In the corner of Kevin Tracey’s office, behind a long shelf lined with medical books, rests “Rosie,” a pink cane adorned with roses. It once belonged to Kelly Owens, who spent her teens and 20s crippled by inflammatory arthritis and Crohn’s disease. Today, she no longer needs Rosie’s help.
Silicon wafers of thin-film microfabricated electrodes can be implanted into a patient to record from or stimulate the nervous system—the vagus nerve in particular. Image credit: The Feinstein Institutes for Medicine Research.
The turning point for Owens came just over two years ago when a research team implanted a small device inside her chest to stimulate her vagus nerve. She celebrated two years of clinical remission last month. “I used to have to think about every movement my body was making, whether washing a dish or putting on a shirt on or bending my arms enough to put on deodorant,” says Owens, who recently gifted the cane as a thank you to Tracey. “My life is so normal now.”
Hundreds of clinical trials are now underway to investigate how harnessing the body’s peripheral wiring might help broadly in the treatment of acute and chronic disease. The results so far appear promising. “I hope many more people will benefit like Owens has,” says Tracey, president and CEO of the Feinstein Institutes for Medical Research in New York and a pioneer in the field of bioelectronic medicine.
That field, which also goes by neuromodulation, biostimulation, or electroceuticals, is emerging as an alternative or add-on to costly chemical and biologic drugs. Dysfunctional neural circuits give rise to dysfunctional organs. The goal of bioelectronic medicine is to restore healthy patterns of electrical impulses—adjusting how neurons fire and, thereby, changing the concentrations of neurotransmitters traveling through those circuits.
Driving growth in bioelectronic medicine is a convergence of advances in neuroscience, electronics, materials science, molecular medicine, and biomedical engineering, alongside more than a billion dollars of investments from government and industry. Within the next decade, researchers say, modulating the body’s neural networks could become a mainstream therapy for many of today's greatest health issues—from arthritis (1), asthma (2), and Alzheimer’s disease (3) to depression (4), diabetes (5), and digestive disorders (6, 7). Stimulating nerves also shows promise in treating cardiovascular disease (8) and septic shock (9), even in improving cognition (10).
What Happens in Vagus
The human body is electric. Peripheral nerves connect all organs to the central nervous system, and those nerves are packaged in various bundles. The vagus nerve, for example, carries about 100,000 nerve fibers. It’s also the longest nerve in the body, linking the brain to organs from the esophagus to the intestines while controlling breathing and heart rate. Further evidence suggests it may be the direct link for that well-known gut-brain connection (11).
Because of its size and breadth, as well as recent progress in understanding exactly how it influences various parts of the body, the vagus nerve has become a major target for the burgeoning field (12). The word vagus comes from the same root as the word vagrant, meaning something that wanders. “The vagus nerve goes everywhere, so it’s an easy way to get access to a bunch of different targets,” says Timir Datta-Chaudhuri, an investigator at the Feinstein Institutes for Medical Research. “It’s like going to the main fuse box.”
Datta-Chaudhuri is among researchers who believe the greatest promise for bioelectronic medicine is in manipulating the vagus nerve to control inflammation and the immune response—both of which drive most chronic disease. In a groundbreaking study published in Nature in 2000, Tracey and his colleagues showed that stimulating the vagus nerve could significantly reduce inflammation in rats (13). Acetylcholine, the principal neurotransmitter that stems from the vagus nerve, inhibited the production of cytokines such as tumor necrosis factor (TNF), an inflammatory molecule involved in rheumatoid arthritis. His team followed up with studies in humans and found the same (14).
“We have known that the vagus nerve is a fundamental component of reflexes that control the physiology of organs—the heart, the intestines, the liver,” says Tracey. “But we never thought it would control the immune system.”
A sure sign that the nonpharmacologic approach may be for real: The pharmaceutical industry is on board. Johnson & Johnson’s portfolio now includes a number of bioelectronic devices. And GlaxoSmithKline has made a big bet on bioelectronic medicine by investing—alongside Verily, formerly Google Life Sciences—$715 million in Galvani Bioelectronics, whose focus is on the design and development of neuromodulation systems. “They are investing in something that would clearly be a disruptive technology,” says Datta-Chaudhuri.
The National Institutes of Health (NIH) and the Defense Advanced Research Projects Agency (DARPA) have also invested significantly in the field through programs such as Stimulating Peripheral Activity to Relieve Conditions, or SPARC (14), and Electrical Prescriptions, or ElectRx. One recent study funded by DARPA’s ElectRx, for example, found that sacral nerve stimulation decreased inflammation in the colon (15). Market intelligence firms predict the bioelectronic device market will reach between $16 and $60 billion annually within the next 5 to 10 years (16, 17). “We’ve already hit $10 billion a year,” says Kip Ludwig, who leads the Bioelectronic Medicines Laboratory at the University of Wisconsin–Madison. “It now looks like we're at the slope in an exponential curve.”
The Body Electric
About four times a day, Owens swipes a magnet over her chest to activate the device implanted under her collar bone. For the next five minutes after each swipe, the device sends electrical currents to her vagus nerve. That’s enough to turn down her inflammation and keep her debilitating symptoms at bay. “Before, I was pumping heavy duty drugs into my body,” says Owens, who was recently hired as director of education and outreach at the Feinstein Institutes for Medical Research. “None worked.” What’s more, those drugs plagued her with severe side effects including osteoporosis.
The idea of electrically stimulating the body to alleviate illness is not new. Doctors have implanted patients with pacemakers, deep-brain stimulators, and other electrical devices for decades. Vagus nerve stimulation itself is currently US Food and Drug Administration (FDA)-approved for epilepsy and treatment-resistant depression. But these were “crude initial attempts” in communicating electrically with the body, says Datta-Chaudhuri.
The mechanisms at play remained mostly mysterious. A device would work for some patients but not others. And no one quite knew why. Devices were often clunky and triggered unwanted side effects. “An era before, we made a device and said, ‘This is cool, so let’s stick it in people and see what happens,’” says Tracey, who is also a co-founder of Setpoint Medical, a bioelectronic device company in Valencia, CA. “That was the old model of medical devices. Now we’re coming from a different direction.”
Today’s implants are down to about the size of a fish oil pill—far smaller even than Owens’ device. And rather than blindly zapping a large bundle of nerves, researchers and engineers are collaborating on the basic physiology and high-tech tools needed to zero in on the specific subset of fibers known to innervate the organ of interest. Further adjustments can be made to personalize a device such as changing the pulse width, amplitude, or frequency of stimulation. “Unlike with many pharmacologic agents, we can be pretty selective not only with therapeutic effects but with reduced side effects,” says Charles Horn, a neuroscientist at the University of Pittsburgh in Pennsylvania. “This is really, really huge, this mindset to use neural devices to treat disease. We’re pretty close to getting some of these to the clinic.”
Bioelectronic medicine wouldn’t necessarily replace drugs; in some cases, it could serve as a complement to traditional therapies. “We can use devices in harmony with lower levels of drugs so they don't have side effects and also to prevent habituation,” says Ludwig.
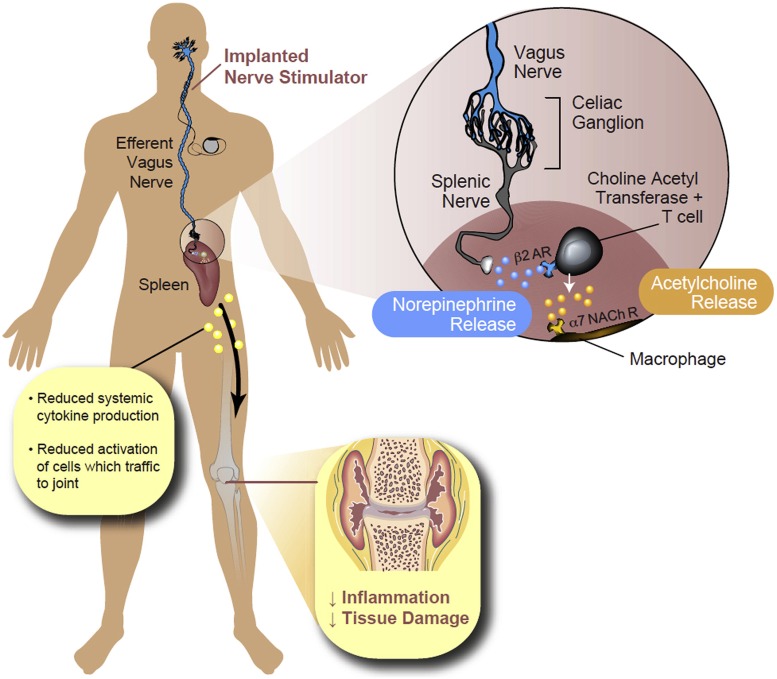
Researchers have found that electrical stimulation of the vagus nerve triggers a cascade of effects that can result in reduced inflammation, a key driver in many chronic diseases. AR, adrenergic receptor; NACh R, nicotinic acetylcholine receptors. Image credit: Reprinted from ref. 21, with permission from Elsevier.
Keeping Pace
One of the holy grails in bioelectronic medicine, says Tracey, is closed-loop therapy. Such a device could not only talk to the nerves but also listen in on their conversations with the rest of the body to decipher how best to respond in real-time—whether by stimulating or blocking nerve signals. In the case of diabetes, that might entail telling the pancreas to make more insulin when it’s needed. In the gastrointestinal system, an electrode might sense the motility rate of the gut and then determine the optimal frequency of pulses to speed it up or slow it down. The goal is to restore a healthy pattern of electrical pulses.
“In a healthy body, all the parts are there and talking to each other like they should be,” says Datta-Chaudhuri. “In a diseased body, either the parts there are not functioning as they should or they are not communicating to each other as they should. So a good approach is to listen in on the messages sent between different parts of the body and insert information.”
Take, for example, the implantable neurostimulator for obstructive sleep apnea, developed by Inspire Medical Systems, Inc. The FDA-approved device connects directly to the hypoglossal nerve, which controls the movement of the tongue and the other airway muscles, and constantly monitors a patient’s airway while he or she is asleep. Whenever a blockage is detected, it emits an electrical stimulation to the nerve, which automatically opens up the tongue.
Of course, a bioelectronic device is not likely to be a panacea. The Inspire system requires an invasive surgery and carries a high price—between $40,000 and $100,000 including the requisite surgery. Ludwig notes that this one-time investment is often offset by the cost savings of not having to pop expensive pills. But there may be additional drawbacks. For example, some Inspire models need to be removed before an MRI scan. And then there are the unknown risks associated with any new technology.
Implanted devices will likely become less invasive with increased miniaturization and more sophisticated electronics. “This field is governed by Moore’s Law,” says Ludwig. “The devices will get smaller and smaller to the point where they will be as invasive as a tattoo.”
While a short, maybe 30-minute surgical procedure would be expected for such an implant today, Ludwig suggests that technological advances will soon allow wireless devices to be injected under lidocaine, eliminating the need for invasive surgery. “That’s when this starts taking over for drugs,” he says. “It changes the regulatory pathway so that it is much less expensive and quicker to market.” Sidestepping surgery and avoiding wires would likely allow a company to submit a 510(k) premarket notification instead of a premarket approval to the FDA. “That's the difference between costing your company 7 years and $150 million versus and a 6-month study and $5 million,” he says.
Noninvasive bioelectronic devices can also take the form of handheld devices, which are generally used for short-term illnesses. In January, the FDA approved a noninvasive bioelectronic treatment for sinus pain. Tivic Health’s ClearUP Sinus opens up the sinuses via transcutaneous nerve stimulation on the cheeks, nose, and brow bones (18). Researchers are also investigating the potential health benefits of stimulating the vagus nerve through the outer ear (19). One study published in July found the self-administered stimulation boosted the quality of life, mood, and sleep compared with a sham treatment in older adults (20).
Bioelectric Barriers
Despite all the promise, it won’t be easy to bring bioelectronic medicine into the mainstream. Gene Civillico, the Program Manager for NIH’s SPARC, notes some resistance to the devices. “When a lot of people think about therapies, they first think, ‘Is there a drug?’ And if that drug didn’t work, ‘Is there another drug?’” says Civillico. “That thinking pervades the ecosystem, from the way therapies are prescribed to how research is supported.”
“I really think this is going to be an enlightened moment when more of the community recognizes the importance of the interactions between the immune system and the nervous system.”
—Marthe Howard
Another key obstacle is the lack of interoperability of data and tools. “This limits our ability, and perhaps more importantly, software’s ability, to tell stories across labs and data sets,” says Civillico.
A better understanding of nerve signals and their exact effects, alongside improved technology to target specific nerve fibers, is also critical to broader adoption of bioelectronic medicine. Toward that end, many more basic science and translational efforts are in the works, thanks in part to SPARC.
“I really think this is going to be an enlightened moment when more of the community recognizes the importance of the interactions between the immune system and the nervous system,” says Marthe Howard, a SPARC investigator and neuroscientist at the University of Toledo in Ohio. Howard is collecting data to help bioengineers develop an electrode array that, when placed on the gut, could optimally hit neurons or the synapses between them based on the treatment goal.
A high priority for the seven-year, $240 million SPARC program, says Ludwig, is a detailed map of the nervous system and how it influences and regulates organs throughout the body. Without a map, future devices might simply not work or, worse, they could end up interfering with the wrong signals, resulting in pain or other problems. “Right now we’re just banging our forearm on a really complex keyboard and hoping that it helps,” says Ludwig. “As it becomes more precise, we hope to play Beethoven’s 5th.”
References
- 1.Genovese M. C., et al. , LB009 First-in-human study of novel implanted vagus nerve stimulation device to treat rheumatoid arthritis. Ann. Rheum. Dis. 78, 264 (2019). [Google Scholar]
- 2.Staats P., Emala C., Simon B., Errico J. P., “Neurostimulation for asthma” in Neuromodulation, Krames E. S., Peckham H., Rezai A. R., Eds. (Academic Press, Cambridge, MA: ), ed. 2, 2008), pp. 1339–1345. [Google Scholar]
- 3.Kaczmarczyk R., Tejera D., Simon B. J., Heneka M. T., Microglia modulation through external vagus nerve stimulation in a murine model of Alzheimer’s disease. J. Neurochem. 146, 76–85 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Sackeim H. A., et al. , Vagus nerve stimulation (VNS) for treatment-resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology 25, 713–728 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Masi E. B., et al. , Identification of hypoglycemia-specific neural signals by decoding murine vagus nerve activity. Bioelectron. Med. 5, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne S. C., et al. , Anti-inflammatory effects of abdominal vagus nerve stimulation on experimental intestinal inflammation. Front. Neurosci. 13, 418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosmans G., et al. , Vagus nerve stimulation dampens intestinal inflammation in a murine model of experimental food allergy. Allergy 74, 1748–1759 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou L., et al. , Low-level transcutaneous vagus nerve stimulation attenuates cardiac remodelling in a rat model of heart failure with preserved ejection fraction. Exp. Physiol. 104, 28–38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey K. J., Huston J. M., "Inhibition of inflammatory cytokine production by cholinergic agonists and vagus nerve stimulation." US Patent 1016639B2 (2019).
- 10.Sanders T. H., et al. , Cognition-enhancing vagus nerve stimulation alters the epigenetic landscape. J. Neurosci. 39, 3454–3469 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breit S., Kupferberg A., Rogler G., Hasler G., Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J., Lu K. H., Powley T. L., Liu Z., Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One 12, e0189518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borovikova L. V., et al. , Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Koopman F. A., et al. , Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 113, 8284–8289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo J., et al. , Sacral nerve stimulation improves colonic inflammation mediated by autonomic-inflammatory cytokine mechanism in rats. Neurogastroenterol. Motil. 31, e13676 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Simmons C., Neurotech Reports: New market research report on bioelectronic medicine forecasts $16.6B market. California Newswire. https://californianewswire.com/neurotech-reports-new-market-research-report-on-bioelectronic-medicine-forecasts-16-6b-market/. Accessed 31 October 2019.
- 17.IDTechEx , Global market report on bioelectronic medicine 2019-2029 from IDTechEx Research. PR Newswire, 21 December 2018. https://www.prnewswire.com/news-releases/global-market-report-on-bioelectronic-medicine-2019-2029-from-idtechex-research-300769900.html. Accessed 31 October 2019.
- 18.Maul X. A., Borchard N. A., Hwang P. H., Nayak J. V., Microcurrent technology for rapid relief of sinus pain: A randomized, placebo-controlled, double-blinded clinical trial. Int. Forum Allergy Rhinol. 9, 352–356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Addorisio M. E., et al. , Investigational treatment of rheumatoid arthritis with a vibrotactile device applied to the external ear. Bioelectron. Med. 5, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bretherton B., et al. , Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: Potential benefits of daily stimulation. Aging (Albany N.Y.) 11, 4836–4857 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koopman F. A., Schuurman P. R., Vervoordeldonk M. J., Tak P. P., Vagus nerve stimulation: A new bioelectronics approach to treat rheumatoid arthritis? Best Pract. Res. Clin. Rheumatol. 28, 625–635 (2014). [DOI] [PubMed] [Google Scholar]