Abstract
Background:
Antiretroviral therapy (ART) adherence is crucial to achieve HIV suppression and to prolong survival of HIV-infected patients. Although monitoring of ART adherence is standard of HIV care, there is yet no optimal method to measure ART adherence. Therefore, it is essential to compare the effectiveness of different adherence measurement tools to predict HIV suppression.
Methods:
In this study, we measured ART adherence using pharmacy refill prescription and self-reported adherence questionnaire. Both the methods were compared for predicting HIV suppression in adult Omani HIV-infected patients attending the outpatient clinics at Sultan Qaboos University Hospital.
Results:
A total of 141 HIV-infected patients were included. The pharmacy refill–based measure showed a median adherence rate of 98.90% (interquartile range [IQR]: 86%-99.45%). The self-report adherence questionnaire revealed a median adherence rate of 100% (IQR: 75-100). A significant positive correlation was found between the adherence rates measured by the 2 methods (r = 0.32, P = .01). The pharmacy refill and self-report questionnaire adherence measures were both negatively correlated with plasma HIV RNA levels (r = −0.20, P = .01 and r = −0.26, P = .04, respectively).
Conclusion:
Collectively, these findings suggest that pharmacy refill measure could serve as a valid and practical tool of ART adherence in routine clinical practice.
Keywords: pharmacy refill, questionnaire, adherence, ART, HIV, patients
What Do We Already Know about This Topic?
Although a number of methods have been proposed to measure antiretroviral therapy (ART) adherence in HIV-positive patients, their performances are still questionable.
How Does Your Research Contribute to the Field?
This article studied 2 ART adherence methods, namely, pharmacy refill prescription and self-reported adherence questionnaire, in order to identify a simple tool that will help evidence-based interventions in Omani adults living with HIV.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
The findings indicated that predictive values for detectable viral loads were similar for both methods, and pharmacy refill adherence measure could be an effective tool to assess ART adherence in HIV-infected Omani patients.
Introduction
The widespread use of antiretroviral therapy (ART) has converted AIDS from a death sentence to a lifelong chronic illness. Antiretroviral therapy has drastically decreased morbidity and mortality as well as transmission of HIV.1 Several studies have shown that maintenance of long-term adherence to ART is a key factor in achieving the desired therapeutic outcome, which is HIV suppression to undetectable level.2,3 Most HIV treatment guidelines currently recommend routine assessment of ART adherence in each HIV-infected patient’s encounter.4,5 Suboptimal adherence to ART could lead to treatment failure, emergence, and transmission of HIV drug-resistant strains, which limit future therapeutic options.6
A variety of methods have been proposed to measure ART adherence, including review of pharmacy refill records, self-reported adherence questionnaires, ART drug blood level monitoring, electronic monitoring devices, and pill count.5 Although there is no best optimal tool to measure ART adherence, self-report adherence is the most commonly used tool.7-9 However, self-report adherence questionnaires need intact patient’s cognitive skills and are subjected to recall and information biases.7-9 The availability of electronic pharmacy records could provide a valid and simple tool to assess adherence through measuring acquisition of prescribed ART.5,10 This study evaluated and compared the correlation of pharmacy refill records and self-reported adherence questionnaire with HIV viral load suppression as proxy for assessing ART adherence. It aimed to provide an evidence to support the use of a simple and effective adherence measurement tool to identify patients who are in great need of adherence support.
Patients and Methods
Study Population
This study was conducted at the infectious disease clinic in the Sultan Qaboos University Hospital (SQUH) in Omani HIV-infected patients from February 1, 2017, to April 30, 2017. The HIV clinic is part of governmental outpatient’s centers that provides free medical care and ART to all Omani HIV-infected patients. The SQUH clinic serves an average of 20 patients per week, and HIV care is provided by highly qualified and HIV-trained infectious disease consultants. Of note, ART in Oman is delivered only through governmental pharmacies, which are always part of the public hospital area. The patients enrolled in this study pick up their ART from SQUH pharmacy during the same HIV clinic visits through electronic prescription. No ART refill is provided from outside or private pharmacies. This study included HIV-infected patients who were on regular clinic follow-up, taking ART for a minimum of 1 year, and had 2 or more plasma HIV RNA measurements 6 months after starting ART. Demographic and clinical data were obtained from electronic medical records and patient charts.
Pharmacy Refill Adherence
The pharmacy refill adherence was retrospectively measured using electronic pharmacy refill records. Usually, patients refilled ART every 3 months, and each patient should have at least 2 refills to be legible for calculation. The pharmacy refill adherence was defined as the proportion (%) of the collected prescribed ART from the SQUH pharmacy. To see whether patients had adequate pills dispensed to cover the period between the clinic visits, the period between the 2 refills was defined as the days between the last prescription of a specific ART drug and the next collected refill of the same or alternative drug. The sum of the total intervals between refills was measured over a minimum period of 3 months for each patient prior to the date of the questionnaire. A 3-month period was chosen to coincide with the adherence period measured by self-report questionnaire. Adherence was measured using the following formula as reported by Grossberg et al.11
Refill adherence was 100% if all pills during the scheduled refill period were dispensed on time. Calculated refill adherence rate values above 100% for patients who refilled earlier than scheduled date were rounded to 100%.
Self-Reported Adherence Questionnaire
The self-reported adherence questionnaire was filled by a random sample of patients who were seen during the study period. The questionnaire used was an Arabic-translated version of Adult AIDS Clinical Trials Group ART Adherence questionnaire.12 It is a validated and commonly used measure of self-report adherence to ART. The validity of the translated Arabic version of the questionnaire was first assessed by Cronbach α test, which was 0.830, indicating excellent reliability. This was conducted via a pilot study in which 15 participants were first included. These participants were later included in the full study. The questionnaire also contained a list of frequently experienced ART side effects and commonly reported reasons for nonadherence. A face-to-face interview was prospectively performed during routine clinic visits. The interview was conducted by the same dedicated trained nonclinical staff throughout the study period. The patients were asked about the number of doses that they have missed in the last 4 days. Taking the numbers of different drugs and the number of doses into consideration, ART compliance was calculated for the last 4 days using the following formula as reported by Grossberg et al.11
Plasma HIV RNA Measurements
The latest 2 plasma HIV RNA measures were used to determine the degree of HIV suppression and ART adherence. The plasma HIV RNA values chosen were the closest to the questionnaire administration time and at the end of the pharmacy refill period. Virological failure was defined as a plasma HIV RNA level >200 copies/mL as defined by US Department of Health and Human Services.5 Although the adherence rate threshold that is needed to achieve HIV suppression is still controversial, several studies have indicated that adherence rates above 85% to 95% strongly correlated with HIV suppression in patients taking non-nucleoside reverse transcriptase inhibitors and protease inhibitors.13,14 In the current study, patients were classified into the adherent group if the measured adherent rate was ≥90% and to nonadherence group if adherent rate was <90%. In addition, adherence rates of 70% and 80% were examined for each adherence tool in predicting viral suppression.
Statistical Analysis
Statistical analysis was performed using STATA version 13 (STATACorp, College Station, Texas). Analysis of categorical variables was carried out using χ2 test and Fisher exact test. Descriptive statistics for continuous variables were measured using the mean and standard deviation for normally distributed data, while the median and interquartile range (IQR) were used for non-normally distributed data. Comparative statistics for continuous variables were measured using Mann-Whitney U test. Univariate logistic regression analysis was used to assess the association among study variables and the adherence rates. Spearman correlation was used to evaluate the relationship between plasma HIV RNA levels and adherence rates in both pharmacy refill and self-reported adherence groups. A P value of <.05 was considered significant. The sensitivities and specificities of both methods at different adherence rate thresholds and at different plasma HIV RNA levels to define viral load suppression, as recommended by the World Health Organization, were also calculated.15
Ethical Approval and Informed Consent
The study was approved by the Sultan Qaboos University, Medical Research Ethics Committee (approval no. 982). A signed informed consent was obtained from all participants before enrollment in the study.
Results
Pharmacy Refill Records
A total of 153 HIV-infected patients followed at the SQUH were enrolled in this study. Of those, 12 patients were excluded for last clinic follow-up (n = 7), not taking ART (n = 2), taking ART for less than 3 months (n = 2), and had multiple refills on biweekly basis during the study period (n = 1). The remaining 141 patients were assessed for ART adherence via pharmacy refill method, and their baseline characteristics are summarized in Table 1. The median age was 44 years, and the median duration of ART was 7 years. The median adherence rate was 98.90% (IQR: 86.23%-99.45%). A total of 39 (28%) participants had pharmacy refill adherence of more than 100%. The most recent median plasma HIV RNA and CD4 count were 20 copies/mL and 476 cells/µL, respectively.
Table 1.
Characteristics of the Study Population.a
| Characteristics | All Patients, N = 141 | Nonadherent to ART, n = 37 | Adherent to ART, n = 104 | P Values |
|---|---|---|---|---|
| Gender | ||||
| Females, n (%) | 55 (39.01) | 12 (32.43) | 43 (41.35) | .34 |
| Males, n (%) | 86 (60.99) | 25 (67.57) | 61 (58.65) | |
| Age, years | ||||
| Median [IQR] | 44 [36-52] | 47 [37-56] | 43 [36-51] | .17 |
| Mean (SD) | 45 (12) | 47 (12) | 44 (12) | |
| Age <35, n (%) | 29 (20) | 4 (10) | 25 (24) | |
| Age 35-45, n (%) | 51 (36) | 14 (37) | 37 (35) | |
| Age > 45, n (%) | 61 (43) | 19 (51) | 42 (40) | |
| Duration of HIV infection | ||||
| Median duration [IQR] | 7 [4-10] | 6 (5-10) | 7 [4-10] | .82 |
| Mean duration (SD) | 6.94 (3.70) | 6.65 (2.79) | 7 (3.98) | |
| Baseline HIV RNA, copies/mL | ||||
| Median [IQR] | 26 880 [940-145 000] | 34 240 [770-157 956] | 25 299 [1192-141 375] | .66 |
| Mean (SD) | 271 354 (964 270) | 335 438 (886 991) | 248 555 (993 402) | |
| Latest HIV RNA, copies/mL | ||||
| Median [IQR] | 20 [0-218] | 55 [0-1634] | 20 [0-1085] | .21 |
| Mean (SD) | 12 678 (58 432) | 29 882 (96 487) | 6558 (35 254) | |
| Baseline CD4+ T cells, /µL | ||||
| Median [IQR] | 171 [61-366] | 163 [60-428] | 176 [61-322] | .73 |
| Mean ( SD ) | 256 (270) | 276 (275) | 249 (269) | |
| Latest CD4, /µL | ||||
| Median [IQR] | 476 [301-662] | 476 [286-700] | 471 [304-654] | .97 |
| Mean (SD) | 508.3071 (292) | 499.1667 (277) | 511 (298) | |
| Virological outcome | ||||
| Virological failure, n (%) | 39 (28) | 15 (40.54) | 24 (23.07) | .04 |
| Virological success, n (%) | 102 (72) | 22 (59.45) | 80 (76.92) | |
| Current ART class | ||||
| NRTI, n (%) | 141 (100) | 37 (100) | 104 (100) | .99 |
| NNRTI, n (%) | 66 (46.81) | 13 (35.14) | 53 (50.96) | .09 |
| PI, n (%) | 66 (46.81%) | 22 (59.46%) | 44 (42.31%) | .07 |
| INSTI, n (%) | 8 (5.67%) | 2 (5.41%) | 6 (5.77%) | .93 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse-transcriptase inhibitors; PI, protease inhibitors; SD, standard deviation.
aVirological success defined as plasma HIV RNA <200 copies/mL. The P value under each variable was obtained from comparing adherent to nonadherent group based on pharmacy refill adherence measure.
Using adherence threshold of ≥90%, 104 (74%) patients were classified as adherent and 37 (26%) patients as nonadherent to ART by the pharmacy refill method (Table 1). Of the total 141 patients, there were 102 (72%) patients who had plasma HIV RNA <200 copies/mL. Of those, 80 (77%) patients were classified as adherent to ART. We found a significant negative correlation between pharmacy refill adherence and plasma HIV RNA (r = −.20, P = .01). Multivariable logistic regression analysis revealed significant associations between nonadherence and plasma HIV RNA >200 copies/mL (odd ratio [OR]: 2.37; 95% confidence interval [CI]: 1.05-5.31; P = .03). In contrast, no significant associations were found between nonadherence and all other tested variables including age, gender, duration of ART or ART class.
The sensitivity and the specificity of pharmacy refill method in predicting suppression of plasma HIV RNA levels below 200 copies/mL are shown in Table 2. Pharmacy refill method sensitivity extended between (35%-41%) using adherence threshold of 70%, 80%, and 90%. Moreover, at different thresholds for adherence rates, the sensitivity remained the same at adherence rates of 90% and 80%, while it decreased at adherence rate of 70% (Table 2).
Table 2.
The Sensitivity and Specificity Measures of Pharmacy Refill Method at HIV RNA Cutoff of 200 Copies/mL.
| Adherence Rate, % | Sensitivity | 95% Confidence Interval | Specificity | 95% Confidence Interval |
|---|---|---|---|---|
| 90 | 0.41 | 0.25-0.58 | 0.79 | 0.70-0.86 |
| 80 | 0.41 | 0.25-0.58 | 0.84 | 0.75-0.90 |
| 70 | 0.35 | 0.21-0.53 | 0.86 | 0.77-0.91 |
Self-Reported Questionnaire
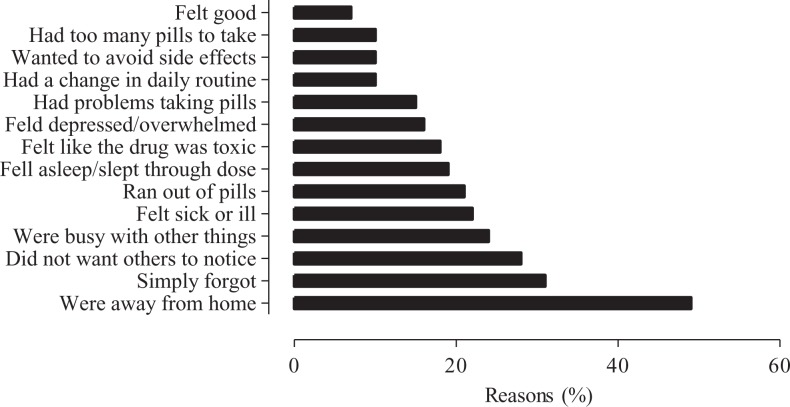
The median ART adherence was 100% (IQR: 75%-100%). As illustrated in Figure 1, the main reason for nonadherence to ART was being away from home. Approximately, 50% of participants forget to take ART when they were away from home. The most frequently reported ART side effects were weight gain, bloating, stomach pain, dizziness, muscle aches, and joint pain.
Figure 1.
Main reasons for nonadherence to antiretroviral therapy among participants.
The self-reported adherence rate was negatively correlated with plasma HIV RNA levels (r = −0.26, P = .04). A significant positive correlation was also evidenced between self-reported and pharmacy refill measures (r = 0.32; P = .01).
Discussion
This study assessed pharmacy refill and self-report as measures of ART adherence and their correlations with plasma HIV RNA suppression. Our data suggest that pharmacy refill is a useful method in our clinical setting to assess ART adherence, and it can predict HIV virologic failure. Although both methods were not highly sensitive, pharmacy refill was effective as self-report for assessing ART adherence. Pharmacy refill has been shown to predict HIV virologic failure in early studies. In a review study by McMahon et al, the pharmacy refills and/or pill counts predicted virologic failure in 88% of studies. In the same review, the studies were unable to predict HIV virologic failure, which was assessed only in ART-experienced patients and included a small sample size.10 Henegar et al reported that pharmacy refill method forecasted virologic failure in ART nonadherence patients,16 and Nieuwkerk and Oort showed a significant association between self-report nonadherence and detectable HIV RNA.17 Our data revealed that ART adherences assessed by pharmacy refill and self-report were positively correlated with each other, while both measures were negatively correlated with plasma HIV RNA <200 copies/mL. These findings are in line with those reported by Fairley et al and Chalker et al who demonstrated a correlation of both methods even in long-term adherence assessments.18,19 In contrast, Grossberg et al found that pharmacy refill method performed better than self-reported adherence measure. However, their study differently defined optimal adherence, virologic suppression, and duration of adherence.11
Interestingly, our data also suggest that pharmacy refill adherence assessment has the same sensitivity when defining virologic failure as HIV RNA >200 copies/mL or > 400 copies/mL. In addition, our study did not detect an adherence threshold effect on virologic suppression when defining optimal rate adherence as >80% or >90%. Nevertheless, pharmacy refill method was less sensitive in detecting HIV suppression at adherence rate of >70%. When comparing pharmacy refill and self-report, in fact each method has its own advantages depending on the cultural contexts, clinical settings, and its usage. Pharmacy refill is a simple standardized, objective and nonjudgmental method. It does not require intact patient’s cognition and it is mainly used to assess whether the increase in viral load is due to nonadherence or ineffective ART. Compared to self-report measure, pharmacy-based method lacks the ability to explore nonadherence barriers and could not identify specific drug nonadherence. In addition, pharmacy refill method is unsuitable when ART refills are submitted to outside pharmacies or unable to track records. In contrast, self-reported measure seems to be the best tool to explore reasons for nonadherence whether it is access to care, stigma, or attitudes toward HIV infection. However, this method requires cognitive skills; therefore, results may vary depending on educational level, mental wellness, and tendency to report socially desired responses.
Our study was unable to demonstrate associations among nonadherent and other patients’ characteristics such as gender, age, and duration of taking ART. These findings could be explained in part by the sample size, which was relatively small, and thereby the results should be interpreted with caution.
This study also explored some barriers to ART adherence using self-report questionnaire. Our results are consistent with those recently reported by Mills et al.20 The main reported reasons for nonadherence to ART in this cohort were being away from home, forgetfulness, and fear of being noticed taking medication. As these factors are modifiable or at least partially modifiable, usage of appropriate interventions could improve ART compliance and ultimately HIV outcomes.
This study has certain limitations. Although there is no gold standard for measuring adherence to ART, demonstrating a negative correlation between pharmacy refill adherence and HIV suppression is useful evidence to support its validity. Second, shorter intervals between pharmacy refills may have overestimated the adherence rate. Third, using nonclinical staff to administer self-report questionnaire might impact on reported compliance. Finally, electronic pharmacy records were needed to apply pharmacy refill measure; therefore, its utility may not be generalizable to resource-limited settings.
In conclusion, given its positive correlation with self-report questionnaire adherence measure and its negative association with plasma HIV RNA levels, pharmacy refill measure could serve as a valid and practical measure of ART adherence in our clinical setting. These results are encouraging and suggest that further investigation of this approach is warranted.
Acknowledgments
The authors are thankful to all participants and also to Drs Ishraq Ali Al-Uwaisi, and Shadin Ibrahim for providing assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the Sultan Qaboos University (IG/MED/MEDE/16/01).
ORCID iD: Mohamed-Rachid Boulassel, DEMS, PhD,  https://orcid.org/0000-0001-5717-7884
https://orcid.org/0000-0001-5717-7884
References
- 1. Giordano TP, Suarez-Almazor ME, Grimes RM. The population effectiveness of highly active antiretroviral therapy are good drugs good enough. Curr HIV/AIDS Rep. 2005;2(4):177–183. [DOI] [PubMed] [Google Scholar]
- 2. Mocroft A, Youle M, Moore A. et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001;15(2):185–194. [DOI] [PubMed] [Google Scholar]
- 3. De Arminio Monforte A, Lepri AC, Rezza G. et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14(5):499–507. [DOI] [PubMed] [Google Scholar]
- 4. WHO HIV/AIDS. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, recommendations for a public health approach. Second edition 2016. 2016. http://www.who.int/hiv/pub/arv/ex-summary.pdf. Accessed January 11, 2018. [PubMed]
- 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Huma Services. 2017. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 12, 2017.
- 6. Hecht FM, Grant RM, Petropoulos CJ. et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med. 1998;339(5):307–311. [DOI] [PubMed] [Google Scholar]
- 7. Chesney MA. The elusive gold standard. Future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(suppl 1):149–155. [DOI] [PubMed] [Google Scholar]
- 8. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mannheimer SB, Morse E, Matts JP. et al. Sustained benefit from a long-term antiretroviral adherence intervention. Results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43(suppl 1):41–47. [DOI] [PubMed] [Google Scholar]
- 10. McMahon JH, Jordan MR, Kelley K. et al. Adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis. 2011;52(4):493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57(10):1107–1110. [DOI] [PubMed] [Google Scholar]
- 12. Chesney M, Ickovics J, Chambers D. et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–266. [DOI] [PubMed] [Google Scholar]
- 13. Maggiolo F, Ravasio L, Ripamonti D. et al. Similar adherence rates favour different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis. 2005;40:158–163. [DOI] [PubMed] [Google Scholar]
- 14. King MS, Brun SC, Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naive, HIV-1-infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191(12):2046–2052. [DOI] [PubMed] [Google Scholar]
- 15. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach second edition; 2016. www.who.int. Accessed in June 3, 2018. [PubMed]
- 16. Henegar CE, Westreich D, Maskew M. et al. Comparison of pharmacy-based measures of adherence to antiretroviral therapy as predictors of virological failure. AIDS Behav. 2015. r;19(4):612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immune Defic Syndr. 2005;38(4):445–448. [DOI] [PubMed] [Google Scholar]
- 18. Fairley CK, Permana A, Read TR. Long-term utility of measuring adherence by self-report compared with pharmacy record in a routine clinic setting. HIV Med. 2005;6(5):366–369. [DOI] [PubMed] [Google Scholar]
- 19. Chalker J, Wagner A, Tomson G. et al. Urgent need for coordination in adopting standardized antiretroviral adherence performance indicators. J Acquir Immune Defic Syndr. 2010;53(2):159–161. [DOI] [PubMed] [Google Scholar]
- 20. Mills EJ, Nachega JB, Bangsberg DR. et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. Plos Med. 2006;3(11):438. [DOI] [PMC free article] [PubMed] [Google Scholar]