Abstract
Background:
Patients treated with peritoneal dialysis (PD) are at increased risk of developing mechanical complications such as dialysate leaks and hernias thought to be partially related to an increase in intra-abdominal pressure (IAP) secondary to dialysate in the abdomen. However, measurement of IAP requires specialized equipment that is not readily available in the home dialysis unit.
Objectives:
To develop a reliable method of measuring IAP in PD patients that could be easily used in the home dialysis unit. We hypothesized that the handheld Stryker pressure monitor would be suitable for this purpose via connection to the PD catheter.
Design:
Cross-sectional.
Setting:
Tertiary Care Hospital, Ottawa, Ontario, Canada.
Patients:
Patients who were having a PD catheter inserted via laparoscopic surgery at The Ottawa Hospital were recruited for the study.
Measurements:
With the patients at end-expiration, the IAP measured with the Stryker monitor connected to the PD catheter was compared with the insufflator pressures of 15, 10, and 5 mm Hg.
Methods:
Bland-Altman plots were constructed and intraclass correlation coefficients were calculated for each pressure.
Results:
Twelve patients participated in the study: 9 men and 3 women. They were on average 53 ± 15 years old and 81 ± 13.4 kg. Two patients had to be excluded from the analysis due to difficulties zeroing the Stryker pressure monitor at the time of surgery. There were also rapid fluctuations in the insufflator pressure recording, creating additional challenges in comparing the 2 measurements at end-expiration. The 95% limits of agreement for the Bland-Altman plots ranged from 7.9 (@15 mm Hg) to 12.2 (@10 mm Hg). The intraclass correlation coefficients for reliability of the individual measurements ranged from 0.015 (10 mm Hg) to 0.634 (15 mm Hg).
Limitations:
Small sample size and lack of a gold standard comparator may have affected our results.
Conclusions:
In our study, we used the operating room insufflator as the gold standard for measuring IAP. By Bland-Altman plots and intraclass correlation coefficients, the pressure values obtained with the Stryker pressure monitor were not a reliable estimate of insufflator IAP especially at lower pressures. Further studies are needed to identify an ideal tool for measurement of IAP to guide PD management.
Keywords: peritoneal dialysis (PD), intra-abdominal pressure, measurement, pilot study
Abrégé
Contexte:
Les patients traités par dialyse péritonéale (DP) sont plus sujets aux complications mécaniques (hernies, fuites de dialysat) attribuées en partie à une augmentation de la pression intra-abdominale (PIA) due à l’accumulation de dialysat dans l’abdomen. La mesure de la PIA requiert toutefois de l’équipement spécialisé difficilement accessible en contexte de dialyse à domicile.
Objectif:
Développer une méthode fiable, et facile à utiliser en contexte de dialyse à domicile, pour mesurer la PIA chez les patients traités par DP. Nous avons émis l’hypothèse qu’un tensiomètre portatif Stryker raccordé au cathéter de DP pourrait convenir à cet usage.
Type d’étude:
Étude transversale
Cadre:
Un centre de soins tertiaires d’Ottawa (Ontario) au Canada.
Sujets:
Des patients de l’hôpital d’Ottawa à qui on avait inséré un cathéter de DP par chirurgie laparoscopique.
Mesures:
La pression intra-abdominale, mesurée en fin d’expiration à l’aide d’un tensiomètre Stryker raccordé au cathéter de DP, a été comparée aux pressions de 15, 10 et 5 mm Hg de l’insufflateur.
Méthodologie:
Des courbes de Bland-Altman ont été établies et des coefficients de corrélation intraclasse ont été calculés pour chaque mesure de pression.
Résultats:
Douze patients, soit neuf hommes et trois femmes, âgés de 53 ± 15 ans et pesant 81 ±13,4 kg en moyenne, ont participé à l’étude. Deux patients ont été exclus de l’analyse en raison de difficultés à remettre le tensiomètre Stryker à zéro au moment de l’intervention. On a observé de rapides fluctuations dans l’enregistrement de la pression avec l’insufflateur, ce qui a compliqué davantage la comparaison des deux mesures en fin d’expiration. Les limites de concordance à 95 % pour les courbes de Bland-Altman se situaient entre 7,9 (15 mm Hg) et 12,2 (10 mm Hg). Les coefficients de corrélation intraclasses pour la fiabilité des mesures individuelles s’échelonnaient entre 0,015 (10 mm Hg) et 0,634 (15 mm Hg).
Limites:
Les résultats sont limités par la faible taille de l’échantillon et l’absence d’étalon-or pour la comparaison.
Conclusion:
Pour cette étude, l’insufflateur de la salle d’opération a servi d’étalon-or pour la mesure de la PIA. Selon les courbes de Bland-Altman et les coefficients de corrélation intraclasses, les valeurs de pression obtenues avec le tensiomètre Stryker n’ont pas constitué une estimation fiable de la PIA de l’insufflateur, particulièrement pour les faibles valeurs de pression. Des études supplémentaires sont nécessaires pour proposer un outil de mesure fiable de la PIA afin de guider la gestion de la DP.
What was known before
Peritoneal dialysis patients are at increased risk to develop dialysate leaks and hernias that are thought to be at least partially due to an increase in intra-abdominal pressure (IAP) secondary to dialysate. Although there are methods to measure IAP, they are not readily applied in an outpatient setting for clinical or research purposes.
What this adds
As assessed in our study, the small handheld Stryker intracompartmental pressure monitor did not provide reliable estimates of IAP when compared with the insufflator during peritoneal dialysis catheter insertion surgery.
Background
Peritoneal dialysis (PD) patients are at increased risk of developing mechanical complications, such as dialysate leaks and hernias that may be related to an increase in intra-abdominal pressure (IAP) secondary to dialysate. Once a PD patient develops one of these complications, they may require a change in their dialysis prescription that could lead to under-dialysis or be required to switch to hemodialysis. For this reason, some physicians advise PD patients to refrain from any strenuous activity that may increase IAP, including resistance exercise.1 To more fully understand the importance of IAP in PD patients, an accurate and easy method of measuring IAP that could be applied in the home dialysis unit would be useful clinically and for research.
Methods to measure IAP have been developed to monitor for the development of intra-abdominal compartment syndrome.2 Patients at high risk of development of the syndrome have IAP measurements done approximately every 8 hours via a Foley catheter. This technique was originally described in 1989 in which IAP was assessed directly via paracentesis or through a Jackson-Pratt drain and compared with values obtained via the Foley catheter.3 Both systems were connected to a monitor via arterial tubing with transducers zeroed at the level of the pubis. The 2 methods of IAP measurement were highly correlated (r = .91). Two other groups of investigators have compared direct IAP measurements to those obtained from the insufflator during surgery.4,5 Similar to the study above, in the first study, the comparator was a round polyvinyl chloride drain connected to an arterial blood pressure set. The correlation between the 2 measurements was excellent (r = .97).4 In the second study, direct IAP measurement was with the Stryker intracompartmental (STIC) pressure monitor which had been developed for diagnosing and managing muscle compartment syndrome.5 The pressure measurements obtained by the STIC monitor and the insufflator correlated very well with a mean difference of 0.04 ± 3.8 mm Hg between the 2 techniques.
Very few studies report IAP measurements in patients treated with PD. Twardowski and colleagues assessed IAP using a central venous pressure monometer connected to an outflow line.6,7 They later modified their technique to include continuous monitoring with a pressure transducer connected to the PD catheter.7 To our knowledge, given the equipment required, IAP is not being routinely measured in any PD program.
The STIC monitor is a simple to use, handheld, portable device that would make it ideal for a measuring IAP in a large number of PD patients in diverse research/clinical environments. Therefore, the objective of this study was to determine whether the Stryker monitor could serve as an easy and reliable method of measuring IAP in PD patients.
Methods
Study Design
We conducted a pilot study to assess the feasibility and accuracy of using the Stryker monitor to measure IAP. The study was conducted according to a prespecified protocol that was approved by the Ottawa Health Science Network Research Ethics Board (20160377-01H; Ottawa, Ontario, Canada).
Patients
All adult (>18 years old) English- or French-speaking patients with chronic kidney disease (CKD) who were referred for assessment at The Ottawa Hospital body access clinic for standard position PD catheter insertion were approached to determine willingness to participate in the study. For logistical reasons, patients who underwent urgent laparoscopic PD catheter insertion (during hospital admission) were excluded.
After providing informed consent, patient demographic information including age, sex, height, weight, medical comorbidities, presurgery dialysis status, and cause of CKD was collected at the time of surgery. Body mass index and Charlson comorbidity index were calculated.
IAP Measurement
Standard cannulation of the abdomen by the surgeon was undertaken followed by insufflation, insertion of trocars, and placement of the PD catheter. The superficial cuff and the remainder of the PD catheter were tunneled under the subcutaneous tissue with an exit site in the right or left lower quadrant, flushed, and then connected to a transfer set. The PD transfer set was connected to the sterile fluid path of the STIC pressure monitor system via extension tubing without using the intervening needle. With the patient at end-expiration, the pressure measured from the PD catheter at the level of the umbilicus in the midaxillary line was compared with the insufflator at 15, 10, and 5 mm Hg as these pressures were felt to be safe in the context of abdominal surgery.
Sample Size and Statistical Analysis
A sample size of 12 was expected to give a 95% confidence interval of ~1 standard deviation for the Bland-Altman method. Bland-Altman plots were constructed and considered acceptable if the mean difference between the readings was 5 mm Hg and 95% of the points fell within 2 standard deviations of the mean difference. Based on previous literature, intraclass correlation coefficients (ICCs) were calculated and considered moderate if reliability values were 0.5 to 0.75, good if 0.75 to 0.9, and excellent if greater than 0.9.8 All analysis was completed with Stata version 15.1 (College Station, TX).
Results
Subjects
A total of 43 patients attended the body access clinic during study recruitment and were screened for participation; 2 patients were too unwell to approach and 1 patient had a language barrier. A further 8 participants declined to participate (Figure 1). Of the 32 patients who consented to the study, measurements were obtained for 12 participants (Figure 1). One investigator (T.T.) had scheduling conflicts such that 11 patients had surgery when she was unavailable, 2 patients’ surgeries were canceled, 1 surgery was put on hold, and 2 patients were scheduled for surgery following study completion. One patient decided to do hemodialysis instead of PD, 1 patient died before surgery, and 1 patient withdrew consent. Battery problems (N = 1) and zeroing errors with the STIC monitor (N = 2) resulted in useful measurements in 10 patients.
Figure 1.
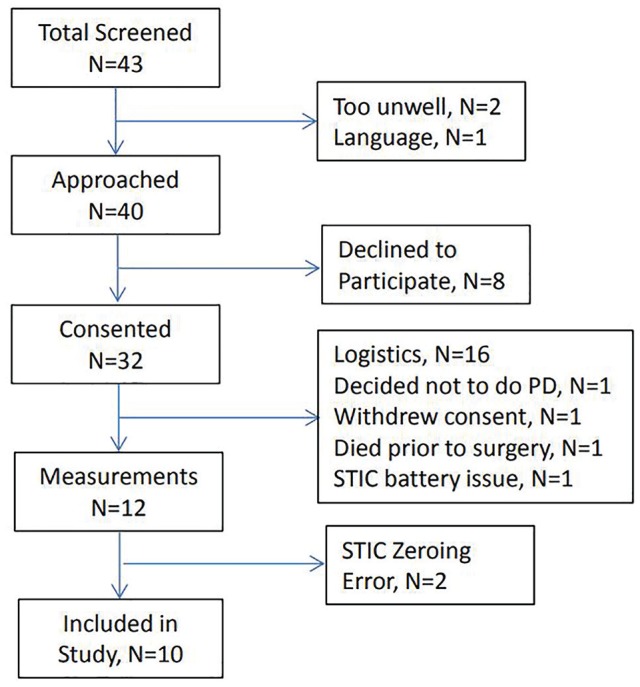
Study flow.
The average age of the included patients was 53 ± 15 years. Participants included 9 men and 3 women with an average height, weight, and body mass index of 1.67 ± 0.08 m, 81.0 ± 13.0 kg, and 28.8 ± 3.9 kg/m2, respectively. The cause of CKD in the patients included IgA nephropathy (4), diabetes (4), polycystic kidney disease (3), and amyloidosis (1) (Table 1). Three of the patients had end-stage kidney disease and were being treated with hemodialysis. The average Charlson comorbidity score was 3.5 ± 2.0.
Table 1.
Participant Characteristics.
| ID | Age | Sex | Height, m | Weight, kg | Body mass index | Comorbidities | Charlson comorbidity score | Dialysis presurgery | Cause of CKD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | M | 1.73 | 96.0 | 32.0 | 12 | 2 | Y | 6 |
| 2 | 43 | M | 1.75 | 83.9 | 27.4 | 9, 12 | 3 | N | 6 |
| 3 | 33 | F | 1.65 | 90.7 | 33.3 | 12 | 2 | N | 4 |
| 4 | 74 | M | 1.68 | 83.0 | 29.4 | 1, 12, 13 | 5 | N | 1 |
| 5 | 66 | M | 1.70 | 88.9 | 30.8 | 1, 2, 3, 13 | 5 | N | 1 |
| 6 | 67 | M | 1.68 | 73.9 | 26.2 | 12, 17 | 5 | Y | 7 |
| 7 | 34 | F | 1.55 | 62.1 | 25.9 | 12 | 2 | N | 6 |
| 8 | 43 | M | 1.69 | 70.0 | 24.5 | 12 | 2 | N | 6 |
| 9 | 46 | F | 1.53 | 78.5 | 33.5 | 12, 13 | 4 | N | 1 |
| 10 | 75 | M | 1.68 | 94.3 | 33.4 | 1, 3, 12, 13, 14 | 8 | N | 1 |
| 11 | 58 | M | 1.60 | 55.3 | 21.6 | 12 | 2 | Y | 4 |
| 12 | 53 | M | 1.85 | 95.7 | 28.0 | 12 | 2 | N | 4 |
Note. CKD = chronic kidney disease; TIA = transient ischemic attack; PCKD = polycystic kidney disease. Comorbidities/Charlson comorbidity scoring: (1 point) 1 = Myocardial infarction, 2 = congestive heart failure, 3 = peripheral vascular disease, 4 = cerebrovascular disease (includes TIA), 5 = dementia, 6 = chronic obstructive pulmonary disease, 7 = connective tissue disease, 8 = peptic ulcer disease, 9 = mild liver disease, 10 = diabetes without end organ damage (2 points), 11 = hemiplegia, 12 = moderate or severe renal disease, 13 = diabetes with end organ damage, 14 = tumor without metastasis (excluded if >5 years), 15 = leukemia, 16 = lymphoma (3 points), 17 = moderate or severe liver disease (6 points), 18 = metastatic liver disease, 19 = AIDS; cause of chronic renal disease: 1 = diabetes mellitus, 2 = ischemic nephropathy, 3 = glomerulonephritis, 4 = PCKD, 5 = obstruction, 6 = other.
IAP Measurement
The average difference and standard deviation between the 2 measurements was −0.5 (2.0), −0.2 (3.1), and −1.2 (2.6) at 15, 10, and 5 mm Hg, respectively. The corresponding 95% limits of agreement for the Bland-Altman plots were 7.9 at 15 mm Hg, 12.2 at 10.0 mm Hg, and 10.3 at 5 mm Hg. The ICCs for reliability of the individual measurements were 0.634 at 15 mm Hg, 0.015 at 10 mm Hg, and 0.212 and at 5 mm Hg (Tables 2 and 3).
Table 2.
Intra-abdominal Pressure Measurements (Insufflator vs Stryker Intracompartmental Monitor).
| ID | Insufflator pressure (15) | Stryker pressure (15) | Insufflator pressure (10) | Stryker pressure (10) | Insufflator pressure (5) | Stryker pressure (5) |
|---|---|---|---|---|---|---|
| 1 | 6 | 5 | 11 | 9 | 14 | 14 |
| 2a | 15 | 24 | 12 | 20 | 7 | 16 |
| 3 | 16 | 15 | 12 | 11 | 6 | 7 |
| 4 | 16 | 18 | 13 | 15 | 8 | 11 |
| 5 | 15 | 13 | 12 | 9 | 7 | 7 |
| 6 | 19 | 21 | 13 | 15 | 8 | 11 |
| 7 | 14 | 14 | 11 | 12 | 6 | 7 |
| 8a | 14 | 11 | 10 | 5 | 5 | 2 |
| 9 | 17 | 19 | 10 | 12 | 5 | 9 |
| 10 | 15 | 17 | 10 | 13 | 5 | 6 |
| 11 | 15 | 18 | 10 | 14 | 5 | 10 |
| 12 | 15 | 12 | 12 | 6 | 6 | 3 |
Zeroing error.
Table 3.
Intraclass Correlation Coefficients for Reliability of the Individual Measurements.
| Pressure | Intraclass correlation coefficients (n = 10) | Intraclass correlation coefficients (n = 12)a |
|---|---|---|
| 15 | .634 | .367 |
| 10 | .015 | .156 |
| 5 | .212 | .230 |
Statistics with the 2 measurements obtained with zeroing errors included.
Discussion
In our study, we compared the IAP readings obtained from the insufflator during surgery to those obtained by the small handheld STIC pressure monitor. The ICC was moderate at the highest test pressure (15 mm Hg) but poor at 10 and 5 mm Hg suggesting that the STIC pressures were not a reliable estimate of the insufflator pressures.
Patients undergoing PD are at risk of developing complications including dialysate fluid leaks, especially around the site of the PD catheter, and/or hernias that may require surgical correction with a temporary or permanent switch to hemodialysis. Most of the hernias develop early after the initiation of the PD catheter raising the possibility of preexisting abdominal wall defects that become obvious with the increase in IAP associated with adding dialysate to the abdomen.9,10 A study by Castellanos et al suggested that patients with an IAP ≥20 cm H2O had more hernias (35% vs 17%) and leakages (21% vs 8%); however, the results were not statistically significant.11 In another study of 142 patients treated with PD more than 5 years, 53 patients developed either a leak or hernia.12 The only independent risk factors for peri-catheter leak and hernias were higher body mass index and polycystic kidney disease, respectively. Higher dialysate volume was not associated with hernias; activities such as lifting that might be associated with an increase in IAP were not assessed. Therefore, although these complications are thought to be related to the increased IAP associated with PD, the evidence remains limited. Despite this, some physicians advise patients not to engage in activities that might increase their IAP such as resistance training which may have potential benefits.1 To clarify this issue, the development of an easy, safe, and accurate method of measuring IAP would facilitate future studies of mechanical complications.
Measuring IAP may also have other benefits in the PD patient population such as determining whether there is any impact on ultrafiltration rates, cardiac filling pressures, or feelings of fullness.13-15 Furthermore, having an easy-to-use method to measure IAP at the bedside might allow for the use of pressure as a therapeutic guide to the PD prescription, as opposed to volume. This might have particular relevance in children.
Despite these potential benefits and the ease of use of the STIC pressure monitor, we were unable to show that the STIC monitor was a reliable way of measuring IAP when compared with the insufflator. However, there were several limitations to our study that should be considered. First, during 2 of the measurement sessions, there were some challenges in properly zeroing the STIC monitor. These patients were excluded from our analysis which decreased our sample size. To protect the sterile surgical field, extension tubing was added to the Stryker monitor that may have affected the measured IAP. In addition, there were some rapid fluctuations in the insufflator pressure recording, creating further challenges in simultaneously recording the 2 measurements at end-expiration. The insufflator may not be a true “gold standard” for comparison as insufflator pressures have been shown to be affected by insufficient patient anesthesia, external pressure on the abdomen, and trocar manipulation.16 We are also unable to comment on IAP during bedside or radiologic insertion of PD catheters as these procedures are not practiced at our center.
Future studies with a larger sample size should be used to explore the possibility of measurement errors in a small number of patients being responsible for our results. We chose to assess the STIC monitor during PD catheter insertion so that a comparator IAP measurement was available. Other opportunities to simultaneous measure IAP with the STIC monitor in chronic PD patients such as during Foley catheter use may be of value.
Conclusions
By Bland-Altman plots and ICCs, the pressure values obtained with the Stryker pressure monitor were not a reliable estimate of insufflator IAP especially at lower pressures. However, there were several limitations to our study, including small sample size and measurement errors that may have contributed to this result. This should be explored in future studies.
Acknowledgments
We thank the staff at the Kidney Research Center at The Ottawa Hospital for their support in conducting this study.
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Ottawa Health Science Network Research Ethics Board (20160377-01H; Ottawa, Ontario, Canada).
Consent for Publication: All co-authors reviewed this final manuscript and consent to its publication.
Availability of Data and Materials: All data collected for this study are available within the written text and tables of this manuscript.
Authors’ Contributions: D.Z. designed the study. T.T. and D.F. identified appropriate patients and obtained consent. H.M. supplied the Stryker monitor and contributed content expertise. T.T., B.B., J.W., and J.L. performed the IAP measurements in the operating room. D.J.C. performed the statistical analysis. T.T., D.Z., and D.J.C. wrote the manuscript which was approved by the other authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: T.T. received a summer studentship for medical students from the University of Ottawa to complete this study. D.Z. receives salary support from the Department of Medicine at The Ottawa Hospital.
ORCID iD: Deborah Zimmerman  https://orcid.org/0000-0003-0000-8806
https://orcid.org/0000-0003-0000-8806
References
- 1. Thangarasa T, Imtiaz R, Hiremath S, Zimmerman D. Physical activity in patients treated with peritoneal dialysis: a systematic review and meta-analysis. Can J Kidney Health Dis. 2018;5:doi: 10.1177/2054358118779821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hemborg B, Moritz U, Hamberg J, Löwing H, Akesson I. Intraabdominal pressure and trunk muscle activity during lifting—effect of abdominal muscle training in healthy subjects. Scand J Rehabil Med. 1983;15(4):183-196. [PubMed] [Google Scholar]
- 3. Iberti TJ, Lieber CE, Benjamin E. Determination of intra-abdominal pressure using a transurethral bladder catheter: clinical validation of the technique. Anesthesiology. 1989;70(1):47-50. doi: 10.1097/00000542-198901000-00011. [DOI] [PubMed] [Google Scholar]
- 4. Risin E, Kessel B, Lieberman N, Schmilovich M, Ashkenazi I, Alfici R. New technique of direct intra-abdominal pressure measurement. Asian J Surg. . 2006;29(4):2472-2450. [DOI] [PubMed] [Google Scholar]
- 5. Brooks AJ, Simpson A, Delbridge M, Beckingham IJ, Girling KJ. Validation of direct intraabdominal pressure measurement using a continuous indwelling compartment pressure monitor. J Trauma. 2005;58(4):83083-83082. [DOI] [PubMed] [Google Scholar]
- 6. Twardowski ZJ, Prowant BF, Nolph KD, Martinez AJ, Lampton LM. High volume, low frequency continuous ambulatory peritoneal dialysis. Kidney Int. 1983;23(1):64-70. [DOI] [PubMed] [Google Scholar]
- 7. Twardowski ZJ, Khanna R, Nolph KD, Scalamogna A, et al. Intraabdominal pressures during natural activities in patients treated with continuous ambulatory peritoneal dialysis. Nephron. 1986;44(2):p129-135. [DOI] [PubMed] [Google Scholar]
- 8. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsang LY, Peng SJ, Ferng SH, Yang CS. Hernia in ESRD patients receiving peritoneal dialysis. Acta Nephrologica. 2002;16:57-61. [Google Scholar]
- 10. Leblanc M, Ouimet D, Pichette V. Dialysate leaks in peritoneal dialysis. Semin Dial. 2001;14(1):50-14. [DOI] [PubMed] [Google Scholar]
- 11. Castellanos LB, Clemente EP, Cabañas CB, et al. Clinical relevance of intraperitoneal pressure in peritoneal dialysis patients. Perit Dial Int. 2017;37(5):562-567. [DOI] [PubMed] [Google Scholar]
- 12. Del Peso GBM, Costero O, Hevia C, et al. Risk factors for abdominal wall complications in peritoneal dialysis patients. Perit Dial Int. 2003;23:249-254. [PubMed] [Google Scholar]
- 13. Paniagua R, Ventura Mde J, Rodríguez E, et al. Impact of fill volume on peritoneal clearances and cytokine appearance in peritoneal dialysis. Perit Dial Int. 2004;24(2):156-162. [PubMed] [Google Scholar]
- 14. Imholz AL, Koomen GC, Struijk DG, Arisz L, Krediet RT. Effect of an increased intraperitoneal pressure on fluid and solute transport during CAPD. Kidney Int. 1993;44(5):1078-1185. [DOI] [PubMed] [Google Scholar]
- 15. Malbrain ML, De Waele JJ, De Keulenaer BL. What every ICU clinician needs to know about the cardiovascular effects caused by abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(4):388-199. [DOI] [PubMed] [Google Scholar]
- 16. Jacobs VR, Morrison JE, Jr, Mundhenke C, Golombeck K, Jonat W. Intraoperative evaluation of laparoscopic insufflation technique for quality control in the OR. JSLS. 2000;4(3):189-195. [PMC free article] [PubMed] [Google Scholar]


