Abstract
One common complication after joint arthroplasty is venous thromboembolism (VTE). Therefore, it is essential to measure the changes in coagulation and fibrinolysis in order to predict VTE among patients who underwent joint arthroplasty. This study aimed to identify potential useful biomarkers for prognosing to VTE. This was a prospective cohort study enrolling 83 patients who underwent joint arthroplasty. The levels of d-dimer, thrombin–antithrombin complex (TAT), plasmin–α2-antiplasmin complex (PIC), soluble thrombomodulin, and tissue plasminogen activator inhibitor complex were measured on day 0 (before surgery) and days 1, 3, and 6 after surgery. Ultrasound examination was used to diagnose VTE on preoperative day 0 and postoperative day 6. A total of 35 patients developed VTE after surgery. Patients with VTE exhibited significantly higher levels of d-dimer and TAT on postoperative days 3 and 6 (all P < .05). The area under curves (AUC) of receiver operating characteristic (ROC) were 0.65 and 0.68 and 0.68 and 0.74 for d-dimer and TAT levels on postoperative days 3 and 6, respectively. The level of TAT/PIC ratio on postoperative day 6 was significantly increased among patients with VTE compared to non-VTE patients (P < .0001). In addition, the AUC of ROC, cutoff level, sensitivity, specificity, positive-predictive value, and negative-predictive value of TAT/PIC ratio were 0.78, 4.03 ng/TU, 97.14%, 33.33%, 51.52%, and 94.12%, respectively. The high sensitivity and negative predictive value of TAT/PIC ratio make it a potential prognostic index for diagnosing VTE during the early phase of postoperative joint arthroplasty.
Keywords: venous thromboembolism, joint arthroplasty, d-dimer, thrombin–antithrombin, soluble thrombomodulin, plasmin-α2–antiplasmin complex, tissue plasminogen activator inhibitor complex
Introduction
One of the most common complications after total joint arthroplasty is venous thromboembolism (VTE), and its incidence rate is relatively high.1–5 Clinical trials show that the rate of deep VTE is 42% to 57% in Western countries and 23% to 42% in Japan among patients who received no antithrombotic prophylaxis after total hip arthroplasty.6,7 The early diagnosis of VTE after joint arthroplasty is of great importance for predicting the prognosis and outcome. Ultrasound imaging has been found to be useful in diagnosing VTE. According to American College of Chest Physicians 9 (ACCP 9), venography and ultrasound are used only when patients had developed VTE and exhibited clinical symptoms, such as pain, swelling, and so on.8 However, during the early phase of postoperative joint surgery, approximately 30% to 50% patients in Western countries and approximately 10% to 30% of patients in Asian countries have small thrombus without any clinical symptoms.1,9–13 These asymptomatic small emboli tend to grow and be fatal if the patients do not receive appropriate precautionary measures.3,14,15 Joint arthroplasty can lead to an unbalance between coagulation and fibrinolysis,6,16 and the disturbances in coagulation and fibrinolysis occur before the formation of emboli.1,9,17–19 Hence, the coagulation and fibrinolysis indices may become abnormal prior to imagological examination. Therefore, it is necessary to identify accurate biomarkers that reflect the changes in coagulation and fibrinolysis in order to predict the occurrence of postoperative VTE, especially symptomatic and fatal pulmonary embolism.17 d-Dimer is a product of cross-linked fibrin clots after lysis via activated plasmin.18,20 Thrombin–antithrombin (TAT) is a complex that reflects the formation of thrombin,18,21–23 while plasmin-α2-antiplasmin complex (PIC) is a fibrinolytic marker that directly reflects the generation of plasmin.16,18,24 In addition, both soluble thrombomodulin (sTM) and tissue plasminogen activator inhibitor complex (t-PAIC) are the markers involved in the endothelial system.23,25 Previous studies have reported that high levels of d-dimer and TAT are correlated with postoperative VTE.1,9,17–19,26 Besides, TM can combine with thrombin to activate protein C and thus prevent coagulation.2,27–30 Research studies have demonstrated that level of sTM increases after joint arthroplasty,23,24 and Kearon et al found that recombinant human sTM is an effective agent to prevent thrombosis after orthopedic surgery.31 McLawhorn et al have demonstrated that the level of PIC increases significantly 4 hours after total knee arthroplasty.24 According the findings of Sharrock et al, the level of t-PAIC increases after joint arthroplasty.32 These research studies illustrate that d-dimer, TAT, sTM, PIC, and t-PAIC are associated with VTE after joint arthroplasty.
According to ACCP, antithrombotic drugs are recommended rather than no antithrombotic prophylaxis for a minimum of 10 to 14 days.8 Studies have demonstrated that aspirin provided comparable VTE prophylaxis compared to factor Xa inhibitors, enoxaparin, and warfarin with the lowest risk of bleeding.33–36 And using antiplatelet therapy does not affect the changes in coagulation and fibrinolysis. In our hospital, surgeons choose aspirin as the antithrombotic treatment. Therefore, this study aimed to identify a prognostic marker for VTE among patients undergoing total joint arthroplasty.
Patients and Methods
Study Design
This cohort study was carried out prospectively.
Patient Recruitment
Patients who had undergone total joint arthroplasty were included in this study. They were confirmed to have no VTE before surgery according to the results of ultrasound scan. For exclusion criteria, those with activated inflammation, cancer, activated bleeding or VTE, atrial fibrillation, pregnancy, thrombophilia, and warfarin or other antithrombotic therapy were excluded from the study. A total of 100 patients who underwent joint arthroplasty were recruited at the Department of Adult Reconstructive Surgery, Beijing Jishuitan Hospital from October 2017 to October 2018. After excluding 17 patients based on our exclusion criteria, 83 patients, including 27 males and 56 females, were ultimately enrolled. All patients received 100 mg of aspirin on day 1 after surgery.
Ultrasound Examination
All patients underwent bilateral lower extremity venous Doppler ultrasonography on preoperative day 0 and postoperative day 6. According to the results of ultrasound scan on day 6, all patients were divided into 2 groups: VTE group and non-VTE group.
Biomarker Analysis
Blood samples were collected from the antecubital vein into a tube containing 3.2% trisodium citrate in the morning of days 0, 1, 3, and 6 before and after the operation. Then, the samples were tested immediately after they were collected. Measurement of d-dimer was performed using an immunoturbidimetry method (Sysmex 5100, corollary regent, Japan), while TAT, PIC, sTM, and t-PAIC were assayed by a chemiluminescence method (Sysmex HISCAL 5000, corollary regent, Japan). The reference ranges of d-dimer, TAT, PIC, and TM are 0.15 to 0.25 mg/L FEU, 0.00 to 4.00 ng/mL, 0.00 to 0.80 μg/mL, and 3.80 to 13.30 TU/mL, respectively. The reference levels of t-PAIC are 0.00 to 10.50 ng/mL in females and 0.00 to 17.00 ng/mL in males, respectively.
Statistical Analysis
Statistical analyses were performed using SPSS 23.0 and Graphpad Prism 7.0. All data were presented as median (2.5th, 97.5th percentiles). The levels of d-dimer, TAT, PIC, TM, t-PAIC, and TAT/PIC ratio were compared between patients with and without VTE on days 0, 1, 3, and 6 days before and after the surgery. Mann-Whitney U test was used to analyze the data of d-dimer, TAT, PIC, TM, t- PAIC, and TAT/PIC. In all figures, the horizontal bars represented the medians and the vertical bars represented the 2.5th and 97.5th percentiles. Data assessment was carried out using a receiver operating characteristic curve (ROC) analysis. P values of less than .05 were considered statistically significant.
Results
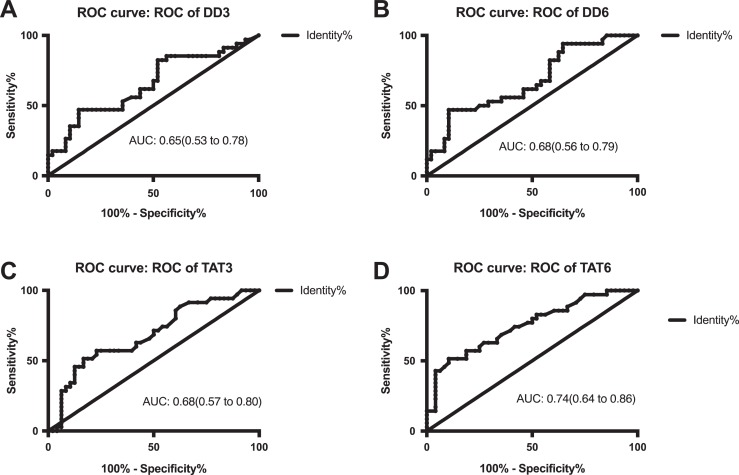
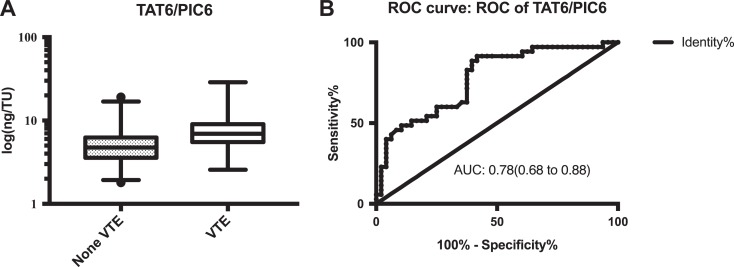
The presence of VTE in 35 of the 83 patients was confirmed by ultrasound examination within the first 7 days after surgery, and their demographic and clinical characteristics are presented in Table 1. The measurement results of d-dimer, TAT, PIC, TM, t-PAIC, and TAT/PIC are shown in Table 2. Notably, the levels of d-dimer on postoperative days 3 and 6 were significantly increased in patients with VTE compared to those without VTE (P = .0172 and P = .0065, respectively). In addition, patients with VTE exhibited higher levels of TAT on postoperative days 3 and 6 (P = .0040 and P < .0001, respectively). Besides, the levels of PIC, TM, and t-PAIC were higher in patients with VTE than those without VTE before surgery and on days 1, 3, and 6 after surgery but did not achieve statistical significance. Furthermore, the level of TAT/PIC ratio on postoperative day 6 was significantly increased in patients with VTE compared to without VTE (P < .0001). As shown in Figure 1, the area under the ROC curves of d-dimer on postoperative day 3 was 0.65, and its cutoff level was 2.26 mg/L FEU, with sensitivity and specificity of 47.06% and 85.42%, respectively. On postoperative day 6, the area under the ROC curves, cutoff level, sensitivity, and specificity of d-dimer were 0.68, 5.21 mg/L FEU, 47.06%, and 89.58%, respectively. Meanwhile, the area under the ROC curves of TAT on postoperative day 3 was 0.68, and its cutoff level was 10.40 ng/mL, with the sensitivity and specificity of 51.43% and 83.33%, respectively. On postoperative day 6, the area under the ROC curves, cutoff level, sensitivity, and specificity of TAT were 0.74, 8.80 ng/mL, 51.43%, and 89.58% (Figure 1). Notably, the ratio of TAT/PIC on postoperative day 6 displayed the largest area under the ROC curves (0.78). In addition, the cutoff level of TAT/PIC was 4.03 ng/TU, along with a sensitivity of 97.14%, a specificity of 33.33%, a positive predictive value of 51.52%, and a negative predictive value of 94.12% (Figure 2).
Table 1.
Demographic and Clinical Characteristics of Patients With VTE and Non-VTE Patients.a
| Items | Non-VTE, n = 48 | VTE, n = 35 |
|---|---|---|
| Male/female, n/n | 19/29 | 8/27 |
| Age, y | 61.00 (40.25-84.20) | 66.00 (53.00-79.00) |
| Basic diseases: AVN/OA/RA/others, n/n/n/n | 8/39/0/1 | 3/32/0/0 |
| Risk factors before operation, n | ||
| Previous VTE | 0 | 0 |
| Previous surgery at past 6 months | 6 | 4 |
| Current smoker | 7 | 3 |
| Hypertension | 18 | 21 |
| Hyperglycemia | 9 | 7 |
| Blood type: O/non-O, n/n | 4/44 | 16/19 |
| C-reactive protein, mg/L | 3.45 (1.27-27.88) | 4.07 (1.58-111.00) |
| Hemoglobin, g/L | 136.00 (86.13-173.13) | 131.00 (111.00-165.00) |
| Platelets, ×109/L | 268.50 (88.13-463.00) | 222.00 (143.00-321.00) |
| Items related to surgery, n/n/n | ||
| Anesthetics: general/regional/combined | 0/1/47 | 0/0/35 |
| Prosthesis: cemented/noncemented/hybrid | 30/18/0 | 26/9/0 |
| Time in operation room, h | 60.00 (50.00-151.00) | 60.00 (48.00-120.00) |
| Estimated blood loss, mL | 50.00 (0.00-887.50) | 50.00 (0.00-1200.00) |
| Surgical approach: anterointernal/anterior longitudinal midline/posterior lateral, n/n/n | 9/21/18 | 5/21/9 |
Abbreviations: AVN, avascular necrosis; OA, osteoarthritis; RA, rheumatic arthritis; VTE, venous thromboembolism.
a Data are expressed as median (2.5th, 97.5th percentiles).
Table 2.
Concentrations of d-Dimer, TAT, TM, PIC, t-PAIC, and TAT/PIC on Day 0 (Before Surgery), 1, 3, and 6 After Surgery.a
| Non-VTE | VTE | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 3 | Day 6 | Day 0 | Day 1 | Day 3 | Day 6 | |
| d-Dimer, mg/L FEU | 0.32 (0.09-5.85) | 2.63 (0.49-17.51) | 1.35 (0.53-4.83)b | 3.95 (1.14-10.28)b | 0.42 (0.17-6.79) | 2.88 (0.41-27.05) | 1.76 (0.53-7.08)b | 4.84 (1.82-20.76)b |
| TAT, ng/mL | 1.45 (0.42-12.65) | 14.35 (3.97-45.24) | 8.00 (2.91-20.38)b | 6.10 (2.15-12.60)b | 1.50 (0.70-12.70) | 17.50 (7.10-37.00) | 10.50 (4.10-19.40)b | 8.90 (3.80-16.70)b |
| TM, μg/mL | 9.90 (6.54-16.20) | 8.65 (5.70-13.24) | 10.30 (6.11-15.63) | 10.20 (7.02-16.36) | 10.30 (7.20-19.40) | 9.10 (6.20-14.70) | 11.00 (7.00-18.20) | 11.50 (7.50-17.40) |
| PIC, TU/mL | 0.57 (0.22-1.52) | 1.21 (0.40-3.99) | 0.63 (0.36-1.59) | 1.17 (0.61-3.69) | 0.57 (0.28-1.36) | 1.50 (0.70-9.99) | 0.79 (0.32-2.39) | 1.18 (0.45-2.35) |
| t-PAIC, ng/mL | 7.85 (2.68-16.02) | 8.50 (4.64-15.98) | 7.60 (3.49-20.76) | 8.60 (2.40-17.20) | 8.20 (2.70-12.00) | 8.70 (3.90-18.80) | 7.90 (3.40-18.70) | 9.20 (3.50-18.20) |
| TAT/PIC, ng/TU | 2.73 (0.88-34.81) | 10.04 (4.58-34.48) | 12.08 (2.90-25.18) | 4.74 (1.93-16.88)b | 2.68 (1.22-16.67) | 10.27 (4.55-32.92) | 14.14 (3.50-35.15) | 6.92 (2.57-28.77)b |
Abbreviations: PIC, plasmin–α2-antiplasmin complex; TAT, thrombin–antithrombin complex; TM, thrombomodulin; t-PAIC, tissue plasminogen activator inhibitor complex; VTE, venous thromboembolism.
a Data are expressed as median (2.5th, 97.5th percentiles).
b P < .05.
Figure 1.
(A-D) Receiver operating characteristic (ROC) curves of DD3, DD6, TAT3, and TAT6. DD3 indicates the level of d-dimer on postoperative day 3; DD 6, the level of d-dimer on postoperative day 6; TAT 3, the level of thrombin–antithrombin complex on postoperative day 3; and TAT 6: the level of TAT on postoperative day 6.
Figure 2.
The level (A) and receiver operating characteristic (ROC) curve (B) of TAT6/PIC6. PIC 6 indicates the level of plasmin–α2-antiplasmin complex on postoperative day 6; TAT 6, the level of thrombin–antithrombin complex on postoperative day 6.
The reference range of d-dimer, TAT, PIC, TM, and t-PAIC (female)/(male) is 0.15 to 0.25 mg/L FEU, 0.00 to 4.00 ng/mL, 0.00 to 0.80 μg/mL, 3.80 to 13.30 TU/mL, and 0.00 to 10.50/0.00 to 17.00 ng/mL, respectively.
Discussions
Venous thromboembolism is a common and serious complication after joint arthroplasty.24,37,38 In this study, nearly 45% of patients who underwent joint arthroplasty had VTE even though they had received antithrombotic prophylaxis. From this point of view, the presence of VTE should be diagnosed as early as possible in order to prevent this severe complication. During the early stage of VTE, the disturbances in coagulation and fibrinolysis occur before the activation of thrombosis.1,9,17–19 Hence, the coagulation indices may become abnormal prior to imagological examination. Moreover, joint arthroplasty can lead to an unbalance between coagulation and fibrinolysis.6,16 The changes in TAT, d-dimer, PIC, TM, and t-PAIC levels may be associated with the occurrence of VTE.26 In the present study, the levels of TAT and d-dimer were significantly different between patients with and without VTE on postoperative days 3 and 6 (both P < .05). Considering that TAT levels can reflect the levels of thrombin formation, the increased levels of TAT indicate the activation of the coagulation system and contribute to the risk of VTE.18,22,23 Yukizawa et al demonstrate that the levels of TAT in patients with VTE are higher than those without VTE after joint arthroplasty, which are consistent with our findings.6 d-Dimer is formed as a result of plasmin digestion of cross-linked fibrin and therefore only occurs when both clotting and fibrinolytic systems are activated.10,18,20,39 A high level of d-dimer indicates the formation of fibrin thrombus.40 The first study by Dunn et al demonstrates that d-dimer level is elevated in patients with VTE on postoperative days 1, 3, and 6.41 Following that, numerous studies have reported that the increased level of d-dimer is correlated with postoperative VTE.1,9,17,18 Taken altogether, d-dimer is considered a good biomarker to diagnose VTE.42–44 Nevertheless, the levels of PIC, sTM, and t-PAIC were not significantly different between the 2 patient groups. Plasmin–α2-antiplasmin complex is a complex of plasmin and α2-antiplasmin, and its levels are increased following the activation of the fibrinolytic system.16,24 Watanabe et al have found that the inactivation of α2-antiplasmin may prevent the occurrence of VTE after joint arthroplasty.9 Besides, sTM and t-PAIC are the 2 markers involved in the endothelial system,23,25 which may be important for VTE risk prediction. Thrombomodulin can combine with thrombin to activate protein C27,28,45 and thus prevent coagulation.29,30,46 According to the findings of Kearon et al,31 recombinant human sTM is an effective agent for preventing thrombosis in patients who have undergone orthopedic surgery. Tissue plasminogen activator inhibitor complex is a complex of tissue plasminogen activator and plasminogen activator inhibitor-1,47,48 in which the t-PA is released from endothelial cells and is involved in the conversion of plasminogen to plasmin.49 Sharrock et al demonstrate that the level of t-PAIC increases after joint arthroplasty.32 However, the exact causes for the nonsignificant association of sTM and t-PAIC with VTE risk in patients undergoing total joint arthroplasty remain unknown.
In this study, we monitor the time series data of coagulation, fibrinolysis, and endothelial-related indices on days 0, 1, 3, and 6 days before and after surgery. Although the levels of d-dimer, TAT, and PIC were elevated after joint arthroplasty, not all time points showed significant differences between VTE group and non-VTE group. After surgery, the concentrations of TAT, d-dimer, and PIC were higher than the baseline before operation but not sTM and t-PAIC. Our data revealed that both coagulation and fibrinolysis are activated following joint arthroplasty. This is probably due to the vascular and bone injuries that trigger the release of tissue factor–bearing microparticle, leading to the activated coagulation cascade (data not published). As a consequence, the routine coagulation tests, such as prothrombin time and active partial thromboplastin time, are not able to reflect these changes.
As aforementioned, the occurrence of VTE is caused by an unbalance between coagulation and fibrinolysis. Thus, we designed a new index of TAT/PIC6 to comprehensively explain the ratio of procoagulation/fibrinolysis on the sixth day after surgery. The area under the ROC curves of TAT/PIC6 was 0.78 and its cutoff level was 4.03 ng/TU, with the sensitivity and specificity of 97.14% and 33.33%, respectively. Moreover, its positive and negative predictive values were 51.52% and 94.12%, respectively. The relatively high sensitivity and negative predictive values make it a useful index for prognosing VTE. However, imaging is still needed to confirm the presence of VTE. Indeed, TAT/PIC6 index can help identify potentially high-risk patients with VTE who need antithrombotic therapy and coagulation monitoring. In addition, these biomarkers are easier and more convenient to be measured compared to ultrasound and venography. In clinical practice, it is of particular significance to prevent VTE in patients undergoing joint arthroplasty at the earliest.3 In view of this, the early diagnosis of VTE is of particular importance for the patients who are asymptomatic following joint arthroplasty. According to ACCP 9, routine ultrasound screening is not recommended if the patients have asymptomatic VTE.8 Alternatively, TAT/PIC6 may be a great option for VTE prognosis, and it can provide guidance to surgeons to prevent VTE after joint arthroplasty.
Although the research has reached its aims, there are some unavoidable limitations. In this study, we only monitor 7 days of coagulation indices and imagological examination during patient hospitalization. However, VTE may occur within the first 3 months after joint arthroplasty. Besides, the number of participants is small. However, our study demonstrates the importance of TAT/PIC, and TAT/PIC is a prognostic index for VTE in joint arthroplasty. Thus, the next step of our research is to recruit more patients and follow up these patients in order to establish a complete perspective model of VTE in patients undergoing joint arthroplasty.
In conclusion, patients who received joint arthroplasty tend to have a high risk of VTE, which is caused by the unbalance between coagulation and fibrinolysis. In addition to TAT and d-dimer, TAT/PIC6 can serve as an ideal indicator to identify VTE during the early phase of postoperative joint arthroplasty.
Footnotes
Authors’ Note: Yuying Chen, Jian Liu, and Jun Wu designed and performed the research. Yuying Chen, Yu Su, Huiru Zhao, Yujing Zhao, Meng Wen, Shan Lu, and Wenjie Zhang acquired the data. Yu Su, Huiru Zhao, Yujing Zhao, Meng Wen, and Shan Lu provided valuable technical assistance. Yuying Chen, Jian Liu, and Jun Wu analyzed and interpreted the data and wrote the manuscript. All authors reviewed and made critical revisions and approved final version of the manuscript. Yuying Cheng and Jian Liu contributed equally. The study was conducted with approval of the Institution’s Ethics Committee (project number 201904-06).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jun Wu  https://orcid.org/0000-0001-5178-0906
https://orcid.org/0000-0001-5178-0906
References
- 1. Hasegawa M, Wada H, Miyazaki S, et al. The evaluation of fibrin-related markers for diagnosing or predicting acute or subclinical venous thromboembolism in patients undergoing major orthopedic surgery. Clin Appl Thromb Hemost. 2018;24:107–114. doi:10.1177/1076029616674824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378:699–707. doi:10.1056/NEJMoa1712746. [DOI] [PubMed] [Google Scholar]
- 3. Geerts WH, Berggvist D, Pineo GF, et al. Prevention of venous thromboembolism: american college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:381S–453S. doi:10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Zheng J, Zhao Y, Xiang Y, Chen X, Jin Y. Effectiveness and tolerability of anticoagulants for thromboprophylaxis after major joint surgery: a network meta-analysis. Cell Physiol Biochem. 2017;42(5):1999–2020. doi:10.1159/000479840. [DOI] [PubMed] [Google Scholar]
- 5. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2010;66(11):1099–1108. doi:10.1007/s00228-010-0889-z. [DOI] [PubMed] [Google Scholar]
- 6. Yukizawa Y, Inaba Y, Watanabe S, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthopaedica. 2012;83(1):14–21. doi:10.3109/17453674.2011.652886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuji T, Fujita S, Ochi T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. Int Orthop. 2008;32(4):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):e278S–e325S. doi:10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Watanabe H, Kikkawa I, Madoiwa S, Sekiya H, Hayasaka S, Sakata Y. Changes in blood coagulation–fibrinolysis markers by pneumatic tourniquet during total knee joint arthroplasty with venous thromboembolism. J Arthroplasty. 2014;29(3):569–573. doi:10.1016/j.arth.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 10. Mitani G, Takagaki T, Hamahashi K, et al. Associations between venous thromboembolism onset, D-dimer, and soluble fibrin monomer complex after total knee arthroplasty. J Orthop Surg Res. 2015;10:172 doi:10.1186/s13018-015-0315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaguchi T, Hasegawa M, Niimi R, Sudo A. Incidence and time course of asymptomatic deep vein thrombosis with fondaparinux in patients undergoing total joint arthroplasty. Thromb Res. 2010;126(4):e323–e326. doi:10.1016/j.thromres.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 12. Sun Y, Chen D, Xu Z, et al. Incidence of symptomatic and asymptomatic venous thromboembolism after elective knee arthroscopic surgery: a retrospective study with routinely applied venography. Arthroscopy. 2014;30(7):818–822. doi:10.1016/j.arthro.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 13. Song K, Xu Z, Rong Z, et al. The incidence of venous thromboembolism following total knee arthroplasty: a prospective study by using computed tomographic pulmonary angiography in combination with bilateral lower limb venography. Blood Coagul Fibrinolysis. 2016;27(3):266–269. doi:10.1097/MBC.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu CT, Chen B, Wang JW, et al. Plasma D-dimer is not useful in the prediction of deep vein thrombosis after total knee arthroplasty in patients using rivaroxaban for thromboprophylaxis. J Orthop Surg Res. 2018;13(1):173 doi:10.1186/s13018-018-0883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borgen PO, Dahl OE, Reikeras O. Biomarkers of coagulation and fibrinolysis during cemented total hip arthroplasty with pre-versus postoperative start of thromboprophylaxis. Thrombosis. 2013;2013:563217 doi:10.1155/2013/563217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watanabe H, Madoiwa S, Sekiya H, et al. Predictive blood coagulation markers for early diagnosis of venous thromboembolism after total knee joint replacement. Thromb Res. 2011;128(6):e137–e143. doi:10.1016/j.thromres.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 18. Inaba Y, Yukizawa Y, Saito T. Coagulation and fibrinolysis markers and their use for the prediction of high risk patients with venous thromboembolism following total hip arthroplasty. Fibrinolysis Thrombolysis Chapter. 2014;7:163–176. doi:10.5772/57248. [Google Scholar]
- 19. Watanabe H, Inoue H, Murayama A, et al. Prediction of venous thromboembolism after total knee arthroplasty using blood coagulation-fibrinolysis markers: a systematic review. Int Orthop. 2015;2:280–283. doi:10.17554/j.issn.2311-5106.2015.02.83. [Google Scholar]
- 20. Huisman MV, Klok FA. Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 2013;11:412–422. doi:10.1111/jth.12124. [DOI] [PubMed] [Google Scholar]
- 21. Lee SY, Niikura T, Iwakura T, Sakai Y, Kuroda R, Kurosaka M. Thrombin-antithrombin III complex tests. J Orthop Surg (Hong Kong). 2017;25:170840616684501 doi:10.1177/0170840616684501. [DOI] [PubMed] [Google Scholar]
- 22. Omote M, Asakura H, Takamichi S, et al. Changes in molecular markers of hemostatic and fibrinolytic activation under various sampling conditions using vacuum tube samples from healthy volunteers. Thromb Res. 2008;123(2):390–395. doi:10.1016/j.thromres.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 23. Yu X, Tian Y, Wang K, Wang YL, Lv GY, Tian GG. Effect of ulinastatin combined rivaroxaban on deep vein thrombosis in major orthopedic surgery. Asian Pac J Trop Med. 2014;7(11):918–921. doi:10.1016/s1995-7645(14)60162-0. [DOI] [PubMed] [Google Scholar]
- 24. McLawhorn AS, Beathe J, YaDeau J, et al. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Res. 2015;33:412–416. doi:10.1002/jor.22776. [DOI] [PubMed] [Google Scholar]
- 25. Lindberg-Larsen V, Ostrowski SR, Lindberg-Larsen M, et al. The effect of pre-operative methylprednisolone on early endothelial damage after total knee arthroplasty: a randomised, double-blind, placebo-controlled trial. Anaesthesia. 2017;72(10):1217–1224. doi:10.1111/anae.13983. [DOI] [PubMed] [Google Scholar]
- 26. Lippi G, Cervellin G, Franchini M, Favaloro EJ. Biochemical markers for the diagnosis of venous thromboembolism: the past, present and future. J Thromb Thrombolysis. 2010;30(4):459–471. doi:10.1007/s11239-010-0460-x. [DOI] [PubMed] [Google Scholar]
- 27. Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304(12):H1585–H1597. doi:10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anastasiou G, Gialeraki A, Merkouri E, Politou M, Travlou A. Thrombomodulin as a regulator of the anticoagulant pathway. Blood Coagul Fibrinolysis. 2012;23(1):1–10. doi:10.1097/MBC.0b013e32834cb271. [DOI] [PubMed] [Google Scholar]
- 29. Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130 doi:10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouwens EA, Stavenuiter F, Mosnier LO. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost. 2013;11:242–253. doi:10.1111/jth.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kearon C, Comp P, Douketis J, Royds R, Yamada K, Gent M. Dose-response study of recombinant human soluble thrombomodulin (ART-123) in the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2005;3(5):962–968. [DOI] [PubMed] [Google Scholar]
- 32. Sharrock NE, Go G, Williams-Russo P, et al. Comparison of extradural and general anaesthesia on the fibrinolytic response to total knee arthroplasty. Br J Anaesth. 1997;79(1):29–34. doi:10.1093/bja/79.1.29. [DOI] [PubMed] [Google Scholar]
- 33. Hood BR, Cowen ME, Zheng HT, Hughes RE, Singal B, Hallstrom BR. Association of aspirin with prevention of venous thromboembolism in patients after total knee arthroplasty compared with other anticoagulants: a noninferiority analysis. JAMA Surg. 2019;154(1):65–72. doi:10.1001/jamasurg.2018.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bala A, Huddleston JI, 3rd, Goodman SB, Maloney WJ, Amanatullah DF. Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor Xa inhibitors? Clin Orthop Relat Res. 2017;475:2205–2213. doi:10.1007/s11999-017-5394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kapoor A, Ellis A, Shaffer N, et al. Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: a network meta-analysis. J Thromb Haemost. 2017;15(2):284–294. doi:10.1111/jth.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah SS, Satin AM, Mullen JR, Merwin S, Goldin M, Sgaglione NA. Impact of recent guideline changes on aspirin prescribing after knee arthroplasty. J Orthop Surg Res. 2016;11:123 doi:10.1186/s13018-016-0456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Green L, Lawrie AS, Patel R, et al. The effect of total hip/knee replacement surgery and prophylactic dabigatran on thrombin generation and coagulation parameters. Thromb Res. 2012;130(5):775–779. doi:10.1016/j.thromres.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 38. Rafee A, Herlikar D, Gilbert R, Stockwell RC, McLauchlan GJ. D-Dimer in the diagnosis of deep vein thrombosis following total hip and knee replacement: a prospective study. Ann R Coll Surg Engl 2008;90:123–126. doi:10.1308/003588408x261627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Refaai MA, Riley P, Mardovina T, Bell PD. The clinical significance of fibrin monomers. Thromb Haemost. 2018;118(11):1856–1866. doi:10.1055/s-0038-1673684. [DOI] [PubMed] [Google Scholar]
- 40. Bytniewski P, Machala W, Romanowski L, et al. The dynamics of D-dimer level fluctuation in patients after the cemented and cementless total hip and total knee replacement. J Orthop Surg Res. 2014;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dunn ID, Hui AC, Triffitt PD, et al. Plasma D-dimer as a marker for postoperative deep venous thrombosis. A study after total hip or knee arthroplasty. Thrombosis and Haemostasis. 1994;71:663–665. [PubMed] [Google Scholar]
- 42. Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–3073. doi:10.1016/s0140-6736(16)30514-1. [DOI] [PubMed] [Google Scholar]
- 43. Rong Z, Yao Y, Chen D, et al. The incidence of deep venous thrombosis before arthroscopy among patients suffering from high-energy knee trauma. Knee Surg Sports Traumatol Arthrosc. 2016;24:1717–1721. doi:10.1007/s00167-016-4026-0. [DOI] [PubMed] [Google Scholar]
- 44. Geersing GJ, Janssen KJ, Oudega R, et al. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ. 2009;339:b2990 doi:10.1136/bmj.b2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets. 2012;13(3):421–431. [DOI] [PubMed] [Google Scholar]
- 46. Dahlbäck B. Pro- and anticoagulant properties of factor V in pathogenesis of thrombosis and bleeding disorders. Int J Lab Hematol. 2016;38(suppl 1):4–11. doi:10.1111/ijlh.12508. [DOI] [PubMed] [Google Scholar]
- 47. Yasar Yildiz S, Kuru P, Toksoy Oner E, Agirbasli M. Functional stability of plasminogen activator inhibitor-1. ScientificWorldJournal. 2014;2014:1–11. doi:10.1155/2014/858293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burleson A, Guler N, Banos A, et al. Perioperative factors and their effect on the fibrinolytic system in arthroplasty patients. Clin Appl Thromb Hemost. 2016;22(3):274–279. doi:10.1177/1076029615611251. [DOI] [PubMed] [Google Scholar]
- 49. Kruithof EK, Dunoyer-Geindre S. Human tissue-type plasminogen activator. Thromb Haemost. 2014;112:243–254. doi:10.1160/TH13-06-0517. [DOI] [PubMed] [Google Scholar]