ABSTRACT
Background
Low calcium intake during pregnancy may cause maternal skeletal calcium mobilization to meet fetal needs. The Recommended Dietary Allowance (RDA) for calcium in nonpregnant, pregnant, or lactating women aged 19–50 y is 1000 mg/d; most women in the United States report consuming 60–80% of the calcium RDA. An insufficient calcium intake could increase maternal bone loss during pregnancy and reduce bone recovery postpartum.
Objectives
The aim of this study was to determine the effect of maternal calcium supplementation on peripheral cortical and trabecular bone loss during pregnancy and bone gain postpartum.
Methods
A total of 64 women were enrolled in the study at 16 wk of gestation and randomly assigned to receive 1000 mg Ca/d or placebo for the remainder of the pregnancy. Measurements were performed at 16, 26, and 36 wk of pregnancy and at 4 and 12 mo postpartum for serum 25-hydroxyvitamin D and markers of bone turnover. Trabecular and cortical bone mineral density (BMD) and content were assessed at the tibia and radius by peripheral quantitative computed tomography.
Results
Mean ± SD daily calcium intake at baseline was 733 ± 350 mg; only 25% of the women met the RDA. Thirty women (47% of those enrolled) remained in the study at 12 mo postpartum. After controlling for baseline bone value, serum 25-hydroxyvitamin D concentrations, length of breastfeeding, and body mass index, the calcium group had significantly greater increases in radial total BMD (P = 0.02) and tibial cortical BMD (P = 0.03) at 12 mo postpartum than the placebo group. Trabecular and total BMD at the tibia trended toward higher values (P < 0.06) in the calcium group than in the placebo group in the same models.
Conclusions
These data show that supplemental calcium provided during pregnancy may improve bone recovery postpartum in women consuming a typical US diet. A larger study is warranted to solidify the conclusions. This trial was registered at clinicaltrials.gov as NCT01145573.
Keywords: calcium, pregnancy, bone, bone mineral density, dual-energy X-ray absorptiometry, peripheral quantitative computed tomography
Introduction
The recommended calcium intake was lowered for pregnant women in 1997 to the amounts recommended for nonpregnant women (1). The Institute of Medicine Expert Committee concluded that any calcium deficit not negated by an increased efficiency in calcium absorption during pregnancy could be supplied by mobilizing maternal bone calcium (2). Based on data from one cross-sectional study of postpartum women (3), it was assumed that maternal bone losses to support the demands of both pregnancy and lactation are recovered by 1 y postpartum. A re-evaluation of the Calcium and Vitamin D Dietary Reference Intakes in 2011 retained the 1997 recommendations for calcium intake during pregnancy and lactation (2).
Longitudinal studies of calcium metabolism during pregnancy show that maternal calcium absorption increases significantly during the second and third trimesters (merged) (4–6). However, even with these high rates of absorption, fetal needs, which peak in the third trimester at 350 mg/d (7, 8), may not be met in women with chronically low calcium consumption (<600 mg/d) (6) because the total absorbed calcium is less than the amount required to replace losses.
Maternal bone turnover increases during pregnancy to meet fetal demands as evidenced by an increase in bone formation and bone resorption markers. Circulating concentrations of biochemical markers of bone turnover increase gradually, with the highest concentrations measured in the third trimester (9–14). Markers of both bone formation and resorption increase significantly (P < 0.001) from the first to the third trimester, demonstrating the likely increase in both maternal bone turnover and fetal bone development (13).
Although bone markers of formation and resorption increase during pregnancy, it has been unclear how maternal bone density responds. Dual-energy X-ray absorptiometry (DXA) and peripheral quantitative computed tomography (pQCT) are the most common methods for assessing bone mineral content (BMC), density, and geometry. In addition, pQCT assesses volumetric density and theoretical strength and distinguishes between trabecular and cortical bone. The majority of studies have used DXA to measure bone mineral density (BMD) at preconception and early in the postpartum period. Advantages to using pQCT to assess bone density are that it can be used during pregnancy because peripheral sites are assessed (the abdomen can be shielded), the radiation dose per measurement is <0.1 mSv, and trabecular and cortical bone are measured separately, which allows for changes to be more clearly seen. To date, Wisser et al. (15) are the only investigators to use pQCT for measuring gestational changes in trabecular and cortical bone; only 1 skeletal site was assessed, a distal location in the nondominant radius. A significant decrease in radial trabecular bone density (median: −1.6%) was observed.
Pregnant women consuming low-calcium diets (<500 mg/d) have higher rates of bone resorption as measured by cross-linked C-terminal telopeptide of type 1 collagen (CTx) (14, 16); a low calcium intake is correlated significantly with higher CTx concentrations [CTx R2: −0.47, P < 0.03; N-terminal telopeptide cross-links (NTx) R2: −0.41, P < 0.05] and a negative calcium balance (13). Women with higher dietary calcium intakes (mean: 1015 mg/d) had lower concentrations of the bone resorption marker NTx (r = −0.015, P < 0.005), suggesting that higher calcium intakes reduce bone resorption in late pregnancy (16). An additional 1200 mg Ca supplementation/d to pregnant Mexican women normally consuming 1000 mg/d reduced the urinary NTx concentrations by 20% at 1 mo postpartum (8).
It is not known if the current calcium Recommended Dietary Allowance (RDA) is sufficient to meet maternal reproductive demands by 1 y postpartum when menses has resumed and a second pregnancy could occur. The objective of this study was to determine the effect of maternal calcium supplementation on peripheral cortical and trabecular bone loss, bone turnover during pregnancy, and bone gain postpartum. The hypothesis was that the current dietary calcium RDA is insufficient to prevent maternal bone loss during pregnancy and to achieve full recovery of the bone lost by 1 y postpartum as measured by pQCT. This study is novel in that it uses pQCT to study BMD along with biomarkers of bone turnover to explore the response to calcium supplementation during gestation.
Methods
The Bone Health in Pregnancy (BHIP) Study (NCT01145573) was a double-blind, longitudinal, randomized controlled trial of calcium supplementation from 16 wk of pregnancy to term with follow-up measurements of bone mineralization and metabolism until 1 y postpartum. The clinical trial was reviewed and approved by the Children's Hospital and Research Center at Oakland, the Alameda County Medical Center Institutional Review Boards, and the University of California, San Francisco, Clinical and Translational Science Institute. All measurements were performed between March 2011 and December 2013.
The primary outcome was cortical and trabecular bone loss and regain from 16 wk of pregnancy to 1 y postpartum as measured by pQCT. Secondary outcomes were changes in the bone turnover markers CTx, osteocalcin, osteoprotegerin, and C-terminal propeptide of type 1 procollagen (CICP) from 16 wk of pregnancy to 12 mo postpartum.
Participants
Women were recruited via postings on electronic mailing lists targeting Oakland and East Bay Area parents. Women were also recruited from local prenatal clinics. Women who initially expressed an interest in the study were screened further by telephone. Those who passed the telephone screen and who were interested in participating were scheduled for the first study visit. Inclusion criteria included the following: age 19–45 y, ≥12 wk pregnant, nonsmoker, English as the primary language, able to give written informed consent, and no plans to move from the area in the next 1.5 y. Exclusion criteria included prepregnancy BMI (in kg/m2) >35.0, presence of a current metabolic bone disease or other disorder known to affect calcium or bone metabolism, a malabsorption disorder, hyperemesis gravidarum, or current use of bisphosphonates.
The BHIP study was initially designed to have 3 arms: calcium supplementation (1000 mg/d), vitamin D supplementation (2000 IU/d), or placebo. Slow recruitment of potential study subjects required reducing the study size to 2 arms: calcium supplementation or placebo. Before this decision was made, 2 women were randomly assigned to the vitamin D supplement group and received vitamin D supplements for ∼12 wk (until 28 wk of gestation). These women were moved into the placebo group.
Study visits
Women reported to the clinical study center at 5 time points for measurements: baseline/first trimester (mean ± SD: 16 ± 2 wk, visit 1), second trimester (26 ± 2 wk, visit 2), third trimester (36 ± 2 wk, visit 3), and 4 mo (visit 4) and 12 mo (visit 5) postpartum. Dietary calcium and vitamin D intake and blood and urine chemistry indexes were measured at each time point. Bone measurements were taken at baseline and visits 3–5.
At baseline, the women were randomly assigned to receive a daily supplement of 1000 mg calcium carbonate or placebo. All study participants received a daily prenatal vitamin mineral supplement (RiteAid Brand) to take during pregnancy, which contained 200 mg Ca, 400 IU vitamin D, 27 mg Fe, 25 mg Zn, and 800 μg folate along with other essential vitamins and minerals. Participants were instructed to discontinue other supplements and to take 2 calcium or placebo tablets daily, 1 in the morning and 1 in the evening, for the duration of their pregnancy. Using NHANES data, we estimated that prestudy usual dietary intake of our study participants would be ∼800 mg Ca/d. We estimated that women in the calcium group would consume approximately twice as much calcium as the placebo group.
Supplement and adherence
The calcium supplement contained 500 mg Ca as calcium carbonate in each capsule (Tischon Corporation; certificate of analysis, 548.4 mg). The placebo capsule was indistinguishable from the calcium supplement in size, color, and taste. Both kinds of tablets were dispensed from the Children's Hospital and Research Center at Oakland clinical pharmacy. A 3-mo supply of capsules (100 capsules/bottle) was provided to each study participant at baseline (visit 1) and in the second trimester (visit 2). A study calendar was also provided to each subject with a study-specific logo (BHIP) to remind subjects to take their supplements. Pill counts were made at each visit by study staff to assess adherence. Due to poor return of study bottles at the end of gestation, primarily due to lack of a defined study time point, the adherence data were not considered reliable and thus intention-to-treat analysis was used. Upon completion of the trial, capsules were analyzed by inductively coupled plasma atomic emission spectroscopy by the Elemental Analysis Facility at the Children's Hospital Oakland Research Institute (placebo: 0.0 mg; calcium: 556 ± 1.9 mg; n = 4 capsules in duplicate).
Subjects were recruited, stratified according to race, and randomly assigned to the calcium or placebo group (in a 1:1 ratio). The study statistician provided a block-randomization table to the clinical pharmacy before study initiation. All investigators and subjects were blinded to study group. The randomization code was provided to the principal investigator by the clinical pharmacy after the last subject completed the final postpartum visit.
Diet analysis
To assess patterns of dietary calcium and vitamin D intake, a food-frequency questionnaire (FFQ) was administered at visits 1–3 during the intervention period. This combined calcium and vitamin D–focused FFQ included 58 food items high in calcium or vitamin D. Two validated questionnaires were combined and used (17, 18). Frequency of consumption for each food in the questionnaire was queried as servings per day, week, or month during the past 3 mo. Intake was also assessed at the baseline visit by 24-h dietary recall. Dietary data were analyzed using Food Processor version 10.1 (ESHA Research).
Blood assessments and markers of bone turnover
Nonfasting blood was sampled from the antecubital vein of participants using the Safety Lok blood collection set (Becton Dickenson) and appropriate evacuated tubes. Serum separator tubes were used for collecting serum for use in analysis of bone markers (CTx, osteocalcin, CICP, and osteoprotegerin), parathyroid hormone, and 25-hydroxyvitamin D [25(OH)D]. Blood for bone marker assays was separated from serum using a Sorvall RC-5C centrifuge. Serum samples were frozen at −80°C until assays were performed. Samples were analyzed in duplicate. If the CV was >20% the sample analysis was repeated.
Serum osteocalcin, a marker of bone formation, was measured using a MicroVue Osteocalcin enzyme immunoassay kit (Quidel Corp.). The intra- and interassay CVs were 10% and 9.8%, respectively. Serum CTx is a marker of bone resorption. Osteomark CTx serum (Wampole Laboratories) is a competitive-inhibition ELISA. The intra- and interassay CVs were 13.9% and 6.9%, respectively. Serum osteoprotegerin, a negative regulator of bone resorption that acts as a decoy receptor for receptor activator of nuclear factor κB ligand (RANKL), was measured by enzyme immunoassay (ALPCO). The intra- and interassay CVs were 10.1% and 9.9%, respectively. Serum CICP, a potential biochemical indicator of collagen production and growth, was measured using a MicroVue enzyme immunoassay kit (Quidel Corp.). The intra- and interassay CVs were 5.5% and 7.1%, respectively.
To measure the serum concentration of 25(OH)D, samples were sent to ARUP National Laboratories (Salt Lake City, UT) in batches; quantitative HPLC tandem mass spectrometry was used to assay both vitamin D2 and D3 isoforms (no. 2002348). The kit, developed by ARUP Laboratories, had a sensitivity of 1.0 ng/mL (ARUP, personal communication, 2016). An internal standard was sent with each batch. Sufficiency was defined as a total 25(OH)D concentration >20 ng/mL (2).
Bone measurements
pQCT was performed using a Norland XCT 2000 pQCT X-Ray Bone Densitometer with Windows-based software (version 6.0); pQCT is a Food and Drug Administration–approved densitometric device. A Stratec cone phantom and a cortical phantom (Bone Diagnostics) were scanned before each subject measurement to verify instrument calibration (19). The reproducibility of primary and derived pQCT parameters [trabecular volumetric BMD (vBMD) and total vBMD] was determined in a group of 30 healthy volunteers by performing the tibial measurement in duplicate on one day. Precision error was calculated as root-mean-square SDs of the duplicate measurements (20). Reproducibility was 0.8% for trabecular vBMD at the 3% site of the distal tibia. The device was calibrated against an original hydroxyapatite European Forearm Phantom initially at the factory.
The left radius (nondominant arm) and tibia (weight-bearing site) were each scanned at 2 locations measured proximal to the end plate of the bone. Tibial length was obtained in duplicate using a segmometer (Rosscraft-Campbell Caliper 20) and the average was used for measurement. Similarly, radial length was obtained in duplicate using a ruler and the average was used for measurement purposes. A pQCT “scout” scan was performed first to determine the anatomical landmark for the end plate reference line placement as previously described (21). The software was then programmatically set to scan each subject at 3% and 38% of the tibial length proximal to the reference line, using a 0.4-mm voxel size and 25-mm/s scan speed. Total and trabecular vBMD (in milligrams per cubic centimeter) were analyzed at the 4% radial and 3% tibial sites. The 50% radius and 38% tibia are midshaft sites analyzed for cortical thickness (in millimeters), cortical BMC, vBMD, and endosteal and periosteal circumferences. Analytical thresholds for analysis of the distal metaphyseal sites (3% tibial and 4% radial) included bone thresholds of 200 and 600 mg/mm3, with contour mode 1 and peel mode 4. For the diaphyseal sites (38% tibial and 50% radial), a bone threshold of 710 mg/mm3 was used; for Stress Strain Index (SSI) a threshold of 300 mg/mm3 and separation mode 2 were used. SSI (in mm3), an in vivo measure of bone strength, was also calculated at the 38% and 50% sites. Bone Strength Index (BSI) was calculated using the equation: total area in mm2 × (total density in mg/cm3)2. Section modulus (in cubic millimeters), a parameter reflecting resistance to bending forces, was also measured midshaft.
The left tibia or radius was scanned unless there was an artifact that would affect the results (e.g., metal implant); in such cases, the contralateral limb was used. Each scan was ∼90 s in duration with an effective dose to the patient of <2 µSv/scan. For scans performed during gestation time points, all participants wore an abdominal lead apron during the duration of the pQCT procedure (10 min) to minimize the amount of radiation scatter that might reach the fetus.
Qualitative movement was assessed in the pQCT radial and tibial scans using the method of Blew et al. (21). Two reviewers (EBF, A Simonsen) independently reviewed each scan and scored them using a 5-item Likert scale (1 = no movement, 5 = significant movement). An average score for each scan was calculated and considered unusable if ≥4. A total of 197 radial and 197 tibial scans were scored, of which 8.6% of radial and 3% of tibial scans had moderate movement (score = 3) but were usable, whereas 1.5% of radial and 1% of tibial scans were removed due to significant movement (score = 4 or 5).
Statistical analysis
Descriptive statistics (frequency distributions or means ± SDs, as appropriate) were computed for each independent variable by group; the study groups were compared with respect to these variables at different time points using generalized estimating equations to account for repeated measures on each subject by day. The primary and secondary hypotheses of the group intervention effects were tested using linear models to assess outcome changes from baseline to postintervention days within and between groups. Covariates that were initially explored and included in the models were based on those that were observed to be different between the intervention groups at baseline (age, vitamin D, BMI for height and weight), as well as those that may be associated with the primary bone outcomes (e.g., length of breastfeeding). BMI was chosen to be included in the model in the place of weight and height. Race did not prove to be significant in our models and so was excluded. All linear regression models of the outcomes were adjusted for baseline values. When appropriate, we conducted Tukey-Kramer follow-up tests of multiple comparisons to determine which groups and time periods were significantly different. Data were analyzed using SAS version 9.4 (SAS Institute), and the value of P = 0.05 was used as the cutoff level for statistical significance; a level of 0.05 < P < 0.1 was considered a trend.
Sample size was based on the mean ± SD change in trabecular spine BMD (−2.84% ± 4.2%) obtained from 34 women from prepregnancy to postpartum (22) and the mean ± SD of the distal tibia (3% site by pQCT) in 34 healthy nonpregnant Caucasian females (aged 19–28 y) measured at our center (EB Fung, 2008, unpublished data). Sample size estimates were based on t tests contrasting changes over time and differences in these changes between 2 groups (calcium compared with placebo) for the targeted bone outcome: trabecular vBMD. Using these data, we estimated that a sample size of 18/group would have the power to detect a 4% difference in the change in vBMD between groups at any one time point. In addition, a sample size of 18/group would also have the power to detect a 3% longitudinal change over time. Previously published data supported these estimates. Ritchie et al. (5) observed a 9.3% decrease in spine BMD (a region high in trabecular bone) by pQCT between prepregnancy and 2 mo postpartum. Moreover, at the initiation of this study, the only published report of bone density using pQCT during pregnancy, by Wisser et al. (15), observed a median 1.6% loss in trabecular vBMD at the distal radius from the first to the third trimester of gestation, with large individual variability (+1.3% to −20.7%). All sample size estimates assumed 0.80 power and α = 0.05 with a 2-sided test.
Results
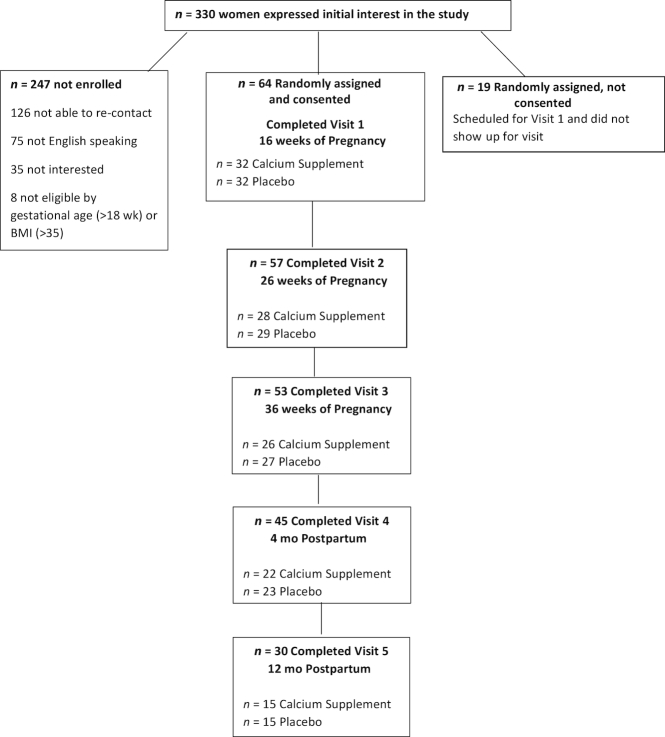
A total of 64 women consented and were randomly assigned to receive the calcium supplement (n = 32) or placebo (n = 32). Contact information was received for 330 women initially interested in participating in the trial (Figure 1). Of these, 83 women passed the initial phone screen and were assigned a study ID number, of whom 64 women consented to participate in the study and completed visit 1. Of the 64 women enrolled in the study, 20 self-classified as Caucasian, 21 as African American, and 23 as other race/ethnicity. This was the first pregnancy for half of the women. Of the initial cohort, 84% completed the intervention (through visit 3), and 47% were retained until the final visit at 12 mo postpartum, equally divided between the calcium and placebo groups (n = 15/group). Those subjects who dropped out were more likely to have participated in the Special Supplemental Nutrition Program for Women, Infants, and Children (P = 0.007) but did not differ with respect to age, race, BMI, weight change during pregnancy, calcium intake, serum 25(OH)D status, or any of the baseline bone variables.
FIGURE 1.
Flowchart of subject recruitment, enrollment, and retention from early pregnancy to 12 mo postpartum.
Baseline characteristics did not differ between the calcium and placebo groups (Table 1), except that the placebo group was, on average, 2 y younger, ∼3 cm taller, and 10 kg heavier than the calcium group. According to both FFQs and 24-h dietary recalls, dietary calcium and vitamin D intakes for both groups were below the current RDA; the mean ± SD intakes for the entire cohort were 733 ± 350 mg/d and 262 ± 158 IU/d using a validated FFQ, respectively, and 918 ± 589 mg/d and 146 ± 142 IU/d by recall, respectively (Figure 2). The mean serum 25(OH)D concentrations were >30 ng/mL for both groups at 16 wk of pregnancy (calcium: 31.3 ± 8.7 ng/mL; placebo: 32.0 ± 8.5 ng/mL), with only 34% of participants having a serum 25(OH)D concentration <30 ng/mL; of those, 22% had a concentration <20 ng/mL. Vitamin D concentrations decreased during gestation and increased postpartum but did not return to baseline concentrations by 12 mo postpartum in either group. These changes did not differ between the 2 groups (Figure 3). Weight gain during pregnancy (the supplementation period) did not differ between groups (8.9 ± 4.7 and 9.7 ± 5.5 kg; data not shown).
TABLE 1.
Descriptive characteristics of women at baseline (16 wk of pregnancy)1
| Calcium supplement (n = 32) | Placebo (n = 32) | P | |
|---|---|---|---|
| Age, y | 31.4 ± 6.4 | 28.2 ± 6.0 | 0.04 |
| Race, % | |||
| Caucasian | 22 | 41 | NS |
| African American | 37 | 28 | |
| Other | 41 | 31 | |
| FFQ2 | |||
| Calcium, mg/d | 708 ± 380 | 757 ± 323 | NS |
| Vitamin D, IU/d | 249 ± 188 | 273 ± 125 | NS |
| Servings of dairy/wk3 | 14.3 ± 8.57 | 14.9 ± 8.6 | NS |
| PAQ, METs/d | 1925 ± 2416 | 2160 ± 2356 | NS |
| Serum 25(OH)D, ng/mL | 31.3 ± 8.7 | 32.0 ± 8.5 | NS |
| Serum calcium, mg/dL | 9.1 ± 0.4 | 8.9 ± 0.3 | NS |
| PTH, pg/mL | 24.1 ± 9.8 | 26.2 ± 17.2 | NS |
| Weight, kg | 70.0 ± 14.3 | 80.2 ± 19.4 | 0.02 |
| Height, cm | 162.8 ± 5.9 | 165.3 ± 5.2 | 0.08 |
| BMI, kg/m2 | 26.3 ± 4.7 | 29.3 ± 6.8 | 0.05 |
| Primiparous, % | 47 | 53 | NS |
| WIC participants, % | 59 | 59 | NS |
Values are means ± SDs unless otherwise indicated. P values are based on Student's t test for continuous variables or chi-square tests for categorical variables. P < 0.05 was considered significant. FFQ, food-frequency questionnaire; MET, metabolic equivalent; PAQ, Physical Activity Questionnaire; PTH, parathyroid hormone; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children; 25(OH)D, 25-hydroxyvitamin D.
Average dietary calcium and vitamin D intake determined by validated FFQ.
Servings of dairy (per week) based on USDA serving sizes.
FIGURE 2.
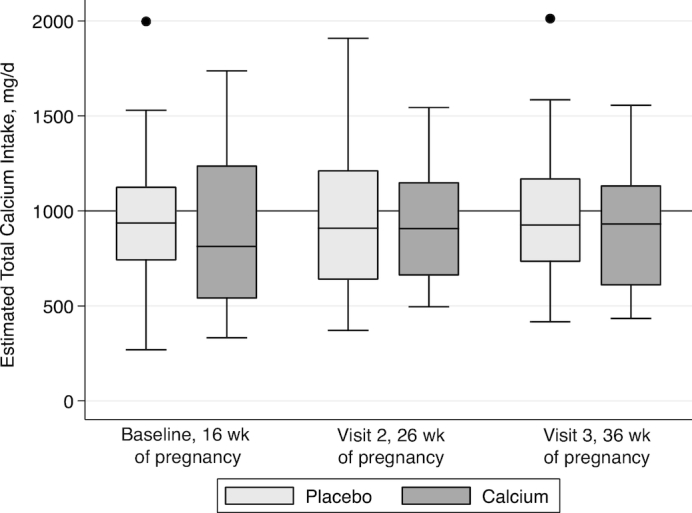
Total calcium intake (dietary and prenatal supplementation) during pregnancy in subjects provided placebo or calcium supplementation. In each box plot, the line represents the median, the outside edges of each box represent the IQR, hash marks represent the 5–95% range, and any values outside are represented by single points. The line in the graph represents the Recommended Dietary Allowance for calcium intake for pregnant women aged 19–50 y. Calcium intake (milligrams per day) was estimated by validated FFQ. Total intake = calcium by FFQ + prenatal supplemental intake (200 mg/d). These estimates do not include the calcium intervention supplement (1000 mg/d) provided to those in the calcium intervention group. No significant difference in total calcium intake was observed by group at any time point. Baseline visit: calcium, n = 32; placebo, n = 32; visit 2: calcium, n = 28; placebo, n = 29; visit 3: calcium, n = 26; placebo, n = 27. FFQ, food-frequency questionnaire.
FIGURE 3.
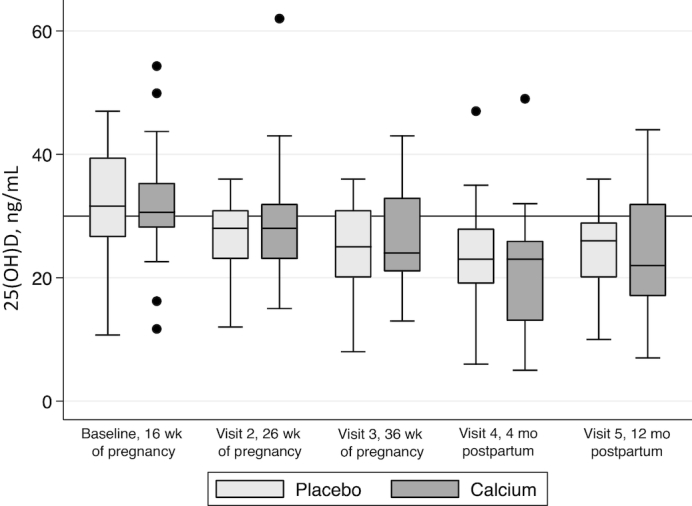
Serum 25(OH)D status from baseline to 12 mo postpartum. In each box plot, the line represents the median, the outside edges of each box represent the IQR, hash marks represent the 5–95% range, and any values outside are represented by single points. 25(OH)D decreased by 17.7% from baseline to 36 wk of gestation and increased slightly postpartum, although 12-mo-postpartum vitamin D concentrations never returned to baseline concentrations (P < 0.001, repeated-measures ANOVA). These changes did not differ by supplementation group. Baseline visit: calcium, n = 32; placebo, n = 32; visit 2: calcium, n = 28; placebo, n = 29; visit 3: calcium, n = 26; placebo, n = 27; visit 4: calcium, n = 22; placebo, n = 23; visit 5: calcium, n = 15; placebo, n = 15. 25(OH)D, 25-hydroxyvitamin D.
Repeated-measures ANOVA revealed significant group-by-time effects between the calcium and placebo groups from baseline to 12 mo postpartum (Table 2). Specifically, cortical vBMD at the tibial proximal diaphyseal and total vBMD at the radial proximal diaphyseal sites were significantly greater in the calcium group than in the placebo group (both P < 0.05). A number of trends were also observed in the bone outcome models (P < 0.1). The radial diaphyseal BSI, tibial metaphyseal BSI (data not shown), trabecular metaphyseal density, and total diaphyseal density variables all trended higher in the calcium group than in the placebo group, consistent with the hypothesis that supplemental calcium improved BMD compared with placebo by 12 mo postpartum.
TABLE 2.
Trabecular and cortical bone at the metaphyseal (3%) and diaphyseal (38%) tibia and metaphyseal (4%) and diaphyseal (50%) radius assessed by peripheral quantitative computed tomography from baseline to 12 mo postpartum in women receiving calcium supplementation or placebo during pregnancy1
| Visit 1 (baseline) | Visit 3 (36 wk of pregnancy) | Visit 4 (4 mo postpartum) | Visit 5 (12 mo postpartum) | |||||
|---|---|---|---|---|---|---|---|---|
| Calcium (n = 32) | Placebo (n = 32) | Calcium (n = 26) | Placebo (n = 27) | Calcium (n = 22) | Placebo (n = 23) | Calcium (n = 15) | Placebo (n = 15) | |
| Tibial distal metaphyseal site (3%), g/cm3 | ||||||||
| Total vBMD | 291 ± 28 | 296 ± 38 | 288 ± 28 | 298 ± 39 | 287 ± 27 | 292 ± 40 | 296 ± 24 | 277 ± 31 |
| Trabecular vBMD | 245 ± 28 | 253 ± 36 | 243 ± 27 | 255 ± 34 | 240 ± 24 | 251 ± 35 | 250 ± 202 | 236 ± 272 |
| Tibial proximal diaphyseal site (38%), g/cm3 | ||||||||
| Total BMD | 922 ± 57 | 932 ± 62 | 924 ± 49 | 938 ± 54 | 920 ± 52 | 935 ± 69 | 913 ± 442 | 921 ± 792 |
| Cortical vBMD | 1197 ± 22 | 1197 ± 15 | 1195 ± 20 | 1196 ± 16 | 1200 ± 17 | 1197 ± 18 | 1195 ± 182 | 1193 ± 112 |
| Radial distal metaphyseal site (4%), g/cm3 | ||||||||
| Total vBMD | 386 ± 45 | 386 ± 52 | 378 ± 43 | 389 ± 49 | 381 ± 52 | 387 ± 62 | 365 ± 35 | 379 ± 39 |
| Trabecular vBMD | 194 ± 38 | 192 ± 32 | 213 ± 37 | 219 ± 31 | 216 ± 37 | 212 ± 28 | 220 ± 49 | 202 ± 34 |
| Radial proximal diaphyseal site (50%), g/cm3 | ||||||||
| Total vBMD | 1013 ± 69 | 1002 ± 76 | 1007 ± 77 | 1003 ± 81 | 1013 ± 73 | 996 ± 87 | 1029 ± 552 | 975 ± 1002 |
| Cortical vBMD | 1209 ± 20 | 1205 ± 19 | 1199 ± 30 | 1206 ± 23 | 1206 ± 21 | 1204 ± 20 | 1214 ± 16 | 1196 ± 25 |
Values are means ± SDs for the n at each time point. Calcium: 1000-mg Ca supplement/d from 16 wk of gestation to delivery. Placebo: placebo daily from 16 wk of gestation to delivery. BMD, bone mineral density; vBMD, volumetric bone mineral density.
Group × time effects from baseline to 12 mo postpartum (visit 5) were observed for 2 variables (tibia diaphyseal cortical BMD: P = 0.015; radial diaphyseal total BMD: P = 0.029). Tibial metaphyseal trabecular BMD and diaphyseal total BMD trended (P < 0.10) toward significantly higher values in the calcium group compared with the placebo group by visit 5.
Absolute change in bone variables from baseline was also assessed (Table 3). These analyses further adjusted for BMI, baseline vitamin D status, and length of time breastfeeding for both the tibia and radius. Significant differences in cortical vBMD at the tibial proximal diaphyseal site were seen between baseline and 12 mo postpartum, with the calcium group gaining 2.3 g/cm3 (or +1.6%) and the placebo group losing −3.6 g/cm3 (−3.0%). Total proximal radial vBMD at the diaphyseal site changed significantly from baseline to 12 mo postpartum, with the calcium group gaining 6.7 g/cm3 (+1.6%) and the placebo group losing −4.2 g/cm3 (−2.8%). Finally, trends were observed in the group × time effect at the tibial distal metaphyseal site for trabecular vBMD, with a slight gain of +1.8% in the calcium group and a loss of −6.6% in the placebo group from baseline to 12 mo postpartum; gains in the total proximal tibial vBMD and radial diaphyseal site for cortical vBMD also tended to occur.
TABLE 3.
Absolute change from baseline to 12 mo postpartum in tibial and radial bone variables assessed by peripheral quantitative computed tomography in women receiving calcium supplementation or placebo during pregnancy1
| Time of study | P | |||||
|---|---|---|---|---|---|---|
| Dependent variable and supplement group | Visit 3 (36 wk of pregnancy; n = 53) | Visit 4 (4 mo postpartum; n = 45) | Visit 5 (12 mo postpartum; n = 30) | Group | Time | Group × time2 |
| Tibial distal metaphyseal site (3%), g/cm3 | ||||||
| Total vBMD | 0.087 | NS | NS | |||
| Calcium | −0.66 ± 2.13 | −2.34 ± 1.53 | −1.42 ± 2.19 | |||
| Placebo | −3.30 ± 1.26 | −6.48 ± 1.87 | −6.61 ± 2.53 | |||
| Trabecular vBMD | 0.067 | NS | 0.058 | |||
| Calcium | −1.41 ± 0.83 | −1.05 ± 1.18 | 0.26 ± 2.07 | |||
| Placebo | −2.58 ± 1.28 | −5.41 ± 1.77 | −6.71 ± 2.34 | |||
| Tibial proximal diaphyseal site (38%), g/cm3 | ||||||
| Total vBMD | NS | NS | 0.062 | |||
| Calcium | −3.78 ± 1.84 | −4.01 ± 6.07 | −0.71 ± 2.04 | |||
| Placebo | 1.15 ± 1.58 | 1.81 ± 1.54 | −1.51 ± 1.67 | |||
| Cortical vBMD | NS | 0.021 | 0.034 | |||
| Calcium | −1.33 ± 2.02 | 4.33 ± 1.49 | 2.27 ± 1.75 | |||
| Placebo | 0.39 ± 1.36 | 1.51 ± 1.46 | −3.55 ± 2.06 | |||
| Radial distal metaphyseal site (4%), g/cm3 | ||||||
| Total vBMD | NS | NS | NS | |||
| Calcium | −5.33 ± 6.38 | −2.82 ± 6.62 | −10.67 ± 6.34 | |||
| Placebo | −0.49 ± 4.89 | −3.43 ± 6.71 | 0.77 ± 6.51 | |||
| Trabecular vBMD | NS | 0.087 | NS | |||
| Calcium | −2.10 ± 1.35 | −1.86 ± 1.32 | −5.32 ± 1.74 | |||
| Placebo | −0.62 ± 1.02 | −1.20 ± 1.06 | −3.42 ± 2.45 | |||
| Radial proximal diaphyseal site (50%), g/cm3 | ||||||
| Total vBMD | NS | NS | 0.018 | |||
| Calcium | −7.01 ± 3.75 | −1.74 ± 2.97 | 6.74 ± 4.89 | |||
| Placebo | 1.04 ± 2.19 | 0.48 ± 2.85 | −4.23 ± 3.69 | |||
| Cortical vBMD | NS | NS | 0.053 | |||
| Calcium | −6.75 ± 4.60 | −0.83 ± 3.12 | 6.69 ± 5.74 | |||
| Placebo | −0.52 ± 3.08 | 0.09 ± 2.73 | −7.47 ± 5.01 | |||
Values are adjusted means ± SEs. Baseline values are not included in the table because they were adjusted for in the statistical models: values shown represent the change from baseline. Calcium: 1000-mg Ca supplement/d from 16 wk of gestation to delivery. Placebo: placebo daily from 16 wk of gestation to delivery. vBMD, volumetric bone mineral density.
Linear mixed models for longitudinal data were used to derive the P values after adjusting for baseline bone values, serum 25-hydroxyvitamin D, length of breastfeeding (in months), and BMI (in kg/m2).
CTx gradually increased from 16 to 36 wk of pregnancy; the gain reached significance at 4 mo postpartum in both groups (P < 0.05; Table 4). Within-group longitudinal differences were observed in each of these bone markers (data not shown in the table); the only significant group-by-time effect that was observed was for CTx between the calcium and placebo groups from baseline to 12 mo postpartum (P = 0.044). The timing of bone loss differed between groups. Bone resorption was greatest for the calcium group at the third trimester time point (visit 3) but was highest for the placebo group at 4 mo postpartum (visit 4).
TABLE 4.
Bone markers of resorption and deposition from baseline to 12 mo postpartum in women taking calcium supplements or placebo1
| Visit 1 (baseline; 16 wk of pregnancy) | Visit 2 (26 wk of pregnancy) | Visit 3 (36 wk of pregnancy) | Visit 4 (4 mo postpartum) | Visit 5 (12 mo postpartum) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calcium (n = 32) | Placebo (n = 32) | Calcium (n = 28) | Placebo (n = 29) | Calcium (n = 26) | Placebo (n = 27) | Calcium (n = 22) | Placebo (n = 23) | Calcium (n = 15) | Placebo (n = 15) | |
| Osteocalcin, ng/mL | 6.9 ± 1.9 | 7.7 ± 2.0 | 6.9 ± 1.7 | 7.1 ± 2.5 | 9.1 ± 3.1 | 9.3 ± 3.5 | 14.2 ± 2.5 | 17.3 ± 3.9 | 11.3 ± 3.6 | 13.9 ± 3.4 |
| CICP, pmol/L | 6.4 ± 1.9 | 6.67 ± 2.2 | 7.2 ± 2.7 | 9.1 ± 5.0 | 9.7 ± 2.8 | 13.9 ± 13.8 | 9.9 ± 3.3 | 16.5 ± 14.4 | 8.8 ± 2.9 | 10.3 ± 7.8 |
| CTx, nM BCE/L | 0.2 ± 0.1 | 0.3 ± 0.5 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.4 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.5 ± 0.3 | 0.2 ± 0.12 | 0.3 ± 0.22 |
| Osteoprotegerin, pmol/L | 9.0 ± 3.8 | 10.1 ± 5.9 | 12.9 ± 5.4 | 13.4 ± 7.5 | 17.4 ± 6.7 | 20.2 ± 14.5 | 6.9 ± 1.9 | 5.9 ± 3.2 | 6.9 ± 3.9 | 4.9 ± 2.7 |
Values are means ± SDs. Statistical analysis: group (calcium compared with placebo) × time effects reported from baseline (visit 1) to 12 mo postpartum (visit 5) using general linear models. Calcium: 1000 mg Ca/d from 16 wk of pregnancy to delivery. Placebo: placebo capsules daily from 16 wk of pregnancy to delivery. BCE, bone collagen equivalent; CICP, C-terminal propeptide of type 1 procollagen; CTx, cross-linked C-terminal telopeptide of type 1 collagen.
P = 0.044, group × time effect from baseline to 12 mo postpartum.
Discussion
Previous research has shown that women consuming inadequate amounts of calcium benefit from calcium supplementation during pregnancy (12, 22, 23). In this randomized, placebo-controlled calcium supplementation trial, we found that women consuming a mean of 700 mg Ca/d plus supplemental calcium (1000 mg/d) showed some improvements in bone recovery at diaphyseal sites at 12 mo postpartum as measured by pQCT.
The majority of previous studies of gestational bone changes have used DXA to assess BMD at preconception and again early in postpartum (5, 24–31). Early postpartum bone measurements may be confounded by the bone loss that normally occurs in early lactation (5, 32). One study (5) found reductions in maternal bone, with the biggest changes occurring at the lumbar spine and the trochanteric region of the hip (3–4.5% loss) at 8- to 10-wk postpartum. Without baseline preconception measures for comparison, the true extent of bone loss cannot be measured by DXA during gestation because of the potential to cause harm to the fetus. Therefore, assessing via pQCT peripheral sites that are rich in both cortical and trabecular bone is a potential alternative.
We hypothesized that calcium supplementation would improve bone recovery postpartum at the metaphyseal sites (3% tibia and 4% radius) that are predominantly trabecular bone. Our results showed that calcium supplementation during pregnancy tended to improve trabecular bone density at the tibia until 1 y postpartum. We did not expect an improvement in cortical bone, given that it is a pool of bone that is very slow to turn over in adults. However, cortical bone density changes, as measured by pQCT, were also seen previously with increases in physical activity but not with changes in calcium intake (33, 34). In our study, no differences in reported physical activity levels were observed between the calcium and placebo groups during pregnancy. Because our physical activity questionnaire was not administered in the postpartum period, it is not known if differences in activity levels contributed to the increase in cortical bone density in the calcium-supplemented group. Although our women gained a mean of 9 kg in weight during pregnancy, we did not observe an effect of gestational weight gain on the postpartum increase in cortical density as has been seen in nonpregnant adults (35).
Trabecular BMD increased in the calcium-supplemented group between week 36 of pregnancy and 12 mo postpartum (i.e., visits 3 and 5), but no significant group × time effects were observed between the calcium and placebo groups. The lack of a difference between the 2 groups may reflect our limited sample size, the subjects’ adequate calcium intake prepregnancy, or the high variability in the forearm pQCT scans (CV: 18% radius compared with 12% for tibia). Brembeck et al. (35) measured BMD using high-resolution pQCT in lactating women and controls. They reported a loss in cortical thickness at the ultra-distal tibia (similar to our metaphyseal site) at 12 mo (−2.48% ± 0.41%) and trabecular thickness at 18 mo (−2.25% ± 1.25%) postpartum if the women lactated for >4 mo. In our study, women breastfed for a mean of 5.9 mo, and cortical density at the tibial diaphyseal site increased 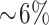 from baseline to 12 mo postpartum in the calcium compared with the placebo group (Table 3).
from baseline to 12 mo postpartum in the calcium compared with the placebo group (Table 3).
Our study is unique in that bone was measured during pregnancy using pQCT, which provides an analysis of trabecular and cortical bone parameters. Measurements of BMD using DXA at pre- and postpartum time points have reported decreases in BMD at the lumbar spine, hip, and whole body both during pregnancy and through lactation (24, 27, 29, 36). Møller et al. (36) reported that among Danish women consuming adequate dietary calcium, only 24% had recovered their prepregnancy whole-body BMD by 12 mo postpartum. Wei et al. (31) evaluated BMD using DXA in the first trimester of pregnancy and again at postpartum and found no relation between BMD, BMC, and serum 25(OH)D status. However, African-American women and those who were obese at 12 wk of pregnancy had a greater loss of BMD at the femoral neck and spine than did Caucasian and nonobese women. In our study, BMD recovery occurred at both peripheral weight-bearing (tibial) and non–weight-bearing (radius) bone sites when supplemental calcium was provided.
Women in the calcium group had a different pattern of bone resorption (as characterized by CTx) compared with the placebo group (see Table 4). Using NTx, Ettinger et al. (8) reported a decrease in bone resorption at 1 mo postpartum in Mexican women who received 1200 mg supplemental Ca/d during pregnancy compared with a placebo. We did not observe significant change in our bone turnover markers between 16 wk of gestation and 12 mo postpartum. These divergent findings may reflect the heterogeneity of our women in terms of ethnicity, parity, breastfeeding patterns, and dietary intakes. For example, in our initial sample of 64 women, one-third identified as Caucasian, one-third African American, and one-third as “other,” which included Asian descent and Latinas.
Limitations of our study include the lack of a measure of adherence to the intervention, subject attrition, and differences in body weight between groups at baseline. Participants were provided a study calendar and a 75-d supply of study supplements at the 16- and 26-wk study visits. They were asked to return the pill bottles and calendars at the subsequent visit. Only one-third of the women returned the empty pill bottles or calendars. Thus, the study staff only recorded a subject's verbal statement that they adhered to the study pill regimen. Other investigators have reported that assessing compliance to a vitamin D supplementation by pill counts was an unreliable predictor of compliance; participant adherence to supplements was 82.9% when serum metabolites were measured but it was only 46.6% when determined by pill counts (37). Loss of participants before the final time point (i.e., 17 mo after baseline) was another limitation. Attrition during pregnancy was only 18%, but it increased to 47% by 12 mo postpartum. During the postpartum period, study staff called the study participants every 2 mo to ask about infant feeding practices and resumption of menses, but disconnected phone lines made this task challenging. Fortunately, an equal number of women were lost from each group. Another limitation was the differences in body weight between the 2 groups at baseline; the placebo group was 10 kg heavier than the calcium group. These differences were included in the longitudinal bone models (baseline BMI), but the confounding effect of increased weight bearing on the skeleton could have had a positive influence on bone retention in the placebo group.
In sum, this is the first study, to our knowledge, to assess the effect of calcium supplementation during pregnancy on measures of changes in cortical and trabecular bone from 16 wk of pregnancy to 1 y postpartum. At baseline, the women consumed close to the calcium Dietary Reference Intake for pregnancy. Those receiving 1000 mg supplemental Ca had a 4–5% greater recovery of cortical and total bone density at a weight-bearing site postpartum. Thus, 1000 mg supplemental Ca/d during pregnancy may improve bone recovery during the first 12 mo postpartum in women with calcium intakes averaging ∼700 mg/d.
ACKNOWLEDGEMENTS
We thank Mickey Adams and her colleagues at Highland Hospital for assistance in recruitment and Aenor Sawyer for assistance with procurement of the pQCT. We also thank Leonid Ber at Now Foods for donating prenatal supplements. Unfortunately, because the study design changed, we were unable to use them in this study, but his generosity and scientific curiosity were an inspiration. Finally, we thank Anja Simonsen for her assistance with independent re-analysis of the pQCT scans for movement.
The authors’ responsibilities were as follows—JCK, EBF, AC, and MVL: designed the research; AC and EBF: conducted the research; GG, EBF, and AC: analyzed the data; EBF and AC: wrote the manuscript and had primary responsibility for the final content; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported in part by a USDA grant and by the National Center for Advancing Translational Sciences of the NIH, University of California San Francisco Clinical and Translational Science Institute grant UL1 TR000004.
The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Present address for AC: Missouri Southern State University, 3950 E Newman Rd, Joplin, MO 64801.
Abbreviations used: BHIP, Bone Health in Pregnancy; BMC, bone mineral content; BMD, bone mineral density; BSI, Bone Strength Index; CICP, C-terminal propeptide of type 1 procollagen; CTx, cross-linked C-terminal telopeptide of type 1 collagen; DXA, dual-energy X-ray absorptiometry; FFQ, food-frequency questionnaire; Ntx, N-terminal telopeptide cross-links; pQCT, peripheral quantitative computed tomography; RDA, Recommended Dietary Allowance; SSI, Stress Strain Index; vBMD, volumetric bone mineral density; 25(OH)D, 25-hydroxyvitamin D.
References
- 1. Institute of Medicine. Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC):National Academies Press; 1997. [PubMed] [Google Scholar]
- 2. Institute of Medicine (IOM). Dietary Reference Intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 3. Kalkwarf HJ, Specker BL, Heubi JE, Vieira NE, Yergey AL. Intestinal calcium absorption of women during lactation and after weaning. Am J Clin Nutr. 1996;63(4):526–31. [DOI] [PubMed] [Google Scholar]
- 4. Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61(3):514–23. [DOI] [PubMed] [Google Scholar]
- 5. Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. 1998;67(4):693–701. [DOI] [PubMed] [Google Scholar]
- 6. Vargas Zapata CL, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. Am J Clin Nutr. 2004;80(2):417–22. [DOI] [PubMed] [Google Scholar]
- 7. Sparks JW. Human intrauterine growth and nutrient accretion. Semin Perinatol. 1984;8(2):74–93. [PubMed] [Google Scholar]
- 8. Ettinger AS, Lamadrid-Figueroa H, Mercado-García A, Kordas K, Wood RJ, Peterson KE, Hu H, Hernández-Avila M, Téllez-Rojo MM. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican women. Nutr J. 2014;13(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hellmeyer L, Ziller V, Anderer G, Ossendorf A, Schmidt S, Hadji P. Biochemical markers of bone turnover during pregnancy: a longitudinal study. Exp Clin Endocr Diabetes. 2006;114(9):506–10. [DOI] [PubMed] [Google Scholar]
- 10. Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15(3):557–63. [DOI] [PubMed] [Google Scholar]
- 11. Yamaga A, Taga M, Hashimoto S, Ota C. Comparison of bone metabolic markers between maternal and cord blood. Horm Res Paediat. 1999;51(6):277–9. [DOI] [PubMed] [Google Scholar]
- 12. Bezerra FF, Mendonça LM, Lobato EC, O'Brien KO, Donangelo CM. Bone mass is recovered from lactation to postweaning in adolescent mothers with low calcium intakes. Am J Clin Nutr. 2004;80(5):1322–6. [DOI] [PubMed] [Google Scholar]
- 13. Zeni SN, Ortela Soler CR, Lazzari A, López L, Suarez M, Di Gregorio S, Somoza JI, de Portela ML. Interrelationship between bone turnover markers and dietary calcium intake in pregnant women: a longitudinal study. Bone. 2003;33(4):606–13. [DOI] [PubMed] [Google Scholar]
- 14. Yamaga A, Taga M, Minaguchi H. Changes in urinary excretions of C-telopeptide and cross-linked N-telopeptide of type I collagen during pregnancy and puerperium. Endocr J. 1997;44(5):733–8. [DOI] [PubMed] [Google Scholar]
- 15. Wisser J, Florio I, Neff M, König V, Huch R, Huch A, von Mandach U. Changes in bone density and metabolism in pregnancy. Acta Obstet Gynecol Scand. 2005;84(4):349–54. [DOI] [PubMed] [Google Scholar]
- 16. Avendaño-Badillo D, Hernández-Avila M, Hernández-Cadena L, Rueda-Hernández G, Solano-González M, Ibarra LG, Hu H, Téllez-Rojo MM. High dietary calcium intake decreases bone mobilization during pregnancy in humans. Salud Pública de México. 2009;51(Suppl 1):S100–7. [DOI] [PubMed] [Google Scholar]
- 17. Hacker-Thompson A, Schloetter M, Sellmeyer DE. Validation of a dietary vitamin D questionnaire using multiple diet records and the block 98 health habits and history questionnaire in healthy postmenopausal women in northern California. J Am Diet Assoc. 2012;112(3):419–23. [DOI] [PubMed] [Google Scholar]
- 18. Hacker-Thompson A, Schloetter M, Sellmeyer D. Validation of two food frequency questionnaires for dietary calcium assessment. J Am Diet Assoc. 2009;109(7):1237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5(4):262–70. [DOI] [PubMed] [Google Scholar]
- 20. Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34(6):1044–52. [DOI] [PubMed] [Google Scholar]
- 21. Blew RM, Lee VR, Farr JN, Schiferl DJ, Going SB. Standardizing evaluation of pQCT image quality in the presence of subject movement: qualitative versus quantitative assessment. Calcif Tissue Int. 2014;94(2):202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr. 2003;78(6):1188–93. [DOI] [PubMed] [Google Scholar]
- 23. Sowers MF, Scholl T, Harris L, Jannausch M. Bone loss in adolescent and adult pregnant women. Obstet Gynecol. 2000;96(2):189–93. [DOI] [PubMed] [Google Scholar]
- 24. Olausson H, Laskey MA, Goldberg GR, Prentice A. Changes in bone mineral status and bone size during pregnancy and the influences of body weight and calcium intake. Am J Clin Nutr. 2008;88(4):1032–9. [DOI] [PubMed] [Google Scholar]
- 25. Kaur M, Pearson D, Godber I, Lawson N, Baker P, Hosking D. Longitudinal changes in bone mineral density during normal pregnancy. Bone. 2003;32(4):449–54. [DOI] [PubMed] [Google Scholar]
- 26. Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34(3):570–8. [DOI] [PubMed] [Google Scholar]
- 27. Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr. 2008;88(2):500S–6S. [DOI] [PubMed] [Google Scholar]
- 28. Ulrich U, Miller PB, Eyre DR, Chesnut CH 3rd, Schlebusch H, Soules MR. Bone remodeling and bone mineral density during pregnancy. Arch Gynecol Obstet. 2003;268(4):309–16. [DOI] [PubMed] [Google Scholar]
- 29. Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R. The effect of pregnancy on bone density and bone turnover. J Bone Miner Res. 2000;15(1):129–37. [DOI] [PubMed] [Google Scholar]
- 30. Wei W, Shary JR, Garrett-Mayer E, Anderson B, Forestieri NE, Hollis BW, Wagner CL. Bone mineral density during pregnancy in women participating in a randomized controlled trial of vitamin D supplementation. Am J Clin Nutr. 2017;106(6):1422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jarjou LMA, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr. 2010;92(2):450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shedd KM, Hanson KB, Alekel DL, Schiferl DJ, Hanson LN, Van Loan MD. Quantifying leisure physical activity and its relation to bone density and strength. Med Sci Sports Exerc. 2007;39(12):2189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaffney-Stomberg E, Lutz LJ, Rood JC, Cable SJ, Pasiakos SM, Young AJ, McClung JP. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: a randomized, double-blind, placebo controlled trial. Bone. 2014;68:46–56. [DOI] [PubMed] [Google Scholar]
- 34. Kuh D, Wills AK, Shah I, Prentice A, Hardy R, Adams JE, Ward K, Cooper C; National Survey for Health and Development (NSHD) Scientific and Data Collection Team. Growth from birth to adulthood and bone phenotype in early old age: a British birth cohort study. J Bone Miner Res. 2014;29(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brembeck P, Lorentzon M, Ohlsson C, Winkvist A, Augustin H. Changes in cortical volumetric bone mineral density and thickness, and trabecular thickness in lactating women postpartum. J Clin Endocrinol Metab. 2015;100(2):535–43. [DOI] [PubMed] [Google Scholar]
- 36. Møller UK, við Streym S, Mosekilde L, Rejnmark L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos Int. 2012;23(4):1213–23. [DOI] [PubMed] [Google Scholar]
- 37. Schneider P, Butz S, Allolio B, Borner W, Klein K, Lehmann R, Petermann K, Tysarczyk-Niemeyer G, Wuster C, Zander C et al.. Multicenter German reference data base for peripheral quantitative computer tomography. Technol Health Care. 2015;3(2):69–73. [PubMed] [Google Scholar]