 327.Kurokawa T., Fukami T., Yoshida T., Nakajima M. Arylacetamide deacetylase is responsible for activation of prasugrel in human and dog. Drug Metab Dispos. 2016;44:409–416. doi: 10.1124/dmd.115.068221. [DOI] [PubMed] [Google Scholar]
- 328.Jiang J., Chen X., Zhong D. Arylacetamide deacetylase is involved in vicagrel bioactivation in humans. Front Pharmacol. 2017;8:846. doi: 10.3389/fphar.2017.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Liu C., Chen X.Y., Zhong D. Metabolism and pharmacokinetics of vicagrel, a novel thienopyridine P2y12 inhibitor, compared with clopidogrel in healthy Chinese subjects. Drug Metab Pharmacokinet. 2017;32:S93–S94. [Google Scholar]
- 330.Fleming F.F., Yao L., Ravikumar P.C., Funk L., Shook B.C. Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. J Med Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kong F., Pang X., Zhao J., Deng P., Zheng M., Zhong D. Hydrolytic metabolism of cyanopyrrolidine DPP-4 inhibitors mediated by dipeptidyl peptidases. Drug Metab Dispos. 2019;47:238–248. doi: 10.1124/dmd.118.084640. [DOI] [PubMed] [Google Scholar]
- 332.Gao R., Li L., Xie C., Diao X., Zhong D., Chen X. Metabolism and pharmacokinetics of morinidazole in humans: identification of diastereoisomeric morpholine N+-glucuronides catalyzed by UDP glucuronosyltransferase 1A9. Drug Metab Dispos. 2012;40:556–567. doi: 10.1124/dmd.111.042689. [DOI] [PubMed] [Google Scholar]
- 333.Zhou D., Guo J., Linnenbach A.J., Booth-Genthe C.L., Grimm S.W. Role of human UGT2B10 in N-glucuronidation of tricyclic antidepressants, amitriptyline, imipramine, clomipramine, and trimipramine. Drug Metab Dispos. 2010;38:863–870. doi: 10.1124/dmd.109.030981. [DOI] [PubMed] [Google Scholar]
- 334.Kaivosaari S., Finel M., Koskinen M. N-Glucuronidation of drugs and other xenobiotics by human and animal UDP-glucuronosyltransferases. Xenobiotica. 2011;41:652–669. doi: 10.3109/00498254.2011.563327. [DOI] [PubMed] [Google Scholar]
- 335.Xue P., Liu D., Wang J., Zhang N., Zhou J., Li L. Redox-sensitive citronellol-cabazitaxel conjugate: maintained in vitro cytotoxicity and self-assembled as multifunctional nanomedicine. Bioconjug Chem. 2016;27:1360–1372. doi: 10.1021/acs.bioconjchem.6b00155. [DOI] [PubMed] [Google Scholar]
- 336.Yamaguchi T., Nakajima Y., Nakamura Y. Possible mechanism for species difference on the toxicity of pivalic acid between dogs and rats. Toxicol Appl Pharm. 2006;214:61–68. doi: 10.1016/j.taap.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 337.Qiu F., Cui L., Chen L., Sun J., Yao X. Two novel creatinine adducts of andrographolide in human urine. Xenobiotica. 2012;42:911–916. doi: 10.3109/00498254.2012.680619. [DOI] [PubMed] [Google Scholar]
- 338.von Richter O., Massimini G., Scheible H., Udvaros I., Johne A. Pimasertib, a selective oral MEK1/2 inhibitor: absolute bioavailability, mass balance, elimination route, and metabolite profile in cancer patients. Br J Clin Pharmacol. 2016;82:1498–1508. doi: 10.1111/bcp.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yin W., Doss G.A., Stearns R.A., Kumar S. N-Acetylation of the glutamate residue of intact glutathione conjugates in rats: a novel pathway for the metabolic processing of thiol adducts of xenobiotics. Drug Metab Dispos. 2004;32:43–48. doi: 10.1124/dmd.32.1.43. [DOI] [PubMed] [Google Scholar]
- 340.Savage R.E., Tyler A.N., Miao X.S., Chan T.C. Identification of a novel glucosylsulfate conjugate as a metabolite of 3,4-dihydro-2,2-dimethyl-2H-naphtho[1,2-b]pyran-5,6-dione(ARQ 501, beta-lapachone) in mammals. Drug Metab Dispos. 2008;36:753–758. doi: 10.1124/dmd.107.018655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.