Abstract
Cerebral toxoplasmosis is the most common cause of expansive brain lesions in people living with HIV/AIDS (PLWHA) and continues to cause high morbidity and mortality. The most frequent characteristics are focal subacute neurological deficits and ring-enhancing brain lesions in the basal ganglia, but the spectrum of clinical and neuroradiological manifestations is broad. Early initiation of antitoxoplasma therapy is an important feature of the diagnostic approach of expansive brain lesions in PLWHA. Pyrimethamine-based regimens and trimethoprim-sulfamethoxazole (TMP-SMX) seem to present similar efficacy, but TMP-SMX shows potential practical advantages. The immune reconstitution inflammatory syndrome is uncommon in cerebral toxoplasmosis, and we now have more effective, safe, and friendly combined antiretroviral therapy (cART) options. As a consequence of these 2 variables, the initiation of cART can be performed within 2 weeks after initiation of antitoxoplasma therapy. Herein, we will review historical and current concepts of epidemiology, diagnosis, and treatment of HIV-related cerebral toxoplasmosis.
Keywords: cerebral toxoplasmosis, toxoplasmic encephalitis, toxoplasmosis, central nervous system, acquired immunodeficiency syndrome
What Do We Already Know About This Topic?
Cerebral toxoplasmosis remains the most common cause of expansive brain lesions in people living with HIV/AIDS (PLWHA). This disease presents high morbidity and mortality, particularly in low- and middle-income countries. In daily practice, clinical and neuroradiological features establish the presumptive diagnosis. Pyrimethamine-based schemes are classically preferred when available. Brain biopsy is usually reserved for patients who fail to respond to 10-14 days of antiparasitic therapy.
How Does Your Research Contribute to the Field?
This article is a critical and historical review of the terminology, epidemiology, case definition, diagnosis, treatment, and pharmacological prophylaxis of cerebral toxoplasmosis in PLWHA. In addition, an algorithm for the management of patients with suspicion of cerebral toxoplasmosis is proposed.
What Are Your Research’s Implications Toward Theory, Practice, or Policy?
This article shows the broad clinical and neuroradiological spectrum of cerebral toxoplasmosis in PLWHA. In this scenario, early treatment is key for a good outcome. If safe and feasible, polymerase chain reaction of Toxoplasma gondii on cerebrospinal fluid should be performed. Early brain biopsy should be strongly considered if there is a high index of suspicion of an alternative diagnosis to cerebral toxoplasmosis. Local algorithm is important in the management of PLWHA-related expansive brain lesions. Currently, if we consider the available evidence and the potential practical advantages, trimethoprim-sulfamethoxazole can be considered the preferred treatment of cerebral toxoplasmosis. The immune reconstitution inflammatory syndrome is not a major concern with cerebral toxoplasmosis and cART can be started within two weeks after the initiation of antiparasitic treatment.
Introduction
Toxoplasma gondii is a ubiquitous, intracellular protozoan parasite that causes cosmopolitan zoonotic infection. Acute T gondii infection is usually subclinical in the vast majority of immunocompetent individuals, and it is very rarely associated with severe clinical manifestations.1 On the other hand, cerebral toxoplasmosis is caused almost exclusively due to reactivation of latent brain cysts2 and can cause devastating consequences in host immunocompromised patients, particularly in people living with HIV/AIDS (PLWHA). If untreated, cerebral toxoplasmosis is uniformly fatal.1,3
Prior to 1980, cerebral toxoplasmosis was a rarely encountered complication of immunosuppression. The first cases of cerebral toxoplasmosis at the beginning of the AIDS epidemic were described between 1982 and 1983.4-6 As the number of PLWHA increased, cerebral toxoplasmosis became one of the most frequent opportunistic infections and the most common causes of focal brain lesions in this population.7 The most accepted explanation to the strong association between reactivated cerebral toxoplasmosis and AIDS is that these immunocompromised patients have a disturbed antiparasitic T-cell response and therefore fail to control this intracellular persistent parasite.8
Despite an important decline in both morbidity and mortality from countries with widespread access to combination antiretroviral therapy (cART), the occurrence of cerebral toxoplasmosis still represents a poor prognostic determinant in the natural history of PLWHA.9-11
Terminology
The predominant neuropathological hallmark of cerebral toxoplasmosis in PLWHA is multifocal necrotizing encephalitis.12,13 However, the term “toxoplasma abscess” has been used by several authors from the beginning of the epidemic4,14-17 to more recent years.18,19 Brain abscess is classically defined as intraparenchymal collection of pus,20-22 but HIV-related cerebral toxoplasmosis does not present pus. Brain abscess development can be divided into 4 histopathological stages: (1) early cerebritis, (2) late cerebritis, (3) early capsule formation, and (4) late capsule formation, pus can be observed in the last 3 stages.23 In addition, a significant delay in the evolution of the stages of brain abscess can occur in an immunocompromised host.24 Thus, early cerebritis that correlates better with the necrotizing encephalitis is characteristically observed in toxoplasmosis.23 For this reason, the terms ”toxoplasmic encephalitis,”7,25-27 “toxoplasma encephalitis,”28,29 or “cerebral toxoplasmosis”30-33 seem to be more appropriate than “toxoplasma abscess.”
Epidemiology
Toxoplasmosis is one of the most common infections in humans with a worldwide distribution, and it is estimated that about one-third of the global population is infected with latent toxoplasmosis.34 The prevalence and burden of T gondii infection in PLWHA shows geographic variability and usually follows the prevalence of T gondii in general population. The prevalence of coinfection in low-income countries, middle-income countries, and high-income countries is 55%, 34%, and 26%, respectively.35 Foci of high prevalence exist in Latin America, parts of Eastern/Central Europe, the Middle East, parts of Southeast Asia, and Africa.36
Availability of cART significantly reduced the incidence of cerebral toxoplasmosis in PLWHA from high-income countries and middle-income countries.9,10,37,38 In Brazil, where the prevalence of HIV in the general population was 0.4% in 2014, there has been an impressive decrease in the incidence rates of cerebral toxoplasmosis (43.6/1000 people-year [PY] between 1987 and 1990 and 4.0/1000 PY between 2009 and 2012; incidence rate ratio = 0.09, 2009-2012 versus 1987-1990; P < .001)10 and in its case fatality rate from pre-cART era (∼90%) to the cART era (∼15%-30%).39 However, the incidence rates of cerebral toxoplasmosis observed in Brazil are higher than those observed in other studies both from other middle- and high-income countries.10 High prevalence of T gondii infection in the general population and specifically in PLWHA explains this scenario.40 Other variables to persistent elevated incidence rates of cerebral toxoplasmosis observed in some settings include late HIV diagnosis, nonadherence to cART, failure of retention in care, and antiretroviral drug resistance.10,39,41
Nowadays, HIV-related cerebral toxoplasmosis continues to be the most common cause of focal brain lesion and an important cause of morbidity and mortality in PLWHA in several low- and middle-income countries.10,11,39,41-43
Case Definition
The use of diagnostic categories has been widely implemented in some opportunistic neurological diseases (ie, tuberculous meningitis, progressive multifocal leukoencephalopathy [PML]).44-46 The case definition for cerebral toxoplasmosis can contribute to the design and comparison of studies, to scientific communication, and even to the management of the disease. The following diagnostic categories are proposed:
Histology-confirmed cerebral toxoplasmosis requires a compatible clinical syndrome, identification of one or more expansive focal brain lesions by imaging, and brain biopsy (or postmortem examination) showing evidence of T gondii. Histopathological demonstration most commonly is obtained by a stereotactic computed tomography (CT)-guided needle biopsy. Hematoxylin and eosin stains can be used to demonstrate tachyzoites of T gondii, mainly in the periphery of the lesions, but sensitivity is significantly increased if immunoperoxidase staining is used.47
Laboratory-confirmed cerebral toxoplasmosis requires a compatible clinical syndrome, identification of one or more expansive focal brain lesions by imaging, and evidence of T gondii DNA in cerebrospinal fluid (CSF) by nucleic acid amplification assays.
Probable cerebral toxoplasmosis requires a compatible clinical syndrome, identification of one or more mass lesions by imaging, and unequivocal radiological response to 10 to 14 days of empiric antitoxoplasma therapy.
Possible cerebral toxoplasmosis requires a compatible clinical syndrome, identification of one or more mass lesions by imaging, presence of serum T gondii immunoglobulin G (IgG) antibodies, and no other alternative diagnosis.
The first two categories—histology-confirmed and laboratory-confirmed cerebral toxoplasmosis—can be considered a “definite” diagnosis. Probable cases correlate with “presumptive” diagnosis in clinical practice, considering the unnecessary use of histopathological studies in the vast majority of cases with cerebral toxoplasmosis and the unavailability of molecular diagnosis in most low- and middle-income countries. The possible cases category can be useful in some scenarios, for example, when patients eventually died or left the hospital within the first days of hospitalization without radiological control.
Syndromic Approach
The clinical presentations of neurological diseases in PLWHS are notably heterogeneous, nonspecific, and overlapping, but a correct diagnosis is essential for timely intervention.7,48
There are at least 2 main ways to classify the AIDS-related neurological complications: (1) according to their etiological agent—primary (caused by HIV itself) or secondary (opportunistic infections or neoplasms) diseases, and (2) by their neuroanatomical localization—central nervous system (CNS) and peripheral nervous system. These 2 classifications are complementary and help order diagnostic probabilities. Among CNS diseases, brain neurological diseases can be classified according to the predominant neurological syndrome in meningitis or encephalitis. The encephalic syndrome can be classified into focal or diffuse brain lesion. Finally, focal brain lesions may or may not show expansive effect.49-52
Complementary to the syndromic diagnosis, 3 aspects are relevant to establish the most probable etiologies of expansive focal brain lesions in PLWHA: (1) local neuroepidemiology (ie, tuberculomas is usually more common than primary central nervous system lymphoma [PCNSL] in low- and middle-income countries)53-56; (2) degree of immunosuppression (ie, lymphocyte CD4 count <200 cells/mm3 suggests opportunistic diseases; PCNSL usually occurs with lymphocyte CD4 count <50 cells/mm3)57,58; and (3) individual clinical, laboratorial, and neuroradiological features.51
The management of expansive focal brain lesions in PLWHA in high-income countries and in some middle-income countries has undergone several changes in approaches throughout the AIDS epidemic. Initially, the main strategy was to try to perform a biopsy of all patients; subsequently, the importance of antitoxoplasma “empirical” therapy was established; finally, molecular diagnosis and functional neuroradiology were incorporated in some referral centers.7,13,14,18,26,29,59-61 Despite this chronology, all these tools continue to be important in managing focal brain lesions in PLWHA. However, the availability of resources is highly heterogeneous and generally scarce or absent where opportunistic diseases are most prevalent at present.
The risk of cerebral toxoplasmosis is markedly increased in PLWHA who are seropositive for T gondii IgG antibody, have a lymphocyte CD4 count <100 cells/mm3, and are not receiving regular and effective prophylaxis.9,62 However, the absence of any combination of these variables does not rule out the possibility of cerebral toxoplasmosis. For example, between 3% and 15% of cases with cerebral toxoplasmosis are seronegative for T gondii IgG antibody,9,47,61,63 and 10% to 25% of cases have a lymphocyte CD4 count >100 cells/mm3.41,61,64
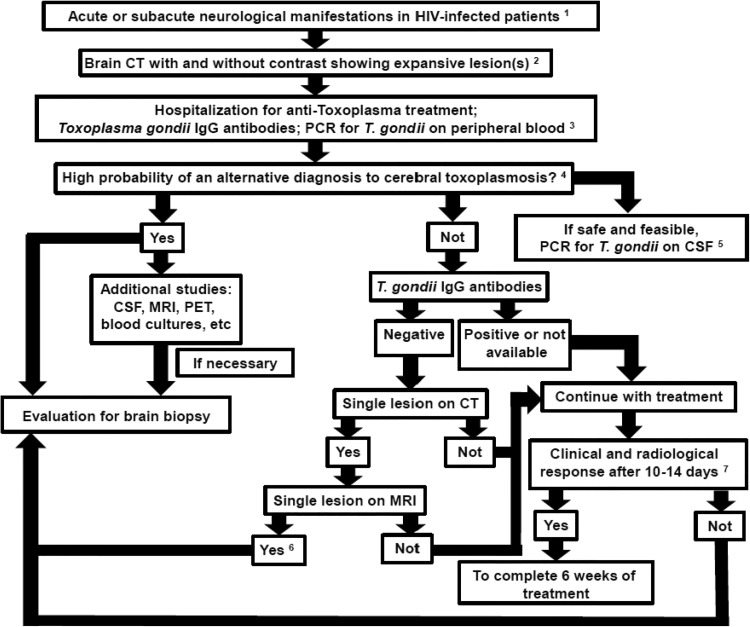
In clinical practice, severe immunocompromised PLWHA (lymphocyte CD4 count <200 cells/mm3) with compatible clinical and radiological findings of cerebral toxoplasmosis should receive antitoxoplasma therapy. Early suspicion and prompt treatment during the initial phase of cerebral toxoplasmosis reduce the risk of neurological sequelae and death. If no clinical and radiological improvement is seen within 10 to 14 days of antitoxoplasma therapy, alternative diagnoses to cerebral toxoplasmosis should be considered.18,25,27,47,65-67 Figure 1 shows a proposed algorithm for the management of suspected cases of cerebral toxoplasmosis in PLWHA.
Figure 1.
Proposed algorithm for the management of suspicion cases of cerebral toxoplasmosis in people living with HIV/AIDS (PLWHA). This algorithm is meant as guidance, and individual clinical situation may render deviation from this algorithm preferable; expert opinion remains vital.1 Up to 4 weeks of suggestive clinical manifestations of expansive brain lesions, such as headache, motor focal deficit, or altered mental status.2 Most common patterns in brain computed tomography (CT) scan are ring-enhancing lesions with perilesional edema, nodular-enhancing lesions with perilesional edema, and nonenhancing lesions with expansive effect. Magnetic resonance imaging (MRI) should be obtained in patients with equivocal or negative CT scans. If available, MRI is the imaging modality of choice for evaluating PLWHA with expansive brain lesions.3 Antitoxoplasma therapy is a tool for the diagnosis of expansive brain lesions in PLWHA. Therefore, close clinical follow-up is imperative. The absence of Toxoplasma gondii immunoglobulin G (IgG) antibodies and the negative polymerase chain reaction (PCR) in blood samples do not rule out the possibility of cerebral toxoplasmosis.4 This step is very important to suspect early an alternative diagnosis to cerebral toxoplasmosis. The main issues to be evaluated are: local neuroepidemiology; degree of immunosuppression; and individual clinical, laboratorial, and neuroradiological features.5 We have carefully evaluated whether there is a risk of cerebral herniation that may contraindicate lumbar puncture. A negative polymerase chain reaction (PCR) for T gondii in cerebrospinal fluid (CSF) does not rule out the possibility of cerebral toxoplasmosis.6 The absence of T gondii IgG antibodies and the presence of a single lesion on MRI suggest an alternative diagnosis to cerebral toxoplasmosis and a brain biopsy is usually indicated. However, if the patient demonstrates clinical improvement, a new brain imaging can be performed with 1 to 2 weeks of antitoxoplasma therapy, and if there is radiological improvement, biopsy will not be necessary.7 Clinical improvement usually precedes radiological improvement, but always consider the impact of corticosteroids regardless of the cause of the disease.
Diagnosis
Clinical Manifestations
Cerebral toxoplasmosis usually presents neurological subacute manifestations. However, the disease can show a rapidly progressing disease and fatal diffuse encephalitis or ventriculitis without evidence of focal brain lesions in imaging studies7,68,69 or even a stroke-like presentation.70
Clinical manifestations of the disease depend mainly on topography and number of lesions. The most common signs and symptoms are headache (38%-93%), focal neurological deficit (22%-80%), fever (35%-88%), mental confusion (15%-52%), seizures (19%-58%), psychomotor or behavioral changes (37%-42%), cranial nerve palsy (12%-28%), ataxia (2%-30%), and visual abnormalities (8%-19%). Patients may also present intracranial hypertension syndrome and involuntary movements.17,25,47,61,63,67,71 Patients can present any pattern of headache with or without other manifestation. A high index of suspicion is necessary, particularly when less common and isolated nonspecific manifestations are observed (ie, hemichorea, behavioral modification). Thus, a broad spectrum of clinical manifestations is possible, and brain imaging is indicated in order to evaluate empirical therapy.72 In the absence of treatment, progression of neurological abnormalities results in stupor, coma, and death. As expected, Glasgow coma scale ≤8 in patients with cerebral toxoplasmosis admitted at intensive care units is an independently associated variable with a poor outcome.72 Because cerebral toxoplasmosis predominantly causes encephalitis with little or no meningeal involvement, meningismus is rare.7
Pneumonia, chorioretinitis, and evidence of other multifocal organ system involvement can occur but are infrequently diagnosed in PLWHA. In addition, toxoplasmosis with multi-organ involvement manifesting with severe diffuse bilateral pneumonia and hemodynamic abnormalities, similar to septic shock, has been reported. These cases may or may not have concomitant cerebral toxoplamosis.73,74 Although cerebral toxoplasmosis is the most common presentation and apparently the single clinical complication in most PLWHA, autopsy studies of patients with toxoplasmosis showed 3 scenarios: (1) patients with only cerebral toxoplasmosis (∼65%); (2) patients with cerebral toxoplasmosis and extra-CNS involvement (∼25%); and (3) patients with only extra-CNS involvement (∼10%).75
Imaging
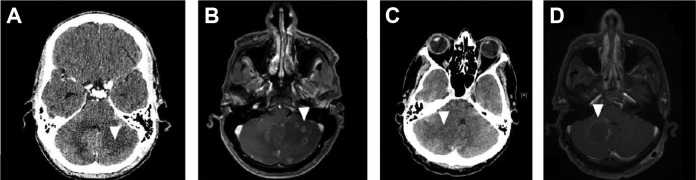
Magnetic resonance imaging (MRI) is the preferred modality for evaluating expansive brain lesions in PLWHA. Magnetic resonance imaging has sensitivity superior to that of CT scan for radiological diagnosis of cerebral toxoplasmosis and can impact the diagnosis and treatment of a subset of patients (Figure 2).76,77 However, CT is the most commonly available technique in most emergency department services. In this scenario, MRI should be obtained in patients with equivocal or negative CT scans. In addition, MRI does not appear to be more specific compared to CT in the differentiation of the etiologies of expansive brain lesions in PLWHA.62
Figure 2.
Contrast-enhanced computed tomography (CT) scan and magnetic resonance imaging (MRI) of an HIV-infected patient with cerebral toxoplasmosis (A and B). At admission, a hypodense lesion without contrast-enhancing in the left cerebellar hemisphere (A). After 3 days, an MRI showed several ring- or heterogeneous-enhancing cerebellar lesions associated with perilesional edema. Computed tomography scan and MRI of an HIV-infected patient with cerebral toxoplasmosis (C and D). At admission, a hypodense lesion without contrast enhancing in the right cerebellar hemisphere associated with perilesional edema and deviation of the fourth ventricle (C). After 5 days, an MRI showed a ring-enhancing cerebellar lesion associated with perilesional edema and lesser deviation of the fourth ventricle (D).
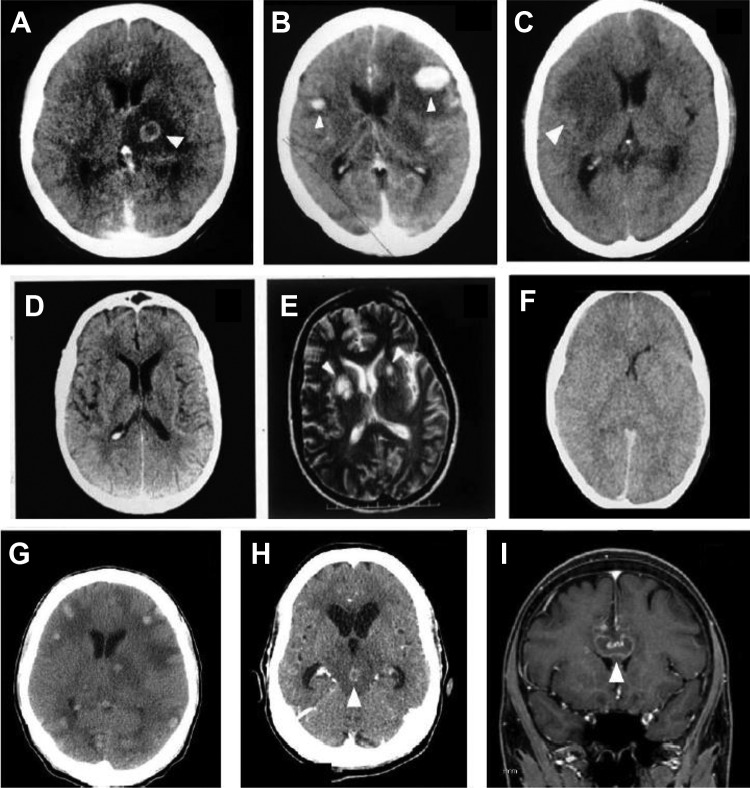
The typical CT and MRI findings in patients with cerebral toxoplasmosis are multiple ring-enhancing lesions in basal ganglia (48%), frontal lobe (37%), and parietal lobe (37%) with surrounding edema.63 In addition, occipital lobe (19%), temporal lobe (18%), and brain stem/cerebellum (5-15%) can be affected.63,78 Approximately 30% to 40% and 15% of patients with cerebral toxoplasmosis have a single lesion seen in CT and MRI studies, respectively.41,63,78, Cerebral toxoplasmosis is the most frequent cause of supratentorial or infratentorial single lesion in PLWHA. In CT images, despite ring-enhancing lesions with perilesional edema being the most common pattern (44%-82%), nodular-enhancing lesions with perilesional edema (3-33%) and nonenhancing lesions with expansive effect (6%-20%) can be observed.41,63,71,78 These radiological patterns depict high sensitivity but low specificity. Less frequent findings include diffuse cerebral edema without visible focal lesions (3%-15%) and CT without alterations but MRI demonstrating focal lesions (3%).41,61,78,79 Hemorrhagic alterations related to cerebral toxoplasmosis have been rarely reported in CT studies but are more frequent in MRI,80 particularly when a susceptibility-weighted imaging sequence is used.81 In most reports, cerebral toxoplasmosis showed small isolated areas of hemorrhagic foci or within the lesions and multiple cerebral ring hemorrhagic lesions. However, multiple rounded hemorrhages associated with perilesional edema can be a unique radiological finding.82,83 Figure 3 shows the spectrum of neuroradiological findings of cerebral toxoplasmosis in PLWHA.
Figure 3.
Brain computed tomography (CT) images showing the spectrum of neuroradiological findings of cerebral toxoplasmosis in people living with HIV/AIDS. Hypodense lesion with ring-enhancing and perilesional edema (A); nodular-enhancing and perilesional edema (B); expansive hypodense lesion without contrast enhancing and with mass effect (C); contrast-enhanced CT scan without abnormalities (D) and corresponding T2-weighted magnetic resonance imaging (MRI) showing multiple basal ganglia lesions, with high-intensity signals (E); contrast-enhanced CT scan showing diffuse edema (F). Non-contrast-enhanced CT scan of an HIV-infected patient showing several spontaneous hyperdense lesions associated with perilesional edema. Histopathology study confirmed the diagnosis of hemorrhagic toxoplasmosis (G). Contrast-enhanced CT scan of an HIV-infected patient showing a ring-enhancing lesion in the mesencephalon associated with perilesional edema and hydrocephalus. This lesion showed complete resolution after 4 weeks of antitoxoplasma therapy (H). Sagittal contrast-enhanced T1-weighted MRI shows a single ring-enhancing lesion crossing the corpus callosum (I). This patient underwent brain biopsy with the suspicion of primary central nervous system (CNS) lymphoma, but the histopathology study confirmed the diagnosis of cerebral toxoplasmosis. The arrows show the abnormalities.
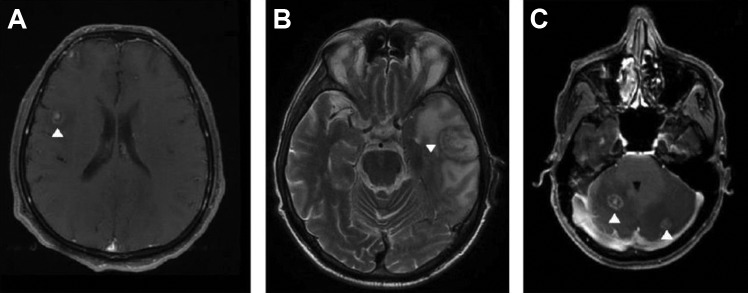
There are 2 imaging signs with low sensitivity but high specificity for the diagnosis of cerebral toxoplasmosis in PLWHA. First, the “eccentric target sign,” a ring-shaped zone of peripheral enhancement (on postcontrast CT or T1-weighted MRI) with a small eccentric nodule along the wall, is observed in <30% of cases.84,85 This sign needs to be differentiated from the “target sign” that has been defined as a central nidus of calcification or central enhancement surrounded by a ring of enhancement and has been considered a characteristic finding of CNS tuberculoma.86 However, the “target signal” is rarely found in other diseases, including cerebral toxoplasmosis.86 Second, the “concentric target sign” is a recently described MRI sign on T2-weighted imaging of cerebral toxoplasmosis with concentric alternating zones of hypo- and hyperintensities. It is believed to be more specific than the “eccentric target sign” in the diagnosis of cerebral toxoplasmosis in PLWHA.87 Figure 4 shows examples of cerebral toxoplasmosis lesions with the “eccentric target sign,” the “concentric target sign,” and the “target sign.”
Figure 4.
Magnetic resonance imaging (MRI) of HIV-infected patients with cerebral toxoplasmosis. T1-weighted imaging showed ring-enhancing brain lesion with a small, enhancing asymmetric nodule along the wall of the lesion (the “eccentric target sign; A). T2-weighted imaging showed a lesion with concentric alternating zones of hypo- and hyperintensities (the “concentric target sign”; B). T1-weighted imaging showed ring-enhancing brain lesion with a small, enhancing central nodule (the “target sign”; C). The arrows show the abnormalities.
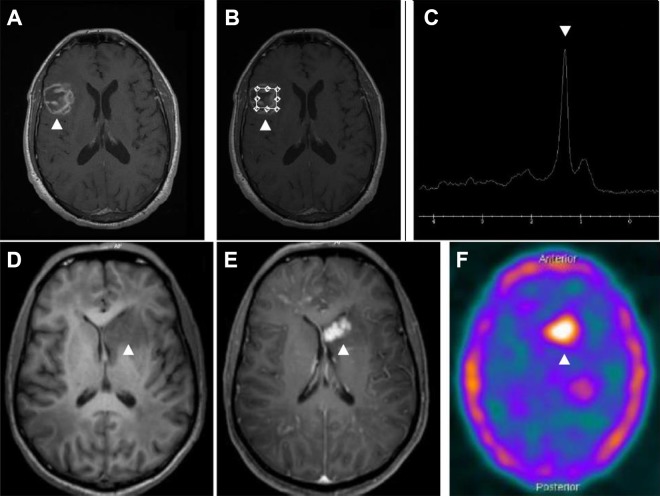
Although certain imaging features favor PCNSL over cerebral toxoplasmosis (ie, single lesion, periventricular lesion, subependymal spread, and homogenous rather than ring enhancement), considerable overlap occurs. Thus, the clinical and routine radiological techniques (CT or MRI) are not sufficiently specific to reliably differentiate PCNSL from cerebral toxoplasmosis. Over the past few decades, noninvasive functional nuclear imaging modalities such as magnetic resonance spectroscopy (MRS), 201Thallium single-photon emission computed tomography (SPECT), and positron emission tomography (PET) have been used to differentiate PCNSL from other expansive brain lesions in PLWHA, but the results are variable. A recent systematic review of MRS in the diagnosis of PCNSL (3 studies, 96 patients) shows sensitivity of 50% to 100% and specificity of 27% to 84%. In this study, meta-analysis could not be performed due to a limited number of eligible studies.88 In this study, systematic review and meta-analysis of 26 studies evaluated the performance of SPECT (18 studies, 667 patients) in the diagnosis of PCNSL in PLWHA. The pooled sensitivity and specificity were 92% and 84%, respectively.88 Figure 5 shows examples of MRS in cerebral toxoplasmosis and SPECT in PCNSL. Interestingly, CSF Epstein-Barr virus (EBV) polymerase chain reaction (PCR) shows sensitivity of 83% to 100% and specificity of 93% to 100% in patients with PCNSL but does not have high diagnostic accuracy to identify PCNSL.89 Neither SPECT nor CSF EBV PCR is able to separately identify PCNSL in PLWHA. In contrast, a study evaluated the combined use of SPECT with CSF EBV PCR for the diagnosis of PCNSL in PLWHA. When the SPECT was compatible with PCNSL and CSF EBV PCR was positive, the results of sensitivity, specificity, positive predictive value, and negative predictive value were 100%, 89%, 87%, and 100%, respectively. The authors concluded that combined SPECT and CSF EBV PCR showed a very high diagnostic accuracy for PCNSL in PLWHA.60 The systematic review of PET in the diagnosis of PCNSL (6 studies, 108 patients) showed a sensitivity of 100% and a specificity of 75% to 100% (4/6 had specificity of 100%), but meta-analysis could not be performed because no false negatives were reported for any of the 6 studies.88 Positron emission tomography seems to be superior than isolated SPECT but has less supporting clinical data and is more expensive.88
Figure 5.
Magnetic resonance imaging (MRI) and spectroscopy of an HIV-infected patient with cerebral toxoplasmosis (A-C). Gadolinium T1-weighted imaging showing a heterogeneous ring-enhancing brain lesion in right temporoparietal region (B). Spectroscopy image showing increased lipid peak and diminution of other metabolic activity corresponding to the known lesion (C). Magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose positron emission tomography–computed tomography (18F-FDG PET-CT) image of an HIV-infected patient with histopathologically confirmed primary central nervous system lymphoma (D-F). Gadolinium T1-weighted imaging showed irregular and nodular-enhancing brain lesion in left nucleocapsular region (E). 18F-FDG PET-CT image showing foci of increased metabolic activity corresponding to the known lesion (F). The arrows show the abnormalities.
Cerebrospinal Fluid
HIV-related cerebral toxoplasmosis usually has little or no meningeal involvement. For this reason, basic CSF characteristics are usually not relevant and only subtle abnormalities such as cell count and protein values normal or mildly elevated are described.90,91 Interestingly, eosinophils can be occasionally found in the CSF of patients with cerebral toxoplasmosis,92 and only one case of eosinophilic meningitis has been reported.93
Molecular Assays
The first PCR assay for T gondii detection was established 3 decades ago as an alternative to the more time-consuming and less direct procedures used at that time.94-96 However, the contribution of molecular techniques in the diagnosis of HIV-related cerebral toxoplasmosis is lower compared to other clinical situations caused by T gondii (ie, congenital toxoplasmosis)52,97 or other opportunistic neurological diseases (ie, PML or CNS cytomegalovirus).89
In patients with suspicion of cerebral toxoplasmosis, a lumbar puncture should only be performed if safe and feasible.98 Sensitivity of detection of T gondii DNA in the CSF of PLWHA shows results between 11% and 100% (∼50%-60%), specificity of 96% to 100%, positive predictive value of 100%, and negative predictive values of 71% to 92%.61,96,99-109 Despite its moderate sensitivity in most studies, the high specificity and positive predictive value of PCR assay has made it a useful tool in the diagnosis of cerebral toxoplasmosis. A positive CSF T gondii PCR assay result establishes the diagnosis of cerebral toxoplasmosis, but a negative result does not exclude it. Therefore, a negative result does not contraindicate the introduction or indicate discontinuation of antitoxoplasma therapy. Antiparasitic treatment significantly reduces the sensitivity of molecular diagnosis,100,110 and the first week is the period with the best performance.100,111 Although there is little information about it in the literature, CSF T gondii PCR seems to be useful in monitoring treatment efficacy.109 Simple anti-T gondii primary prophylaxis did not seem to reduce the sensitivity of PCR assay.112
In PLWHA with expansive brain lesions that contraindicate lumbar puncture, T gondii PCR assay in blood samples may be an alternative tool. However, the DNA concentration of T gondii is very low in the blood of patients with cerebral toxoplasmosis, and this feature appears to affect the sensitivity of the test.113 The sensitivity of T gondii PCR assay in blood samples of PLWHA shows a broad range of results between 1% and 86% (∼ 30%-50%).96,110,113-124 The performance of the T gondii PCR assay in blood samples of HIV-related cerebral toxoplasmosis seems to increase with the number of expansive brain lesions and particularly in the most severe forms with altered levels of consciousness.113,123 Although controversial, some T gondii strains may have a higher capacity of dissemination in blood, which may enhance the sensitivity of PCR in this compartment.124,125 In addition, the concomitant performance of CSF and blood sample of T gondii PCR seems to increase the individual sensitivity of each assay.118,126
Most molecular diagnostic studies of HIV-related cerebral toxoplasmosis were conducted in the 1990s with conventional PCR (nPCR),96,127 and this technique continues to be used as in-house assays in several laboratories. However, real-time quantitative PCR (qPCR) assay is currently the state-of-the-art molecular technique. Although nPCR and qPCR appear to show similar performances, the advantages of qPCR include rapid DNA detection, less risk of laboratory contamination with amplicons (which is an important source of false-positive reactions), robustness, reproducibility, and DNA quantification of T gondii.128-131 The use of commercial PCR kits appears to be an attractive approach, as they tend to be generally easy to use and standardized.132 However, commercial PCR assays generally exhibit equivalent or inferior performances to carefully developed laboratory tests (nPCR or qPCR).132-134 Thus, the proficiency of the laboratory performing the molecular diagnosis and the need for optimization of PCR conditions are crucial.135
Brain Biopsy
Histopathological diagnosis obtained by stereotactic brain biopsy is the classical and standard procedure for the etiological identification of focal brain lesions in PLWHA.61,136 The number of biopsies declined dramatically in the cART era,137 but this procedure still has relevant uses.
A recent meta-analysis (19 studies, 820 PLWHA underwent stereotactic brain biopsy) found a diagnostic success rate of 92%.138 This rate for brain biopsy compares favorably to other patient populations. Another meta-analysis (26 studies, 1209 PLWHA underwent stereotactic biopsy—24 studies, open brain biopsy—1 study, or both techniques—7 studies) showed a diagnostic success rate significantly higher in the post-cART than the pre-cART era (97.5% versus 91.9%, respectively, P = .047).139 This finding may be secondary at least in part due to improvement in surgical techniques, but probably the change in HIV-related disease epidemiology by introduction of cART is an important explanation for this difference.139 The rates of morbidity and mortality of PLWHA who underwent stereotactic brain biopsy are 5% and 0.7%, respectively.138 Management change was 60% and clinical improvement was 34%, presumably as a direct result for new information obtained from stereotactic brain biopsy.138
The 3 most common diagnoses obtained from stereotactic or open brain biopsies of focal brain lesion in PLWHA are PCNSL (15%-28%), PML (21-22), and cerebral toxoplasmosis (19%-20%).138,139
Stereotactic brain biopsy is a safe and effective way of diagnosing focal brain lesions in PLWHA and has therapeutic and clinical impact. The outcomes of brain biopsy are usually better in centers with higher technical expertise. Barriers to use of biopsy include clinical condition of the patients, topography of the lesion, its invasiveness, elevated costs, and structural requirements.62,140
The American Academy of Neurology published guidelines for the evaluation and management of AIDS-related intracranial mass lesions in 1998, which have not been revised since then.59 These guidelines recommend brain biopsy in adult patients having (1) large lesions with mass effect and impending brain herniation; (2) a combination of a single contrast-enhancing lesion and negative anti-T gondii IgG antibodies; (3) a cerebral lesion and increased uptake of 201Thallium SPECT; and (4) failure to respond after 10 to 14 days of antitoxoplasma therapy, indicated by persistence or worsening of either clinical symptomatology or the mass lesions observed in CT or MRI. Nowadays, there is some disagreement about these recommendations. First, this algorithm was devised as a means to indicate the need for early biopsy in a patient most likely to have PCNSL,141 the most common differential diagnosis of cerebral toxoplasmosis in high-income countries but not in most low- and middle-income countries. As a consequence, the elaboration of specific algorithms is necessary for the function of local neuroepidemiology and of the diagnostic tools available. Second, there is anecdotical evidence of decompressive craniectomy in HIV-related cerebral toxoplasmosis with impending brain herniation. In this situation, the postsurgical outcome seems to be almost uniformly fatal, and clinical management with neurointensive care may be a better option. Third, in settings with high prevalence of toxoplasmosis in the general population, patients with cerebral toxoplasmosis can present a single lesion and negative anti-T gondii IgG antibodies. Thus, antitoxoplasma therapy can be an initial approach in this scenario. Fourth, isolated SPECT showed lower specificity and lower positive predictive value for PCNSL diagnosis in the cART era.88,142 However, compatible SPECT associated with positive CSF EBV PCR had excellent accuracy for PCNSL diagnosis.60
Differential Diagnosis
Historically, the 2 leading causes of expansive focal brain lesions in high-income countries were cerebral toxoplasmosis and PCNSL. In low- and middle-income countries, tuberculomas are common and PCNSL is rarely reported. A myriad of etiologies may occasionally present with expansive brain lesions in PLWHA: Nocardia species, varicella zoster virus, Aspergillus species, Listeria monocytogenes, Treponema pallidum, Histoplasma capsulatum, and Cryptococcus neoformans. Eventually, bacterial,52,143 mycobacterial,144 or fungal145 abscesses, particularly in early stages of evolution in CT scan, may be misdiagnosed as cerebral toxoplasmosis. However, neuroradiological features of brain abscess are usually well characterized, and immediate neurosurgical evaluation is imperative.19
Although some features of lesions can suggest the presence of PCNSL (ie, a single bihemispherical lesion involving the corpus callosum) or CNS tuberculomas (ie, multiple nodular-enhancing lesions in a patient with pulmonary tuberculosis), clinical and radiological characteristics do not discriminate well enough in most cases between cerebral toxoplasmosis, PCNSL, and tuberculomas. The presence of typical and atypical presentations of cerebral toxoplasmosis is challenging, particularly in countries with a high incidence of T gondii infection. In this line, it is important to note that a common presentation of a rare disease is less likely than a rare presentation of a common disease.146 Early initiation of “empirical” anti-T gondii therapy is an important diagnostic tool in the management of expansive brain lesions in PLWHA. Figure 1 shows a proposed algorithm for the management of suspected cases of HIV-related cerebral toxoplasmosis.
Concomitant Neurological Diseases in PLWHA
Concomitant neurological diseases in PLWHA is a challenging subject that has not been sufficiently discussed in the literature. In some reports, the frequency of coexistence of neurological diseases in PLWHA ranged from 5% to 35% in neuropathological studies16,78,147-152 and between 9% and 24% in clinical studies.27,153-156 These clinical studies were carried out in referral centers of low- and middle-income countries, had different case definitions, and the access to diagnostic tools was heterogeneous.153-156 It is well known that there is a dramatic decrease in the incidence of AIDS-defining neurological diseases in the cART era in middle- and high-income countries.10,38 As expected, although information is scarce, the frequency of concomitant neurological diseases also appears to have decreased in the cART era. In a high-income country, the frequency of multiple concomitant neurological diseases due to different infectious agents and/or tumors decreased from 21% between 1984 and 1992 to 11% between 1996 and 1999.150 In addition, 2 prospective cohort studies carried out in a referral center from São Paulo, Brazil, found similar frequencies of concomitant neurological diseases, 14% in 2007 and 15% in 2017,157,158 showing the persistence of this problem at least in some settings. Subsets of PLWHA with prolonged and severe immunodepression (ie, late presenters, patients discontinuing cART, or drug resistance) seem to reflect a condition to concomitant neurological diseases. Spectrum of local neuroepidemiology, detailed neurological evaluation, close follow-up, and appropriate diagnostic tools seems to be other important aspects involved in the management of concomitant neurological diseases in PLWHA. This coexistence may have implications for the diagnosis, treatment, and outcome of PLWHA.
Treatment
Over the last 3 decades of the AIDS epidemic, approximately 50% to 90% of patients with cerebral toxoplasmosis demonstrate response with antitoxoplasma regimes.3,9,41,159-163 Nowadays, ∼80% to 90% of patients receiving antitoxoplasma therapy demonstrate clinical and radiological improvement. Case fatality rate of HIV-related cerebral toxoplasmosis significantly decreased in the cART era. For example, during hospitalization, case fatality rate varied from >90% in the pre-cART era to ∼30% in recent years in São Paulo, Brazil, and was ∼15% at a tertiary referral center in this state.39 These different results suggest the heterogeneity of results among the several facilities. The most important outcome variables seem to be severe neurological manifestations at admission, timely diagnosis and treatment, greater experience in managing this disease, and availability of appropriate resources (eg, imaging, intensive care unit).41,72 Nevertheless, the long-term outcome is challenging,9,64 and the rate of neurological sequelae due to cerebral toxoplasmosis continues to be high in the cART era (∼30%-40%)41,72 and has important impact in the quality of life of PLWHA.64
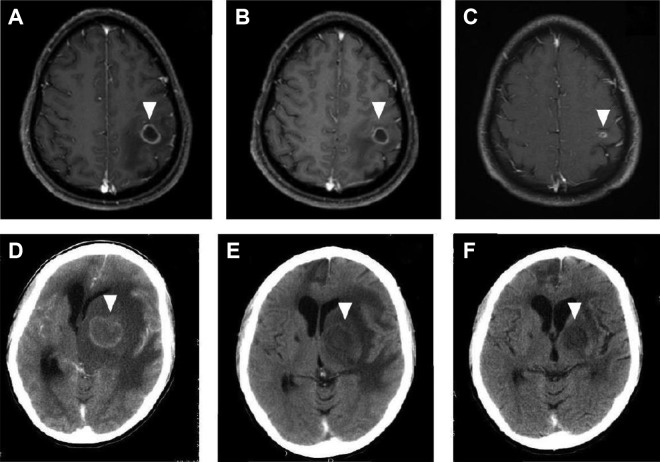
The median time of neurological response to HIV-related cerebral toxoplasmosis is 5 days.3 Of the patients who eventually improve, 86% show clinical improvement by day 7 of treatment and 95% show radiographic improvement by day 14 of treatment.3,63 Figure 6 shows examples of cerebral toxoplasmosis lesions during antitoxoplasma therapy.
Figure 6.
Magnetic resonance imaging (MRI) of an HIV-infected patient with cerebral toxoplasmosis (A-C). At admission, single lesion was observed in the left parietal lobe (A). After 2 weeks of antitoxoplasma therapy without corticosteroids, partial reduction in both size and perilesional edema was observed (B). After 6 weeks of antitoxoplasma therapy, marked decrease in lesion size and perilesional edema was seen (C). Contrast-enhanced computed tomography (CT) imaging of an HIV-infected patient with cerebral toxoplasmosis (D-F). At admission, extensive single lesion in the left basal ganglia causing brain herniation was seen (D). After 4 weeks of antitoxoplasma therapy with corticosteroids, marked reduction was observed in both size and perilesional edema (E). After 8 weeks of antitoxoplasma therapy, another CT scan showed residual alterations only (F). The arrows show the abnormalities.
Pyrimethamine plus sulfadiazine (P-S) has been used since the first described cases of cerebral toxoplasmosis in the AIDS epidemic.6 At that time, P-S had shown to be significantly more active on T gondii than trimethoprim-sulfamethoxazole (TMP-SMX) in experimental models (in vitro and in vivo),164 but there were no data on the use of TMP-SMX in human cerebral toxoplasmosis. In this scenario, P-S was consolidating as the preferred scheme for treating cerebral toxoplasmosis in PLWHA.
Pyrimethamine plus sulfadiazine acts synergistically by inhibiting T gondii proliferation and survival through inhibiting the folate metabolic pathway. The drugs inhibit dihydrofolate reductase and dihydropteroate synthase, respectively, and consequently block the synthesis of tetrahydrofolate, which is required by the parasite for DNA synthesis. Trimethoprim-sulfamethoxazole presents a similar mechanism of action. However, trimethoprim, unlike pyrimethamine, is a highly selective inhibitor of dihydrofolate reductase of T gondii. This feature explains the lower hematological toxicity caused by trimethoprim compared to pyrimethamine and why the use of folic acid is only necessary with the latter drug.165-167 Clindamycin, a lincomycin, inhibits T gondii by an unknown mechanism that involves the parasite organelle apicoplast.168
The preferred initial therapy for cerebral toxoplasmosis in Department of Health and Human Services of the United States,98 European AIDS Clinical Society,169 and British HIV Association170 guidelines is the combination of P-S. For patients with a history of sulfa allergy, sulfa desensitization should be attempted. If desensitization is not possible, pyrimethamine plus clindamycin (P-C) is the preferred alternative regimen in most guidelines98,169,170 although it is less effective in preventing relapses compared to P-S.159,161 Trimethoprim-sulfamethoxazole usually appears as alternative in these 3 guidelines, but other recommendations include TMP-SMX in the first-line therapies.171-173 In clinical practice, however, TMP-SMX is infrequently used when P-C and P-S are available. In contrast, TMP-SMX is the first choice in Africa and in other low- and middle-income countries, particularly where pyrimethamine-based regimens are not available or where there is experience with TMP-SMX.
Pyrimethamine-based regimens are beset by important limitations, including, adverse events, poor tolerability, complex posology, and the absence of parenteral formulations, a major problem in patients with alteration in mental status.174,175 Case series and clinical trials reported toxicity led to discontinuation of pyrimethamine-based regimens in approximately one-third of patients.63,159,161 A systematic review of adverse events associated with P-S or P-C in the treatment of toxoplasmosis (7 studies: 2 randomized clinical trials, 4 retrospective cohort studies, 1 prospective cohort study; 687 patients) reported 11% to 32% of discontinuation or change in pyrimethamine-based therapies because of adverse events. Bone marrow suppression and dermatologic complications were the most frequent adverse events.174
Recently, a systematic review and meta-analysis of relative efficacy and safety of treatment regimens for HIV-related cerebral toxoplasmosis (9 studies: 5 randomized clinical trials, 3 retrospective cohort studies, 1 prospective cohort study; 692 patients) was performed. In comparison to P-S, treatment with P-C or TMP-SMX was associated with similar rates of partial or complete clinical response, radiological response, and drug discontinuation because of adverse events.175 The current evidence fails to identify a superior regimen in terms of relative efficacy or safety for the treatment of cerebral toxoplasmosis. Real-world considerations are relevant when TMP-SMX is evaluated as a preferred treatment for cerebral toxoplasmosis. Potential advantages of TMP-SMX over P-S or P-C include (1) the convenience of the lower pill burden and dosing frequency and the availability of intravenous formulations; current guidelines suggest the option of intravenous TMP-SMX as initial treatment in severely ill patients98; (2) the availability of several generic TMP-SMX formulations with the consequent impact on cost-effectiveness and increased accessibility; (3) prevention of Pneumocystis jirovecii pneumonia, other bacterial infections, and malaria98,176; and (4) the convenience of use simplifying the early initiation of cART, which is associated with increased survival of HIV-infected patients with most opportunistic diseases.
There is no randomized clinical trial evaluating the efficacy of adjunctive steroids in HIV-related cerebral toxoplasmosis, but observational data showed no benefit with these drugs to treat cerebral edema in severe disease.72 Despite these limitations, the recommendation is to use steroids only when cerebral toxoplasmosis lesions have significant mass effect or when diffuse brain edema is observed.67 A suggestion can be to administer 1.5 mg/kg/d prednisone or dexamethasone equivalent for approximately 2 to 3 weeks and then steroid taper (if more than 2 weeks was used). The indiscriminate administration of steroids may result in a transient improvement in lesions of other etiologies, for example, PCNSL or tuberculomas, and complicates the evaluation of the response to anti-T. gondii treatment. Anticonvulsivants should be administered in the occurrence of seizures, but its prophylactic use should be discouraged.67
Immune Reconstitution Inflammatory Syndrome
The spectrum of immune reconstitution inflammatory syndrome (IRIS) includes: (1) paradoxical IRIS: worsening of symptoms of a previously diagnosed opportunistic infection for which the patient is receiving treatment, and (2) unmasking IRIS: diagnosis of a new opportunistic infection with inflammatory characteristics after the initiation of cART.177,178 Unmasking IRIS is difficult to differentiate from a classical presentation of cerebral toxoplasmosis. In paradoxical IRIS, alternative explanations for the worsening include failure of opportunistic disease treatment, failure of cART due to lack of adherence or drug resistance, and onset of other disease.
The incidence of IRIS varies with each pathogen, and its influence on the immune system is specific. Tuberculous meningitis, cryptococcal meningitis, and PML present the higher rates of CNS-related IRIS in PLWHA.179 In contrast, T gondii is an unusual cause of IRIS probably due to the mechanisms of immune evasion by this parasite.2,180,181 The first clinical case of cerebral toxoplasmosis–related IRIS was published in 2001,182 and the first pathological-proven case was reported in 2009.183 There are few case reports and 2 epidemiological studies about cerebral toxoplasmosis–related IRIS. In the first, 3 (4.6%) of 65 cases of cerebral toxoplasmosis were classified as unmasking IRIS,184 and no cases of paradoxical IRIS were reported. In the second, 5 (3.5%) cases of paradoxical IRIS of 143 cases at risk of paradoxical IRIS were identified, and 8 (0.4%) cases of unmasking IRIS of 2228 patients who started CART while having a CD4 count <200 cells/mL were described.185 Considering the abovementioned information, cerebral toxoplasmosis–related IRIS is rare but should be considered in the appropriate clinical and laboratory context.
No clinical trials are available on the management of IRIS-related cerebral toxoplasmosis. However, similar to other IRIS-related neurological opportunistic infections, steroids are the mainstay of therapy since they restore the blood–brain barrier, decrease T-cell activation, and prevent influx of inflammatory cells.186 Dosage and duration of steroids are partially based on clinical experience and extrapolated from other diseases.187 A recommendation can be (1) for mild forms of IRIS: 1 mg/kg/d prednisone (or dexamethasone equivalent) for 1 to 2 weeks; (2) for moderate forms of IRIS: 1.5 mg/kg/d prednisone (or dexamethasone equivalent) for 2 weeks followed by 2 weeks of 0.75 mg/kg/d (or dexamethasone equivalent) and then steroid taper; and (3) for severe forms of IRIS (severe edema or impending herniation): 1 g methylprednisone for 3 to 5 days followed by oral steroid taper. In patients who reside or have lived in endemic areas of Strongyloides stercoralis, empirical eradication with ivermectin before high corticosteroid regimens should be strongly considered.178 Discontinuation of cART is not a standard approach in the management of IRIS and should be considered only in life-threatening cases or following poor response to steroids.178
Timing of ART Initiation
A substantial reduction in risk of HIV-related cerebral toxoplasmosis was reported from the pre-cART to the cART era, demonstrating the importance of cART in the burden of this opportunistic disease.10,64,188-190 However, when this opportunity is lost and cerebral toxoplasmosis occurs, the next step is to decide when to start cART. There is no conclusive information regarding this issue.191,192 A randomized controlled trial of 282 patients with opportunistic infections other than tuberculosis (∼5% with cerebral toxoplasmosis) showed that early cART (median 12 days after initiation of opportunistic infection therapy) versus deferred initiation of cART (median 45 days after initiation of opportunistic infection therapy) had significantly lower incidence of AIDS progression or death (a secondary study end point).193 As discussed earlier, IRIS is not a major concern with cerebral toxoplasmosis, and a study demonstrated that there is no relationship between the timing of cART initiation and the occurrence of cerebral toxoplasmosis–related paradoxical IRIS.185 In contrast to cryptococcal meningitis194 or tuberculous meningitis,195 many physicians would start cART within 2 weeks after the initiation of cerebral toxoplasmosis treatment. More controlled data are needed to address this controversial issue.
Pharmacologic Prophylaxis
Primary Prophylaxis
Current recommendations indicate primary prophylaxis for cerebral toxoplasmosis in Toxoplasma IgG-positive patients with lymphocyte CD4 count <100 cells/mm3 or <200 cells/mm3.97,168 Because ∼25% of cases with cerebral toxoplasmosis occur with lymphocyte CD4 count >100 cells/mm3, in some reports,42 <200 cells/mm3 could be a more appropriate threshold. Patients with lymphocyte CD4 count >200 cells/mm3 for >3 to 6 months in response to cART are recommended to discontinue primary prophylaxis.169,196-198 Patients with lymphocyte CD4 count between 100 and 200 cells/mm3 and HIV viral load below limits of detection can be considered for discontinuing primary prophylaxis.199 The minimal period with undetectable viremia is not clear, but >3 to 6 months seems reasonable.98 For patients with lymphocyte CD4 count between 100 and 200 cells/mm3 with HIV viral load above detection limits, primary prophylaxis should be reintroduced.98 Daily TMP-SMX is the preferred regimen.200 If patients cannot tolerate TMP-SMX, the alternative regimens are dapsone-pyrimethamine plus leucovorin or atovaquone with or without pyrimethamine/leucovorin.201-203 All these options are also effective against pneumonia for Pneumocystic jirovecii.
Secondary Prophylaxis
Because all antitoxoplasma therapies used in clinical practice are active against the tachyzoite form of T gondii, but not on the tissue cyst form, discontinuation of therapy after the induction phase of treatment usually results in recrudescence of the disease.7 Current preferred recommendations for secondary prophylaxis of cerebral toxoplasmosis consist of a combination of pyrimethamine with sulfadiazine and leucovorin.98 Pyrimethamine combined with clindamycin is commonly used for patients with intolerance to sulfa drugs, but this scheme does not provide protection against pneumonia due to P jirovecii. Typically, sulfadiazine should be taken every 6 hours. However, in patients with adherence difficulties, an alternative regimen includes the same daily total dose of sulfadiazine every 12 hours,204 but the clinical experience of this scheme is limited.205 On the other hand, a dose of 600 mg clindamycin every 8 hours is recommended because of the high failure rate observed with lower doses.161
Similarly, with initial therapy of cerebral toxoplasmosis, TMP-SMX is an interesting alternative to secondary prophylaxis. Systematic reviews and meta-analysis of secondary prophylaxis for the prevention of HIV-related cerebral toxoplasmosis relapse using pyrimethamine-based therapy (24 studies—5 randomized clinical trials, 19 observational studies; 1596 patients)206 and TMP-SMX (6 studies—1 randomized clinical trial, 5 observational studies; 235 patients)207 found a similar relapse rate. Despite few studies and limited clinical experience with TMP-SMX in developed countries, this combination has been used in settings without pyrimethamine (ie, several African countries) and in some centers in Brazil where pyrimethamine is available. Interesting, a French cohort study of 83 patients with HIV-related cerebral toxoplasmosis treated with TMP-SMX reported effectiveness of 85.5%, with a relatively low incidence of side effects (22%; 7.4% requiring treatment interruption). Relapse occurred in 30% of the patients, but the most important risk factor for a new episode of cerebral toxoplasmosis was poor adherence to secondary prophylaxis with TMP-SMX. Despite this, patients with relapses received TMP-SMX, and the treatment was effective in 91% of cases.208 The recommended dose of TMP-SMX for secondary prophylaxis is 50% of a daily initial dosage taken every 12 hours.162,169 Patients with lymphocyte CD4 count >200 cells/mm3 for >6 months in response to cART are recommended to discontinue secondary prophylaxis.98,169,198,209,210 Patients with lymphocyte CD4 count <200 cells/mm3 should restart secondary prophylaxis regardless of the HIV viral load.98
Conclusion
The introduction of cART markedly decreased the incidence of cerebral toxoplasmosis in PLWHA. However, this disease remains the most common cause of expansive brain lesions and causes high morbidity and mortality in persons with advanced immunosuppression, particularly from low- and middle-income countries. Cerebral toxoplasmosis presents a wide spectrum of clinical and neuroradiological manifestations and a timely high index of suspicion is vital. Antitoxoplasma therapy is an important component of the diagnostic approach to expansive brain lesions in PLWHA. Local neuroepidemiology, the degree of immunosuppression, and individual clinical, laboratory, and neuroradiological features are important for the timely evaluation of alternative diagnoses. The use of local algorithms is important. Trimethoprim-sulfamethoxazole can be used for primary prophylaxis, initial therapy, and secondary prophylaxis of HIV-related cerebral toxoplasmosis. Currently, early initiation of cART is possible because more effective, safe, and friendly therapeutic options are available.
Acknowledgments
Thanks to Dr René Rivero, of the Department of Radiology of the Instituto de Infectologia Emílio Ribas, São Paulo, Brazil, and Dr Fernando Vilar, of the Department of Infectious Diseases of the University of São Paulo Medical School, Ribeirão Preto, Brazil, for providing gently Figure 5A–C and 5D–F, respectively.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: José Ernesto Vidal  https://orcid.org/0000-0001-7830-8716
https://orcid.org/0000-0001-7830-8716
References
- 1. Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev. 2018;31(4):pii:e00057–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18(5):198–201. [DOI] [PubMed] [Google Scholar]
- 3. Luft BJ, Hafner R, Korzun AH. et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. Members of the ACTG 077p/ANRS 009 Study Team. N Engl J Med. 1993;329(14):995–1000. [DOI] [PubMed] [Google Scholar]
- 4. Vilaseca J, Arnau JM, Bacardi R, Mieras C, Serrano A, Navarro C. Kaposi’s sarcoma and Toxoplasma gondii brain abscess in a Spanish homosexual. Lancet. 1982;1(8271):572. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control (CDC). Opportunistic infections and Kaposi’s sarcoma among Haitians in the United States. MMWR Morb Mortal Wkly Rep. 1982;31(26):353–354, 360-361. [PubMed] [Google Scholar]
- 6. Luft BJ, Conley F, Remington JS. et al. Outbreak of central-nervous-system toxoplasmosis in western Europe and North America. Lancet. 1983;1(8328):781–784. [DOI] [PubMed] [Google Scholar]
- 7. Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211–222. [DOI] [PubMed] [Google Scholar]
- 8. Schlüter D, Barragan A. Advances and challenges in understanding cerebral toxoplasmosis. Front Immunol. 2019;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antinori A, Larussa D, Cingolani A. et al. Italian Registry Investigative NeuroAIDS. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis. 2004;39(11):1681–1691. [DOI] [PubMed] [Google Scholar]
- 10. Coelho L, Cardoso SW, Amancio RT. et al. Trends in AIDS-defining opportunistic illnesses incidence over 25 years in Rio de Janeiro, Brazil. PLoS One. 2014;9(6):e98666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ford N, Shubber Z, Meintjes G. et al. Cause of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2(10):e438–e444. [DOI] [PubMed] [Google Scholar]
- 12. Sher JH. Cerebral toxoplasmosis (letter). Lancet. 1983;1(8335):1225. [DOI] [PubMed] [Google Scholar]
- 13. Levy RM, Bredesen DE, Rosenblum ML. Opportunistic central nervous system pathology in patients with AIDS. Ann Neurol. 1988;23(suppl):S7–S12. [DOI] [PubMed] [Google Scholar]
- 14. Snider WD, Simpson DM, Nielsen S. et al. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14(4):403–418. [DOI] [PubMed] [Google Scholar]
- 15. Levy RM, Pons VG, Rosenblum ML. Central nervous system mass lesions in the acquired immunodeficiency syndrome (AIDS). J Neurosurg. 1984;61(1):9–16. [DOI] [PubMed] [Google Scholar]
- 16. Navia BA, Petito CK, Gold JW, Cho ES, Jordan BD, Price RW. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19(3):224–238. [DOI] [PubMed] [Google Scholar]
- 17. Rosenblum ML, Levy RM, Bredesen DE. Neurosurgical implications of the acquired immunodeficiency syndrome (AIDS). Clin Neurosurg. 1988;34:419–445. [PubMed] [Google Scholar]
- 18. Moulignier A. HIV and the central nervous system. Rev Neurol (Paris). 2006;162(1):22–42. [DOI] [PubMed] [Google Scholar]
- 19. Brouwer MC, Tunkel AR, McKhann GM II, van de Beek D. Brain abscess. N Engl J Med. 2014;371(5):447–456. [DOI] [PubMed] [Google Scholar]
- 20. Muzumdar D, Jhawar S, Goel A. Brain abscess: an overview. Int J Surg. 2011;9(2):136–144. [DOI] [PubMed] [Google Scholar]
- 21. Chen M, Low DCY, Low SYY, Muzumdar D, Seow WT. Management of brain abscesses: where are we now? Childs Nerv Syst. 2018;34(10):1871–1880. [DOI] [PubMed] [Google Scholar]
- 22. Chow F. Brain and spinal epidural abscess. Continuum (Minneap Minn). 2018;24(5):1327–1348. [DOI] [PubMed] [Google Scholar]
- 23. Britt RH, Enzmann DRJ. Clinical stages of human brain abscesses on serial CT scans after contrast infusion. Computerized tomographic, neuropathological, and clinical correlations. J Neurosurg. 1983;59(6):972–989. [DOI] [PubMed] [Google Scholar]
- 24. Obana WG, Britt RH, Placone RC, Stuart JS, Enzmann DR. Experimental brain abscess development in the chronically immunosuppressed host. Computerized tomographic and neuropathological correlations. J Neurosurg. 1986;65(3):382–391. [DOI] [PubMed] [Google Scholar]
- 25. Mamidi A, DeSimone JA, Pomerantz RJ. Central nervous system infections in individuals with HIV-1 infection. J Neurovirol. 2002;8(3):158–167. [DOI] [PubMed] [Google Scholar]
- 26. Manzardo C, Del Mar Ortega M, Sued O, García F, Moreno A, Miró JM. Central nervous system opportunistic infections in developed countries in the highly active antiretroviral therapy era. J Neurovirol. 2005;11(suppl 3):72–82. [DOI] [PubMed] [Google Scholar]
- 27. Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet Neurol. 2012;11(7):605–617. [DOI] [PubMed] [Google Scholar]
- 28. Bilgrami M, O’Keefe P. Neurologic diseases in HIV-infected patients. Handb Clin Neurol. 2014;121:1321–1344. [DOI] [PubMed] [Google Scholar]
- 29. Marra CM. Central nervous system infection with Toxoplasma gondii . Handb Clin Neurol. 2018;152:117–122. [DOI] [PubMed] [Google Scholar]
- 30. Chirch LM, Luft BJ. Cerebral toxoplasmosis in AIDS. Handb Clin Neurol. 2007;85:147–158. [DOI] [PubMed] [Google Scholar]
- 31. Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol. 2016;12(11):662–674. [DOI] [PubMed] [Google Scholar]
- 32. Le LT, Spudich SS. HIV-Associated neurologic disorders and central nervous system opportunistic infections in HIV. Semin Neurol. 2016;36(4):373–381. [DOI] [PubMed] [Google Scholar]
- 33. Sonneville R, Magalhaes E, Meyfroidt G. Central nervous system infections in immunocompromised patients. Curr Opin Crit Care. 2017;23(2):128–133. [DOI] [PubMed] [Google Scholar]
- 34. Hill DE, Chirukandoth S, Dubey JP. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev. 2005;6(1):41–61. [DOI] [PubMed] [Google Scholar]
- 35. Wang ZD, Wang SC, Liu HH. et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4(4):e177–e188. [DOI] [PubMed] [Google Scholar]
- 36. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–1394. [DOI] [PubMed] [Google Scholar]
- 37. Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl. 2):115–121. [DOI] [PubMed] [Google Scholar]
- 38. d’Arminio Monforte A, Cinque P, Mocroft A. et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55(3):320–328. [DOI] [PubMed] [Google Scholar]
- 39. Vidal JE, Oliveira AC. AIDS-related cerebral toxoplasmosis in São Paulo State, Brazil: marked improvements in the highly active antiretroviral therapy-era but the challenges continue. Braz J Infect Dis. 2013;17(3):379–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xavier GA, Cademartori BG, Cunha Filho NA, Farias NA. Evaluation of seroepidemiological toxoplasmosis in HIV/AIDS patients in the south of Brazil. Rev Inst Med Trop Sao Paulo. 2013;55(1):25–30. [DOI] [PubMed] [Google Scholar]
- 41. Vidal JE, Hernandez AV, de Oliveira AC, Dauar RF, Barbosa SP, Jr, Focaccia R. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the CART era. AIDS Patient Care STDS. 2005;19(10):626–634. [DOI] [PubMed] [Google Scholar]
- 42. Oliveira JF, Greco DB, Oliveira GC, Christo PP, Guimarães MD, Oliveira RC. Neurological disease in HIV-infected patients in the era of highly active antiretroviral treatment: a Brazilian experience. Rev Soc Bras Med Trop. 2006;39(2):146–151. [DOI] [PubMed] [Google Scholar]
- 43. Miro J, Murray H. Toxoplasmosis In: Dolin R, Masur H, Saag M, eds. AIDS Therapy. 3rd ed Philadelphia, PA: Churchill Livingstone; 2008;659–681. [Google Scholar]
- 44. Cinque P, Koralnik IJ, Clifford DB. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J Neurovirol. 2003;9(Suppl 1):88–92. [DOI] [PubMed] [Google Scholar]
- 45. Marais S, Thwaites G, Schoeman JF. et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–812. [DOI] [PubMed] [Google Scholar]
- 46. Berger JR, Aksamit AJ, Clifford DB. et al. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology. 2013;80(15):1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Collazos J. Opportunistic infections of the CNS in patients with AIDS. CNS Drugs. 2003;17(12):869–887. [DOI] [PubMed] [Google Scholar]
- 48. Price RW. Neurological complications of HIV infection. Lancet. 1996;348(9025):445–452. [DOI] [PubMed] [Google Scholar]
- 49. Vidal JE, Penalva de Oliveira AC. Alterações neurológicas. Parte I. Em: Infectologia ambulatorial. Diagnóstico e Tratamento. Lindoso JAL, da Eira M, Casseb J, Silva ACCM, eds. São Paulo, Brazil: Sarvier; 2008:67–79. [Google Scholar]
- 50. Vidal JE, Dauar RF, de Oliveira AC. Utility of brain biopsy in patients with acquired immunodeficiency syndrome before and after introduction of highly active antiretroviral therapy. Neurosurgery. 2008;63(6):E1209. [DOI] [PubMed] [Google Scholar]
- 51. Penalva de Olivera AC, Casseb J, Annes M. et al. Manifestações neurológicas In: Focaccia R Editor Científico. Diament D Ferreira MS Siciliano RF, eds. Tratado de Infectologia. 4a Edição São Paulo, Brazil: Editora Atheneu; 2009:184–200. [Google Scholar]
- 52. Oliveira AP, Pappalardo MC, Dantas D, Lins D, Vidal JE. Brain abscess due to Staphylococcus aureus of cryptogenic source in an HIV-1 infected patient in use of antiretroviral therapy. Rev Inst Med Trop Sao Paulo. 2016;58:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trujillo JR, Garcìa-Ramos G, Novak IS, Rivera VM, Huerta E, Essex M. Neurologic manifestations of AIDS: a comparative study of two populations from Mexico and the United States. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(1):23–29. [PubMed] [Google Scholar]
- 54. Lanjewar DN, Jain PP, Shetty CR. Profile of central nervous system pathology in patients with AIDS: an autopsy study from India. AIDS. 1998;12(3):309–313. [DOI] [PubMed] [Google Scholar]
- 55. Modi M, Mochan A, Modi G. Management of HIV-associated focal brain lesions in developing countries. QJM. 2004;97(7):413–421. [DOI] [PubMed] [Google Scholar]
- 56. Pillay S, Ramchandre K. Audit of computed tomography brain findings in HIV-infected patients with space occupying infective lesions at a regional level hospital in KwaZulu-Natal. SAGE Open Med. 2018;6:2050312118801242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bower M, Fife K, Sullivan A. et al. Treatment outcome in presumed and confirmed AIDS-related primary cerebral lymphoma. Eur J Cancer. 1999;35(4):601–604. [DOI] [PubMed] [Google Scholar]
- 58. Brandsma D, Bromberg JEC. Primary CNS lymphoma in HIV infection. Handb Clin Neurol. 2018;152:177–186. [DOI] [PubMed] [Google Scholar]
- 59. American Academy of Neurology. Evaluation and management of intracranial mass lesions in AIDS. Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 1998;50(1):21–26. [DOI] [PubMed] [Google Scholar]
- 60. Antinori A, De Rossi G, Ammassari A. et al. Value of combined approach with thallium-201 single-photon emission computed tomography and Epstein-Barr virus DNA polymerase chain reaction in CSF for the diagnosis of AIDS-related primary CNS lymphoma. J Clin Oncol. 1999;17(2):554–560. [DOI] [PubMed] [Google Scholar]
- 61. Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2002;34(1):103–115. [DOI] [PubMed] [Google Scholar]
- 62. Antinori A, Ammassari A, De Luca A. et al. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology. 1997;48(3):687–694. [DOI] [PubMed] [Google Scholar]
- 63. Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327(23):1643–1648. [DOI] [PubMed] [Google Scholar]
- 64. Martin-Iguacel R, Ahlström MG, Touma M. et al. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect. 2017;75(3):263–273. [DOI] [PubMed] [Google Scholar]
- 65. Cohn JA, McMeeking A, Cohen W, Jacobs J, Holzman RS. Evaluation of the policy of empiric treatment of suspected Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Am J Med. 1989;86(5):521–527. [DOI] [PubMed] [Google Scholar]
- 66. Holloway RG, Mushlin AI. Intracranial mass lesions in acquired immunodeficiency syndrome: using decision analysis to determine the effectiveness of stereotactic brain biopsy. Neurology. 1996;46(4):1010–1015. [DOI] [PubMed] [Google Scholar]
- 67. Pereira-Chioccola VL, Vidal JE, Su C. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 2009;4(10):1363–1379. [DOI] [PubMed] [Google Scholar]
- 68. Gray F, Gherardi R, Wingate E. et al. Diffuse “encephalitic” cerebral toxoplasmosis in AIDS. Report of four cases. J Neurol. 1989;236(5):273–277. [DOI] [PubMed] [Google Scholar]
- 69. Mauhin W, Demoule A, Leclercq D. et al. Toxoplasmic ventriculitis. Med Mal Infect. 2016;46(2):100–103. [DOI] [PubMed] [Google Scholar]
- 70. Philip-Ephraim EE, Charidimou A, Williams E, Kajogbola G. Stroke-like presentation of cerebral toxoplasmosis: two HIV-infected cases. Cerebrovasc Dis Extra. 2015;5(1):28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Luma HN, Tchaleu BC, Temfack E. et al. HIV-associated central nervous system disease in patients admitted at the Douala General Hospital between 2004 and 2009: a retrospective study. AIDS Res Treat. 2013;2013:709810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sonneville R, Schmidt M, Messika J. et al. Neurologic outcomes and adjunctive steroids in HIV patients with severe cerebral toxoplasmosis. Neurology. 2012;79(17):1762–1766. [DOI] [PubMed] [Google Scholar]
- 73. Oksenhendler E, Cadranel J, Sarfati C. et al. Toxoplasma gondii pneumonia in patients with the acquired immunodeficiency syndrome. Am J Med. 1990;88(5N):18N–21N. [PubMed] [Google Scholar]
- 74. Pastorello RG, Costa ADCL, Sawamura MVY, Nicodemo AC, Duarte-Neto AN. Disseminated toxoplasmosis in a patient with advanced acquired immunodeficiency syndrome. Autops Case Rep. 2018;8(1):e20180 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jautzke G, Sell M, Thalmann U. et al. Extracerebral toxoplasmosis in AIDS. Histological and immunohistological findings based on 80 autopsy cases. Pathol Res Pract. 1993;189(4):428–436. [DOI] [PubMed] [Google Scholar]
- 76. Ciricillo SF, Rosenblum ML. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg. 1990;73(5):720–724. [DOI] [PubMed] [Google Scholar]
- 77. Levy RM, Mills CM, Posin JP, Moore SG, Rosenblum ML, Bredesen DE. The efficacy and clinical impact of brain imaging in neurologically symptomatic AIDS patients: a prospective CT/MRI study. J Acquir Immune Defic Syndr. 1990;3(5):461–471. [PubMed] [Google Scholar]
- 78. Renold C, Sugar A, Chave JP. et al. Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Medicine (Baltimore). 1992;71(4):224–239. [DOI] [PubMed] [Google Scholar]
- 79. Knobel H, Guelar A, Graus F, Miró JM, Padro S, Mercader JM. Toxoplasmic encephalitis with normal CT scan and pathologic MRI. Am J Med. 1995;99(2):220–221. [DOI] [PubMed] [Google Scholar]
- 80. Bhagavati S, Choi J. Frequent hemorrhagic lesions in cerebral toxoplasmosis in AIDS patients. J Neuroimaging. 2009;19(2):169–173. [DOI] [PubMed] [Google Scholar]
- 81. Benson JC, Cervantes G, Baron TR. et al. Imaging features of neurotoxoplasmosis: a multiparametric approach, with emphasis on susceptibility-weighted imaging. Eur J Radiol Open. 2018;5:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wijdicks EFM, Borleffs JCC, Hoepelman AIM, Jansen GH. Fatal disseminated hemorrhagic toxoplasmic encephalitis as the initial manifestation of AIDS. Ann Neurol. 1991;29(6):683–686. [DOI] [PubMed] [Google Scholar]
- 83. Pellegrino D, Picciarelli de Lima P, Penalva de Oliveira AC, Vidal JE. Hemorrhagic brain lesions in a newly diagnosed HIV-1 infected patient [published online ahead of print June 3, 2019]. Int J STD AIDS. 2019. [DOI] [PubMed] [Google Scholar]
- 84. Ramsey RG, Gean AD. Neuroimaging of AIDS. I. Central nervous system toxoplasmosis. Neuroimaging Clin N Am. 1997;7(2):171–186. [PubMed] [Google Scholar]
- 85. Kumar GG, Mahadevan A, Guruprasad AS. et al. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31(6):1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bargalló J, Berenguer J, García-Barrionuevo J. et al. The “target sign”: is it a specific sign of CNS tuberculoma? Neuroradiology. 1996;38(6):547–550. [DOI] [PubMed] [Google Scholar]
- 87. Mahadevan A, Ramalingaiah AH, Parthasarathy S, Nath A, Ranga U, Krishna SS. Neuropathological correlate of the “concentric target sign” in MRI of HIV-associated cerebral toxoplasmosis. J Magn Reson Imaging. 2013;38(2):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang M, Sun J, Bai HX. et al. Diagnostic accuracy of SPECT, PET, and MRS for primary central nervous system lymphoma in HIV patients: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(19):e6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Portegies P, Solod L, Cinque P. et al. Guidelines for the diagnosis and management of neurological complications of HIV infection. Eur J Neurol. 2004;11(5):297–304. [DOI] [PubMed] [Google Scholar]
- 90. Berger JR, Cohen BA. Opportunistic infections of the nervous system in AIDS In: Gendelman HE, Grant I, Everall IP, Lipton SA, Swindells S, eds. The Neurology of AIDS. Oxford, United Kingdom: Oxford University Press; 2005:485–529. [Google Scholar]
- 91. Montoya JG, Boothroyd JC, Kovacs JA. Toxoplasma gondii In: Mandell G, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:3495–3526. [Google Scholar]
- 92. de Almeida SM, de Souza CV, Pletsch L. et al. Diagnostic importance of eosinophilic meningitis in HIV-positive and HIV-negative patients. J Neurovirol. 2019;25(3):331–341. [DOI] [PubMed] [Google Scholar]
- 93. Vidal JE, Alves M, Kassab M, Dauar R, Vasconcellos D. First case report of eosinophilic meningitis associated with cerebral toxoplasmosis in an HIV-infected patient [published online ahead of print] Int J STD AIDS. 2019. [DOI] [PubMed] [Google Scholar]
- 94. Burg JL, Grover CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27(8):1787–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weiss JB. DNA probes and PCR for diagnosis of parasitic infections. Clin Microbiol Rev. 1995;8(1):113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bastien P. Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S205–S215. [DOI] [PubMed] [Google Scholar]
- 97. Murat JB, Hidalgo HF, Brenier-Pinchart MP, Pelloux H. Human toxoplasmosis: which biological diagnostic tests are best suited to which clinical situations? Expert Rev Anti Infect Ther. 2013;11(9):943–956. [DOI] [PubMed] [Google Scholar]
- 98. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed April 25, 2019.
- 99. Lebech M, Lebech AM, Nelsing S, Vuust J, Mathiesen L, Petersen E. Detection of Toxoplasma gondii DNA by polymerase chain reaction in cerebrospinal fluid from AIDS patients with cerebral toxoplasmosis. J Infect Dis. 1992;165(5):982–983. [DOI] [PubMed] [Google Scholar]
- 100. Novati R, Castagna A, Morsica G. et al. Polymerase chain reaction for Toxoplasma gondii DNA in the cerebrospinal fluid of AIDS patients with focal brain lesions. AIDS. 1994;8(12):1691–1694. [DOI] [PubMed] [Google Scholar]
- 101. Schoondermark-van de Ven E, Galama J, Kraaijeveld C, van Druten J, Meuwissen J, Melchers W. Value of the polymerase chain reaction for the detection of Toxoplasma gondii in cerebrospinal fluid from patients with AIDS. Clin Infect Dis. 1993;16(5):661–666. [DOI] [PubMed] [Google Scholar]
- 102. Eggers C, Gross U, Klinker H, Schalke B, Stellbrink HJ, Kunze K. Limited value of cerebrospinal fluid for direct detection of Toxoplasma gondii in toxoplasmic encephalitis associated with AIDS. J Neurol. 1995;242(10):644–649. [DOI] [PubMed] [Google Scholar]
- 103. Ammassari A, Murri R, Cingolani A, De Luca A, Antinori A. AIDS-associated cerebral toxoplasmosis: an update on diagnosis and treatment. Curr Top Microbiol Immunol. 1996;219:209–222. [DOI] [PubMed] [Google Scholar]
- 104. Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS. 1997;11(1):1–17. [DOI] [PubMed] [Google Scholar]
- 105. Vidal JE, Colombo FA, Penalva de Oliveira AC, Focaccia R, Pereira-Chioccola VL. PCR assay using cerebrospinal fluid for diagnosis of cerebral toxoplasmosis in Brazilian AIDS patients. J Clin Microbiol. 2004;42(10):4765–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Alfonso Y, Fraga J, Fonseca C. et al. Molecular diagnosis of Toxoplasma gondii infection in cerebrospinal fluid from AIDS patients. Cerebrospinal Fluid Res. 2009;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nogui FL, Mattas S, Turcato Júnior G, Lewi DS. Neurotoxoplasmosis diagnosis for HIV-1 patients by real-time PCR of cerebrospinal fluid. Braz J Infect Dis. 2009;13(1):18–23. [DOI] [PubMed] [Google Scholar]
- 108. Mikita K, Maeda T, Ono T, Miyahira Y, Asai T, Kawana A. The utility of cerebrospinal fluid for the molecular diagnosis of toxoplasmic encephalitis. Diagn Microbiol Infect Dis. 2013;75(2):155–159. [DOI] [PubMed] [Google Scholar]
- 109. Robert-Gangneux F, Belaz S. Molecular diagnosis of toxoplasmosis in immunocompromised patients. Curr Opin Infect Dis. 2016;29(4):330–339. [DOI] [PubMed] [Google Scholar]
- 110. Foudrinier F, Aubert D, Puygauthier-Toubas D. et al. Detection of Toxoplasma gondii in immunodeficient subjects by gene amplification: influence of therapeutics. Scand J Infect Dis. 1996;28(4):383–386. [DOI] [PubMed] [Google Scholar]
- 111. Anselmo LM, Vilar FC, Lima JE, Yamamoto AY, Bollela VR, Takayanagui OM. Usefulness and limitations of polymerase chain reaction in the etiologic diagnosis of neurotoxoplasmosis in immunocompromised patients. J Neurol Sci. 2014;346(1-2):231–234. [DOI] [PubMed] [Google Scholar]
- 112. Cingolani A, De Luca A, Ammassari A. et al. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45(6):472–476. [DOI] [PubMed] [Google Scholar]
- 113. Ajzenberg D, Lamaury I, Demar M. et al. Performance testing of PCR assay in blood samples for the diagnosis of toxoplasmic encephalitis in AIDS patients from the French departments of America and genetic diversity of Toxoplasma gondii: a prospective and multicentric study. PLoS Negl Trop Dis. 2016;10(6):e0004790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ho-Yen DO, Joss AW, Balfour AH, Smyth ET, Baird D, Chatterton JM. Use of the polymerase chain reaction to detect Toxoplasma gondii in human blood samples. J Clin Pathol. 1992;45(10):910–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dupouy-Camet J, de Souza SL, Maslo C. et al. Detection of Toxoplasma gondii in venous blood from AIDS patients by polymerase chain reaction. J Clin Microbiol. 1993;31(7):1866–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Filice GA, Hitt JA, Mitchell CD. et al. Diagnosis of Toxoplasma parasitemia in patients with AIDS by gene detection after amplification with polymerase chain reaction. J Clin Microbiol. 1993;31(9):2327–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Khalifa KE, Roth A, Roth B, Arasteh KN, Janitschke K. Value of PCR for evaluating occurrence of parasitemia in immunocompromised patients with cerebral and extracerebral toxoplasmosis. J Clin Microbiol. 1994;32(11):2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dupon M, Cazenave J, Pellegrin JL. et al. Detection of Toxoplasma gondii by PCR and tissue culture in cerebrospinal fluid and blood of human immunodeficiency virus-seropositive patients. J Clin Microbiol. 1995;33(9):2421–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Robert F, Ouatas T, Blanche P, Tourte-Schaefer C, Sicard D, Dupouy-Camet J. Retrospective evaluation of the detection of Toxoplasma gondii by polymerase chain reaction in AIDS patients. Presse Med. 1996;25(11):541–545. [PubMed] [Google Scholar]
- 120. Lamoril J, Molina JM, de Gouvello A. et al. Detection by PCR of Toxoplasma gondii in blood in the diagnosis of cerebral toxoplasmosis in patients with AIDS. J Clin Pathol. 1996;49(1):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Franzen C, Altfeld M, Hegener P. et al. Limited value of PCR for detection of Toxoplasma gondii in blood from human immunodeficiency virus-infected patients. J Clin Microbiol. 1997;35(10):2639–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Colombo FA, Vidal JE, Penalva de Oliveira AC. et al. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J Clin Microbiol. 2005;43(10):5044–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Correia CC, Melo HR, Costa VM. Influence of neurotoxoplasmosis characteristics on real-time PCR sensitivity among AIDS patients in Brazil. Trans R Soc Trop Med Hyg. 2010;104(1):24–28. [DOI] [PubMed] [Google Scholar]
- 124. Cardona N, Basto N, Parra B. et al. Detection of Toxoplasma DNA in the peripheral blood of HIV-positive patients with neuro-opportunistic infections by a real-time PCR assay. J Neuroparasitology. 2011;2:1–6. [Google Scholar]
- 125. Ajzenberg D. Is PCR testing on blood samples useful or not in the diagnosis of Toxoplasma encephalitis? Trans R Soc Trop Med Hyg. 2010;104(8):569–570. [DOI] [PubMed] [Google Scholar]
- 126. Joseph P, Calderón MM, Gilman RH. et al. Optimization and evaluation of a PCR assay for detecting toxoplasmic encephalitis in patients with AIDS. J Clin Microbiol. 2002;40(12):4499–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bretagne S. Molecular diagnostics in clinical parasitology and mycology: limits of the current polymerase chain reaction (PCR) assays and interest of the real-time PCR assays. Clin Microbiol Infect. 2003;9(6):505–511. [DOI] [PubMed] [Google Scholar]
- 128. Costa JM, Pautas C, Ernault P, Foulet F, Cordonnier C, Bretagne S. Real-Time PCR for diagnosis and follow-up of toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probe. J Clin Microbiol. 2000;38(8):2929–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Menotti J, Vilela G, Romand S. et al. Comparison of PCR enzyme-linked immunosorbent assay and real-time PCR assay for diagnosis of an unusual case of cerebral toxoplasmosis in a stem cell transplant recipient. J Clin Microbiol. 2003;41(11):5313–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bastien P, Procop GW, Reischl U. Quantitative real-time PCR is not more sensitive than “conventional” PCR. J Clin Microbiol. 2008;46(6):1897–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Morelle C, Varlet-Marie E, Brenier-Pinchart MP. et al. Comparative assessment of a commercial kit and two laboratory-developed PCR assays for molecular diagnosis of congenital toxoplasmosis. J Clin Microbiol. 2012;50(12):3977–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Filisetti D, Sterkers Y, Brenier-Pinchart MP. et al. Multicentric comparative assessment of the bio-evolution Toxoplasma gondii detection kit with eight laboratory-developed PCR assays for molecular diagnosis of congenital toxoplasmosis. J Clin Microbiol. 2015;53(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Robert-Gangneux F, Brenier-Pinchart MP, Yera H. et al. Molecular Biology Study Group of the French National Reference Center for Toxoplasmosis. Evaluation of toxoplasma ELITe MGB real-time PCR assay for diagnosis of toxoplasmosis. J Clin Microbiol. 2017;53(5):1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sterkers Y, Varlet-Marie E, Cassaing S. et al. Multicentric comparative analytical performance study for molecular detection of low amounts of Toxoplasma gondii from simulated specimens. J Clin Microbiol. 2010;48(9):3216–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gildenberg PL, Gathe JC, Jr, Kim JH. Stereotactic biopsy of cerebral lesions in AIDS. Clin Infect Dis. 2000;30(3):491–499. [DOI] [PubMed] [Google Scholar]
- 137. Rosenow JM, Hirschfeld A. Utility of brain biopsy in patients with acquired immunodeficiency syndrome before and after introduction of highly active antiretroviral therapy. Neurosurgery. 2007;61(1):130–140. [DOI] [PubMed] [Google Scholar]
- 138. Zhang J, Liu X, Fu K. et al. Diagnostic value and safety of stereotactic biopsy in acquired immune deficiency syndrome patients with intracranial lesions: systematic review and meta-analysis. World Neurosurg. 2017;98:790–799. [DOI] [PubMed] [Google Scholar]
- 139. Lee AM, Bai HX, Zou Y. et al. Safety and diagnostic value of brain biopsy in HIV patients: a case series and meta-analysis of 1209 patients. J Neurol Neurosurg Psychiatry. 2016;87(7):722–733. [DOI] [PubMed] [Google Scholar]
- 140. Levy RM, Russell E, Yungbluth M, Hidvegi DF, Brody BA, Dal Canto MC. The efficacy of image-guided stereotactic brain biopsy in neurologically symptomatic acquired immunodeficiency syndrome patients. Neurosurgery. 1992;30(2):186–189. [DOI] [PubMed] [Google Scholar]
- 141. Berger JR. Mass lesions of the brain in AIDS: the dilemmas of distinguishing toxoplasmosis from primary CNS lymphoma. AJNR Am J Neuroradiol. 2003;24(4):554–555. [PMC free article] [PubMed] [Google Scholar]
- 142. Giancola ML, Rizzi EB, Schiavo R. et al. Reduced value of thallium-201 single-photon emission computed tomography in the management of HIV-related focal brain lesions in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2004;20(6):584–588. [DOI] [PubMed] [Google Scholar]
- 143. Vidal JE, Tuon FF. Brain abscess due to viridans streptococci in a severely immunosuppressed HIV-infected patient. Int J STD AIDS. 2009;20(9):654–656. [DOI] [PubMed] [Google Scholar]
- 144. Vidal JE, Penalva de Oliveira AC, Bonasser Filho F. et al. Tuberculous brain abscess in AIDS patients: report of three cases and literature review. Int J Infect Dis. 2005;9(4):201–207. [DOI] [PubMed] [Google Scholar]
- 145. Vidal JE, Dauar RF, Melhem MS. et al. Cerebral aspergillosis due to Aspergillus fumigatus in AIDS patient: first culture-proven case reported in Brazil. Rev Inst Med Trop Sao Paulo. 2005;47(3):161–165. [DOI] [PubMed] [Google Scholar]
- 146. Clarke P, Glick S, Reilly BM. Clinical problem-solving. On the threshold—a diagnosis of exclusion. N Engl J Med. 2005;352(9):919–924. [DOI] [PubMed] [Google Scholar]
- 147. Chimelli L, Rosemberg S, Hahn MD, Lopes MB, Netto MB. Pathology of the central nervous system in patients infected with the human immunodeficiency virus (HIV): a report of 252 autopsy cases from Brazil. Neuropathol Appl Neurobiol. 1992;18(5):478–488. [DOI] [PubMed] [Google Scholar]
- 148. Martínez AJ, Sell M, Mitrovics T. et al. The neuropathology and epidemiology of AIDS. A Berlin experience. A review of 200 cases. Pathol Res Pract. 1995;191(5):427–443. [DOI] [PubMed] [Google Scholar]
- 149. Kibayashi K, Mastri AR, Hirsch CS. Neuropathology of human immunodeficiency virus infection at different disease stages. Hum Pathol. 1996;27(7):637–642. [DOI] [PubMed] [Google Scholar]
- 150. Jellinger KA, Setinek U, Drlicek M, Böhm G, Steurer A, Lintner F. Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol. 2000;100(2):213–220. [DOI] [PubMed] [Google Scholar]
- 151. Vago L, Bonetto S, Nebuloni M. et al. Pathological findings in the central nervous system of AIDS patients on assumed antiretroviral therapeutic regimens: retrospective study of 1597 autopsies. AIDS. 2002;16(14):1925–1928. [DOI] [PubMed] [Google Scholar]
- 152. Silva AC, Rodrigues BS, Micheletti AM. et al. Neuropathology of AIDS: an autopsy review of 284 cases from Brazil comparing the findings pre- and post-HAART (highly active antiretroviral therapy) and pre- and postmortem correlation. AIDS Res Treat. 2012;2012:186850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Satishchandra P, Nalini A, Gourie-Devi M. et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96). Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- 154. Subsai K, Kanoksri S, Siwaporn C, Helen L. Neurological complications in AIDS patients: the 1-year retrospective study in Chiang Mai University, Thailand. Eur J Neurol. 2004;11(11):755–759. [DOI] [PubMed] [Google Scholar]
- 155. Siddiqi OK, Ghebremichael M, Dang X. et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis. 2014;58(12):1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yang R, Zhang H, Xiong Y. et al. Molecular diagnosis of central nervous system opportunistic infections and mortality in HIV-infected adults in central China. AIDS Res Ther 2017;14:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Vidal JE, Penalva de Oliveira AC, Pellegrino D. et al. Complicações neurológicas em pacientes infectados pelo HIV e fatores associados a óbito na era CART: estudo observacional prospectivo no Instituto de Infectologia Emílio Ribas. Braz J Infect Dis. 2011;15(Suppl 1):23. [Google Scholar]
- 158. Telles JP, Fernandes R, Barros TD. et al. The continuous challenging of neuroepidemiology in people living with HIV/AIDS in the late antiretroviral therapy in Brazil Poster presented at: 29th European Congresso of Clinical Micriobiology and Infectios Diseases, 2019. Amsterdam, The Netherlands: Poster P0724. [Google Scholar]
- 159. Dannemann B, McCutchan JA, Israelski D. et al. Treatment of toxoplasmic encephalitis in patients with AIDS: a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann Intern Med. 1992;116(1):33–43. [DOI] [PubMed] [Google Scholar]
- 160. Bedos JP, Chastang C, Lucet JC, Kalo T, Gachot B, Wolff M. Early predictors of outcome for HIV patients with neurological failure. JAMA. 1995;273(1):35–40. [DOI] [PubMed] [Google Scholar]
- 161. Katlama C, De Wit S, O’Doherty E, Van Glabeke M, Clumeck N. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis. 1996;22(2):268–275. [DOI] [PubMed] [Google Scholar]
- 162. Torre D, Casari S, Speranza F. et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Italian Collaborative Study Group. Antimicrob Agents Chemother. 1998;42(6):1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pellegrino D, Gryschek R, Penalva de Oliveira AC, Marcusso R, Correia A, Vidal JE. Efficacy and safety of trimethoprim-sulfamethoxazole in HIV-infected patients with cerebral toxoplasmosis in Brazil: a single-arm open-label clinical trial [published online ahead of print] Int J STD AIDS. 2019. [DOI] [PubMed] [Google Scholar]
- 164. Grossman PL, Remington JS. The effect of trimethoprim and sulfamethoxazole on Toxoplasma gondii in vitro and in vivo. Am J Trop Med Hyg. 1979;28(3):445–455. [DOI] [PubMed] [Google Scholar]
- 165. Holliman RE. Folate supplements and the treatment of cerebral toxoplasmosis. Scand J Infect Dis. 1989;21(4):475–476. [DOI] [PubMed] [Google Scholar]
- 166. Niyongabo T, Leport C, Vilde JL. Usefulness of folinic acid in cytopenia by antiparasitic drugs in AIDS patients. Presse Med. 1991;20(34):1677–1681. [PubMed] [Google Scholar]
- 167. Georgiev VS. Management of toxoplasmosis. Drugs. 1994;48(2):179–188. [DOI] [PubMed] [Google Scholar]
- 168. Fichera ME, Bhopale MK, Roos DS. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii . Antimicrob Agents Chemother. 1995;39(7):1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. European AIDS Clinical Society. Guidelines. Version 9.1. 2018. http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf. Accessed May 1, 2019.
- 170. Nelson M, Dockrell D, Edwards S. et al. British HIV Association and British Infection Association Guidelines for the treatment of opportunistic infection in HIV-seropositive individuals 2011. HIV Med. 2011;12(suppl 2):1–140. [DOI] [PubMed] [Google Scholar]
- 171. Gilbert DN, Chambers HF, Eliopoulos GM, Saag MS. The Sanford Guide to Antimicrobial Therapy. 45th ed Sperryville, VA: Antimicrobial Therapy, Inc; 2015. [Google Scholar]
- 172. World Health Organization. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Geneva, Switzerland: World Health Organization; 2017. [PubMed] [Google Scholar]
- 173. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção pelo HIV em Adultos / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância, Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Brasília, Brazil: Ministério da Saúde; 2018. [Google Scholar]
- 174. Ben-Harari RR, Goodwin E, Casoy J. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: a systematic review. Drugs R D. 2017;17(4):523–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hernandez AV, Thota P, Pellegrino D. et al. A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: it is trimethoprim-sulfamethoxazole a real option? HIV Medicine. 2017;18(2):115–124. [DOI] [PubMed] [Google Scholar]
- 176. Saadani Hassani A, Marston BJ. Impact of cotrimoxazole and insecticide-treated nets for malaria prevention on key outcomes among HIV-infected adults in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr. 2015;68(suppl 3): S306–S317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bahr N, Boulware DR, Marais S, Scriven J, Wilkinson RJ, Meintjes G. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep. 2013;15(5):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Manzardo C, Guardo AC, Letang E, Plana M, Gatell JM, Miro JM. Opportunistic infections and immune reconstitution inflammatory syndrome in HIV-1-infected adults in the combined antiretroviral therapy era: a comprehensive review. Expert Rev Anti Infect Ther. 2015;13(6):751–767. [DOI] [PubMed] [Google Scholar]
- 179. Müller M, Wandel S, Colebunders R. et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Farkash AE, Maccabee PJ, Sher JH, Landesman SH, Hotson G. CNS toxoplasmosis in acquired immune deficiency syndrome: a clinical-pathological-radiological review of 12 cases. J Neurol Neurosurg Psychiatry. 1986;49(7):744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Blader IJ, Saeij JP. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS. 2009;117(5-6):458–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Tsambiras PE, Larkin JA, Houston SH. Case report: toxoplasma encephalitis after initiation of CART. AIDS Read. 2001;11(12):608–610. [PubMed] [Google Scholar]
- 183. Pfeffer G, Prout A, Hooge J, Maguire J. Biopsy-proven immune reconstitution syndrome in a patient with AIDS and cerebral toxoplasmosis. Neurology. 2009;73(4):321–322. [DOI] [PubMed] [Google Scholar]
- 184. Martin-Blondel G, Alvarez M, Delobel P. et al. Toxoplasmic encephalitis IRIS in HIV-infected patients: a case series and review of the literature. J Neurol Neurosurg Psychiatry. 2011;82(6):691–693. [DOI] [PubMed] [Google Scholar]
- 185. van Bilsen WPH, van den Berg CHSB, Rijnders BJA. et al. Immune reconstitution inflammatory syndrome associated with toxoplasmic encephalitis in HIV-infected patients. AIDS. 2017;31(10):1415–1424. [DOI] [PubMed] [Google Scholar]
- 186. Bowen L, Nath A, Smith B. CNS immune reconstitution inflammatory syndrome. Handb Clin Neurol. 2018;152:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Meintjes G, Wilkinson RJ, Morroni C. et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Abgrall S, Rabaud C, Costagliola D; Clinical Epidemiology Group of the French Hospital Database on HIV. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clin Infect Dis. 2001;33(10):1747–1755. [DOI] [PubMed] [Google Scholar]
- 189. UK Collaborative HIV Cohort (CHIC) Study Steering Committee; Garvey L, Winston A, Walsh J. et al. HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. Eur J Neurol. 2011;18(3):527–534. [DOI] [PubMed] [Google Scholar]
- 190. Buchacz K, Baker RK, Palella FJ., Jr et al. AIDS defining opportunistic illnesses in US patients, 1994-2007: a cohort. AIDS. 2010;24(10):1549–1559. [DOI] [PubMed] [Google Scholar]
- 191. Lawn SD, Török ME, Wood R. Optimum time to start antiretroviral therapy during HIV-associated opportunistic infections. Curr Opin Infect Dis. 2011;24(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Grant PM, Zolopa AR. When to start ART in the setting of acute AIDS-related opportunistic infections: the time is now. Curr HIV/AIDS Rep. 2012;9(3):251–258. [DOI] [PubMed] [Google Scholar]
- 193. Zolopa A, Andersen J, Powderly W. et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Boulware DR, Meya DB, Muzoora C. et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Török ME, Yen NT, Chau TT. et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)–associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Furrer H, Opravil M, Bernasconi E. et al. Stopping primary prophylaxis in HIV-1-infected patients at high risk of toxoplasma encephalitis. Lancet. 2000;355(9222):2217–2218. [DOI] [PubMed] [Google Scholar]
- 197. Mussini C, Pezzotti P, Govoni A. et al. Discontinuation of primary prophylaxis for Pneumocystis carinii pneumonia and toxoplasmic encephalitis in human immunodeficiency virus type I-infected patients: the changes in opportunistic prophylaxis study. J Infect Dis. 2000;181(5):1635–1642. [DOI] [PubMed] [Google Scholar]
- 198. Miro JM, Lopez JC, Podzamczer D. et al. GESIDA 04/98 Study Group. Discontinuation of primary and secondary Toxoplasma gondii prophylaxis is safe in HIV-infected patients after immunological restoration with highly active antiretroviral therapy: results of an open, randomized, multicenter clinical trial. Clin Infect Dis. 2006;43(1):79–89. [DOI] [PubMed] [Google Scholar]
- 199. Miro JM, Esteve A, Furrer H; on behalf the Opportunistic Infection Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord. Safety of stopping primary T. gondii prophylaxis with suppressed viremia and CD4 >100/mm3 Paper presented at: CROI, 2016, Boston, MA: Abstract #765. [Google Scholar]
- 200. Carr A, Tindall B, Brew BJ. et al. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117(2):106–111. [DOI] [PubMed] [Google Scholar]
- 201. Girard PM, Landman R, Gaudebout C. et al. Dapsone-pyrimethamine compared with aerosolized pentamidine as primary prophylaxis against Pneumocystis carinii pneumonia and toxoplasmosis in HIV infection. The PRIO Study Group. N Engl J Med. 1993;328(21):1514–1520. [DOI] [PubMed] [Google Scholar]
- 202. Podzamczer D, Salazar A, Jimenez J. et al. Intermittent trimethoprim-sulfamethoxazole compared with dapsone-pyrimethamine for the simultaneous primary prophylaxis of Pneumocystis pneumonia and toxoplasmosis in patients infected with HIV. Ann Intern Med. 1995;122(10):755–761. [DOI] [PubMed] [Google Scholar]
- 203. Opravil M, Hirschel B, Lazzarin A. et al. Once-weekly administration of dapsone/pyrimethamine vs. aerosolized pentamidine as combined prophylaxis for Pneumocystis carinii pneumonia and toxoplasmic encephalitis in human immunodeficiency virus-infected patients. Clin Infect Dis. 1995;20(3):531–541. [DOI] [PubMed] [Google Scholar]
- 204. Jordan MK, Burstein AH, Rock-Kress D. et al. Plasma pharmacokinetics of sulfadiazine administered twice daily versus four times daily are similar in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48(2):635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Podzamczer D, Miró JM, Ferrer E. et al. Thrice-weekly sulfadiazine-pyrimethamine for maintenance therapy of toxoplasmic encephalitis in HIV-infected patients. Spanish Toxoplasmosis Study Group. Eur J Clin Microbiol Infect Dis. 2000;19(2):89–95. [DOI] [PubMed] [Google Scholar]
- 206. Connolly MP, Goodwin E, Schey C. et al. Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathog Glob Health. 2017;111(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Connolly MP, Haitsma G, Hernández AV, Vidal JE. Systematic review and meta-analysis of secondary prophylaxis for prevention of HIV-related toxoplasmic encephalitis relapse using trimethoprim-sulfamethoxazole. Pathog Glob Health. 2017;111(6):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Béraud G, Pierre-François S, Foltzer A. et al. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994-2006. Am J Trop Med Hyg. 2009;80(4):583–587. [PubMed] [Google Scholar]
- 209. Soriano V, Dona C, Rodríguez-Rosado R, Barreiro P, González-Lahoz J. Discontinuation of secondary prophylaxis for opportunistic infections in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2000;14(4):383–386. [DOI] [PubMed] [Google Scholar]
- 210. Bertschy S, Opravil M, Cavassini M. et al. Discontinuation of maintenance therapy against toxoplasma encephalitis in AIDS patients with sustained response to anti-retroviral therapy. Clin Microbiol Infect. 2006;12(7):666–671. [DOI] [PubMed] [Google Scholar]