Abstract
IL-17 antagonism is among the most potent treatments for psoriasis. Generally safe, new onset and exacerbations of inflammatory bowel disease may occur in association with IL-17 therapy. We describe a patient with long-standing history of psoriasis and psoriatic arthritis in whom asymptomatic Crohn’s disease was identified during treatment with secukinumab. The patient underwent an elective colonoscopy for colorectal cancer screening which revealed inflammation and multiple ulcers in the terminal ileum suggestive of Crohn’s disease. While the patient did not have any gastrointestinal symptoms, he was diagnosed as having asymptomatic Crohn’s disease. Given the association of inflammatory bowel disease with secukinumab treatment, secukinumab was discontinued. Although in this patient, Crohn’s disease was identified during treatment with secukinumab, a direct causal relationship cannot be assumed. Medications that are effective for both psoriasis and inflammatory bowel disease may be a good choice in patients with psoriasis who have comorbid Crohn’s disease or develop inflammatory bowel disease during treatment with another biologic.
Keywords: Psoriasis, biologic, inflammatory bowel disease, Crohn’s disease
Introduction
Dermatologists and patients with plaque psoriasis have enjoyed the dramatic improvement in outcomes, thanks to a number of biologic treatments. IL-17 antagonism is among the most potent treatments for psoriasis. Generally safe, new onset and exacerbations of inflammatory bowel disease (IBD) can occur in psoriasis patients receiving IL-17 therapy.1 We describe a patient with long-standing history of psoriasis and psoriatic arthritis (PsA) in whom asymptomatic Crohn’s disease (CD) was identified during treatment with secukinumab.
Case report
The subject is a 69-year-old male who was diagnosed with psoriasis and PsA over 45 years ago. Throughout this time period, patient’s psoriasis has been treated with a variety of medications, including methotrexate, etanercept and adalimumab, supplemented with intermittent dexamethasone as needed for arthritis pain; each worked well though appeared to become less effective with time. The patient was then treated with secukinumab for a year and a half, with excellent control of both the psoriasis and PsA. The psoriasis was essentially 100% clear, and the joint pain had resolved to the point that the intermittent dexamethasone was rarely needed. There was no apparent side effect of the secukinumab.
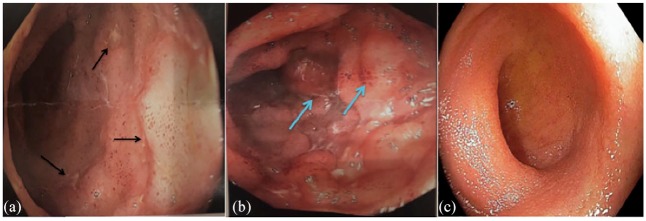
The patient underwent an elective colonoscopy for colorectal cancer screening in February 2019, which revealed inflammation and multiple ulcers in the terminal ileum, suggestive of CD with a normal-appearing colon (Figure 1). Terminal ileum biopsies revealed chronic active ileitis, which is seen in CD; however, there were no granulomas, no crypt abscesses and no evidence of dysplasia or malignancy (Figure 2). While the patient did not have any gastrointestinal symptoms, he was diagnosed as having asymptomatic CD. Given the association of IBD with secukinumab, secukinumab was discontinued and the patient was started on ustekinumab. It was later switched to guselkumab because the psoriasis and joint pain had recurred. No follow-up colonoscopy was done.
Figure 1.
Endoscopy of the terminal ileum. (a) The black arrows show multiple ulcers with regular borders on edematous mucosa seen in the terminal ileum of the patient. (b) The blue arrows show edematous erythematous mucosa with nodularity in patient’s terminal ileum. (c) Endoscopy of healthy terminal ileum from another patient being shown for comparison.
Figure 2.
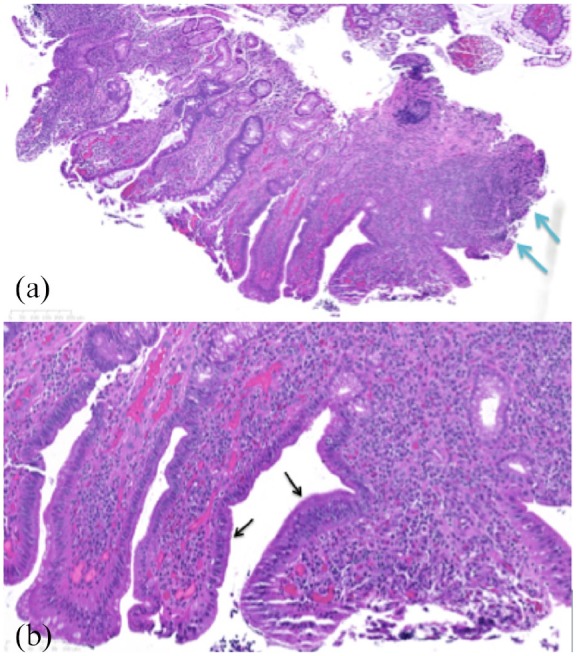
Histopthology of the ileal mucosa biopsy. (a) Histopathology of the ileal mucosa demonstrates there is preservation of the crypt architecture with the presence of mucin depletion and reactive epithelial changes. The lamina propria contains increased numbers of inflammatory cells (lymphocytes and plasma cells) as shown by the blue arrows. (b) On higher magnification, neutrophils are seen superficially with superficial cryptitis (neutrophils infiltrating the crypt epithelium) without crypt abscesses and active inflammation as shown by the black arrows.
Discussion
Psoriasis is a T-cell-mediated immune disease characterized by increased keratinocyte proliferation leading to the formation of well-demarcated erythematous plaques with scaling.2 CD and ulcerative colitis (UC) are parts of the spectrum of IBD. All three conditions result from immune dysregulation due to genetic predisposition and environmental assaults. The anatomical and functional integrity of the tissue environment barriers is compromised in the skin of psoriasis patients and intestinal lumen of IBD patients.3 Psoriasis and IBD are linked epidemiologically as well as genetically. In a population-based nationwide study in Korea, psoriasis patients had a higher risk of IBD than did the general population.4 A meta-analysis of genome-wide association studies recognized seven susceptibility loci shared by psoriasis and CD in addition to the four already established common psoriasis and CD risk loci.3 There is also an association between PsA and IBD.5,6
In addition to the shared epidemiologic and genetic links, these conditions share some therapies. Tumor necrosis factor (TNF) inhibitors adalimumab and infliximab are effective for plaque psoriasis and IBD.7 Ustekinumab, an anti-p40 IL-12/23 humanized monoclonal antibody, is effective for psoriasis and was approved in 2016 by Health Canada and US Food and Drug Administration (FDA) for the treatment of moderate to severe active CD (not for UC).8,9
IL-17 inhibition, highly effective for psoriasis, was expected to improve IBD. However, in controlled trials of patients with active CD, the placebo groups did better than the groups receiving anti-IL-17 (secukinumab) and anti-IL17 receptor (brodalumab) antibodies.10,11 In a retrospective analysis of pooled data from 21 clinical trials, the exposure-adjusted incidence rate for UC and CD was 0.13 and 0.05 per 100 patient-years, respectively.12 One case report described a patient with rapid onset of fulminant IBD after a single infusion of secukinumab, although the authors acknowledged that the patient might have developed IBD even without secukinumab infusion, considering the patient’s positive family history.13
Although in this patient CD was incidentally found during secukinumab therapy, a direct causal relationship cannot be assumed. The patient had previously been on adalimumab, which may have been keeping underlying IBD under control. The ulcers and inflammation may have been present long before treatment with secukinumab, as CD may be asymptomatic. Endoscopically or histologically detected inflammation occurs in a majority of IBD patients whose disease is clinically in remission.14 Without experiencing any gastrointestinal symptoms, patients may not have any testing to detect the IBD.
The overall incidence of IBD in psoriasis patients treated with secukinumab appears rare and does not appear to increase over time. However, caution should be exercised in patients with IBD and possibly in patients with family history of IBD. Medications that are effective for both psoriasis and IBD may be a good choice in patients with psoriasis who have comorbid CD or who develop IBD during treatment with another biologic.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.R.F. has received research, speaking and/or consulting support from a variety of companies, including Galderma, GSK/Stiefel, Almirall, Leo Pharma, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Valeant, Abbvie, Samsung, Janssen, Lilly, Menlo, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is the founder and majority owner of www.DrScore.com and the founder and part owner of Causa Research, a company dedicated to enhancing patients’ adherence to treatment. W.H., S.A.-N., C.S.A. and R.S.B. have no conflicts to disclose.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: All investigators ensure that the planning conduct and reporting of human research are in accordance with the Helsinki Declaration as revised in 2013. The patient provided written informed consent granting permission for patient information and images to be published.
References
- 1. Kamata M, Tada Y. Safety of biologics in psoriasis. J Dermatol 2018; 45(3): 279–286. [DOI] [PubMed] [Google Scholar]
- 2. Das D, Akhtar S, Kurra S, et al. Emerging role of immune cell network in autoimmune skin disorders: an update on pemphigus, vitiligo and psoriasis. Cytokine Growth Factor Rev 2019; 43: 35–44. [DOI] [PubMed] [Google Scholar]
- 3. Vlachos C, Gaitanis G, Katsanos K, et al. Psoriasis and inflammatory bowel disease: links and risks. Psoriasis 2016; 6: 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee JY, Kang S, Bae JM, et al. Psoriasis increases the risk of concurrent inflammatory bowel disease: a population-based nationwide study in Korea. Indian J Dermatol Venereol Leprol 2019; 85(2): 145–152. [DOI] [PubMed] [Google Scholar]
- 5. Manos CK, Xiao R, Brandon TG, et al. Risk factors for arthritis and the development of comorbid cardiovascular and metabolic disease in children with psoriasis. Arthritis Rheumatol 2017; 69(Suppl. 10). https://acrabstracts.org/abstract/risk-factors-for-arthritis-and-the-development-of-comorbid-cardiovascular-and-metabolic-disease-in-children-with-psoriasis/ (accessed 22 September 2019). [Google Scholar]
- 6. Charlton R, Green A, Shaddick G, et al. Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis 2018; 77(2): 277–280. [DOI] [PubMed] [Google Scholar]
- 7. Adegbola SO, Sahnan K, Warusavitarne J, et al. Anti-TNF therapy in Crohn’s disease. Int J Mol Sci 2018; 19(8): 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aggeletopoulou I, Assimakopoulos SF, Konstantakis C, et al. Interleukin 12/interleukin 23 pathway: biological basis and therapeutic effect in patients with Crohn’s disease. World J Gastroenterol 2018; 24(36): 4093–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf 2019; 46: 751–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61(12): 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Targan SR, Feagan B, Vermeire S, et al. A randomised, double blind, placebo-controlled phase 2 study of brodalumab in patients with moderate to severe Crohn’s disease. Am J Gastroenterol 2016; 111(11): 1599–1607. [DOI] [PubMed] [Google Scholar]
- 12. Schreiber S, Colombel JF, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis 2019; 78(4): 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Bhatia A, Krugliak Cleveland N, et al. Rapid onset of inflammatory bowel disease after receiving secukinumab infusion. ACG Case Rep J 2018; 5: e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baars JE, Nuij VJ, Oldenburg B, et al. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis 2012; 18(9): 1634–1640. [DOI] [PubMed] [Google Scholar]