ABSTRACT
Background
High body iron status has been shown to be associated with adverse health outcomes. However, the relation between high body iron status, body mass index (BMI), and cognition is still understudied.
Objective
This study aimed to examine the association between iron intake and cognitive function in Chinese adults and tested the interaction effect of iron intake and BMI on cognition.
Design
Longitudinal study data from a nationwide sample (n = 4852; age ≥55 y) from the China Health and Nutrition Survey during 1991–2006 were used. Of the participants, 3302 had completed cognitive screening tests in ≥2 surveys. Cognitive function was assessed in 1997, 2000, 2004, and 2006. Dietary iron intake was obtained from a 3-d food record during home visits in 1991, 1993, 1997, 2000, 2004, and 2006. Multivariable mixed linear regression and logistic regression were used.
Results
The cumulative mean ± SD iron intake in 1997 of tested subjects was 23.7 ± 11.3 mg/d (25.4 mg/d in men and 22.2 mg/d in women). High iron intake was associated with poor cognition. In fully adjusted models, across the quartiles of iron intake the regression coefficients (95% CIs) were 0, −0.39 (−0.77, −0.01), −0.55 (−0.95, −0.15), and −0.90 (−1.33, −0.47), respectively. Comparing extreme quartiles of iron intake (high), the OR (95% CI) for poor cognitive function was 1.30 (1.04, 1.64). There was a significant interaction between iron intake and BMI. The association between high iron intake and poor cognition was stronger among those with a high BMI than those with a low BMI. Among those with a BMI (kg/m2) >24, across quartiles of iron intake the ORs (95% CIs) for poor cognitive function were 1.00, 1.27 (0.91, 1.78), 1.41 (0.97, 2.04), and 2.04 (1.38, 3.01), respectively.
Conclusion
Higher iron intake is associated with poor cognition in Chinese adults, especially among those with a high BMI.
Keywords: iron intake, cognitive function, obesity, Chinese, adults
Introduction
The prevalence of dementia, a general term used to encompass cognitive decline, is increasing dramatically, especially in developing countries. Dementia was estimated to affect 35.6 million people worldwide in 2010, and this number is expected to double every 20 y, reaching an estimated 115.4 million people by 2050 (1). In China, ∼9.5 million adults aged ≥60 y had dementia (2). Many modifiable risk factors have been associated with dementia, including lower education, hypertension, obesity, hearing loss, diabetes, smoking, depression, physical inactivity, and social isolation (3). Among these, dietary patterns have increasingly received attention (4). For example, it has been shown that adherence to a Mediterranean diet is associated with better cognitive outcomes (4). In addition, in the Whitehall II prospective cohort study, it was shown that a diet characterized by higher intakes of red meat, processed meat, peas and legumes, and fried food and a lower intake of whole grains was associated with faster cognitive decline (5).
There is evidence suggesting that iron accumulation in the brain increases with age (6). There has been evidence of iron accumulation in the brain in patients with Alzheimer, Parkinson, and Huntington disease and amyotrophic lateral sclerosis (6). Higher dietary iron intake has been found to be associated with higher risk for Parkinson disease in the Western population (7, 8). Moreover, high iron concentrations have been shown to be associated with both an increased risk of diabetes among adults in many countries, including China (9), as well as cognitive impairment (10), suggesting that insulin resistance and hyperglycemia and hypoglycemia may play a role in the link (11). Several studies have shown a positive association between iron status and cognitive impairment among older adults (7). However, these studies were mainly conducted in Western countries, where iron status may differ from Asian countries due to differences in diet and regional agriculture.
Increased BMI in mid- and late life has been shown to be positively associated with cognitive function (10, 12). However, a study suggested that this “obesity paradox” is likely due to reverse causation whereby people with preclinical dementia tend to lose weight decades before the diagnosis of dementia (13). Interestingly, accompanied by the increased burden of obesity, the prevalence of anemia decreased dramatically during the past decades in China (14). Anemia was found to be inversely associated with obesity in Chinese women (15). However, no study to our knowledge has assessed the interaction between obesity and iron intake in relation to cognitive function.
China has been experiencing a rapid change in dietary patterns characterized by increased intake of animal-based foods and decreased intakes of plant-based foods (16). In addition, the prevalence of overweight and obese people has increased substantially over the past few decades (17). Therefore, a better understanding of the interactions between these factors and anemia will help to identify the causes of the increasing burden of dementia.
This study aims to assess the longitudinal association between iron intake and cognitive function as well as the interaction between iron intake and BMI and with some other characteristics in Chinese old adults. We used the data collected over a 15-y period from the China Health and Nutrition Survey (CHNS).
Methods
Study design and study sample
This was a longitudinal study based on repeated measurements of dietary intake and cognitive function over 15 y from the CHNS. The CHNS is an ongoing, open, prospective, household-based cohort study conducted in 9 provinces in China (16, 18). The CHNS uses a multistage random-cluster sampling process to select samples in both urban and rural areas. Nine waves of data collection (i.e., 1989, 1991, 1993, 1997, 2000, 2004, 2006, 2009, and 2011) have been conducted. In the 1997, 2000, 2004, and 2006 surveys cognitive screen tests were conducted among those aged >55 y. In total, 4852 participants attended the cognitive screen tests between 1997 and 2006 (Supplemental Figure 1). Of these participants, 3302 attended the screen test in ≥2 surveys. Participants who completed ≥1 cognitive screen test were included in the analysis.
The survey was approved by the institutional review committees of the University of North Carolina and the National Institute of Nutrition and Food Safety (China). Informed consent was obtained from all participants. The response rate based on those who participated in 1989 and remained in the 2006 survey was >60%.
Outcome variable: cognitive function
The cognitive screening items used in the CHNS included a subset of items from the Telephone Interview for Cognitive Status–Modified (19). The tool has been used in other population studies in China to assess cognitive function (20). The cognitive screening included 3 tasks: 1) immediate and delayed recall of a 10-word list, 2) counting backward from 20 to 1, and 3) serial 7 subtraction (5 times). Scores for task 1 ranged from 0 to 20, with each correctly recalled word assigned a score of 1. A total verbal memory score was constructed as the sum of the immediate and delayed 10-word recall. For task 2, a score of 2 was given to those counted backward correctly in the first try. For those only counted backward correctly in the second try, a score of 1 was given. For task 3, a score of 1 was assigned to each of the 5 serial 7 subtractions. Because orientation was only assessed in 1997, 2000, and 2004, we did not include it in the analysis. The total global cognitive score ranges from 0 to 27. A high cognitive score represents better cognition. Based on a study in Shanghai, the prevalence of mild cognitive impairment among people aged ≥60 y was 20% (21). In our study, we chose the first quintile of the cognitive function test score as representing poor cognitive function, which corresponds to a global cognitive function score cutoff of <7.
Exposure variables: cumulative mean iron intake
At each wave, individual dietary intake data were collected by a trained investigator conducting a 24-h dietary recall on each of 3 consecutive days. In addition, foods and condiments in the home inventory, foods purchased from markets or harvested from gardens, and food waste were weighed and recorded by interviewers at the beginning and end of the 3-d survey period. A detailed description of the dietary measurements has been published previously (16). Food-consumption data were converted to nutrient intakes using the Chinese food-composition table (22). The dietary assessment method has been validated for energy intake (23).
We calculated a cumulative average intake of iron for each individual at each time period to reduce variation within individuals and to represent long-term habitual intake (24). For example, the 1991 iron intake was used for the follow-up between 1991 and 1993, the average of the 1991 and 1993 intake was used for the follow-up between 1997 and 2000, and so on. In the sensitivity analysis, we also assessed the association between the most recent iron intake and cognitive function. In sensitivity analyses, we excluded 1991 and 1993 dietary intakes. Because the main findings did not change, we decided to include the 1991 and 1993 dietary data in our analysis and results because they offered more information on 4–6 y of dietary history. Height, weight, and blood pressure were measured at each wave. Overweight or obesity was defined as BMI (kg/m2) ≥24.
Covariates
Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or having known hypertension. Sociodemographic and lifestyle factors were collected in each wave using a structured questionnaire. The following constructed variables were included to reflect socioeconomic status: education (low: illiterate/primary school; medium: junior middle school; and high: high middle school or higher), per capita annual family income (recoded into tertiles as low, medium, and high), urbanization levels (16) (recoded into tertiles as low, medium, and high).
Physical activity level (metabolic equivalents of task) was estimated on the basis of self-reported activities (including occupational, domestic, transportation, and leisure-time physical activity) and duration using a Compendium of Physical Activities (25). Smoking status was categorized into nonsmokers, ex-smokers, and current smokers. Alcohol drinking was categorized as yes or no. Self-reported diabetes and stroke were coded as yes or no.
Statistical analysis
Cumulative mean iron intake was recoded into quartiles. The chi-square test was used to compare differences between groups for categorical variables and ANOVA was used for continuous variables. A mixed-effects model using mixed command in Stata (StataCorp) was used to assess the association between iron intake and cognitive function. A negative regression coefficient represents cognitive function decline. A set of models were used: model 1 adjusted for age, gender, and energy intake; model 2 further adjusted for intake of fat, smoking, alcohol drinking, income, urbanization, education, and physical activity; model 3 further adjusted for intake of fruit and vegetables; and model 4 further adjusted for BMI and hypertension.
In sensitivity analyses, we also stratified our analysis by total meat intake (including pork, beef and poultry; above or below the median). We also excluded those with a global cognitive function score ≤4 and further adjusted for diabetes and stroke. To assess the association between cumulative mean iron intake and the risk of poor cognitive function, we used mixed-effects logistic regression adjusting for the same covariates as in model 4 mentioned above. To test the interaction between iron intake and set of variables including BMI, hypertension, and income, a product term of each pair of variables was put in the regression model. variables was put in the regression model. We used the marginsplot command in Stata 15.1 (StataCorp) to visually present the interaction.
All of the analyses were performed using STATA 15.1 (StataCorp). Significance was considered when P < 0.05 (2-sided).
Results
Descriptive results
The cumulative mean ± SD iron intake in 1997 was 23.7 ± 11.3 mg/d (25.4 mg/d in men and 22.2 mg/d in women). The mean ± SD intake of iron in the high quartile was 36.9 ± 13.3 mg/d.
Table 1 shows the sample characteristics among participants attending the first cognitive function test according to amounts of cumulative iron intake. Across the quartiles of iron intake, the intakes of energy, protein, fat, carbohydrate, and fresh vegetable increased. However, there was no difference in fruit intake across quartiles of iron intake. The prevalence of smoking and alcohol drinking increased across quartiles of iron intake. Physical activity level was higher among those with a high intake of iron than in those with a low intake.
TABLE 1.
Sample characteristics of Chinese adults aged ≥55 y attending the first cognitive function test by quartiles of cumulative iron1
| Iron intake quartiles | |||||
|---|---|---|---|---|---|
| 1 (median: 14.6 mg/d) (n = 1205) | 2 (19.3 mg/d) (n = 1081) | 3 (23.7 mg/d) (n = 1171) | 4 (31.7 mg/d) (n = 1228) | P | |
| Age, y | 66.5 ± 8.7 | 63.6 ± 7.7 | 62.0 ± 7.0 | 61.7 ± 6.5 | <0.001 |
| Sex, n (%) | <0.001 | ||||
| Men | 412 (34.2) | 519 (48.0) | 594 (50.7) | 723 (58.9) | |
| Women | 793 (65.8) | 562 (52.0) | 577 (49.3) | 505 (41.1) | |
| Education, n (%) | <0.001 | ||||
| Low | 759 (75.8) | 690 (70.5) | 755 (69.5) | 863 (74.1) | |
| Medium | 117 (11.7) | 136 (13.9) | 194 (17.9) | 181 (15.5) | |
| High | 125 (12.5) | 153 (15.6) | 137 (12.6) | 120 (10.3) | |
| Urbanization, n (%) | <0.001 | ||||
| Low | 216 (17.9) | 217 (20.1) | 303 (25.9) | 456 (37.1) | |
| Medium | 306 (25.4) | 308 (28.5) | 329 (28.1) | 376 (30.6) | |
| High | 683 (56.7) | 556 (51.4) | 539 (46.0) | 396 (32.2) | |
| Smoking, n (%) | <0.001 | ||||
| Nonsmoker | 898 (74.9) | 753 (69.7) | 760 (65.0) | 738 (60.1) | |
| Ex-smoker | 45 (3.8) | 39 (3.6) | 42 (3.6) | 51 (4.2) | |
| Current smoker | 256 (21.4) | 288 (26.7) | 367 (31.4) | 438 (35.7) | |
| Survey year, n (%) | 0.007 | ||||
| 1997 | 536 (44.5) | 521 (48.2) | 476 (40.6) | 579 (47.1) | |
| 2000 | 187 (15.5) | 161 (14.9) | 208 (17.8) | 218 (17.8) | |
| 2004 | 298 (24.7) | 235 (21.7) | 292 (24.9) | 268 (21.8) | |
| 2006 | 184 (15.3) | 164 (15.2) | 195 (16.7) | 163 (13.3) | |
| Alcohol drinking, n (%) | 273 (23.1) | 335 (31.4) | 387 (33.8) | 439 (36.4) | <0.001 |
| Physical activity, MET-h/wk | 64.2 ± 84.6 | 85.9 ± 97.5 | 94.2 ± 101.4 | 106.2 ± 107.4 | <0.001 |
| BMI, kg/m2 | 23.1 ± 3.8 | 23.2 ± 3.7 | 23.2 ± 3.6 | 22.8 ± 3.4 | 0.015 |
| BMI ≥24, n (%) | 429 (38.6) | 372 (37.2) | 431 (39.4) | 362 (32.6) | 0.004 |
| Energy intake, kcal/d | 1719.1 ± 503.8 | 2087.7 ± 1084.9 | 2180.8 ± 584.6 | 2413.0 ± 829.7 | <0.001 |
| Fat intake, g/d | 60.9 ± 34.9 | 70.0 ± 114.1 | 68.6 ± 36.5 | 72.2 ± 58.6 | <0.001 |
| Protein intake, g/d | 49.5 ± 15.8 | 62.6 ± 18.3 | 67.6 ± 21.3 | 74.2 ± 30.0 | <0.001 |
| Carbohydrate intake, g/d | 239.6 ± 75.0 | 295.4 ± 85.4 | 316.7 ± 99.1 | 358.9 ± 124.9 | <0.001 |
| Intake of fruit, g/d | 24.5 ± 73.5 | 25.8 ± 92.2 | 19.1 ± 70.1 | 23.7 ± 83.1 | 0.21 |
| Intake of fresh vegetable, g/d | 217.2 ± 137.1 | 268.4 ± 159.6 | 292.2 ± 192.8 | 317.4 ± 194.8 | <0.001 |
| Intake of meat, g/d | 62.2 ± 67.2 | 78.2 ± 80.9 | 79.5 ± 86.9 | 72.7 ± 90.8 | <0.001 |
| Most recent iron intake, mg/d | 13.2 ± 3.8 | 18.3 ± 5.2 | 21.1 ± 7.3 | 27.6 ± 18.4 | <0.001 |
| Cumulative iron intake, mg/d | 13.7 ± 2.6 | 19.2 ± 1.2 | 23.8 ± 1.6 | 35.9 ± 12.5 | <0.001 |
| Hypertension, n (%) | 468 (40.8) | 346 (34.1) | 370 (33.2) | 376 (33.2) | <0.001 |
| Diabetes, n (%) | 43 (3.6) | 27 (2.5) | 35 (3.0) | 44 (3.7) | 0.37 |
| Stroke, n (%) | 30 (2.5) | 19 (1.8) | 18 (1.6) | 32 (2.7) | 0.18 |
Values are means ± SDs unless otherwise indicated; n = 4685. P values were calculated by using ANOVA or chi-square test. MET-h, metabolic equivalent of task hours.
The mean ± SD global cognition score was 12.1 ± 6.8 in 1997. The annual cognitive function score decline was 0.1 (95% CI: 0.07, 0.13). The prevalence of global cognitive scores <7 ranged from 19.8% to 23.1% in the 4 survey waves between 1997 and 2006.
Association between iron intake and cognitive function
Iron intake was related to cognitive function decline (Table 2). Comparing extreme quartiles of iron intake, the difference in cognitive function was −1.49 after adjusting for age, gender, and energy intake. In fully adjusted models, across quartiles of iron intake, the regression coefficients (95% CIs) were 0, −0.39 (−0.77, −0.01), −0.55 (−0.95, −0.15), and −0.90 (−1.33, −0.47), respectively. A similar positive association between iron intake and memory decline was observed. The regression coefficients (95% CIs) for memory across quartiles of iron intake were 0, −0.34 (−0.61, −0.06), −0.40 (−0.69, −0.12), and −0.60 (−0.92, −0.29), respectively. In sensitivity analyses, after excluding those with a global cognitive function score <4 or further adjusting for diabetes or stroke the above association between iron intake and cognition did not change. No association between the most recent intake of iron and cognitive function was found in multivariable mixed model (data not shown).
TABLE 2.
Regression coefficients (95% CIs) for cognitive function by quartiles of iron intake among Chinese adults aged ≥55 y who participated in the China Health and Nutrition Survey1
| Iron intake quartiles | |||||
|---|---|---|---|---|---|
| 1 (low intake; median: 14.6 mg/d) | 2 (19.3 mg/d) | 3 (23.7 mg/d) | 4 (high intake; 31.7 mg/d) | P-trend | |
| Global cognitive function | |||||
| Model 1 | 0.00 | −0.26 (−0.61, 0.09) | −0.74 (−1.11, −0.37) | −1.49 (−1.89, −1.09) | <0.001 |
| Model 2 | 0.00 | −0.36 (−0.73, 0.01) | −0.59 (−0.98, −0.20) | −0.90 (−1.32, −0.47) | <0.001 |
| Model 3 | 0.00 | −0.36 (−0.74, 0.01) | −0.58 (−0.97, −0.19) | −0.89 (−1.32, −0.47) | <0.001 |
| Model 4 | 0.00 | −0.39 (−0.77, −0.01) | −0.55 (−0.95, −0.15) | −0.90 (−1.33, −0.47) | <0.001 |
| Sensitivity analysis | 0.00 | −0.39 (−0.75, −0.03) | −0.52 (−0.90, −0.14) | −0.85 (−0.13, −0.44) | <0.001 |
| Verbal memory score | |||||
| Model 1 | 0.00 | −0.31 (−0.55, −0.06) | −0.56 (−0.82, −0.30) | −0.98 (−1.26, −0.69) | <0.001 |
| Model 2 | 0.00 | −0.33 (−0.60, −0.06) | −0.44 (−0.72, −0.16) | −0.61 (−0.91, −0.30) | <0.001 |
| Model 3 | 0.00 | −0.32 (−0.59, −0.05) | −0.43 (−0.72, −0.15) | −0.60 (−0.90, −0.29) | <0.001 |
| Model 4 | 0.00 | −0.34 (−0.61, −0.06) | −0.40 (−0.69, −0.12) | −0.60 (−0.92, −0.29) | <0.001 |
| Sensitivity analysis | 0.00 | −0.32 (−0.58, −0.05) | −0.41 (−0.69, −0.13) | −0.59 (−0.89, −0.28) | <0.001 |
n = 4852. Regression coefficients (95% CIs) were estimated with mixed-effects regression models with different levels of adjustment. Model 1 adjusted for age, gender, and energy intake. Model 2 further adjusted for intake of fat, smoking, alcohol drinking, income, urbanicity, education, and physical activity. Model 3 further adjusted for intakes of fruit and vegetables. Model 4 further adjusted for BMI and hypertension. The sensitivity analysis model 4 further adjusted for diabetes and stroke after excluding those with a global cognitive function score ≤4. All the adjusted variables were treated as time-varying covariates.
Weight status modifies the association between iron intake and cognitive function
A significant interaction (P = 0.008) between iron intake and BMI in relation to cognitive function was found (Figure 1). The positive association between iron intake and cognitive function decline was stronger among those with a high BMI than in those with a low BMI. Among those who were overweight or obese, there was a significant increase in ORs (95% CIs) for global cognition scores <7 across quartiles of iron intake of 1.00, 1.27 (0.91, 1.78), 1.41 (0.97, 2.04), and 2.04 (1.38, 3.01), respectively (Table 3). However, this trend was not observed among those with a normal BMI (P-interaction = 0.038).
FIGURE 1.
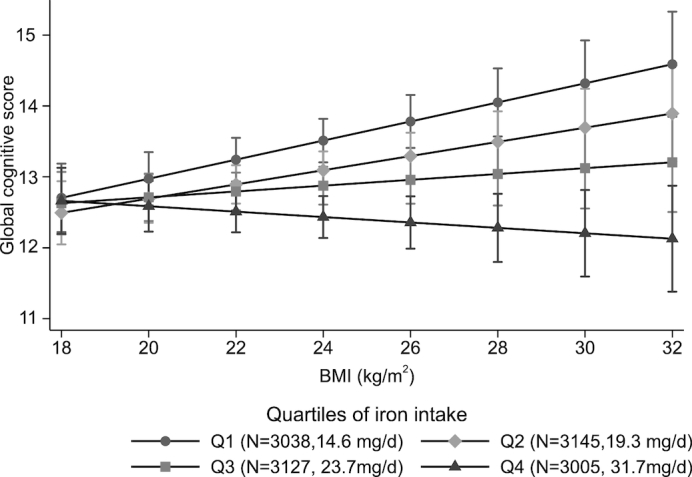
Interaction between iron intake and BMI in relation to global cognitive function. Values are means (95%CI) derived by using the margins command in Stata after running a mixed linear model adjusted for age, gender, intakes of energy and fat, education, urbanicity, smoking, alcohol drinking, physical activity, and intakes of fruit and vegetables. P-interaction between iron intake and BMI = 0.008. Numbers of observations and the median intake of iron are presented in each quartile of iron intake (Q1–Q4). Q, quartile.
TABLE 3.
ORs (95% CIs) for global cognitive scores <7 across quartiles of iron intake among Chinese adults aged ≥55 y by characteristics: China Health and Nutrition Survey1
| Iron intake quartiles | |||||
|---|---|---|---|---|---|
| 1 (low intake; median: 14.6 mg/d) | 2 (19.3 mg/d) | 3 (23.7 mg/d) | 4 (high intake; 31.7 mg/d) | P-interaction | |
| Overall sample | 1.00 | 1.06 (0.87, 1.30) | 1.09 (0.88, 1.35) | 1.30 (1.04, 1.64) | |
| Income | 0.046 | ||||
| Low | 1.00 | 1.15 (0.83, 1.61) | 0.95 (0.67, 1.34) | 0.93 (0.65, 1.34) | |
| Medium | 1.00 | 1.02 (0.72, 1.46) | 1.20 (0.83, 1.75) | 1.59 (1.07, 2.37) | |
| High | 1.00 | 1.04 (0.70, 1.53) | 1.11 (0.73, 1.71) | 1.85 (1.18, 2.91) | |
| Overweight/obesity | 0.038 | ||||
| No | 1.00 | 1.00 (0.78, 1.29) | 0.97 (0.75, 1.26) | 1.06 (0.80, 1.41) | |
| Yes | 1.00 | 1.27 (0.91, 1.78) | 1.41 (0.97, 2.04) | 2.04 (1.38, 3.01) | |
| Hypertension | 0.914 | ||||
| No | 1.00 | 1.11 (0.86, 1.44) | 1.11 (0.85, 1.46) | 1.36 (1.02, 1.80) | |
| Yes | 1.00 | 0.95 (0.69, 1.31) | 1.02 (0.73, 1.43) | 1.15 (0.80, 1.66) | |
| Gender | 0.904 | ||||
| Men | 1.00 | 1.14 (0.78, 1.67) | 1.09 (0.74, 1.62) | 1.25 (0.84, 1.86) | |
| Women | 1.00 | 1.02 (0.80, 1.30) | 1.08 (0.83, 1.40) | 1.34 (1.00, 1.78) | |
| Urbanization | 0.647 | ||||
| Low | 1.00 | 1.33 (0.84, 2.09) | 1.45 (0.92, 2.27) | 1.65 (1.04, 2.61) | |
| Medium | 1.00 | 1.06 (0.72, 1.55) | 1.09 (0.73, 1.63) | 1.06 (0.70, 1.61) | |
| High | 1.00 | 0.97 (0.73, 1.29) | 0.94 (0.68, 1.29) | 1.35 (0.95, 1.93) | |
n = 4852. Mixed-effects logistic modes adjusted for age, gender, intake of energy and fat, smoking, alcohol drinking, income, urbanicity, education, physical activity, intakes of fruit and vegetable, BMI, and hypertension. Stratification variables were not adjusted in the corresponding models. Income was categorized into low, medium, and high on the basis of tertile of year-specific income.
Similarly, there was an interaction between family income and iron intake (P-interaction = 0.046). The positive association between iron intake and low global cognitive score was only found in the medium- and high-income groups but not in the low-income group. There were no significant interactions with urban or rural residence, gender, smoking, alcohol drinking, or hypertension.
When we limited the analyses to those who took the cognitive tests in ≥2 waves of the survey, or stratified the analyses by high or low intakes of meat, the findings remained unchanged. In fully adjusted models, across the quartiles of iron intake the ORs (95% CIs) for global cognition scores <7 were 1.00, 1.06 (0.87, 1.30), 1.09 (0.88, 1.35), and 1.30 (1.04, 1.64), respectively.
Discussion
This longitudinal study in China shows that high iron intake was associated with poor cognition. Those with a high iron intake (mean intake: 36.9 mg/d) were ∼30% more likely to have poor cognitive function than others. In addition, there was a significant interaction between iron intake and BMI. The positive association between iron intake and cognitive decline was stronger among those with a high BMI or a high income. There was no interaction between iron intake and gender and hypertension. The association was independent of lifestyle factors and hypertension.
To the best of our knowledge, this is the first population study to investigate the interaction between iron intake and BMI in relation to cognitive function. We found that iron intake was inversely associated with cognitive function. Interestingly, in high-BMI groups, iron intake was more strongly associated with cognitive decline. It could be hypothesized that, among those with a high BMI, the negative consequences of high iron intake may be exacerbated because of pre-existing elevated inflammatory status in obese individuals (26). It was previously shown in the Austrian Stroke Prevention Study that a higher BMI was associated with elevated brain iron among 314 healthy community-dwelling participants (27). Further research is needed to elucidate the relations between iron, BMI, and cognitive function, including the role of heavy metal contamination.
The findings are supported by a positive association between iron intake and other disorders that induce cognitive decline, such as Parkinson disease, in a Western population (8). However, in Asia, an inverse association between iron intake and Parkinson disease was found in a Japanese case-control study (28). There is a limited number of studies on iron intake and cognitive function with consistent findings. Our findings disagree with a cross-sectional study that reported a positive association between iron intake and cognitive function in a Spanish population aged 65–90 y (29). The reasons for the discrepancies include the differences in study design and exposure classification. In the Spanish study, those receiving diabetes treatment were excluded and the mean intake of iron was ∼12 mg/d in men and 10 mg/d in women (29). In the Japanese study, the mean iron intakes in the cases and controls were only 7.5 and 7.6 mg, respectively (28). Furthermore, the variation in iron intake in the Japanese study was also small (<6.42 compared with >8.73 mg/d in the first and fourth quartiles of iron intake). The iron intake in our study was higher than in the Western population. Nonheme iron is the major source of total iron intake in the Chinese population (30). It has been reported that the mean iron intake ranged from 22.1 to 26.1 between 1991 and 2004 in the CHNS (30). Mean iron intake in the highest quartile in our analysis was 3 times the recommended iron intake (i.e., 12 mg/d) for Chinese adults (31). Although nonheme iron has a lower bioavailability than heme iron, nonheme iron intake but not heme iron intake was associated with Parkinson disease in the United States (8).
The mechanisms linking iron intake and cognitive function have yet to be fully elucidated. In 1956, Harman (32) hypothesized that free nonheme iron is a major factor in neural and cognitive aging. Several reviews showed the important role of iron in neurodegenerative diseases as a redox-active ion that can cause oxidative stress in the cell (7, 33). In animal studies, iron intake is related to iron deposits in the brain (34). Given the redox properties of iron, accumulation of iron in the brain may increase oxidative stress on the brain. Furthermore, in rats, iron-induced cognitive deficits were found to be related to dysfunction of cholinergic neural transmission in the brain (35). Iron chelation has been shown to be effective in treating animal models of neurodegenerative diseases, such as Parkinson and Alzheimer diseases (33).
Human laboratory studies examining the iron-cognition relation are limited and the findings are inconsistent. Although a study in the United Kingdom found no association between iron intake and brain iron deposit (36), another study in the United States found that iron supplementation was associated with increased brain iron (37). Epidemiologic studies have also tested Harman's hypothesis concerning the role of iron in cognitive aging. A 2-y longitudinal study in 78 healthy adults showed that accumulation of subcortical nonheme iron is a risk factor for cognitive decline (38). High iron intake has also been shown to be associated with an increased risk of diabetes (9), Parkinson disease, and other neurodegenerative diseases (7) in adult populations. Brain iron content is associated with cognitive decline (39, 40) and amyloid deposition, which has been associated with neurodegenerative disorders (41). Furthermore, high iron status as indicated by serum ferritin, transferrin, and hemoglobin concentrations has been shown to be positively associated with increased risk of hyperuricemia among participants of the CHNS (42). Moreover, hyperuricemia is related to poor cognitive function in the Chinese population (43). Serum ferritin is also positively associated with build-up of neocortical amyloid-β (44). Further research is needed to elucidate the mechanism behind the iron-cognition association.
The potential adverse health effects of high iron status in the Chinese population have been studied. For example, high iron status was associated with increased risk of mortality in a 10-y follow-up study in Chinese adults (45). Serum ferritin was positively associated with diabetes risk in several large-population studies in China (46–52), which is consistent with the findings among Western populations. Our current study adds cognitive decline to the list of potential high-iron–related health problems. Given the sharply increased burden of both diabetes and dementia in China as well as the positive association between iron and diabetes, an interplay between iron, diabetes, and cognitive function decline is likely. The mediating effect of diabetes between iron and cognitive function decline warrants further exploration. Iron status in the Chinese population has improved substantially because data from the Chinese National Nutrition Survey showed that the prevalence of anemia was reduced by 53% from 20.8% in 2002 to 9.7% in 2012 in rural areas (14). The public health profile shift related to iron intake in the past decades in China calls for timely policy adjustment in high-risk populations (i.e., those at risk of iron overload).
The strong positive association between high iron intake and poor cognitive function among those with overweight or obesity deserves attention and intervention. Contrary to the current practice of iron supplementation and fortification in China, reducing iron intake should be considered among those with a high BMI, although further research is needed. Otherwise, a further increase in the burden of dementia may be expected in China, because with the ongoing increased intake of animal food in the Chinese diet the bioavailability of iron will increase (53).
Key strengths of our study include the longitudinal study design, multiple measurements of dietary intake, and the relatively large sample size. The repeated measures of 3-d dietary intake in combination with household food inventory provide a robust estimate of long-term iron intake. The large variation in iron intake makes it possible to assess the association between high iron intake and cognitive function. The mean cumulative iron intakes were 13.9 and 36.9 mg/d in the first and highest quartiles of iron intake, respectively. However, we were not able to explore the potential mechanisms due to a lack of related biomarkers. Additional forms of iron intake, such as iron supplementation, were not considered. The information on the use of iron-fortified soy sauce was not available in the CHNS because iron-fortified soy sauce was introduced to prevent anemia in some provinces in China in 2003. Although iron-related biomarkers are available in the 2009 survey, cognitive function was not tested in 2009. Given the low cognitive status of the cohort, the use of a 24-h food recall may be biased due to its reliance on memory recall. Cognitive function is multifaceted, and our study only measured cognition as a global measure that relied on auditory and verbal processing skills. We did not have other cognition measures such as speed of processing or visual processing. In the analyses we have adjusted for potential confounders including sociodemographic and lifestyle factors and residual confounding is possible.
In conclusion, our study found that high iron intake was positively associated with poor cognition among Chinese adults, independent of gender and lifestyle and sociodemographic factors. There is a significant interaction between iron intake and BMI, showing increased cognitive decline in those with a high BMI and a high iron intake. This study supports the importance of a balanced diet with appropriate amounts of iron intake.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ZS: contributed to the conception, analysis, and interpretation of data and drafting of the report; YW, ML, JL, and TE-O: contributed to analysis and interpretation of the data, commented on the report, and revised the manuscript; and all authors: read and approved the final manuscript. None of the authors declared a conflict of interest.
Notes
The authors reported no funding received for this study.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
This research used data from the China Health and Nutrition Survey (CHNS). The National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924, T32 HD007168), the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700) and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) provided financial support for the CHNS data collection and analysis files from 1989 to 2015 and future surveys and the China-Japan Friendship Hospital, Ministry of Health, supported the CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011.
References
- 1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. e2. [DOI] [PubMed] [Google Scholar]
- 2. Wu YT, Ali GC, Guerchet M, Prina AM, Chan KY, Prince M, Brayne C. Prevalence of dementia in mainland China, Hong Kong and Taiwan: an updated systematic review and meta-analysis. Int J Epidemiol. 2018;47:(3):709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J et al.. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–734. [DOI] [PubMed] [Google Scholar]
- 4. van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr. 2017;36(2):506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belaidi AA, Bush AI. Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem. 2016;139(Suppl 1):179–97. [DOI] [PubMed] [Google Scholar]
- 7. Agrawal S, Berggren KL, Marks E, Fox JH. Impact of high iron intake on cognition and neurodegeneration in humans and in animal models: a systematic review. Nutr Rev. 2017;75(6):456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Logroscino G, Gao X, Chen H, Wing A, Ascherio A. Dietary iron intake and risk of Parkinson's disease. Am J Epidemiol. 2008;168(12):1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bao W, Rong Y, Rong S, Liu L. Dietary iron intake, body iron stores, and the risk of type 2 diabetes: a systematic review and meta-analysis. BMC Med. 2012;10:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJW, Pocock SJ. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431–6. [DOI] [PubMed] [Google Scholar]
- 11. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim S, Kim Y, Park SM. Body mass index and decline of cognitive function. PLoS One. 2016;11(2):e0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes (Lond). 2015;39(9):1383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Hu Y, Mao D, Wang R, Chen J, Li W, Yang X, Piao J, Yang L. Prevalence of anemia among Chinese rural residents. Nutrients. 2017;9(3):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin Y, Melse-Boonstra A, Pan X, Yuan B, Dai Y, Zhao J, Zimmermann MB, Kok FJ, Zhou M, Shi Z. Anemia in relation to body mass index and waist circumference among Chinese women. Nutr J. 2013;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhai FY, Du SF, Wang ZH, Zhang JG, Du WW, Popkin BM. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes Rev. 2014;15(Suppl 1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu YF. Overweight and obesity in China. Br Med J. 2006;333(7564):362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: the China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol. 2010;39(6):1435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plassman BL, Welsh KA, Helms M, Brandt J, Page WF, Breitner JC. Intelligence and education as predictors of cognitive state in late life: a 50-year follow-up. Neurology. 1995;45(8):1446–50. [DOI] [PubMed] [Google Scholar]
- 20. Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender differences in cognition among older adults in China. J Hum Resour. 2012;47(4):951–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ding D, Zhao Q, Guo Q, Meng H, Wang B, Luo J, Mortimer JA, Borenstein AR, Hong Z. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement. 2015;11(3):300–9., e2. [DOI] [PubMed] [Google Scholar]
- 22. Yang Y. Chinese food composition table 2004. Beijing (China): Peking University Medical Press; 2005. [Google Scholar]
- 23. Yao M, McCrory MA, Ma G, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr. 2003;77(6):1409–16. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 25. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO et al.. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32::S498–504. [DOI] [PubMed] [Google Scholar]
- 26. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010 Available at: https://www.hindawi.com/journals/mi/2010/289645/. A ccessed on 26 October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pirpamer L, Hofer E, Gesierich B, De Guio F, Freudenberger P, Seiler S, Duering M, Jouvent E, Duchesnay E, Dichgans M et al.. Determinants of iron accumulation in the normal aging brain. Neurobiol Aging. 2016;43:149–55. [DOI] [PubMed] [Google Scholar]
- 28. Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N et al.. Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J Neurol Sci. 2011;306(1–2):98–102. [DOI] [PubMed] [Google Scholar]
- 29. Ortega RM, Requejo AM, Andres P, Lopez-Sobaler AM, Quintas ME, Redondo MR, Navia B, Rivas T. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66(4):803–9. [DOI] [PubMed] [Google Scholar]
- 30. Zhai F, Wang H, Du S, He Y, Wang Z, Ge K, Popkin BM. Prospective study on nutrition transition in China. Nutr Rev. 2009;67(Suppl 1):S56–61. [DOI] [PubMed] [Google Scholar]
- 31. Chinese Nutrition Society. Chinese DRIs Handbook. Beijing (China): Standards Press of China; 2013. [Google Scholar]
- 32. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. [DOI] [PubMed] [Google Scholar]
- 33. Li K, Reichmann H. Role of iron in neurodegenerative diseases. J Neural Transm (Vienna). 2016;123(4):389–99. [DOI] [PubMed] [Google Scholar]
- 34. Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127(10):2030–8. [DOI] [PubMed] [Google Scholar]
- 35. Perez VP, de Lima MN, da Silva RS, Dornelles AS, Vedana G, Bogo MR, Bonan CD, Schröder N. Iron leads to memory impairment that is associated with a decrease in acetylcholinesterase pathways. Curr Neurovasc Res. 2010;7(1):15–22. [DOI] [PubMed] [Google Scholar]
- 36. Hernandez Mdel C, Allan J, Glatz A, Kyle J, Corley J, Brett CE, Muñoz Maniega S, Royle NA, Bastin ME, Starr JM et al.. Exploratory analysis of dietary intake and brain iron accumulation detected using magnetic resonance imaging in older individuals: the Lothian Birth Cohort 1936. J Nutr Health Aging. 2015;19(1):64–9. [DOI] [PubMed] [Google Scholar]
- 37. Hagemeier J, Tong O, Dwyer MG, Schweser F, Ramanathan M, Zivadinov R. Effects of diet on brain iron levels among healthy individuals: an MRI pilot study. Neurobiol Aging. 2015;36(4):1678–85. [DOI] [PubMed] [Google Scholar]
- 38. Daugherty AM, Haacke EM, Raz N. Striatal iron content predicts its shrinkage and changes in verbal working memory after two years in healthy adults. J Neurosci. 2015;35(17):6731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, Diouf I, Farquharson S, Fripp J, Ames D et al.. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain. 2017;140(8):2112–9. [DOI] [PubMed] [Google Scholar]
- 40. Ayton S, Faux NG, Bush AI; Alzheimer's Disease Neuroimaging Initiative. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ayton S, Diouf I, Bush AI; Alzheimer's disease Neuroimaging Initiative. Evidence that iron accelerates Alzheimer's pathology: a CSF biomarker study. J Neurol Neurosurg Psychiatry. 2018;89(5):456–60. [DOI] [PubMed] [Google Scholar]
- 42. Li X, He T, Yu K, Lu Q, Alkasir R, Guo G, Xue Y. Markers of iron status are associated with risk of hyperuricemia among Chinese adults: nationwide population-based study. Nutrients. 2018;10(2):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu M, Wang J, Zeng J, He Y. Relationship between serum uric acid level and mild cognitive impairment in Chinese community elderly. BMC Neurol. 2017;17(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goozee K, Chatterjee P, James I, Shen K, Sohrabi HR, Asih PR, Dave P, ManYan C, Taddei K, Ayton SJ et al.. Elevated plasma ferritin in elderly individuals with high neocortical amyloid-beta load. Mol Psychiatry. 2018;. 23(8);1807–12 [DOI] [PubMed] [Google Scholar]
- 45. Shi Z, Zhen S, Zhou Y, Taylor AW. Hb level, iron intake and mortality in Chinese adults: a 10-year follow-up study. Br J Nutr. 2017;117(4):572–81. [DOI] [PubMed] [Google Scholar]
- 46. Gao S, Zhao D, Qi Y, Wang M, Zhao F, Sun J, Liu J. The association between serum ferritin levels and the risk of new-onset type 2 diabetes mellitus: a 10-year follow-up of the Chinese Multi-Provincial Cohort Study. Diabetes Res Clin Pract. 2017;130:154–62. [DOI] [PubMed] [Google Scholar]
- 47. Han LL, Wang YX, Li J, Zhang XL, Bian C, Wang H, Du S, Suo LN. Gender differences in associations of serum ferritin and diabetes, metabolic syndrome, and obesity in the China Health and Nutrition Survey. Mol Nutr Food Res. 2014;58(11):2189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun L, Zong G, Pan A, Ye X, Li H, Yu Z, Zhao Y, Zou S, Yu D, Jin Q et al.. Elevated plasma ferritin is associated with increased incidence of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr. 2013;143(9):1459–65. [DOI] [PubMed] [Google Scholar]
- 49. Guo X, Zhou D, An P, Wu Q, Wang H, Wu A, Mu M, Zhang D, Zhang Z, Wang H et al.. Associations between serum hepcidin, ferritin and Hb concentrations and type 2 diabetes risks in a Han Chinese population. Br J Nutr. 2013;110(12):2180–5. [DOI] [PubMed] [Google Scholar]
- 50. Chen T, Ren Y, Liu Y, Long Y, Zhang X, Yu H, Xu J, Yu T, Tian H. Serum gamma-glutamyl transferase, ferritin and the risk of type 2 diabetes in women from a Chinese minority. Diabetes Res Clin Pract. 2010;90(3):352–7. [DOI] [PubMed] [Google Scholar]
- 51. Shi Z, Zhou M, Yuan B, Qi L, Dai Y, Luo Y, Holmboe-Ottesen G. Iron intake and body iron stores, anaemia and risk of hyperglycaemia among Chinese adults: the prospective Jiangsu Nutrition Study (JIN). Public Health Nutr. 2010;13(9):1319–27. [DOI] [PubMed] [Google Scholar]
- 52. Luan de C, Li H, Li SJ, Zhao Z, Li X, Liu ZM. Body iron stores and dietary iron intake in relation to diabetes in adults in North China. Diabetes Care. 2008;31(2):285–6. [DOI] [PubMed] [Google Scholar]
- 53. Du S, Zhai F, Wang Y, Popkin BM. Current methods for estimating dietary iron bioavailability do not work in China. J Nutr. 2000;130(2):193–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


