Highlights
-
•
Cell ablation mimics wounding on a single cell level.
-
•
Calcium, ROS, and hormone dynamics were described.
-
•
Local, regional, and systemic responses can be distinguished.
Abstract
Plants as sessile organisms are constantly under attack by herbivores, rough environmental situations, or mechanical pressure. These challenges often lead to the induction of wounds or destruction of already specified and developed tissues. Additionally, wounding makes plants vulnerable to invasion by pathogens, which is why wound signalling often triggers specific defence responses. To stay competitive or, eventually, survive under these circumstances, plants need to regenerate efficiently, which in rigid, tissue migration-incompatible plant tissues requires post-embryonic patterning and organogenesis. Now, several studies used laser-assisted single cell ablation in the Arabidopsis root tip as a minimal wounding proxy. Here, we discuss their findings and put them into context of a broader spectrum of wound signalling, pathogen responses and tissue as well as organ regeneration.
Current Opinion in Plant Biology 2019, 52:124–130
This review comes from a themed issue on Cell biology
Edited by Eva Benkova and Yasin Dagdas
For a complete overview see the Issue and the Editorial
Available online 2nd October 2019
https://doi.org/10.1016/j.pbi.2019.08.006
1369-5266/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Well-studied mechanisms of wound healing in animals rely strongly on targeted migration of cells to the wound area. In plant tissues, this is not possible, since plant cells are encapsulated by their rigid cell walls. Thus, regeneration in plants has to rely on oriented cell divisions, acquisition of new cell fates and on directional cell elongation. Early wounding studies in the 19th and beginning of 20th century provided initial phenomenology of regeneration [1,2,3•] but only in the last decade approaches mainly involving the surgical removal of the root tip provided much insight into the mechanism of regeneration and accompanied transcriptional reprograming [4,5••]. However, the cellular processes and, in particular, molecular mechanisms underlying this regeneration response remain poorly characterized. Recent studies employing local, targeted cell elimination in the roots of the model plant Arabidopsis thaliana promise to provide fresh insights into the still mysterious mechanism of wound healing in plants.
Non-targeted wounding studies
Most of the earlier wounding experiments involved surgically induced, rather large-scale injuries in different tissues of various plant models. Originally, these studies involved simple observation of processes following the wounding and, later, mainly with the use of Arabidopsis root, they employed global transcriptome analysis and more sophisticated use of molecular markers and other genetic tools.
Cellular responses during regeneration
The most obvious response of surrounding cells to wounding is (re)entry into mitosis, also in differentiated cells that have left the cell cycle. These cells dedifferentiate, divide, and form the new cell walls parallel to the wound site ultimately filling the wound with new cells [2,6,3•]. In the root meristem, where cells are constantly in the cell cycle, wounding enhances cell divisions in cells close to the wound site; these wound-activated root cells subsequently lose their identity and adopt embryonic/stem cell-like identity (Figure 1a) [5••,7]. Although these processes have been well described, neither the signal that activates the neighboring cells nor the mechanism coordinating which cells are responsive, has been identified.
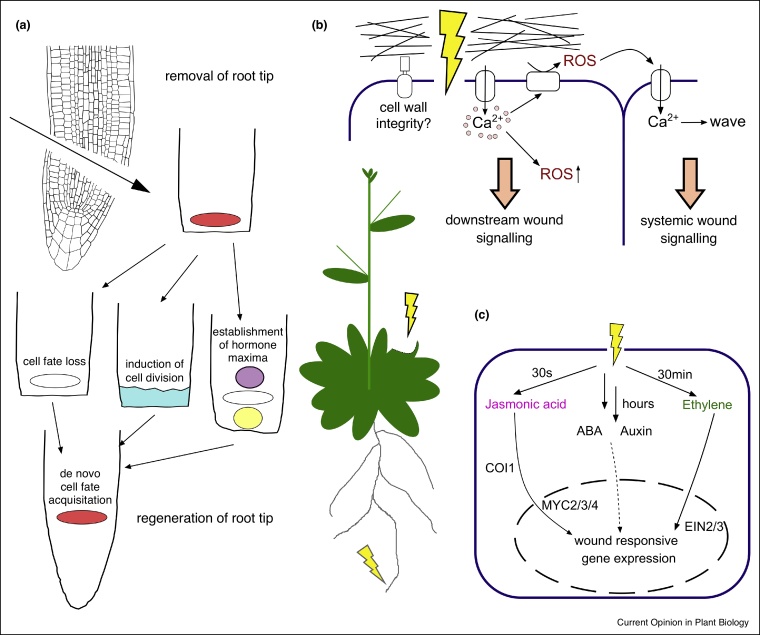
Figure 1.
Wounding triggers primary wounding signals, phytohormone signalling and complex regeneration responses. (a) Cutting off the root tip including the stem cell niche leads to a complete rebuilding of the missing structures by the following processes: (i) Dedifferentiation in cells close to the wound and adoption of embryonic/stem cell programs [5••,7]; (ii) Increase in division rates in cells close to the wound and switch in division planes [7]; (iii) Establishment of new accumulation zones for the phytohormones cytokinin (purple) and auxin (yellow) to define the new stem cell niche [5••]; (iv) Finally, de novo establishment of correct cell types in newly generated cells to restore the original tissue pattern [4,5••]. (b) Wounding on a cellular level means the disruption of the cellular envelope – cell wall (black) and plasma membrane (blue). Cell wall integrity sensing is presumably involved in wound signalling [20•,21]. Wound signalling quickly manifests as a Ca2+ wave which spreads through neighboring tissues [11,12]. The Ca2+ wave relates to the production of ROS in the apoplast and causes itself an oxidative burst inside and outside the cells [13, 14, 15]. Together, Ca2+ and ROS trigger multiple downstream signalling events at the wound site and in distal organs to induce immune responses [12,14,20•]. (c) Wounding induces production of various phytohormones with different dynamics. Jasmonate accumulation starts seconds after the wounding [50] and is perceived by CORONATINE INSENSITIVE1 (COI1) [51]. This leads to the activation of MYC2/3/4 transcription factors regulating downstream genes [52]. Ethylene accumulates 30 min after wounding by an increased activity of its biosynthesis genes [24] and acts through ETHYLENE-INSENSITIVE PROTEIN 2/3 (EIN2/3) transcription factors [53,20•]. ABA accumulation after wounding occurs after several hours in desiccated tissues and presumably functions in maintaining healthy plant physiology rather than immune responses [26]. Wounding induces changes in auxin accumulation and signalling after removal of the whole root tip; this involves induction of YUCCA biosynthetic components that play an important role in rebuilding destroyed structures [25,46].
Notably, even when the whole stem cell niche of the root is removed, the root meristem pattern is re-established de novo with correct arrangement of the lost cell types (Figure 1a) [4]. Single cell sequencing revealed that the newly generated cells quickly adopt the required new cell types, and this is partly dependent on the spatially separated maxima of two major phytohormones, auxin and cytokinin (Figure 1a). However, this de novo cell fate acquisition occurred (albeit with less efficiency) also when these maxima were disrupted, which suggests so far unknown intercellular positional signalling that coordinates the re-patterning of the root tip [5••]. This highlights the superior ability of plant organs to fully regenerate and restore correct tissue patterns.
Primary wound signalling
For the efficient initiation of defence responses and regeneration, plants need to quickly recognize the invaders or the induced destruction and signal to the immediate surroundings and the rest of the plant [8•,9,10]. The first known downstream signalling events that occur after herbivore attack or wounding are Ca2+ wave initiation [11,12] and an accumulation of reactive oxygen species (ROS) (Figure 1b) [13, 14, 15]. Wounding and pathogen associated elicitors also induce the production of small peptides that act as defence activators [16], for example, Pep1 and Pep2, which activate downstream immune responses against root pathogens [17, 18, 19].
Although these processes are well established to occur after the wounding/herbivore attack and mediate immune responses in plants (for a detailed review see Ref.: [20•]), they are triggered by an initial wound signal that is still unknown. Cell wall integrity sensing by constant measurement of the wall composition [20•,21] is thought to be a crucial element of wound detection. However, no direct, mechanistic connection between the known components of the cell wall integrity sensing and the wound/herbivore responses has been established.
Unsurprisingly, phytohormones, as universal endogenous signals, are induced with different dynamics after attack to contribute to the balance of growth and immunity/defence [22]. Historically, by extracting organic compounds from wound sites, the signalling compound traumatin was isolated which accelerates the wound healing when exogenously applied [3•]. Similarly, wounding induces jasmonic acid (JA) [23], ethylene (Et) [24] and less directly, auxin [25] and abscisic acid (ABA) (Figure 1c) [26,27]. While the biosynthetic pathways for most of these phytohormones are known, the exact production sites and the signalling mechanism underlying their activation, have not been investigated.
Wounding by targeted cell elimination
Recent reports have made use of targeted elimination of a single cell or small group of cells coupled with state-of-the-art live imaging allowing for more precise characterization of the wound responses and regeneration processes.
Laser ablation technique
In the 90 s, the UV laser ablation technique was introduced allowing for elimination of single cells. Originally, this was used to study cell-to-cell signalling and patterning mechanisms rather than as a tool to induce wounding and study regeneration. This technique has the advantage of removing a cell with spatial and temporal preciseness [28•,29•], in contrast to genetic [30] or chemical ablations [31••,32••]. Different types of lasers on different imaging setups [28•,29•,31••,33,34••] have been used with propidium iodide staining which stains cell walls, allow identification of dead cells and also pre-sensitizes cells for ablation [35]. This allowed the first live observation of wound healing responses in real time and in situ [32••].
Cellular responses during regeneration
The root meristem is a tissue where cells are constantly in the cell cycle to proliferate for a sustained growth. Cell elimination dramatically accelerates division rates of adjacent cells predominately at its inner adjacent side, as the time required for one division is reduced from 18 to 5–12 hours (depending on the cell type) (Figure 2c). These ‘restorative divisions’ involve a change in division planes from anticlinal (perpendicular to the growth axis) to periclinal (parallel to the growth axis) allowing for efficient replacement of the dead cells in the wound from the inside. Earlier studies showed that also in the stem cell niche, ablated cells are replaced by irregular divisions of adjacent cells [28•]. Outside of the stem cell niche, in differentiating cells, stem cell programs aid the regeneration process as seen by the re-activation of the endodermis/cortex (SHR/SCR and CYCD6;1) or the lateral root cap/epidermis (FEZ and SMB) stem cell regulators (Figure 2c) [32••]. Additionally, PLETHORA transcription regulators expressed in a decreasing gradient from the stem cell niche and associated with root stem cell activity [36] appear to endow cells with the competence to induce restorative divisions outside of the stem cell niche [32••].
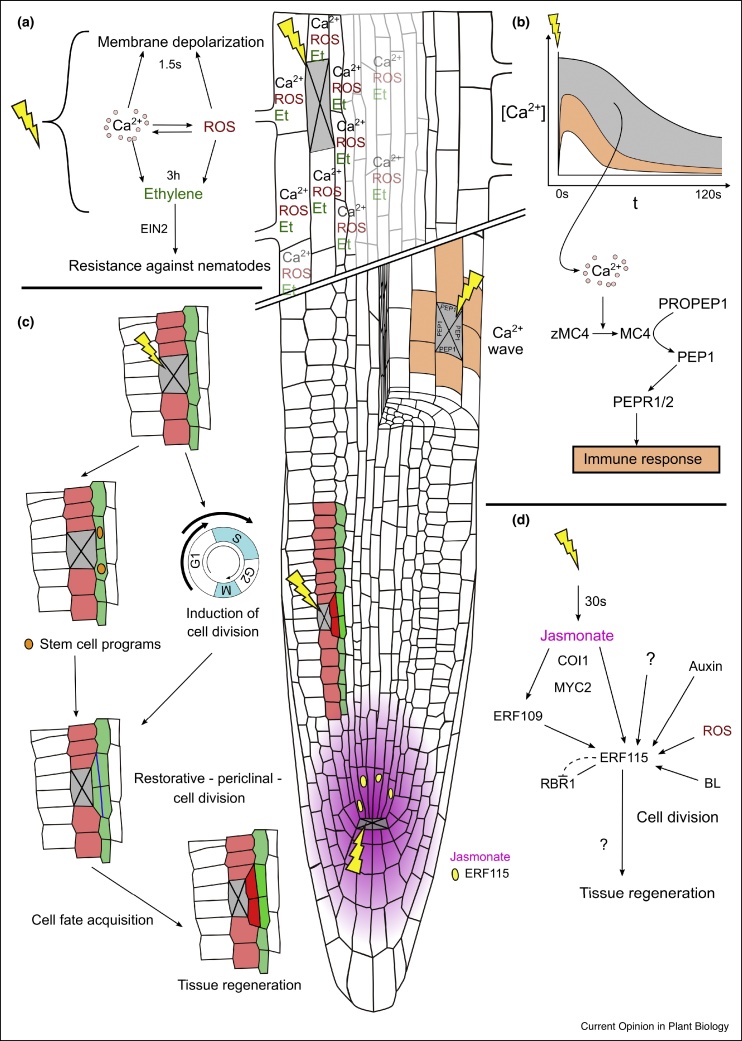
Figure 2.
Single cell ablation in the Arabidopsis root meristem triggers multiple local and regional wounding responses. (a) Ablation of cortex cells in the elongation zone triggers the induction of Ca2+, ROS, ethylene, and membrane depolarization. The increase in Ca2+ influx after ablation is dependent on ROS production in the apoplast by RBOH enzymes and allows the fast change in membrane polarization (1.5 s after ablation). Additionally, it induces an accumulation of ROS around the wound that occurs ∼6 min after the ablation. Both, Ca2+ influx and ROS production contribute to the ethylene signalling induction by an increased ACC SYNTHASE 6 (ACS6) expression starting three hours after ablation. Eventually, ethylene signalling via EIN2 increases the resistance against nematode infection [37••]. (b) Laser ablation of epidermis cells in the transition zone triggers a Ca2+ influx that spreads throughout the adjacent tissue but results in different amplitudes depending on the distance from the harmed cell. Harmed cells (grey) exhibit a stronger Ca2+ influx than those directly adjacent to the eliminated cells (orange) and cells further away (white). Strong influx and complete destruction of membrane integrity activate METACASPASE4 (MC4) from inactive zMC4, which cleaves the PRECURSOR OF PEP1 (PROPEP1) into Pep1. By this, it becomes translocated from the vacuolar membrane to the cytosol to be perceived by the PEPR1 and PEPR2 receptors at the cell surface of neighboring (orange) cells [34••]. (c) Ablation in the root meristem triggers restorative divisions to replace the eliminated cells. These divisions happen predominately in the inner adjacent cells. They are induced by the activation of stem cell programs (orange nuclei; here: SHR – CYCD6;1) and an accelerated progression through the cell cycle. They include the switch of the division plane from anticlinal to periclinal, and the newly generated outer daughter cells adopt the cell fate of the eliminated cells to eventually regenerate the disrupted tissue pattern [32••]. (d) Ablations in the stem cell niche trigger a jasmonate induction within 30 s which is perceived by COI1 to activate MYC2, a JA-dependent transcription factor. MYC2 binds to the promoter of ERF115 to enhance its expression around the wound site [41••]. ERF115 is also activated by its JA/MYC2-dependent homologue ERF109 [41••] and by downstream signalling of auxin [41••], ROS [49], and brassinosteroids (BL) [47,48]. In ablations outside the stem cell niche, ERF115 expression is confined to cells directly adjacent to the killed cell [31••,32••]. ERF115 can bind to RETINOBLASTOMA-RELATED1 (RBR1) and inhibit its activity to regulate the division rate in the quiescent centre and the stem cell niche [41••]. Few downstream targets of ERF115 have been identified. One of them, PSK5, might be involved in the acceleration of the cell cycle progression [47]. Eventually, ERF115 transcription factor activity contributes greatly to tissue regeneration after single cell ablation as well as whole root tip removal [31••,32••,41••]. Yellow thunderbolts indicate UV laser ablation.
Already the earlier ablation experiments suggested that cells in the root adopt their fate depending on the tissue context [28•,29•]. This is manifested dramatically during restorative divisions of any cell type. After the division plane switch, the inner daughter cell, which stays in the cell file it originated from, retains its identity. Remarkably, the outer daughter cell rapidly adopts the cell identity of the eliminated cell, which it replaces (Figure 2c) [32••].
The restorative divisions, which require accelerated cell cycle progression, division plane switch and finally cell fate change of the daughter cells, appear to be very robust and likely dependent on multiple redundant stem cell program-dependent and independent mechanisms. However, what signal triggers these divisions and what mechanism restricts them to cells only directly adjacent to the wound, remains elusive.
Primary wound signalling
Similar to herbivore attacks, wounding of single cells in the root meristem induces Ca2+ waves in the surrounding tissue. However, harmed cells exhibit a greater Ca2+ influx with an increased duration which is translated by a novel Ca2+-responsive protease, metacaspase MC4, into the rapid processing and release of Pep1 peptide. Eventually, the secreted Pep1 reaches the surface of neighboring cells and starts signalling through PEPR1/2 receptors to activate defence-related genes (Figure 2b) [34••].
Ablation of cells outside the root meristem (in the elongation zone) also triggers a Ca2+ wave and an increase in ROS accumulation in cells close to the wound site. Similar to previous studies [13], this Ca2+ wave and its propagation partly depend on enzymatic ROS production in the apoplast [37••]. These phenomena also coincide with a membrane depolarization close to the ablation site which probably comes from changed ion fluxes, like Ca2+and other available ions (Figure 2a) [37••].
Ablation experiments in the shoot apical meristem induce similar Ca2+ waves, which are required for the repolarization of the auxin efflux transporter PIN1 away from the wounded tissues [38], consistent with previously established importance of Ca2+ signalling for PIN polarity in roots [39]. Additionally, microtubules rearrange in the same cells after ablation as a consequence of a changed mechanical stresses, but this seems to be independent of the Ca2+ waves, indicating more complex and yet unknown mechanosensitive signalling mechanisms responsive to wounding [38].
Involvement of phytohormones
As expected, multiple phytohormones are involved in coordinating regenerative processes following wounding but their exact role and interactions are far from clarified. Cell ablation or infection with root-invading nematodes, which can lead to the specific removal of single cells in the root, leads to the increase of the transcriptional ethylene response marker ACS6 as early as three hours after ablation. Defence against these invaders depends on ethylene signalling through EIN2 [37••,40] and this triggering of the ethylene signalling partly depends on the Ca2+ wave and ROS production by apoplast-localized oxidases. Overall, these observations reveal an important role of ethylene in the root immune and wound response (Figure 2a) [37••].
Jasmonates (JA), phytohormones typically associated with plant immunity, are induced around wounds specifically in the central root meristem as early as 30 s after the ablation (Figure 2d). Similarly, nematode infestation or root growing through rough soil inducs JA [41••]. Pending evidence to the contrary, it seems JA response is not induced in root tissues other than the root meristem [37••].
Auxin has been implicated among many other processes, also in regulation of division plane orientation, cell fate (re)specification [42] and for the maintenance of the stem cell niche in the root meristem centre [43]. Removal of the root tip triggers a strong auxin accumulation above the ablated cells, presumably due to a disruption of the intercellular auxin flow, to induce replacement of the meristem centre [44]. Chilling stress induces natural death in root tip cells, which thereby block auxin transport anatomically. The resulting auxin accumulation helps maintaining the meristem centre during the stress [45]. Increased auxin biosynthesis, in contrast, occurs in wounded leaves [25] and root stumps after meristem removal [46] and is crucial for the efficient tissue re-establishment. However, it remains unknown how wound-responsive auxin transport, biosynthesis or signalling play a role in local regenerative processes.
Downstream transcriptional regulations
Besides the above-mentioned glimpses into wound-triggered signalling processes, little is known about the downstream mechanisms leading to regeneration. One of the few identified components is the ETHYLENE RESPONSE FACTOR 115 (ERF115), a transcription factor required for the efficient initiation of restorative divisions [31••,32••], and its close homologue and upstream regulator ERF109 [41••]. Without wounding, the ERF115 expression domain is usually restricted to the rarely occurring cell divisions in the quiescent centre, where it is controlled by brassinosteroids [47,48], but it can be slightly increased by exogenous application of ROS, auxin, and JA [41••,49]. In some cell types after wounding, ERF115 becomes upregulated in a JA-dependent manner during restorative divisions in cells directly adjacent to the wound (Figure 2d) [41••,31••]. It remains a mystery how such spatially restricted ERF115 induction is achieved by rather broadly spreading signals, exactly which factors are involved in cell types where ERF115 is not induced and which downstream targets of ERF115 mediate the regeneration.
Conclusions
Several recent studies using the single cell ablation allowed identifying wound response processes at different levels: (i.) local – cells directly adjacent to the wounds, (ii.) regional – cell groups in close proximity, or (iii.) systemic – the whole tissue in the same organ or in completely different parts of the plants. Comparable responses after cell ablation, nematode infestation or naturally occurring wounding suggest that laser-assisted cell elimination can be used to study mechanism of wound healing.
Multiple signals have been identified to be involved in the response to wounding, but the nature of the primary wound signal which activates the adjacent cells remains completely elusive, along with most of the downstream regeneration mechanisms. Further studies, building up on these initial findings and combining laser-assisted cell elimination with live imaging, forward genetic screens and single cell transcriptomics will allow us to get detailed molecular insights of what is happening at the local, regional, and systemic levels and how different signalling mechanisms cooperatively contribute toward wound healing. These studies not only will reveal mechanisms of tissue regeneration but also help us to understand the general mechanisms of positional information-based tissue patterning.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
Acknowledgement
Research in the J.F. laboratory is funded by the European Union’s Horizon 2020 program (ERC grant agreement no. 742985).
References
- 1.Molisch H. 1888. Zur Kenntniss der Thyllen, nebst Beobachtungen über Wundheilung in der Pflanze. [Google Scholar]
- 2.Hartsema A. 1926. Anatomische und experimentelle Untersuchungen über das Auftreten von Neubildungen an Blättern von Begonia Rex. [Google Scholar]
- 3•.Bloch R. Wound healing in higher plants. Bot Rev. 1941;7:110–146. [Google Scholar]; This review summarizes the discoveries from most wound studies of the 19th and early 20th century and states multiple findings that are still not fully understood today.
- 4.Sena G., Wang X., Liu H.-Y., Hofhuis H., Birnbaum K. Organ regeneration does not require a functional stem cell niche in plants. Nature. 2009;457:1150–1153. doi: 10.1038/nature07597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Efroni I., Mello A., Nawy T., Ip P.-L., Rahni R., DelRose N. Root regeneration triggers an embryo-like sequence guided by hormonal interactions. Cell. 2016;165:1721–1733. doi: 10.1016/j.cell.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses single-cell transcriptomics to describe the process of wound healing after the removal of the root tip. It shows the involvement of embryonic cell programs and auxin/cytokinin distribution in the root during regeneration.
- 6.Hush J., Hawes C., Overall R. Interphase microtubule re-orientation predicts a new cell polarity in wounded pea roots. J Cell Sci. 1990;96 [Google Scholar]
- 7.Barlow P. Regeneration of the cap of primary roots of Zea mays. New Phytol. 1974;73:937–954. [Google Scholar]
- 8•.León J., Rojo E., Sánchez-Serrano J. Wound signalling in plants. J Exp Bot. 2001;52:1–9. doi: 10.1093/jexbot/52.354.1. [DOI] [PubMed] [Google Scholar]; In this review, the knowledge about intercellular and intracellular wound signalling with respect to hormones, calcium, and peptide components is elaborated. Furthermore, it mentions studies with a high variety in model organisms, which allows a broader relevance for the drawn conclusions.
- 9.Heil M., Land W. Danger signals – damaged-self recognition across the tree of life. Front Plant Sci. 2014;5:578. doi: 10.3389/fpls.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henry G., Thonart P., Ongena M. PAMPs, MAMPs, DAMPs and others: an update on the diversity of plant immunity elicitors. Biotechnol Agron Soc Environ. 2012;16:257–268. [Google Scholar]
- 11.Knight M., Read N., Campbell A., Trewavas A. Imaging calcium dynamics in living plants using semi-synthetic recombinant aequorins. J Cell Biol. 1993;121:83–90. doi: 10.1083/jcb.121.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyota M., Spencer D., Sawai-Toyota S., Jiaqi W., Zhang T., Koo A. Glutamate triggers long-distance, calcium-based plant defense signaling. Science (New York, N.Y.) 2018;361:1112–1115. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 13.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V., Vandepoele K. ROS signaling: the new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki N., Mittler R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic Biol Med. 2012;53:2269–2276. doi: 10.1016/j.freeradbiomed.2012.10.538. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M., Ohura I., Kawakita K., Yokota N., Fujiwara M., Shimamoto K. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell Online. 2007;19:1065–1080. doi: 10.1105/tpc.106.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts K. Potential awareness of plants. Nature. 1992;360:14. [Google Scholar]
- 17.Huffaker A., Pearce G., Ryan C. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci U S A. 2006;103:10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krol E., Mentzel T., Chinchilla D., Boller T., Felix G., Kemmerling B. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285:13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartels S., Lori M., Mbengue M., van Verk M., Klauser D., Hander T. The family of Peps and their precursors in Arabidopsis: differential expression and localization but similar induction of pattern-triggered immune responses. J Exp Bot. 2013;64:5309–5321. doi: 10.1093/jxb/ert330. [DOI] [PubMed] [Google Scholar]
- 20•.Savatin D., Gramegna G., Modesti V., Cervone F. Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci. 2014;5:470. doi: 10.3389/fpls.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review gives a detailed overview about signalling components during plant wounding at our current knowledge. It focusses on cell wall integrity sensing, wound-associated DAMPs and signal transduction by ion fluxes, ROS, phosphorylation changes and hormone induction.
- 21.Nühse T. Cell wall integrity signaling and innate immunity in plants. Front Plant Sci. 2012;3:280. doi: 10.3389/fpls.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huot B., Yao J., Montgomery B., He S. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant. 2014;7:1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Creelman R.A., Tierney M.L., Mullet J.E. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci U S A. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boller T., Kende H. Regulation of wound ethylene synthesis in plants. Nature. 1980;286:259–260. [Google Scholar]
- 25.Chen L., Tong J., Xiao L., Ruan Y., Liu J., Zeng M. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J Exp Bot. 2016;67:4273–4284. doi: 10.1093/jxb/erw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkenmeier G., Ryan C. Wound signaling in tomato plants. Plant Physiol. 1998;117:687–693. doi: 10.1104/pp.117.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson S.N., Erb M., Hartley S.E. Roots under attack: contrasting plant responses to below‐and aboveground insect herbivory. New Phytol. 2016;210:413–418. doi: 10.1111/nph.13807. [DOI] [PubMed] [Google Scholar]
- 28•.van den Berg C., Willemsen V., Hage W., Weisbeek P., Scheres B. Cell fate in the Arabidopsis root meristem determined by directional signaling. Nature. 1995;378:62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]; This is the first study in which laser ablation has been used in the root meristem. It shows that killed cells in the stem cell niche can be replenished by adjacent stem cells and that it involves a switch of cell fate in the newly generated daughter cells. This suggests existence of unknown intercellular signaling mechanisms determining different cell fates in the stem cell niche.
- 29•.Berger F., Haseloff J., Schiefelbein J., Dolan L. Positional information in root epidermis is defined during embryogenesis and acts in domains with strict boundaries. Curr Biol. 1998;8:421–430. doi: 10.1016/s0960-9822(98)70176-9. [DOI] [PubMed] [Google Scholar]; This study describes how epidermis cells can adopt their cell fate (to be become root hair or non-root hair cells) according to the presence of their neighbors, as showed by the use of single cell ablation. Additionally, it shows that completely isolated cells keep their cell fate as defined during embryogenesis as long as their cell walls remains intact.
- 30.Weijers D., Hamburg J.-P., Rijn E., Hooykaas P., Offringa R. Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol. 2003;133:1882–1892. doi: 10.1104/pp.103.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Heyman J., Cools T., Canher B., Shavialenka S., Traas J., Vercauteren I. The heterodimeric transcription factor complex ERF115–PAT1 grants regeneration competence. Nat Plants. 2016;2 doi: 10.1038/nplants.2016.165. [DOI] [PubMed] [Google Scholar]; This study describes the wounding-induced expression of ERF115. It shows, that wounding by cutting off the root tip, chemical or laser ablation triggers the exression of ERF115 which acts together with PAT1, its heterodimeric binding partner, to contribute toward wound healing in the root meristem. When these two proteins are overexpressed, they induce strong callus formation interfering with growth and development in the induced seedlings.
- 32••.Marhava P., Hoermayer L., Yoshida S., Marhavý P., Benková E., Friml J. Re-activation of stem cell pathways for pattern restoration in plant wound healing. Cell. 2019;177:957–969. doi: 10.1016/j.cell.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the wound healing process in the root meristem after single cell ablation and describes the phenomenon of ‘restorative divisions and patterning’. These wounding-induced divisions switch division plane from anticlinal to periclinal and occur only in cells directly adjacent to eliminated cells and predominately along the inner wound side. They occur with an increased rate compared to regular divisions in the meristem and re-activate stem cell pathways. The inner daughter cells keep their cell fate while the outer adopt the cell type of the cell they replace. This leads eventually to the healing of the wound.
- 33.Marhavý P., Montesinos J., Abuzeineh A., Van Damme D., Vermeer J., Duclercq J. Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev. 2016;30:471–483. doi: 10.1101/gad.276964.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Hander T., Fernández-Fernández Á., Kumpf R., Willems P., Schatowitz H., Rombaut D. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science (New York, N.Y.) 2019;363 doi: 10.1126/science.aar7486. eaar7486. [DOI] [PubMed] [Google Scholar]; This study shows an ablation-dependent Ca2+ accumulation that activates a newly discovered calcium-dependent metacaspase, MC4. The calcium influx is strongest in harmed cells and their neighbors. There, MC4 induces the cleavage of PROPEP1 into the signal peptide PEP1, which becomes translocated from the vacuolar membrane to the cytosol. From there it can reach the PEP1 receptor in the neighboring cells to activate downstream immune repsonses.
- 35.Lo Schiavo F., Last R., Morelli G., Raikhel N., editors. Cellular Integration of Signalling Pathways in Plant Development. Springer; Berlin, Heidelberg: 1998. [Google Scholar]
- 36.Galinha C., Hofhuis H., Luijten M., Willemsen V., Blilou I., Heidstra R., Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- 37••.Marhavý P., Kurenda A., Siddique S., Tendon V.D., Zhou F., Holbein J. Single-cell damage elicits regional, nematode-restricting ethylene responses in roots. EMBO J. 2019;38 doi: 10.15252/embj.2018100972. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that single cell ablation in the root elognation zone triggers the induction of ethylene signalling which is important for the resistance against infestation by root nematodes. Furthermore, it shows the triggering of a Ca2+ wave, a change in membrane polarization and an oxidative burst in cells close to the wound. All these phenomena are partly dependent on ROS production in the apoplast by RBOH enzymes.
- 38.Li T., Yan A., Bhatia N., Altinok A., Afik E., Durand-Smet P. Calcium signals are necessary to establish auxin transporter polarity in a plant stem cell niche. Nat Commun. 2019;10 doi: 10.1038/s41467-019-08575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Vanneste S., Brewer P.B., Michniewicz M., Grones P., Kleine-Vehn J. Inositol trisphosphate-induced Ca2+ signaling modulates auxin transport and PIN polarity. Dev Cell. 2011;20:855–866. doi: 10.1016/j.devcel.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Bisson M.M., Bleckmann A., Allekotte S., Groth G. EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem J. 2009;424:1–6. doi: 10.1042/BJ20091102. [DOI] [PubMed] [Google Scholar]
- 41••.Zhou W., Lozano-Torres J., Blilou I., Zhang X., Zhai Q., Smant G. A jasmonate signaling network activates root stem cells and promotes regeneration. Cell. 2019;177:942–956.e14. doi: 10.1016/j.cell.2019.03.006. [DOI] [PubMed] [Google Scholar]; This study identified that exogenous application of jasmonates triggers cell divisions in the quiescent center through the activation of ERF115, which binds to RBR1. Similarly, it shows that wounding by laser ablation, sand and nematode infection triggered a fast induction of jasmonates in the root meristem, which activates the expression of ERF115 via COI1 and MYC2. Eventually, it shows that regeneration through the ERF115 is additionally activated by auxin and ERF109, a transcription factor that is also responsive to jasmonate and wounding.
- 42.Vanneste S., Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Ding Z., Friml J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc Natl Acad Sci U S A. 2010;107:12046–12051. doi: 10.1073/pnas.1000672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J., Hofhuis H., Heidstra R., Sauer M., Friml J., Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- 45.Hong J.H., Savina M., Du J., Devendran A., Ramakanth K.K., Tian X. A sacrifice-for-survival mechanism protects root stem cell niche from chilling stress. Cell. 2017;170:102–113. doi: 10.1016/j.cell.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Xu D., Miao J., Yumoto E., Yokota T., Asahina M., Watahiki M. YUCCA9-mediated auxin biosynthesis and polar auxin transport synergistically regulate regeneration of root systems following root cutting. Plant Cell Physiol. 2017;58:1710–1723. doi: 10.1093/pcp/pcx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heyman J., Cools T., Vandenbussche F., Heyndrickx K., Leene J., Vercauteren I. ERF115 controls root quiescent center cell division and stem cell replenishment. Science. 2013;342:860–863. doi: 10.1126/science.1240667. [DOI] [PubMed] [Google Scholar]
- 48.Lee H.-S., Kim Y., Pham G., Kim J., Song J.-H., Lee Y. Brassinazole resistant 1 (BZR1)-dependent brassinosteroid signalling pathway leads to ectopic activation of quiescent cell division and suppresses columella stem cell differentiation. J Exp Bot. 2015;66:4835–4849. doi: 10.1093/jxb/erv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong X., Tian H., Yu Q., Zhang F., Wang R., Gao S. PHB3 maintains root stem cell niche identity through ROS-responsive AP2/ERF transcription factors in Arabidopsis. Cell Rep. 2018;22:1350–1363. doi: 10.1016/j.celrep.2017.12.105. [DOI] [PubMed] [Google Scholar]
- 50.Glauser G., Dubugnon L., Mousavi S.A., Rudaz S., Wolfender J.L., Farmer E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J Biol Chem. 2009;284:34506–34513. doi: 10.1074/jbc.M109.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- 52.Kazan K., Manners J.M. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 53.Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]