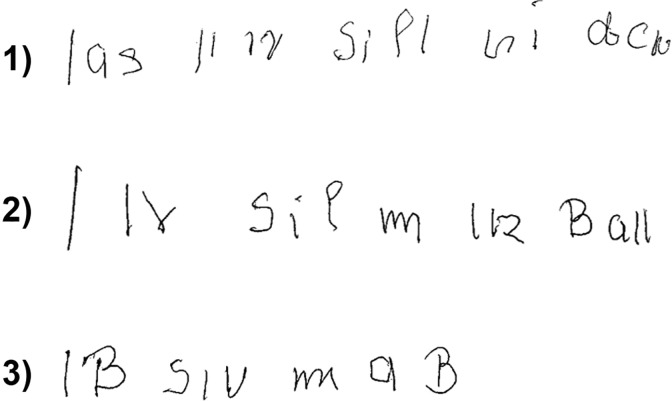Abstract
Posterior cortical atrophy (PCA) describes a rare heterogenous neurodegenerative syndrome with early visuospatial and visuoperceptual deficits due to atrophy of parieto-occipital brain regions. Here, we describe the case of a 62-year-old woman showing severe cognitive impairments as well as hemianopsia and all core symptoms of Bálint's syndrome. Years ago, the patient had complained about a “tunnel view” and concentration problems. The diagnostic results point to a case of PCA with underlying Alzheimer pathology. The disease course until diagnosis lasted for 7 years, reflecting the diagnostic difficulties with this still largely unknown syndrome. The unfamiliar symptom presentation including fluctuations in cognitive performance, affective symptoms, cerebrospinal fluid (CSF) biomarkers, which were at first inconspicuous, and a former suspected diagnosis of dissociative pseudodementia, altogether brought considerable uncertainty to the involved health-care professionals. We conclude that cases of “atypical dementia” presenting with visual symptoms, even if appearing unspecific at first, are suspect of PCA. This case report provides an ostensive overview of PCA, including imaging data, CSF-findings, original drawings and handwriting samples from the patient.
Keywords: posterior cortical atrophy, Alzheimer’s disease, Bálint’s syndrome, hemianopsia
Background
Posterior cortical atrophy (PCA) is a rare neurodegenerative syndrome with an early age of onset in the mid 50s to early 60s. Initially described as visual Alzheimer variant, the underlying pathology, however, in some cases, can also be attributable to other neurodegenerative entities.1 The pattern of cortical atrophy shows an early accentuation of parieto-occipital brain regions involving important fields of cortical visual representation, whereas areas affected early on in Alzheimer disease (AD), like the middle temporal lobes, often are spared for a relatively long period.2,3 Accordingly, the first symptoms predominantly concern visuospatial and visuoperceptual functions, whereas higher mnestic functions are normally affected later in disease course.4,5 Patients often have a good insight into their symptoms, whereas AD often is associated with anosognosia.6 Together with the logopenic variant of primary progressive aphasia and early-onset AD, PCA might be part of a clinical continuum of early-onset dementias that share clinical and neuroanatomical features.7 The consensus criteria from 2017 suggest a 3-step diagnostic approach with diagnosis of the clinical–radiological syndrome requiring at least 3 core symptoms of visuospatial or visuoperceptual impairment at disease onset in contrast to relatively spared memory, executive, and speech functions as well as preserved personality and behavior.8 While these criteria discriminate against AD, as well as language and behavioral variants of frontotemporal lobar degeneration, additional exclusion criteria account for possible somatic causes like strokes, lesions of the visual pathway, or brain tumors. The radiological signs, however, are facultative, which makes PCA an all-clinical diagnosis. When clinical criteria are met, in a second step it shall be checked if the disease is associated with a neurodegenerative syndrome other than AD (ie, corticobasal syndrome, Lewy body dementia, or prion disease). Finally, the underlying pathology shall be clarified by biomarker analysis in cerebrospinal fluid (CSF), which nowadays is only possible for Alzheimer pathology (Aβ 1-42, Tau, p-Tau) and prion disease (14-3-3 protein, RT-QuIC method).
Case Description
A 62-year-old married woman was admitted to our Neurogerontopsychiatric Day Care Unit due to cognitive deficits. The first abnormalities dated back 7 years, when the patient still worked as a physiotherapist, but developed problems with reading and writing professional letters as well as with performing simple calculations. Due to these issues, the patient had to quit her job 1 year later. Five years ago, the patient complained about a “tunnel view” which led to repeated consultations of opticians to adjust new glasses without any contacts to ophthalmologists. Spatial disorientation, being helpless in unknown places, and not being able to drive any longer occurred 3 years later. For 2 years now, the patient has disorientation in time, and daily life has been impaired by loss of practical abilities, like handling the cooker, the washing machine, the telephone, or the TV remote control. Today, the patient needs personal assistance for dressing (wears clothes inside out or is not able to put them on) and eating (leaves food on the plate). Also, difficulties in grasping things like cutlery are described. Concentration problems were accompanying these symptoms, severe memory deficits, however, being described for the last 2 years only. Symptoms and results of cognitive screening tests are listed in Table 1. The first professional appointment was 5 years ago, when the patient was examined in a neurologic hospital, which resulted in a suspected diagnosis of beginning dementia of unclear entity and prescription of donepezil. The CSF biomarkers were normal then (Table 2). During another hospital stay half a year later, the amnestic deficits were diagnosed as mild cognitive impairment which was attributed to an “atypical depressive syndrome” and even a dissociative etiology has been discussed due to the symptom presentation leaving a “demonstrative” impression and a long-lasting marital conflict as a potential psychogenic factor. In the mini-mental state examination (MMSE), the patient scored 24/30 points then, remarkably with the deficits occurring in the visuoconstructive (−1) and the calculation (−5) task without memory impairment (Table 1). Neurologic examinations had been described as unremarkable so far. The medical record showed a history of arterial hypertension and Hashimoto’s thyroiditis. There were no relevant childhood diseases or pre/perinatal complications. Twice, at the age of 26 (postpartum) and 34, the patient had an acute polymorphic psychosis. Both episodes remitted after a few days of antipsychotic medication with no need of medicamentous recidive prophylaxis. The patient had no history of alcohol or drug abuse. The family history was empty of neuropsychiatric disorders.
Table 1.
Symptoms and Results of Cognitive Screening Tests in 2013 and 2018.a
| Symptom/Test | 2013 | 2018 |
|---|---|---|
| MMSE | 24 | 9 |
| MoCA | NA | 4 |
| Concentration problems | + | + |
| Disorientation in time/place | − | + |
| Depressed mood | + | − |
| Memory deficits | − | + |
| Acalculia | + | + |
| Agraphia | − | + |
| Alexia | + | + |
| Visual problems, “tunnel view” | + | + |
| Constructional dyspraxia | + | + |
| Simultagnosia | NA | + |
| Optic ataxia | NA | + |
| Oculomotor apraxia | − | + |
| Hemianopsia | − | + |
| Finger agnosia | NA | + |
| Spatial disorientation | − | + |
| Dressing apraxia | − | + |
Abbreviations: NA, not available (not performed and/or not described); MoCA, montreal cognitive assessment test; MMSE, mini-mental state examination; PCA, posterior cortical atrophy; +: present; −: not present.
aWith more than 2 symptoms indicative for visuospatial and visuoperceptual deficits at first consultation in 2013 with relatively spared memory functions, the clinical core criteria for PCA according to the Consensus classification from 20178 are fulfilled. The low MMSE score of 24 in 2013 is explained by the patients’ inability to calculate 100-7 (and likewise to spell words backward) and visuoconstructional problems whilst drawing the 2 overlapping pentagons.
Table 2.
Cerebrospinal Fluid Findings.a
| Biomarkers | 01/2013 | 09/2018 | Reference |
|---|---|---|---|
| Aβ 1-42 | 581 | 307 | > 450 [pg/mL] |
| Tau | 299 | 228 | < 450 [pg/mL] |
| p-tau | 48 | 40 | < 61 [pg/mL] |
| tau/Aβ 1-42 | 0.51 | 0.74 | < 0.52 |
a Cerebrospinal fluid biomarker analysis from 2013 and 5 years later. Whereas total values for Aβ 1-42, tau, and phospho-tau were still in the reference of the assay (Innotest) in 2013, 5 years later a marked decrease in Aβ 1-42 was found. The ratio for tau to Aβ 1-42 which exhibits a higher diagnostic sensitivity than the Aβ 1-42 value alone (0.93 vs 0.82, see study by Duits et al9) was closely under the cutoff value in 2013 but clearly pathological in 2018 (values outside the reference bold).
Investigations
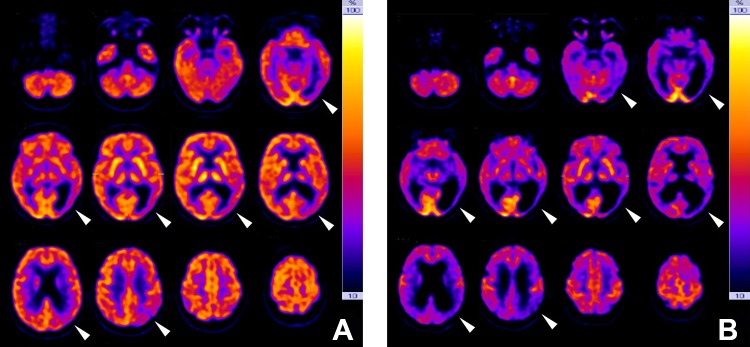
In our examination, the patient was disoriented in time, place, and situation. Concentration and short- and long-term memory were severely impaired. The patient could not subtract 7 from 100, could not spell short words, could not recall any of 3 words after 2 minutes, and had difficulties remembering the names of her 2 sons. Severe word finding difficulties appeared. Apart from that, the speech was fluent with no signs of agrammatism, semantic, or phonemic paraphasia. Insight into the deficits was preserved by the patient, being described as feeling of an “empty head with nothing left inside.” The overall mood was good, the affect, however, being slightly labile, quickly responding to both positive and negative emotional stimulation. Additionally, the patient was very irritable in new situations, then she sometimes reacted short-tempered and seemed to be helpless. Formal thinking was a bit slow but sorted. There were no delusions and no hallucinations. The neurologic examination showed hemianopsia to the right. Eye saccades were uncontrolled and apractic, but there was no gaze palsy. Otherwise, the brain nerves were inconspicuous. The right-handed patient had difficulties imitating hand movements with no side preference as a sign of ideomotoric apraxia. The description of Binet's images and Navon figures was hampered due to simultagnosia. The clock drawing test10 as well as free drawing attempts showed severe visuospatial deficits (Figure 1). The patient had agraphia (Figure 2) and alexia. The drawing of complex figures was disabled (Figure 3). Furthermore, we found an optic ataxia on the right side (atactic grasping of objects when coming into the visual field from the right). Apart from that, there were no focal neurologic defects, particularly no Parkinsonism and postural stability was fine. The neuropsychologic screening tests pointed to severe cognitive impairments (mini-mental state examination [MMSE] 9 points, montreal cognitive assessment test [MoCA] 4 points). The MRI brain scans showed a ballooning of the ventricular system, which was most pronounced in the left posterior horn with strong atrophy of left parieto-occipital cortices (Figure 4). In the fluorodeoxyglucose-positron emission tomography (FDG-PET) investigation a generalized cerebral hypometabolism was evident, most distinctive in left parieto-occipital cortex regions (Figure 5). The electroencephalography recording showed a permanent slowing with generalized theta, sometimes also delta-activity without any epileptic potentials or focal abnormalities. The CSF showed no signs of central nervous system inflammation or blood–brain barrier impairment. Tau and Phospho-Tau protein were normal, β-amyloid 1-42 was markedly decreased (Table 1) at the current investigation, whereas in 2013, it still was above cutoff. The results of the routine blood test were inconspicuous.
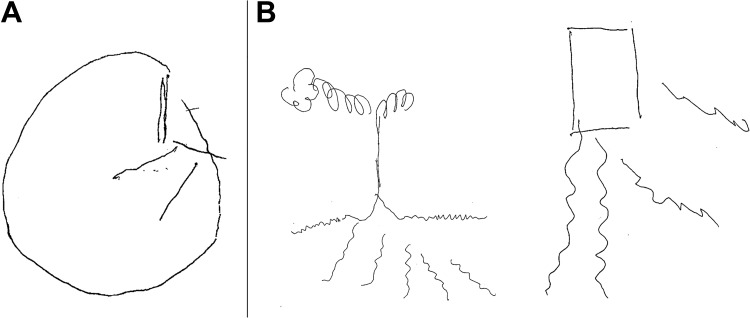
Figure 1.
Visuospatial disorganization. Clock drawing (A): Task to draw the conture, all numbers, and the hands at 10 past 11. The performance hints to severe visuospatial disorganization (score = 5). Free drawing attempts (B), a tree, and a house, are 2-dimensional, poor in details, and leave some sections unconnected to the rest of the image illustrating the patient’s visuospatial deficits.
Figure 2.
Agraphia. Performance on the task to write the sentence “Das Kind spielt mit dem Ball” (German for “the child plays with the ball”) on 3 different occasions (1-3). The patient had the written sentence lying on the table in front of her.
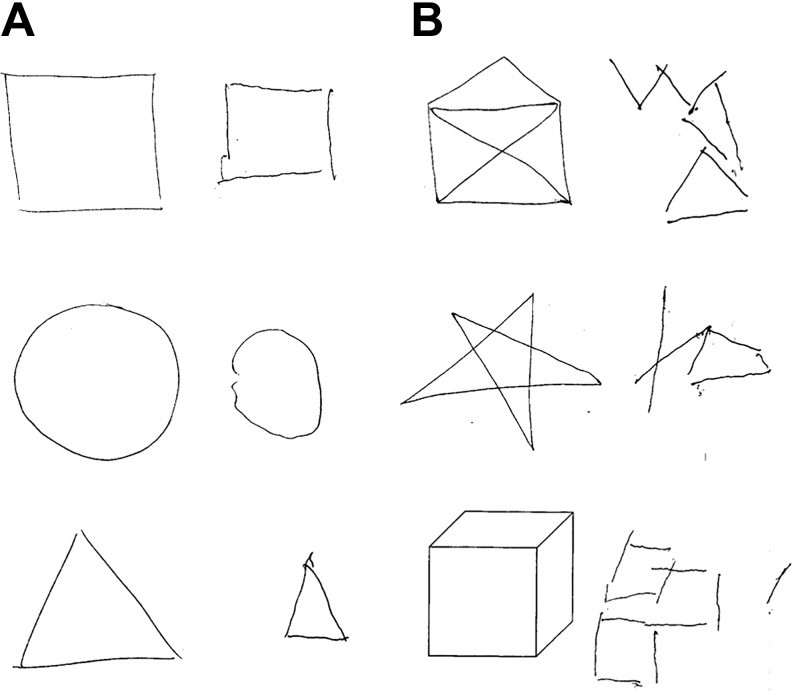
Figure 3.
Visuoconstructional impairment. While drawing basic geometric figures (A) was still possible, the patient had difficulties drawing more complex figures (B), hinting to a visuoconstructional impairment.
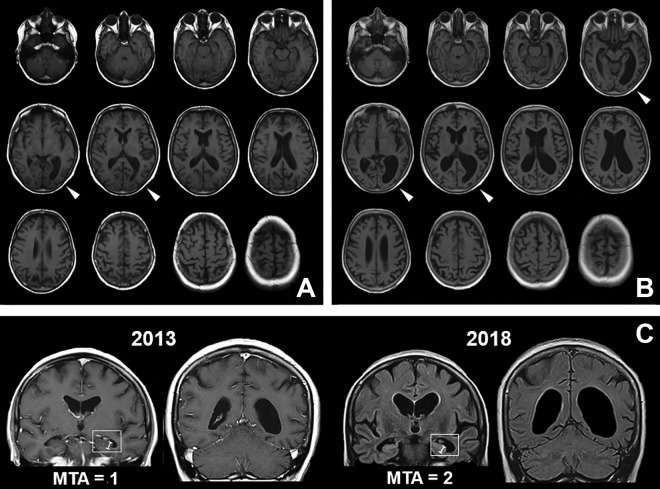
Figure 4.
Brain MRI scans with progressive left parieto-occipital atrophy. In the first brain MRI scans 5 years ago (A), a dilatation of the posterior horn of the left lateral ventricle, and discrete atrophy of the surrounding cortical area (white arrows) was detected. In the recent MRI images (B), a marked ballooning of the ventricular system was found, which again appears to be most pronounced in the left posterior horn. The left parieto-occipital cortices now are shrunken immensely (white arrows). Also the coronal view (C) depicts the pronounced dilatation of the left posterior horn with only slight medial temporal atrophy due to widening of the choroidal fissure (medial temporal atrophy score [MTA] = 1) in 2013, whereas in 2018 a global atrophy with widening also of the temporal horns but no decrease in hippocampal height (white indicators) is visible (MTA = 2). MRI indicates magnetic resonance imaging.
Figure 5.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) investigations. In the first FDG-PET investigation in 2013 (A), a reduced tracer uptake in the left parieto-occipital regions could be found (white arrows), whereas otherwise the tracer distribution was still normal. Five years later (B), a generalized cerebral hypometabolism was evident, but still most distinctive in left parieto-occipital cortex regions (white arrows). All images are displayed in radiological orientation.
Treatment
Due to the rareness of the disorder, evidence for beneficial effects of antidementive medication in PCA is scarce. It is common practice, however, to try antidementive medication whenever PCA is associated with amyloid pathology.11,12 This approach is supported by single cases which show improvements of visuoconstructive functioning under cholinesterase inhibitor treatment,13 as well as by a small double-blind placebo-controlled crossover trial which indicates a slight amelioration of cognitive functions under donepezil treatment.14 In the meanwhile, first efforts have been made to specifically address visuospatial deficits in PCA by nonpharmacological interventions: reading accuracy can be assisted by software-based reading aids,15 and navigational cues might countervail the patients’ spatial disorientation.16 As a clinical disease progress was reported, with memory functions now also being clearly impaired, we added memantine to the cholinergic medication. Otherwise, we recommended occupational therapy for training of practical abilities and coping with visuospatial deficits as well as speech therapy for practicing communication skills that have been impaired by word finding problems. Additionally, we gave psychoeducative information to the patient and her husband.
Discussion
Here, we describe the case of a 62-year-old woman having hemianopsia and complex visual deficits with all core symptoms of Bálint’s syndrome, including optic ataxia, simultagnosia, and oculomotor apraxia. The clinical presentation together with repeated brain scans (Figure 4) showing progredient atrophy of the parieto-occipital cortices stressed on the left side, points to a case of PCA. This conclusion is supported by the FDG-PET findings (Figure 5), depicting a corresponding pattern of glucose hypometabolism. The asymmetric atrophy of visual cortex areas explains the also asymmetric visuospatial and visuoperceptual deficits with hemianopsia to the contralateral side, and right-handed optic ataxia. The ideomotoric apraxia as well as the alexia and agraphia hint to parietal deficits, whereas the higher cognitive dysfunctions with severe memory impairment point to the underlying Alzheimer pathology with additional affection of mid-temporal areas at a later stage (Figure 4C). The disentanglement of all those symptoms, and putting together the pieces of the puzzle to the most probable diagnosis of PCA with amyloid pathology, has lasted more than 7 years from the first occurrence of symptoms, despite repeated contacts to professionals of neurology and psychiatry. Therefore, the question comes up, if the visuospatial deficits have occurred later during disease course questioning the diagnosis of PCA, or if they were overseen in the first place. The anamnesis and review of medical reports point to the latter, as parieto-occipital dysfunctions anamnestically have been among the first clinical symptoms in this case. Nonetheless, even a dissociative pseudodementia was discussed during a past hospital stay due to affective symptoms and an “atypical” symptom presentation. Namely, the former investigators described a lack of anosognosia and significant psychological strain but unluckily misinterpreted symptoms as “demonstrative” attention seeking and suspected a chronic marital conflict as psychoreactive fundament of the disorder. This misdiagnosis, together with the initially inconspicuous CSF biomarkers, has brought some uncertainty to the involved health-care professionals. The relatively well-preserved self-reflection and only mild amnestic symptoms for many years after symptom onset, however, are typical for PCA cases and important distinctive features to discriminate PCA from classical AD. Also, psychiatric symptoms like depressed mood and anxiety can be observed regularly in PCA and should be considered both in the diagnostic process and in treatment, as depressive symptoms at disease onset are negatively correlated with quality of life in later disease stages.17,18 Cognitive behavioral therapy and support groups with the chance to exchange both professional information and to get peer support in an empathic environment can be effective interventions in this regard.19 Despite normal total values of CSF biomarkers in the initial investigation, the ratio of tau/Aβ 1-42 would have been already borderline, then. Therefore, whenever feasible, a combined analysis of biomarkers should be performed to obtain higher diagnostic sensitivity.9 We conclude that due to the significance of a life-changing diagnosis of a neurodegenerative disease, and implications for social and financial support, it is crucial to carefully discuss all differential diagnoses after a rigorous neurological examination and thoughtful anamnesis. This is all the more important, when the clinical symptoms are atypical for AD. The early occurrence of visual symptoms, even if appearing unspecific, should lead to the consideration of a PCA case.
Acknowledgments
The authors are grateful to the patient and her guardian to give informed consent to the publication of this case report, helping to enlighten the diagnosis of a rare disease with new aspects. Sincere thanks go to Martin Lauer for many long and fruitful discussions of the etiology and treatment of rare neurodegenerative syndromes and to Marina Ziegler for her diligent proofreading of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Georg C. Ziegler  https://orcid.org/0000-0001-9411-3169
https://orcid.org/0000-0001-9411-3169
References
- 1. Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. [DOI] [PubMed] [Google Scholar]
- 2. Whitwell JL, Jack CR, Jr, Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051–1061. doi:10.1016/j.neurobiolaging.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng G, Wang J, Feng Z, et al. Clinical and neuroimaging differences between posterior cortical atrophy and typical amnestic Alzheimer’s disease patients at an early disease stage. Sci Rep. 2016;6:29372 doi:10.1038/srep29372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. De Renzi E. Slowly progressive visual agnosia or apraxia without dementia. Cortex. 1986;22(1):171–180. [DOI] [PubMed] [Google Scholar]
- 5. Cogan DG. Visual disturbances with focal progressive dementing disease. Am J Ophthalmol. 1985;100(1):68–72. [DOI] [PubMed] [Google Scholar]
- 6. Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33–40. doi:10.1159/000058331. [DOI] [PubMed] [Google Scholar]
- 7. Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571–1578. doi:10.1212/WNL.0b013e3181c0d427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crutch SJ, Schott JM, Rabinovici GD, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13(8):870–884. doi:10.1016/j.jalz.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duits FH, Teunissen CE, Bouwman FH, et al. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10(6):713–723 e712. doi:10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 10. Shulman KI, Gold DP, Cohen CA, Zucchero CA. Clock-drawing and dementia in the community: a longitudinal study. Int J Geriatr Psychiatry. 1993;8:487–496. [Google Scholar]
- 11. Maia da Silva MN, Millington RS, Bridge H, James-Galton M, Plant GT. Visual dysfunction in posterior cortical atrophy. Front Neurol. 2017;8:389 doi:10.3389/fneur.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ortner M, Kurz A. [Posterior cortical atrophy. Pathology, diagnosis and treatment of a rare form of dementia]. Nervenarzt. 2015;86(7):833–839. doi: 10.1007/s00115-015-4265-1. [DOI] [PubMed] [Google Scholar]
- 13. Kim E, Lee Y, Lee J, Han SH. A case with cholinesterase inhibitor responsive asymmetric posterior cortical atrophy. Clin Neurol Neurosurg. 2005. 108(1):97–101. doi:0.1016/j.clineuro.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14. Ridha BH, Crutch S, Cutler D, et al. A double-blind placebo-controlled cross-over clinical trial of DONepezil In Posterior cortical atrophy due to underlying Alzheimer’s Disease: DONIPAD study. Alzheimers Res Ther. 2018;10(1):44 doi:10.1186/s13195-018-0363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yong KXX, Rajdev K, Shakespeare TJ, Leff AP, et al. Facilitating text reading in posterior cortical atrophy. Neurology. 2015;85(4):339–348. doi:10.1212/Wnl.0000000000001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yong KXX, McCarthy ID, Poole T, et al. Navigational cue effects in Alzheimer’s disease and posterior cortical atrophy. Ann Clin Transl Neurol. 2018;5(6):697–709. doi:10.1002/acn3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hvidsten L, Engedal K, Selbaek G, Wyller TB, Benth JŠ, Kersten H. Quality of life in people with young-onset dementia: a Nordic two-year observational multicenter study. J Alzheimers Dis. 2019;67(1):197–210. doi:10.3233/JAD-180479. [DOI] [PubMed] [Google Scholar]
- 18. Suarez-Gonzalez A, Crutch SJ, Franco-Macias E, Gil-Neciga E. Neuropsychiatric symptoms in posterior cortical atrophy and Alzheimer disease. J Geriatr Psychiatry Neurol. 2016;29(2):65–71. doi:10.1177/0891988715606229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suarez-Gonzalez A, Henley SM, Walton J, Crutch SJ. Posterior cortical atrophy: an atypical variant of Alzheimer disease. Psychiatr Clin North Am. 2015;38(2):211–220. doi:10.1016/j.psc.2015.01.009. [DOI] [PubMed] [Google Scholar]