Abstract
Major medical illnesses place patients at risk of venous thromboembolism (VTE). Some risk factors including age ≥75 years or history of cancer place them at increased risk of VTE that extends for at least 5 to 6 weeks following hospital admission. Betrixaban thromboprophylaxis is now approved in the United States for this indication. We estimated the annual number of acutely ill medical patients at extended risk of VTE discharged from US hospital. Major medical illnesses (stroke, respiratory failure/chronic obstructive pulmonary disease, heart failure, pneumonia, other infections, and rheumatologic disorders) and 2 common risk factors for extended VTE risk, namely, age ≥75 years and history of cancer (active or past) were examined in 2014 US hospital discharges using the first 3 discharge diagnosis codes in the National Inpatient Sample (database of acute-care hospital discharges from the US Agency for Health Care Quality and Research). In 2014, there were 20.8 million discharges with potentially at risk of nonsurgical-related VTE. Overall, 7.2 million (35%) discharges corresponded to major medical illness that warranted thromboprophylaxis according to 2012 American College of Chest Physicians (ACCP) guideline. Among them, 2.79 million were aged ≥75 years and 1.36 million had a history of cancer (aged 40-74 years). Overall, 3.48 million discharges were at extended risk of VTE. Many medical inpatients at risk of VTE according to 2012 ACCP guideline might benefit from the awareness of continuing risk and some of these patients might benefit from extended thromboprophylaxis, depending on the risk of bleeding and comorbidities.
Keywords: acute medical illness, epidemiology, extended thromboprophylaxis, risk factors, venous thromboembolism
Introduction
Hospitalized acutely ill medical patients have an increased risk of venous thromboembolism (VTE) including deep vein thrombosis and pulmonary embolism, leading to substantial morbidity and mortality.1 The guideline recommends standard duration thromboprophylaxis up to 21 days, but it is usually terminated at the time of discharge.2 However, despite the administration of standard duration thromboprophylaxis, some acutely ill medical patients with specific risk factors remain at high risk for VTE that extends for at least 5 to 6 weeks following hospital admission. Furthermore, the majority of VTE now occur postdischarge.3–5
Previous trials with extended thromboprophylaxis, using different anticoagulant drugs showed some promise in reducing VTE burden but failed to achieve a net clinical benefit because of an increase in major bleeding.2,3 However, these trials in immobilized hospitalized medical patients identified factors associated with an extended risk of VTE, such as advanced age, a past history of cancer or VTE, elevated d-dimer, or multiple medical comorbidities.3,6
These risk factors were utilized to select the population included in the APEX trial.7 In this study, extended thromboprophylaxis with betrixaban, a direct factor Xa inhibitor, reduced asymptomatic and symptomatic VTE events by up to 45% at the end of follow-up (day 77) compared with standard duration enoxaparin, without increasing major bleeding. This result was associated with reduction in symptomatic VTE including VTE-related hospitalizations, fatal and irreversible events, and reduction in ischemic strokes.8–11 Betrixaban is now approved in the United States for extended thromboprophylaxis in hospitalized acutely ill medical patients at extended risk for VTE. Using a US-wide data set of acute-care hospital medical patients, we estimated the annual number of patients at extended risk for VTE according to the selected APEX trial criteria.
Methods
Annual estimates of the number of US hospitalized patients at risk of VTE according to 2012 American College of Chest Physicians (ACCP) guideline were derived from the 2014 US National Inpatient Sample (NIS), a data set available from the Healthcare Cost and Utilization Project (HCUP) sponsored by the US Agency for Healthcare Research and Quality (AHRQ).2 The NIS data set includes all US states participating in HCUP, representing acute hospital care received by more than 95% of the US population.12,13 The NIS databases are stratified probability samples of US community hospitals.12 By definition, out-of-hospital events are not included.
Study Population
All inpatients aged ≥18 years who had a length of stay ≥2 days were included. Agency for Healthcare Research and Quality Clinical Classification System (CCS) codes were used to define the type of surgical procedure and the diagnosis of medical illness, as described in our previous publication.12,13 Surgical patients were defined by inpatients who underwent a surgical procedure in an operating room. Primary procedure codes were used to characterize the type of surgery. According to the 2012 ACCP guideline, surgical patients with a Caprini score ≥3 (based mainly on the type of surgery and a length of stay ≥4 days as a surrogate for severe immobility) were considered at risk for VTE.14 Surgical patients at no/low risk for VTE were reassessed according to their medical conditions and added to the medical population (inpatients who did not have a procedure in an operating room). For the purpose of our analysis, surgical patients at moderate, high, or highest risk for VTE were excluded.
Number of Patients at Extended Risk for VTE
Major medical illnesses, included stroke, respiratory failure/chronic obstructive pulmonary disease, heart failure, pneumonia, other infections, and rheumatologic disorders (arthropathy/spondylopathy) placed hospitalized patients at risk for VTE. The 2 more common risk factors for VTE easily identified in the data set, namely age ≥75 years and a history of cancer (active and past) were considered. Indeed, other risk factors such as a history of VTE and elevated d-dimer are not comprehensively collected in the data set. Moreover, in keeping with the 2012 ACCP guideline based on the Padua Prediction Score risk assessment model which does not include inflammatory bowel disease as a risk factor for VTE, this medical condition was not included in our analysis.2 Therefore, patients at extended risk for VTE were recognized by those aged ≥75 years or having a history of cancer (aged 40-74 years) admitted with a major medical illness. The first 3 CCS diagnostic codes were analyzed to determine the types of medical illness used for risk assessment. AHRQ definitions were used to identify comorbidities.
Outcomes
Outcomes included the number and percent of medically ill patients at risk for posthospital discharge VTE, eligible for extended thromboprophylaxis.
Analyses
All analyses were descriptive and were performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina). Categorical variables are reported as frequencies and percentages.
Results
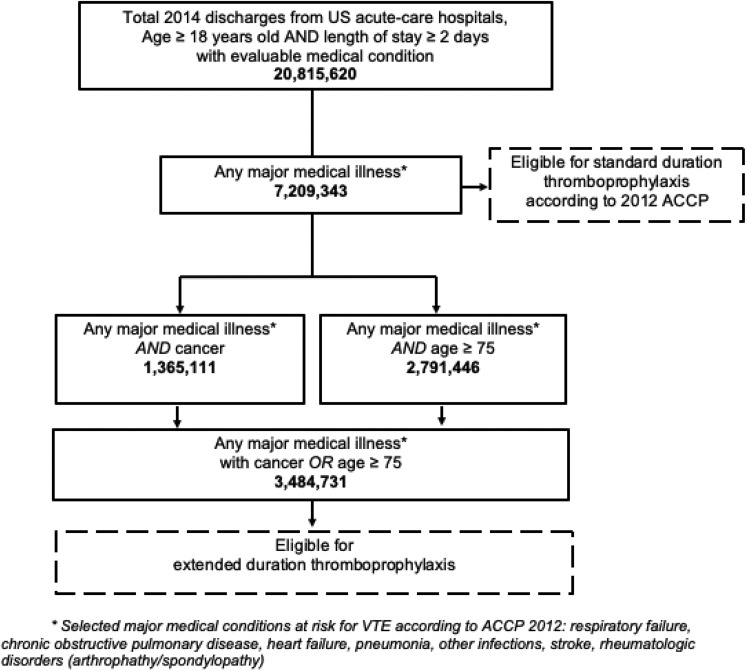
In 2014, an estimated 20.8 million adult medical patients with length of stay ≥2 days were discharged from US acute-care hospitals (Figure 1). Respiratory failure (12%), heart failure (11%), and infections, classified as pneumonia and other infections (15%) were the commonest conditions (Table 1). Overall, 7.2 million (35%) discharges presented with a major medical diagnosis associated with risk for VTE. Among them, 1.36 million patients had a history of cancer and 2.79 million were aged ≥75 years.
Figure 1.
Flow chart and estimated discharges at-risk for standard and extended duration thromboprophylaxis.
Table 1.
Major Medical Illnesses Discharge Diagnoses in 2014 by Diagnosis, Age, and History of Cancer.
| Overall Discharges | Age Group in Year (n) | History of Cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 18-39 | Row % | 40-59 | Row % | 60-74 | Row % | ≥75 | Row % | ||||
| Number (n) | 20 815 620 | % of All | 5 684 465 | 27.3% | 4 867 461 | 23.4% | 4 902 047 | 23.5% | 5 361 647 | 25.8% | |
| Stroke | 574 850 | 2.8% | 15 675 | 2.7% | 118 960 | 20.7% | 187 240 | 32.6% | 252 975 | 44.0% | 92 790 |
| Respiratory failure/COPD | 2 567 991 | 12.3% | 115 325 | 4.5% | 592 080 | 23.1% | 946 425 | 36.9% | 914 160 | 25.6% | 483 350 |
| Heart failure | 2 338 371 | 11.2% | 69 890 | 3.0% | 413 485 | 17.7% | 727 450 | 31.1% | 1 127 546 | 48.2% | 402 395 |
| Pneumonia | 1 653 541 | 7.9% | 107 505 | 6.5% | 344 915 | 20.9% | 509 035 | 30.8% | 692 085 | 41.9% | 382 025 |
| Infections other than pneumonia | 1 621 960 | 7.8% | 157 105 | 9.7% | 390 595 | 24.1% | 502 095 | 31.0% | 572 165 | 35.2% | 353 820 |
| Arthropathy/spondylopathy | 545 560 | 2.6% | 70 850 | 13.0% | 187 495 | 33.8% | 144 510 | 26.5% | 142 705 | 26.2% | 60 410 |
| Any of above | 7 209 343 | 34.6% | 457 865 | 6.4% | 1 653 601 | 22.9% | 2 306 431 | 32% | 2 791 446 | 38.7% | 1 365 111 |
| Age ≥75 or history of cancer (40-74)a | 3 484 731 | 16.7% | – | 207 895 | 485 390 | 2 791 446 | 1 336 801 | ||||
Abbreviation: COPD, chronic obstructive pulmonary disease.
a Limited APEX trial criteria (2 of the 4 main criteria for inclusion into the APEX study).
Discussion
Our main finding was that among inpatients with medical conditions such as stroke, respiratory failure/chronic obstructive pulmonary disease, heart failure, pneumonia or other infections, or arthropathy/spondylopathy, almost half were either aged ≥75 years or aged 40 to 74 years with a history of cancer, representing 3.5 million patients of 7.2 million. These patients are considered to have an extended duration of high risk for VTE beyond the hospital stay. According to the APEX trial findings, these patients might benefit from thromboprophylaxis with betrixaban for up to 42 days, rather than standard duration thromboprophylaxis.
Current risk assessment tools, especially Padua Prediction Score mentioned in the ACCP guidelines, only identify patient at risk for VTE who require standard duration thromboprophylaxis but do not recognize high-risk patients who could benefit from extended prophylaxis.2 Patients meeting the inclusion criteria of the APEX trial, namely hospitalized for major medical illness leading to severe immobility with advanced age (68% of the overall cohort), prior cancer (12%), prior VTE (8%), or elevated d-dimers, are at extended duration of high risk for VTE and benefit from extended thromboprophylaxis.
Age is recognized as an important risk factor for VTE in hospital and postdischarge, however significant 6-month frequencies of clinical VTE, around 4.5%, were seen in all age groups (<65, 65-75, and >75 years) in a recent US observational study.5 Notably, in this study, most VTE occurred in outpatients and less than 10% of patients received post-discharge thromboprophylaxis. Another recent study has examined the population of US hospitalized medically ill patients who might qualify for extended thromboprophylaxis according to the modified IMPROVE score used in the MARINER study.15 This score did not perform as well as expected by the trialists and identified a lower risk population resulting in an underpowering of the trial despite a 50% increase in the sample size. Rivaroxaban is undergoing regulatory approval for this indication. In comparison, in our study, the APEX criteria applied to the US hospitalized population recognized more patients. These patients were shown to be at high risk in the trial and betrixaban has Food and Drug Administration approval for this indication.
Our analysis estimates the potential clinical impact of extended thromboprophylaxis in hospitalized acutely ill medical patients on the reduction of VTE burden. Based on the APEX trial findings, and applied to our US data in 2014, extended thromboprophylaxis would have avoided 20 000 additional symptomatic VTE (number needed to treat [NNT] = 167), 15 000 all-cause strokes (NNT = 233), and 16 000 additional preventable deaths (NNT = 223) compared with current standard enoxaparin.7,8,10,11 These data may serve as a basis to estimate the related economic impact of this strategy.16
The major limitation was that the number of patients eligible for extended thromboprophylaxis described here is an overestimation and actually reflects a “maximum case scenario.” Indeed, many reasons, including exclusion criteria from the APEX trial, especially risk factors for bleeding or polypharmacy with strong P-glycoprotein inhibitors, or costs, might preclude the actual prescription of extended betrixaban. Other limitations included that the extent of immobility was not captured in HCUP, thus our data potentially again overestimates the eligible population. Moreover, other known risk factors for VTE including 2 major risk factors recognized in the APEX trial, namely, prior VTE and d-dimer, were not comprehensively captured in the data set which would on the contrary underestimate the extended risk population by 10% to 15%. Finally, the data set does not collect data on medication utilization. Therefore, our study was not able to estimate neither the proportion of at-risk patients who received thromboprophylaxis nor whether antithrombotic therapies, when used, were given appropriately.
Conclusion
Based on this “maximum case scenario,” almost half of the high-risk hospitalized acutely ill medical patients currently eligible for standard duration thromboprophylaxis are at extended risk and might benefit from the awareness of continuing risk. Some of these patients might benefit from extended thromboprophylaxis, depending on the risk of bleeding and comorbidities.
Footnotes
Authors’ Note: The article was presented orally at the ISTH congress, in 2017, in Berlin, Germany.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.-C.M. has received consulting fees and advisory board fees from Bristol-Myers Squibb/Pfizer, Bayer, and Boehringer-Ingelheim. W.H. has no conflict of interest. S.Z.G. has received research support from Boehringer-Ingelheim, BMS, BTG EKOS, Daiichi-Sankyo, Janssen, NHLBI, and Thrombosis Research Institute; consulting fees from Bayer, Boehringer-Ingelheim, BMS, Daiichi-Sankyo, Janssen, and Portola. R.D.H. has received consulting fees from Portola and Sanofi, and research grant from Portola. A.F.H. has received research fees from Portola Pharmaceuticals and consulting fees from Bayer, Boehringer-Ingelheim, Janssen, Merck, and Novartis. C.-M.G. has received consulting fees and research support from Johnson & Johnson, Janssen, Bayer, Portola, and research support from BMS. F.A.A. has served as a consultant to Portola Pharmaceuticals, United Bioscience Corporation and Millennium Pharmaceuticals. A.T.C. has received consulting fees from AbbVie, ACI Clinical, Aspen, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Boston Scientific, CSL Behring, Daiichi-Sankyo, GlaxoSmithKline, GLG, Guidepoint Global, Johnson and Johnson, Leo Pharma, Medscape, McKinsey, Navigant, ONO, Pfizer, Portola, Sanofi, Takeda, Temasek Capital, and TRN; advisory board membership with Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Johnson and Johnson, ONO, Pfizer, Portola, and Sanofi; payments for lectures including speakers bureau services, preparation of reports and development of educational presentations from Aspen, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi, GlaxoSmithKline, Johnson and Johnson, Medscape, Pfizer, and Portola.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by Portola Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsor.
ORCID iD: Anne-Céline Martin  https://orcid.org/0000-0002-6148-3523
https://orcid.org/0000-0002-6148-3523
References
- 1. Heit JA, Melton LJ, III, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76(11):1102–1110. [DOI] [PubMed] [Google Scholar]
- 2. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen AT, Spiro TE, Spyropoulos AC, et al. MAGELLAN Study Group. D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost. 2014;12(4):479–487. [DOI] [PubMed] [Google Scholar]
- 4. Amin AN, Varker H, Princic N, et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7(3):231–238. [DOI] [PubMed] [Google Scholar]
- 5. Amin A, Neuman WR, Lingohr-Smith M, Menges B, Lin J. Venous thromboembolism prophylaxis and risk for acutely medically ill patients stratified by different ages and renal disease status. Clin Appl Thromb Hemost. 2019;25:1076029618823287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. [DOI] [PubMed] [Google Scholar]
- 7. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375(6):534–544. [DOI] [PubMed] [Google Scholar]
- 8. Gibson CM, Nafee T, Yee MK, et al. Symptomatic event reduction with extended-duration betrixaban in acute medically ill hospitalized patients. Am Heart J. 2018;198:84–90. [DOI] [PubMed] [Google Scholar]
- 9. Chi G, Yee MK, Amin AN, et al. Extended-duration betrixaban reduces the risk of rehospitalization associated with venous thromboembolism among acutely ill hospitalized medical patients: findings from the APEX trial (acute medically ill venous thromboembolism prevention with extended duration betrixaban trial). Circulation. 2018;137(1):91–94. [DOI] [PubMed] [Google Scholar]
- 10. Gibson CM, Korjian S, Chi G, et al. Comparison of fatal or irreversible events with extended-duration betrixaban versus standard dose enoxaparin in acutely ill medical patients: an APEX trial substudy. J Am Heart Assoc. 2017;6(7):e006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gibson CM, Chi G, Halaby R, et al. Extended-duration betrixaban reduces the risk of stroke versus standard-dose enoxaparin among hospitalized medically ill patients: an APEX trial substudy (acute medically ill venous thromboembolism prevention with extended duration betrixaban). Circulation. 2017;135(7):648–655. [DOI] [PubMed] [Google Scholar]
- 12. Huang W, Cohen AT, Martin AC, Anderson FA. Magnitude of venous thromboembolism risk in US hospitals: impact of evolving national guidelines for prevention of venous thromboembolism. Am J Med. 2019;132(5):588–595. [DOI] [PubMed] [Google Scholar]
- 13. Anderson FA, Jr, Zayaruzny M, Heit JA, Fidan D, Cohen AT. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82(9):777–782. [DOI] [PubMed] [Google Scholar]
- 14. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miao B, Chalupadi B, Clark B, et al. Proportion of US hospitalized medically ill patients who may qualify for extended thromboprophylaxis. Clin Appl Thromb Hemost. 2019;25(4):1076029619850897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guy H, Laskier V, Fisher M, et al. Cost-effectiveness of betrixaban compared with enoxaparin for venous thromboembolism prophylaxis in nonsurgical patients with acute medical illness in the United States. Pharmacoeconomics. 2019;37(5):701–714. [DOI] [PubMed] [Google Scholar]