Short abstract
Glässer’s disease, caused by Haemophilus parasuis, is a chronic disease related to an inflammatory immune response. Baicalin exerts important biological functions. In this study, we explored the protective efficacy of treatment with baicalin and the potential mechanism of activation of the MAPK signaling pathway in porcine aortic vascular endothelial cells (PAVECs) induced by H. parasuis. H. parasuis stimulated expression of receptor for advanced glycation end products, induced a significant increase in the level of protein kinase-α and protein kinase-δ phosphorylation, and significantly up-regulated ERK, c-Jun N-terminal kinase, and p38 phosphorylation in PAVECs. H. parasuis also up-regulated the levels of apoptotic genes (Bax, C-myc, and Fasl) and the expression levels of c-Jun and c-Fos, and induced S-phase arrest in PAVECs. However, treatment with baicalin inhibited expression of RAGE, suppressed H. parasuis-induced protein kinase-α and protein kinase-δ phosphorylation, reduced ERK, c-Jun N-terminal kinase, and p38 phosphorylation, down-regulated apoptotic genes (Bax, C-myc, and Fasl), attenuated phospho-c-Jun production from the extracellular to the nuclei, and reversed S-phase arrest in PAVECs. In conclusion, baicalin treatment inhibited the MAPK signaling pathway, thereby achieving its anti-inflammatory responses, which provides a new strategy to control H. parasuis infection.
Keywords: Baicalin, Haemophilus parasuis, apoptosis, vascular endothelial cells, invasion
Introduction
Haemophilus parasuis colonizes in the upper respiratory tract of pigs and is the causative agent of Glässer’s disease.1 The typical characteristics of Glässer’s disease are polyserositis, meningitis, and arthritis.2 So far, at least 15 serovars of H. parasuis have been identified using heat-stable Ag extracts,3 but up to 20% of isolates cannot be serotyped. In China, serovars 4, 5, and 13 were thought to be the most frequently occurring.4 In general, serovars are considered to be virulence markers of H. parasuis.5 Serovar 5 is thought to be highly virulent, resulting in high mortality, and serovar 4 is considered to be moderately virulent in swine.6 Because the pathogenic mechanism of H. parasuis infection is not clear and there is a lack of cross-immunity protection between different serovars, controlling the infection caused by H. parasuis has become more difficult.
Baicalin is the major bioactive compound extracted from the traditional Chinese medicinal herb Baikal skullcap (Scutellaria baicalensis Georgi), known as Huang qin.7 It has been found that baicalin has important biological functions. Baicalin reduces biofilm formation, attenuates the quorum sensing-controlled virulence, and enhances clearance of Pseudomonas aeruginosa from mice.8 Baicalin significantly improves the survival of mice with Escherichia coli–induced sepsis and inhibits activation of NLRP3 inflammasome through augmenting protein kinase (PK) A signaling.9 It has also been shown that baicalin inhibits PKC and receptor for advanced glycation end products (RAGE) expression in streptozotocin-induced diabetic rats.10 Baicalin treatment reduces the high phosphorylation levels of c-Jun N-terminal kinase (JNK), p65, p-38, and ERK1/2 triggered by atherosclerosis.11 These studies suggest that baicalin acts as an anti-inflammatory regulator and inhibits p38 MAPK signaling pathways in Glässer’s disease.
In this study, we focused on activation of the MAPK signaling pathway in porcine aortic vascular endothelial cells (PAVECs) during H. parasuis infection and the inhibitory effect of baicalin on activation of the MAPK signaling pathway induced by H. parasuis. Our results demonstrate that H. parasuis could trigger the activation of MAPK signaling pathway in PAVECs. Baicalin displayed inhibitory effects on activation of the MAPK signaling pathway induced by H. parasuis, which may provide a new target to control H. parasuis infection.
Materials and methods
Ethics approval
This study was performed in strict accordance with the recommendations of the China Regulations for the Administration of Affairs Concerning Experimental Animals 1988 and Hubei Regulations for the Administration of Affairs Concerning Experimental Animals 2005. The protocols were approved by China Hubei Province Science and Technology Department (permit number SYXK(ER) 2010-0029). All experimental animals were euthanized at the end of the experiments. All experiments were approved by Wuhan Polytechnic University guidelines and regulations.
Bacterial strain, growth conditions, and drug
H. parasuis SH0165 strain, a highly virulent strain of serovar 5, was isolated from the lung of a commercial pig with arthritis, fibrinous polyserositis, hemorrhagic pneumonia, and meningitis.12,13 The SH0165 strain was grown in tryptic soy broth (Difco Laboratories, Detroit, MI) or tryptic soy agar (Difco Laboratories) supplemented with 10 μg/ml NAD (Sigma–Aldrich, St. Louis, MO) and 10% newborn calf serum (Gibco, Gaithersburg, MD) at 37°C. Baicalin was obtained from the National Institutes for Food and Drug Control (Beijing, P.R. China; B110715-201318). Baicalin was dissolved and diluted in RPMI-1640 medium (Gibco).
Isolation and culture of PAVECs
Ten 30-d-old naturally farrowed, early-weaned piglets (Duroc×Landrace×large white) weighing 6–8 kg which were detected to be negative for Ab against H. parasuis by INGEZIM Haemophilus 11. H. parasuis. K1 (INGEZIM, Spain), were obtained from Wuhan Jinying Livestock Co. Ltd. (Wuhan, P.R. China) and used for in vitro experiments.
PAVECs were isolated, cultured, and identified according to a previously established method.14 PAVECs were obtained in small sheets after treatment of the aortic lumen (20 min at 37°C) with 0.1% type I collagenase (Sigma–Aldrich) in M-199 medium (Gibco) containing penicillin-streptomycin solution (Gibco). The suspension was centrifuged at 100 g for 10 min, and the cells from one aorta were re-suspended in 5 ml M-199 containing 20% FBS (Gibco), and then plated in a T-25 tissue-culture plate (Costar, Washington, DC). PAVECs were counted, and their viability was determined by Trypan Blue exclusion. PAVECs were identified by the uptake of acetylated low-density lipoprotein (Ac-LDL). PAVECs were incubated with 10 μg/ml 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI)-labeled Ac-LDL (Invitrogen, Carlsbad, CA) in medium for 12 h at 37°C. The cells were washed three times with PBS, detached by trypsinization, and detected by fluorescence microscopy.
Evaluation of expression of RAGE, apoptosis-related genes, and the anti-apoptotic gene by RT-PCR
To explore the expression levels of RAGE, apoptosis-related genes (Bax, C-myc, and Fasl), and the anti-apoptotic gene (Bcl-xl) in the PAVECs infected with H. parasuis, 2 × 107 cells were seeded onto 24-well plates and treated with baicalin at a concentration of 12.5, 25, 50, or 100 μg/ml for 1 h. N-Acetyl-L-cysteine (NAC; 1 mM/ml) was added as a positive control. Afterwards, 2 × 107 CFU/ml H. parasuis was added to the wells and co-cultured for 6 h. The PAVECs were collected, and total RNA was extracted using TRIzol reagent (Invitrogen). The RNA obtained was reverse transcribed to cDNA using reverse transcriptase (TaKaRa, Dalian, P.R. China). cDNA amplification was carried out by the SYBR Green PCR Kit (Applied Biosystems, Foster City, CA) using an ABI 7500 real-time PCR system (Applied Biosystems). Individual transcripts of each sample were repeated three times and β-actin was used as the internal control. Nucleotide sequences of the primers utilized for RT-PCR are listed in Table 1.
Table 1.
Primers for qRT-PCR.
| Gene | Nucleotide sequence (5′-3′) | Temperature (°C) | Length (bp) | |
|---|---|---|---|---|
| β-actin | Forward | TGCGGGACATCAAGGAGAAG | 57.4 | 216 |
| Reverse | AGTTGAAGGTGGTCTCGTGG | 57.4 | ||
| Bax | Forward | GCCGAAATGTTTGCTGACG | 55.2 | 156 |
| Reverse | GAGCCGATCTCGAAGGAAGT | 57.4 | ||
| Fasl | Forward | GCCAGCCAAAGGCATACAGAAT | 57.7 | 335 |
| Reverse | ATCTTTCCCTCCATCAGCACCA | 57.7 | ||
| Bcl-xl | Forward | GCAACCCATCCTGGCACCT | 59.5 | 136 |
| Reverse | TCAAACTCATCGCCCGCCT | 57.3 | ||
| C-myc | Forward | GGTCTTCCCCTACCCACT | 57.2 | 200 |
| Reverse | CCTCATCCTCTTGTTCTTCC | 55.4 | ||
| Rage | Forward | ATCCTGCCTCTGAACTCA | 52.6 | 159 |
| Reverse | GGTGTCTCCTGGTCTCTT | 54.9 | ||
| c-Fos | Forward | GCTGACAGATACACTCCAAGCGG | 61.3 | 542 |
| Reverse | AGGAAGACGTGTAAGTAGTGCAG | 57.8 | ||
| c-Jun | Forward | CGCCAGTCTACGCTAATC | 55.1 | 288 |
| Reverse | GGTTCCTCATACGCTTCC | 54.8 | ||
Western blotting
PAVECs (2 × 107) pre-treated with baicalin at a concentration of 12.5, 25, 50, or 100 μg/ml for 2 h were co-cultured with 2.0 × 107 CFU/ml H. parasuis for 12 h. PAVECs proteins were extracted using a total protein extraction kit (Beyotime Biotechnology, Shanghai, P.R. China). Total proteins were isolated by 12% SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were blocked with 5% skimmed milk at 37°C for 2 h and washed five times with TBST. The PVDF membranes were incubated with respective Ab, or anti-β-actin Ab (Cell Signaling Technology, Danvers, MA) for 8 h at 4°C. The PVDF membranes were washed five times with TBST and incubated with HRP-linked goat anti-rabbit Ab (Cell Signaling Technology) at 37°C for 2 h and visualized using enhanced chemiluminescence solution (Pierce; Thermo Fisher Scientific, Waltham, MA). The levels of the proteins and β-actin were examined using the FluorChemFC2 AIC system (Alpha Innotech, San Leandro, CA).
Localization of c-Jun with indirect immunofluorescence
The subcellular localization of c-Jun was explored in the PAVECs. PAVECs (2 × 107) were seeded onto 24-well plates and treated with baicalin at a concentration of 12.5, 25, 50, or 100 μg/ml or NAC (1 mM/ml) for 2 h, and 2 × 107 CFU/ml H. parasuis were co-incubated with the PAVECs for 12 h. The cells were fixed with 4% paraformaldehyde for 1 h at 37°C and permeabilized with 0.1% Triton X-100 for 30 min. The PAVECs were incubated with anti-rabbit c-Jun (60A8) rabbit mAb (Cell Signaling Technology) or Phospho-c-Jun (Ser73) (D47G9) XP® Rabbit mAb (Cell Signaling Technology) for 1 h. PAVECs were incubated with Cy3-labeled goat anti-rabbit IgG (H+L; Boster, Wuhan, P.R. China). 4′,6′-diamidino-2-phenylindole (DAPI; 1 μg/ml; Beyotime) was co-cultured with the PAVECs for 30 min. The subcellular localization of c-Jun and phosphor (p)-c-Jun was visualized using a Nikon C2 confocal laser-scanning microscope (Nikon, Tokyo, Japan).
Cell-cycle analysis using flow cytometry
The effects of baicalin on the cell cycle of PAVECs infected by H. parasuis were determined as described previously, with some modifications.15 PAVECs (2 × 107) were seeded onto 24-well plates and treated with baicalin at a concentration of 12.5, 25, 50, or 100 μg/ml for 2 h. H. parasuis (2 × 107 CFU/ml) was added to the plates and co-incubated for 12 h. Cells were washed five times with sterile PBS and stained with PI/RNase Staining Buffer (BD, USA), and the cell cycle was detected by flow cytometry (FC500; Beckman Coulter, USA).
Detection of the effect of baicalin on the interaction between PAVECs and H. parasuis by transmission electron microscopy
The effect of baicalin on the interaction between PAVECs and H. parasuis was examined by transmission electron microscopy (TEM) as described previously, with minor modifications.16 PAVECs (2 × 107) were seeded onto 24-well plates and treated with baicalin at a concentration of 12.5, 25, 50, or 100 μg/ml for 2 h prior to bacterial infection. H. parasuis (2 × 107 CFU/ml) was added to the plates and co-incubated for 12 h. The infected cells were gently washed five times with PBS. PAVECs were fixed with 0.1 M cacodylate buffer (pH 7) including 5% glutaraldehyde and 0.15% ruthenium red at 37°C for 5 h. PAVECs were reacted with polycationic ferritin (1 mg/ml). The thin sections were examined by a Tecnai G2 20 TWIN transmission electron microscope (FEI, Hillsboro, OR).
Statistical analysis
The experimental data are expressed as the mean±SD. The difference among two groups was analyzed using Student’s t-test. P < 0.05 was considered significant.
Results
Baicalin inhibited expression of RAGE in PAVECs triggered by H. parasuis
To determine expression of RAGE, the PAVECs were infected with H. parasuis for 6 h, and RAGE was detected by RT-PCR. H. parasuis stimulated expression of RAGE compared to the control cells (Figure 1). PAVECs were treated with baicalin for 2 h, and mRNA was isolated. NAC significantly suppressed RAGE expression in PAVECs infected with H. parasuis (P < 0.01; Figure 1). Also, 12.5–100 μg/ml baicalin decreased expression of RAGE mRNA in a dose-dependent manner (P < 0.01; Figure 1).
Figure 1.
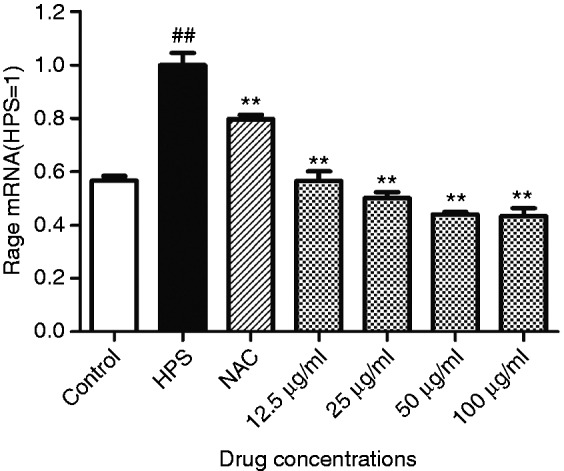
Effect of baicalin on expression of receptor for advanced glycation end products in porcine aortic vascular endothelial cells (PAVECs) infected with Haemophilus parasuis. HPS: H. parasuis. ##P<0.01 versus control; **P < 0.01.
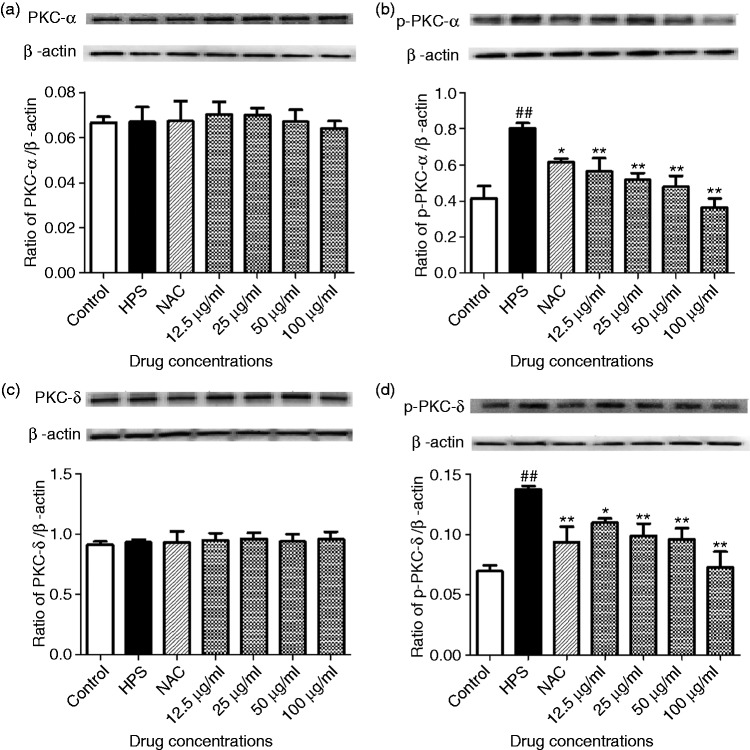
Baicalin suppressed H. parasuis-infected PAVECs PKC-α and PKC-δ phosphorylation
Rage mediates activation of PKC-α and PKC-δ.17 To determine whether PAVECs can respond to RAGE, PAVECs were examined for PKC-α and PKC-δ phosphorylation in response to H. parasuis or baicalin treatment. H. parasuis induced a significant increase in phosphorylation of PKC-α and PKC-δ (P < 0.01; Figure 2b and d). Baicalin treatment at 12.5–100 μg/ml significantly reversed phosphorylation of PKC-α and PKC-δ of PAVECs in a dose-dependent manner (P < 0.05; Figure 2b and d).
Figure 2.
Effect of baicalin on phosphorylation of protein kinase (PKC)-α and PKC-δ of PAVECs. (a) the ratio of PKC-α/β-actin; (b) the ratio of p-PKC-α/β-actin; (c) the ratio of PKC-δ/β-actin; (d) the ratio of p-PKC-δ/β-actin. ##P < 0.01 versus control; *P < 0.05; **P<0.01.
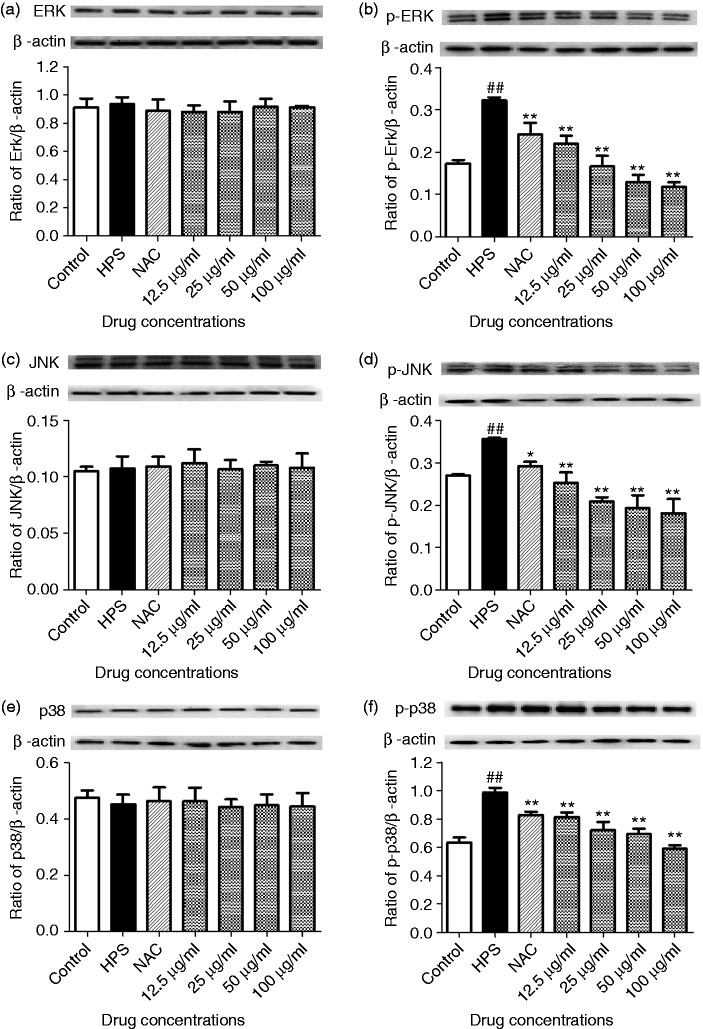
Effect of baicalin on phospho-ERK, -JNK, -p38, and total-ERK, -JNK, -p38 expression in PAVECs
The MAPK signaling pathway plays an important role in vascular damage.18 Therefore, we examined the effect of H. parasuis on activation of the MAPK signaling pathway in PAVECs. The PAVECs were infected with H. parasuis, and phosphorylation of MAPK was measured using phospho-specific Abs. H. parasuis significantly up-regulated ERK, JNK, and p38 phosphorylation in PAVECs (P < 0.01; Figure 3b, d and f). In contrast, ERK, JNK, and p38 phosphorylation in PAVECs triggered by H. parasuis was suppressed by NAC (P < 0.05; Figure 3b, d, and f). Baicalin treatment at a concentration of 12.5–100 μg/ml reduced ERK, JNK, and p38 phosphorylation in PAVECs induced by H. parasuis (P < 0.01) in a dose-dependent manner (Figure 3b, d, and f).
Figure 3.
Effect of baicalin on phospho-ERK, -JNK, and -p38, and total-ERK, -JNK. and -p38 expression in PAVECs by Western blotting. (a) the ratio of Erk/β-actin; (b) the ratio of p-Erk/β-actin; (c) the ratio of JNK/β-actin; (d) the ratio of p-JNK/β-actin; (e) the ratio of p38/β-actin; (f) the ratio of p-p38/β-actin. ##P < 0.01 versus control; *P < 0.05; **P < 0.01.
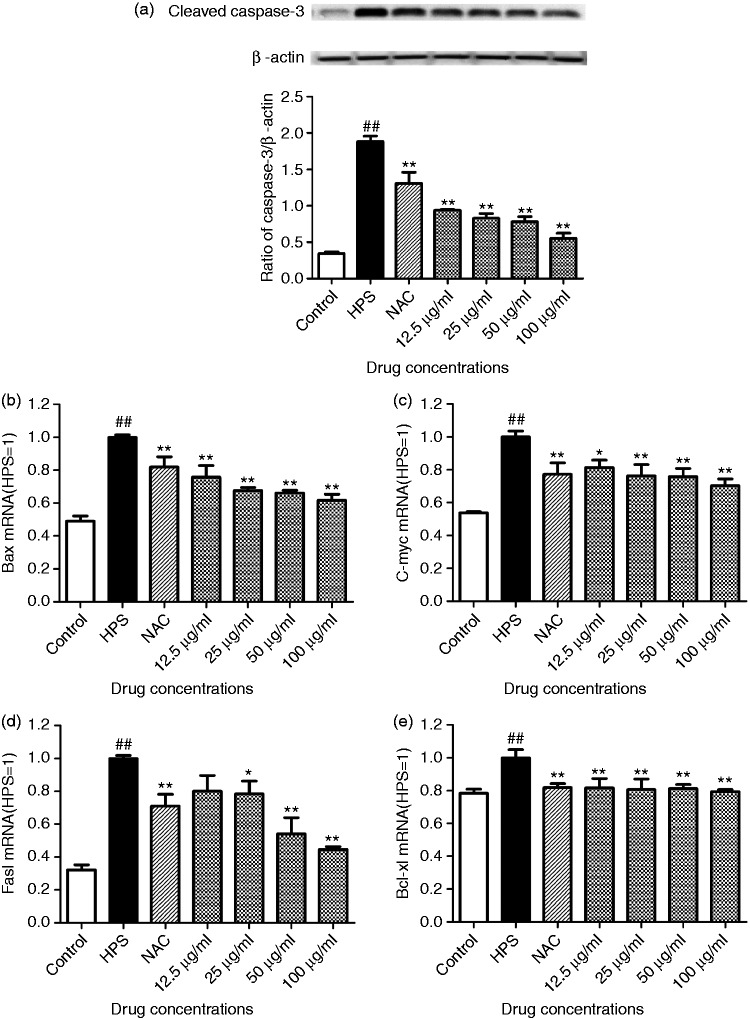
Effect of baicalin on expression of mitochondrial apoptosis-related genes in PAVECs triggered by H. parasuis
Apoptosis is mediated by activation of the caspase cascades. To determine the role of caspase-3 in H. parasuis-induced apoptosis in PAVECs, we examined the level of activated caspase-3 using Western blotting. Higher activity of cleaved caspase-3 in PAVECs was activated by H. parasuis, and the activity of cleaved caspase-3 was significantly inhibited by baicalin at a concentration of 12.5–100 μg/ml (P < 0.01; Figure 4a). To study the molecular mechanism of mitochondria-dependent apoptosis triggered by H. parasuis further, we examined expression of apoptotic genes (Bax, C-myc, and Fasl) and the anti-apoptotic gene (Bcl-xl). H. parasuis up-regulated the levels of apoptotic genes (Bax, C-myc, and Fasl) compared to the controls, and baicalin at a concentration of 25–100 μg/ml down-regulated the apoptotic genes (Bax, C-myc, and, Fasl; P < 0.05; Figure 4b, c, and d). H. parasuis promoted expression of the anti-apoptotic gene (Bcl-xl) compared to the controls (P < 0.01; Figure 4e).
Figure 4.
Effect of baicalin on expression of mitochondrial apoptosis-related genes in PAVECs triggered by H. parasuis. After PAVECs were treated with baicalin and infected with H. parasuis, expression of mitochondrial apoptosis-related genes (Bax, C-myc, Fasl, and Bcl-xl) and apoptosis-related protein (cleaved caspase-3) was measured. β-Actin was used as reference. (a) the ratio of caspase-3/β-actin; (b) the expression of Bax at mRNA level; (c) the expression of C-myc at mRNA level; (d) the expression of Fasl at mRNA level; (e) the expression of Bcl-xl at mRNA level. ##P < 0.01 versus control; *P < 0.05; **P < 0.01.
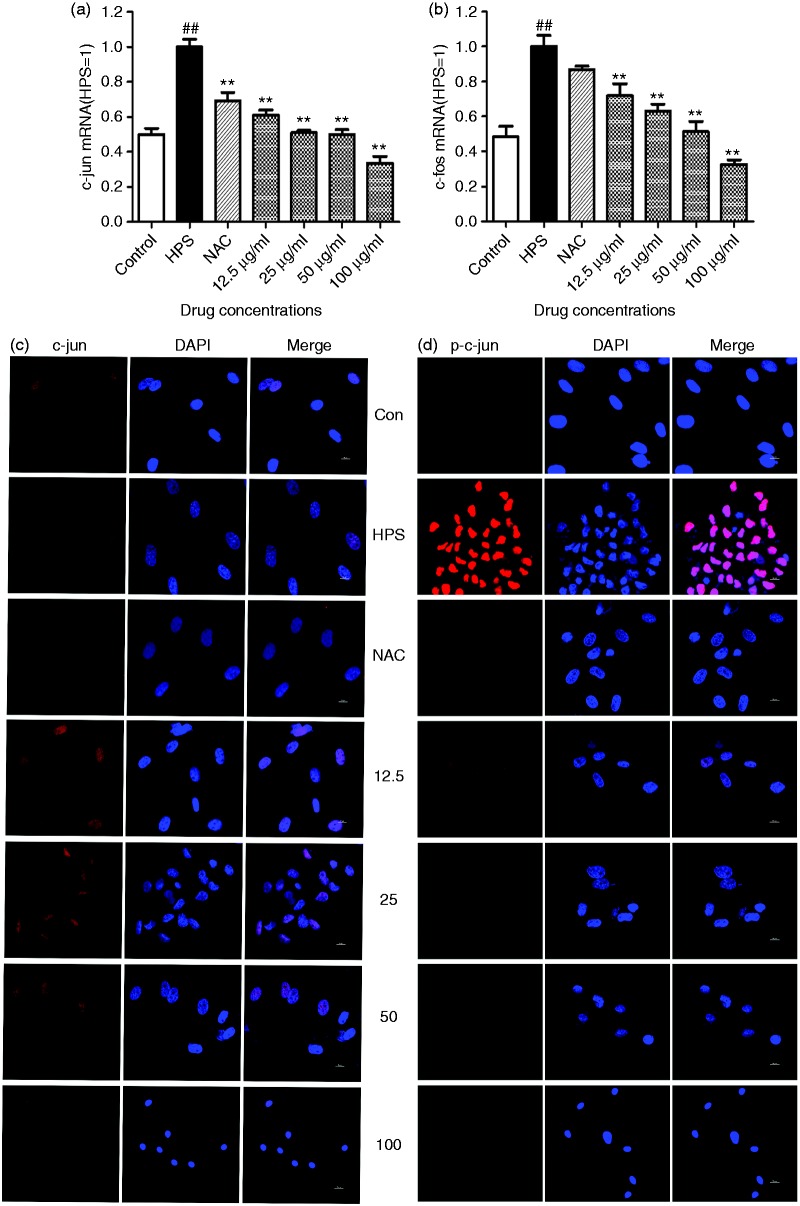
Effect of baicalin on activation of transcription factor activator protein-1 in PAVECs induced by H. parasuis
After the PAVECs were stimulated by H. parasuis, the activator protein (AP)-1 transcription factors c-Jun and c-Fos were determined by RT-PCR. H. parasuis significantly up-regulated expression of c-Jun and c-Fos mRNA compared to the controls (P < 0.01; Figure 5a and b). We also detected the subcellular localization of c-Jun in PAVECs triggered by H. parasuis. Cytoplasmic-to-nuclear translocation of p-c-Jun was detected in the H. parasuis-infected cells, while p-c-Jun was rarely observed in the cytoplasm of NAC-treated cells (Figure 5d; P < 0.05). A high level of production of p-c-Jun was observed in the nuclei of the cells treated with 12.5 μg/ml baicalin. However, 25–100 μg/ml baicalin significantly inhibited p-c-Jun production from the extracellular to the nuclei (Figure 5d; P < 0.05).
Figure 5.
Effect of baicalin on expression of c-Jun and c-Fos in PAVECs triggered by H. parasuis (a and b); and localization of c-Jun by indirect immunofluorescence (c and d). ##P < 0.01 versus control; **P < 0.01.
Effect of baicalin on the cell cycle in PAVECs triggered by H. parasuis
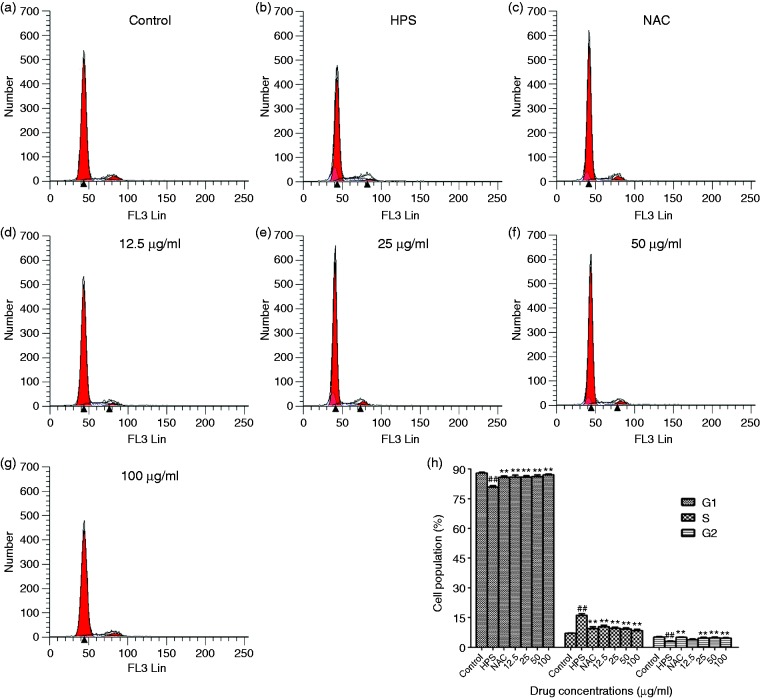
PAVECs were infected by H. parasuis and stained with PI/RNase Staining Buffer, and the cell cycle was measured by flow cytometry. H. parasuis induced S-phase arrest in PAVECs stimulated by H. parasuis compared to the control cells (Figure 6; P < 0.01). We also evaluated the effect of baicalin on cell-cycle distribution in PAVECs. The positive control, NAC, reduced S-phase arrest in the PAVECs (Figure 6; P < 0.01). Baicalin at a concentration of 25–100 μg/ml significantly reversed the S-phase arrest in PAVECs induced by H. parasuis (Figure 6; P < 0.01).
Figure 6.
Effect of baicalin on cell cycle of PAVECs by flow cytometric analysis. (a) control cells; (b) cells infected with H. parasuis; (c–g) cells treated with NAC or baicalin; (h) the effect of baicalin on the cell population of PAVECs. ##P < 0.01 versus control; **P < 0.01.
Effect of baicalin on interaction between PAVECs and H. parasuis
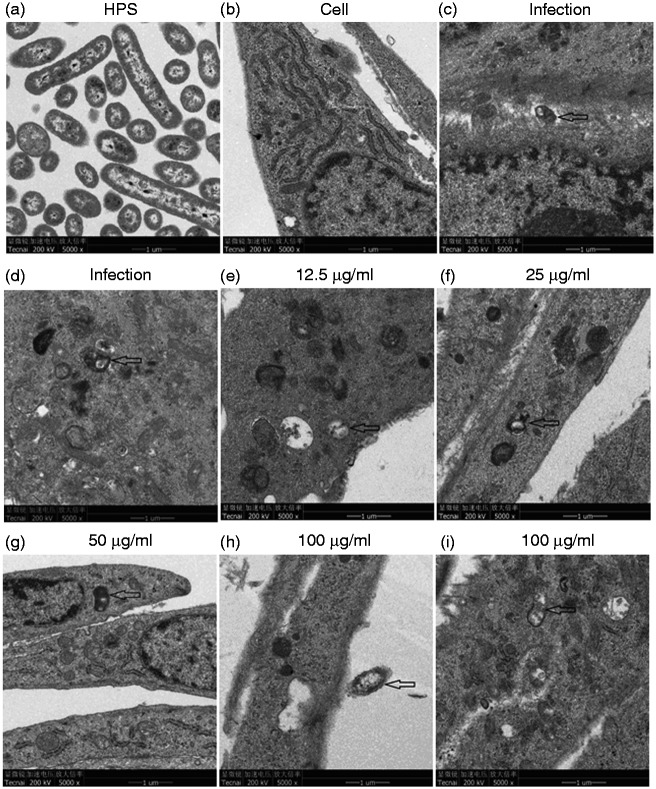
TEM revealed morphological alterations in bacterium–cell interactions. There was a direct interaction between H. parasuis and PAVECs, and adhesion to or invasion of PAVECs elicited the morphological alterations (Figure 7c and d). Baicalin at a concentration of 12.5–100 μg/ml improved morphological damage to PAVECs by H. parasuis, although the bacteria were present in the cells (Figure 7e, f, g, and i) or adhension to the cells (Figure 7h). Lysosomes and mitochondria increased significantly after PAVECs were treated with baicalin at a concentration of 12.5–100 μg/ml (Figure 7e–i).
Figure 7.
Effect of baicalin on the interaction between PAVECs and H. parasuis by transmission electron microscopy. (a) H. parasuis (HPS). (b) PAVECs. (c and d) H. parasuis adhesion to (c) or invasion of (d) PAVECs. (e–i) PAVECs were treated with different concentrations of baicalin. Arrows show the presence of bacteria.
Discussion
Previous research has shown that bacteria adhere to target cells as the first essential event in infection.19,20 If attachment is established, the bacteria may utilize their potential to build a niche in order to be conducive to replication, colonization, and survival.21,22 Then the cell-cycle alternation, apoptosis, could be observed in order to obtain persistent colonization during infection process.23 In the present study, we showed that H. parasuis adhered to or invaded PAVECs, resulting in cell-cycle arrest, apoptosis, and MAPK signaling pathway activation.
RAGE belongs to the immunoglobulin superfamily of cell surface molecules and is considered to be a membrane receptor.24 It has been recorded that activation of RAGE has important effects on the inflammation immune and microorganism infection process. Helicobacter pylori stimulates expression of RAGE in gastric biopsy specimens, which is thought to be related to cancer-mediated inflammation.25 RAGE expression affects the antiviral immunity during paucigranulocytic asthma in early-life infection with respiratory syncytial virus in mice.26 RAGE deficiency impairs clearance of Staphylococcus aureus in sepsis in mice.27 In addition, RAGE activation alters inflammation and bacterial clearance in a murine model of pneumonia caused by Acinetobacter baumannii.28 In this study, we showed that H. parasuis activated expression of RAGE in PAVECs and baicalin reduced RAGE expression, but the mechanism of action of RAGE resulting in inflammation, and further study is needed to determine how baicalin reduces RAGE expression.
It has been reported that activation of inflammatory signaling pathways, including ERK, JNK, and p38, induces secretion of cytokines.29 We showed that H. parasuis activated the inflammatory signaling molecules ERK, JNK, and p38 in PAVECs. Our previous work demonstrated that H. parasuis stimulates the production of IL-6, IL-8, IL-10, and TNF-α.14 These molecules may serve as anti-H. parasuis immune factors.12,30 We also showed that baicalin inhibited ERK, JNK, and p38 phosphorylation. It is suggested that baicalin activates the Th1-induced immune response, which results in the promotion of bacterial clearance.8 Baicalin attenuates the Th17 immune response and reduces silica-induced inflammation and fibrosis.31 Consistent with our previous study, baicalin also reduces the levels of IL-1β, IL-8, and TNF-α in LPS-induced mesenchymal stem cells, and the inflammation response in the mesenchymal stem cells through the MAPK/ERK pathway.32 Baicalin treatment inhibits acetaminophen-induced liver inflammation through down-regulating the ERK signaling pathway.33 These data suggest that baicalin regulates the inflammatory immune response to resist infection and improve treatment.
Endothelial cells are important components of blood vessels. It has been documented that endothelial cells play important roles in regulating inflammation during the inflammatory immune response to bacteria.34 So, we hypothesized that endothelial cells might be key effectors of the inflammatory immune response to bacteria that results in vascular damage during infection. However, PAVECs have not been considered to have important regulatory effects on the inflammatory immune response. Details are limited about how PAVECs are regulated during the inflammatory immune response to H. parasuis and what the functions of cell death are in this process. Our previous studies showed that baicalin could inhibit the activation of NF-κB and NLRP3 inflammasome signaling in PAVECs and piglet monocytes induced by H. parasuis.12,14 Baicalin could also attenuate the activation of PKC-MAPK signaling pathways in piglet monocytes triggered by H. parasuis.35 However, whether baicalin could modulate apoptosis via RAGE, MAPK, and AP-1 in PAVECs during H. parasuis invasion has not been investigated. Previous research found apoptosis in monocytes during Streptococcus pneumoniae infection,36 macrophages infected with Mycobacterium bovis37,38 or Neisseria gonorrhoeae,39 and HeLa cells infected by E. coli.40 Whether PAVECs undergo apoptosis during H. parasuis infection remains unclear. Therefore, we explored whether there was an interaction between PAVECs and H. parasuis during cell death. We showed that cleaved caspase-3 in PAVECs was activated during H. parasuis infection. We further investigated the expression level of the apoptotic genes Bax, C-myc, and Fasl and found that it was up-regulated. The level of the anti-apoptotic gene Bcl-xl was also up-regulated, although the mechanism needs further investigation in our next study.
In the present study, we demonstrated that H. parasuis infection of PAVECs led to alteration of the cell cycle. SH0165, an isolated strain, was used to infect PAVECs. The primary cells were used based on its significant relevance to the monic environment of natural infection of H. parasuis.14 Previous research has shown that bacterial infection can result in cell-cycle modulation, which may be related to pathogenesis.41 Legionella pneumophila challenge of Hela cells in the S phase induces Icm/Dot-dependent cell-cycle arrest.42 Mycobacterium tuberculosis can modulate the immune system through altering host cell-cycle arrest at the G1/S transition to promote long-term persistent infection.43 Neisseria meningitidis can cause G1 cell-cycle arrest in human epithelial cells and Detroit 562 and NP69 cells.44 To our knowledge, the present study is the first report that H. parasuis interferes with cell-cycle regulation in PAVECs.
In conclusion, these findings suggested that H. parasuis induces MAPK signaling pathway activation and baicalin inhibits the MAPK signaling pathway via regulation of the inflammatory immune response. Our study may provide a potential host defense mechanism against H. parasuis, and baicalin could be a therapeutic option in the management of H. parasuis infection.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant Nos. 31572572 and 31601922) and the Natural Science Foundation of Hubei Province, P.R. China (Grant No. 2017CFB446).
References
- 1.Riley MG, Russell EG, Callinan RB. Haemophilus parasuis infection in swine. J Am Vet Med Assoc 1977; 171: 649–651. [PubMed] [Google Scholar]
- 2.Nielsen R. Pathogenicity and immunity studies of Haemophilus parasuis serotypes. Acta Vet Scand 1993; 34: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kielstein P, Rapp-Gabrielson VJ. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol 1992; 30: 862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai X, Chen H, Blackall PJ, et al. Serological characterization of Haemophilus parasuis isolates from China. Vet Microbiol 2005; 111: 231–236. [DOI] [PubMed] [Google Scholar]
- 5.Rosner H, Kielstein P, Müller H, et al. [Relationship between serotype, virulence and SDS-PAGE protein patterns of Haemophilus parasuis.] Dtsch Tierarztl Wochenschr 1991; 98: 327–330. [PubMed] [Google Scholar]
- 6.Amano H, Shibata M, Kajio N, et al. Pathogenicity of Haemophilus parasuis serovars 4 and 5 in contact-exposed pigs. J Vet Med Sci 1996; 58: 559–561. [DOI] [PubMed] [Google Scholar]
- 7.Perez CA, Wei Y, Guo M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J Inorg Biochem 2009; 103: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, Dong B, Wang K, et al. Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model. PLoS One 2017; 12: e0176883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CG, Yan L, Mai FY, et al. Baicalin inhibits NOD-like receptor family, pyrin containing domain 3 inflammasome activation in murine macrophages by augmenting protein kinase A signaling. Front Immunol 2017; 8: 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh J, Chaudhari BP, Kakkar P, et al. Baicalin and chrysin mixture imparts cyto-protection against methylglyoxal induced cytotoxicity and diabetic tubular injury by modulating RAGE, oxidative stress and inflammation. Environ Toxicol Pharmacol 2017; 50: 67–75. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Wang F, Fan L, et al. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed Pharmacother 2018; 97: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 12.Fu S, Xu L, Li S, et al. Baicalin suppresses NLRP3 inflammasome and nuclear factor-kappa B (NF-κB) signaling during Haemophilus parasuis infection. Vet Res 2016; 47: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L, Xu L, Wu T, et al. Evaluation of recombinant protein superoxide dismutase of Haemophilus parasuis strain SH0165 as vaccine candidate in a mouse model. Can J Microbiol 2017; 63: 312–320. [DOI] [PubMed] [Google Scholar]
- 14.Fu S, Liu H, Xu L, et al. Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells Infected by Haemophilus parasuis Causing Glässer’s disease. Sci Rep 2018; 8: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie W, Zhang Z, Song L, et al. Cordyceps militaris fraction induces apoptosis and G2/M arrest via c-Jun N-terminal kinase signaling pathway in oral squamous carcinoma KB cells. Pharmacogn Mag 2018; 14: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peixoto RS, Pereira GA, Sanches Dos Santos L, et al. Invasion of endothelial cells and arthritogenic potential of endocarditis-associated Corynebacterium diphtheriae. Microbiology 2014; 160: 537–546. [DOI] [PubMed] [Google Scholar]
- 17.Kay AM, Simpson CL, Stewart JA, et al. The role of AGE/RAGE signaling in diabetes-mediated vascular calcification. J Diabetes Res 2016; 680973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Nigris F, Rienzo M, Sessa M, et al. Glycoxydation promotes vascular damage via MAPK-ERK/JNK pathways. J Cell Physiol 2012; 227: 3639–3647. [DOI] [PubMed] [Google Scholar]
- 19.Login FH, Jensen HH, Pedersen GA, et al. The soluble extracellular domain of E-cadherin interferes with EPEC adherence via interaction with the Tir:intimin complex. FASEB J. Epub ahead of print 19 June 2018. DOI: 10.1096/fj.201800651. [DOI] [PubMed]
- 20.Sudaryatma PE, Nakamura PE, Mekata H, et al. Bovine respiratory syncytial virus infection enhances Pasteurella multocida adherence on respiratory epithelial cells. Vet Microbiol 2018; 220: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson CJ, Satkovich J, Köseoğlu VK, et al. The ethanolamine permease EutH promotes vacuole adaptation of Salmonella enterica and Listeria monocytogenes during macrophage infection. Infect Immun 2018; 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donaldson GP, Ladinsky MS, Yu KB, et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018; 360: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox AC, McConnell KW, Yoseph BP, et al. The endogenous bacteria alter gut epithelial apoptosis and decrease mortality following Pseudomonas aeruginosa pneumonia. Shock 2012; 38: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langer T, Corvey C, Kroll K, et al. Expression and purification of the extracellular domains of human glycoprotein VI (GPVI) and the receptor for advanced glycation end products (RAGE) from Rattus norvegicus in Leishmania tarentolae. Prep Biochem Biotechnol 2017; 47: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 25.Morales ME, Rojas RA, Monasterio AV, et al. [ Expression of RAGE in Helicobacter pylori infested gastric biopsies]. Rev Med Chil 2013; 141: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 26.Arikkatt J, Ullah MA, Short KR, et al. RAGE deficiency predisposes mice to virus-induced paucigranulocytic asthma. Elife 2017; 6: e21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammad M, Na M, Welin A, et al. RAGE deficiency impairs bacterial clearance in murine staphylococcal sepsis, but has so significant impact on staphylococcal septic arthritis. PLoS One 2016; 11: e0167287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noto MJ, Becker KW, Boyd KL, et al. RAGE-mediated suppression of interleukin-10 results in enhanced mortality in a murine model of Acinetobacter baumannii sepsis. Infect Immun 2017; 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W, Hu S, Du X, et al. Vitamin B5 reduces bacterial growth via regulating innate immunity and adaptive immunity in mice infected with Mycobacterium tuberculosis. Front Immunol 2018; 9: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng Z, Chen X, Yue H, et al. The effect of rfaD and rfaF of Haemophilus parasuis on lipooligosaccharide induced inflammation by NF-κB/MAPKs signaling in porcine alveolar macrophages. J Vet Med Sci 2018; 80: 842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T, Dai W, Li C, et al. Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J Nat Prod 2015; 78: 3049–3057. [DOI] [PubMed] [Google Scholar]
- 32.Zhu L, Liu Y-J, Shen H, et al. Astragalus and baicalein regulate inflammation of mesenchymal stem cells (MSCs) by the mitogen-activated protein kinase (MAPK)/ERK pathway. Med Sci Monit 2017; 23: 3209–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao CC, Day YJ, Lee HC, et al. ERK signaling pathway plays a key role in baicalin protection against acetaminophen-induced liver injury. Am J Chin Med 2017; 45: 105–121. [DOI] [PubMed] [Google Scholar]
- 34.Harding M, Kubes P. Innate immunity in the vasculature: interactions with pathogenic bacteria. Curr Opin Microbiol 2012; 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 35.Ye C, Li R, Xu L, et al. Effects of baicalin on piglet monocytes involving PKC-MAPK signaling pathways induced by Haemophilus parasuis. BMC Vet Res 2019; 15: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daigneault M, De Silva TI, Bewley MA, et al. Monocytes regulate the mechanism of T-cell death by inducing Fas-mediated apoptosis during bacterial infection. PLoS Pathog 2012; 8: e1002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benítez-Guzmán A, Arriaga-Pizano L, Morán J, et al. Endonuclease G takes part in AIF-mediated caspase-independent apoptosis in Mycobacterium bovis-infected bovine macrophages. Vet Res 2018; 49: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang S, Song Z, Wu Y, et al. MicroRNA-27b modulates inflammatory response and apoptosis during Mycobacterium tuberculosis infection. J Immunol 2018; 200: 3506–3518. [DOI] [PubMed] [Google Scholar]
- 39.Deo P, Chow SH, Hay ID, et al. Outer membrane vesicles from Neisseria gonorrhoeae target PorB to mitochondria and induce apoptosis. PLoS Pathog 2018; 14: e1006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Hao L, Liu X, et al. Enhanced anti-proliferative efficacy of epothilone B loaded with Escherichia coli Nissle 1917 bacterial ghosts on the HeLa cells by mitochondrial pathway of apoptosis. Drug Dev Ind Pharm 2018; 44: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 41.Priya A, Kaur K, Bhattacharyya S, et al. Cell cycle arrest and apoptosis induced by enteroaggregative Escherichia coli in cultured human intestinal epithelial cells. J Med Microbiol 2017; 66:217–225. [DOI] [PubMed] [Google Scholar]
- 42.De Jesús-Díaz DA, Murphy C, Sol A, et al. Host cell S phase restricts Legionella pneumophila intracellular replication by destabilizing the membrane-bound replication compartment. MBio 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumming BM, Rahman MA, Lamprecht DA, et al. Correction: Mycobacterium tuberculosis arrests host cycle at the G1/S transition to establish long term infection. PLoS Pathog 2017; 13: e1006490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Von Papen M, et al. Disease and carrier isolates of Neisseria meningitidis cause G1 cell cycle arrest in human epithelial cells. Infect Immun 2016; 84: 2758–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]