Abstract
We present the case of an 18-year-old woman with B-cell acute lymphoblastic leukemia (ALL) who developed hemorrhagic stroke and epilepsia partialis continua due to acute cerebral vein thrombosis (CVT). The patient had 10 risk factors for CVT (including use of asparaginase chemotherapy for the ALL) and also unfortunately had 4 biomarkers for poor prognosis for outcome post-CVT diagnosis. Immediate transfer to a Comprehensive Stroke Center allowed for hyperacute neurointerventional clot extraction with rapid restoration of the patency of the superior sagittal sinus. This resulted in an unexpectedly favorable neurological outcome and simultaneously allowed for early resumption of chemotherapy for ALL after only a 5-day hiatus. Our case highlights the importance of immediate transfer of highest risk patients with multiple biomarkers for poor prognosis to a Comprehensive Stroke Center with endovascular and neurosurgical capabilities and the possibility of overcoming the odds of a poor outcome with venous clot extraction if medical management fails. Neurological deterioration due to escalating intracranial pressure with impending herniation may occur rapidly, and treatment at such facilities can be life-saving.
Keywords: cerebral vein thrombosis, cancer and stroke, endovascular intervention, thrombectomy, transfer
Introduction
Thrombosis of the cerebral veins and sinuses is a distinct cerebrovascular disorder with a predilection for young adults and children, which, in high-risk cases, can lead to hemorrhagic strokes and intracranial hypertension.1,2 The pathogenesis of cerebral vein thrombosis (CVT) is different from ischemic stroke and intraparenchymal hemorrhage (IPH) because of its dual-mechanistic pathophysiology: First, occlusion of the local cerebral veins leads to localized vasogenic edema and the so-called “venous infarction,” and second, thrombosis of the major cerebral sinuses provokes intracranial hypertension due to blockage of the cerebrospinal fluid outflow tract typically draining from the cerebral ventricles through the subarachnoid spaces to the arachnoid villi and then into the superior sagittal sinus (SSS).1 During an acute CVT, 2 forms of cerebral edema exist concurrently: vasogenic edema, due to increased permeability of the blood–brain barrier with extravasation of proteins, electrolytes, and water into the extracellular space, and cytotoxic edema, due to local ischemia with failure of the energy-dependent ion exchange at the cell membrane that leads to intracellular swelling, which can be seen as diffusion restriction on magnetic resonance imaging (MRI).1,3
The occurrence of CVT in patients undergoing asparaginase chemotherapy for acute lymphoblastic leukemia (ALL) is a well-established complication that can cause significant morbidity and neurological sequela.4,5 Here, we report the case of an 18-year-old woman with ALL undergoing induction chemotherapy at a Comprehensive Cancer Center who developed an extensive CVT yet had an unexpectedly favorable neurological outcome despite the 4 biomarkers for poor prognosis for outcome post-CVT diagnosis. This fortunate outcome occurred after immediate transfer to a Comprehensive Stroke Center for emergent neurointerventional clot extraction.
Case
An 18-year-old woman with newly diagnosed B-cell ALL, on day 12 of induction chemotherapy per CALBG protocol (vincristine, daunorubicin, cytarabine, PEG-asparaginase, prednisone and intrathecal methotrexate), woke up at 3:50 am in the Moffitt Comprehensive Cancer Center in Tampa, Florida, with a severe frontal headache and weakness and numbness in the right arm and leg. She was a previously healthy nullipara who had recently endured neutropenic fever and sepsis with pneumonia and urinary tract infection. She was taking a third-generation oral contraceptive (OCP), ethinyl estradiol/levonorgestrel, for intermenstrual bleeding, and was on deep venous thrombosis (DVT) prophylaxis with dalteparin 5000 units daily. She developed pancytopenia from asparaginase chemotherapy.
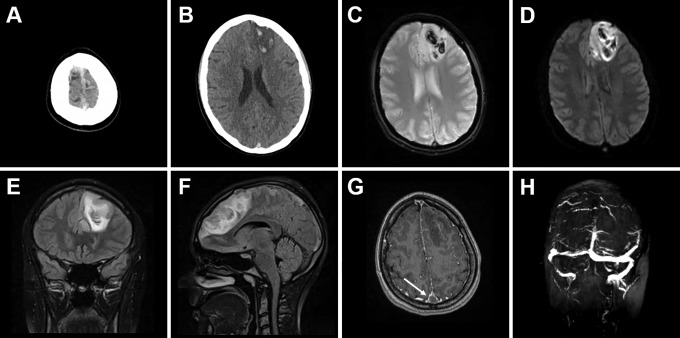
When examined by the on-call neurology resident, she followed most commands but appeared abulic, had right hemiparesis and epilepsia partialis continua (EPC) involving her right foot. National Institutes of Health Stroke Scale (NIHSS) score was 6. STAT computed tomography (CT) of the head (Figure 1A and B) showed a hyperdense signal in the SSS concerning for CVT, a left frontal IPH with edema, and 0.4 cm left-to-right midline shift. The left frontal IPH with mass effect localized appropriately to her focal neurological defects. She was immediately started on heparin drip for the thrombosis, loading doses of levetiracetam and lacosamide for the EPC seizure, and transferred into the intensive care unit. The OCP was discontinued. Both the endovascular neurosurgeon and the vascular neurologist on-call at Tampa General Hospital, the closest regional Comprehensive Stroke Center, were contacted to discuss transfer for possible mechanical thrombectomy. About 30 minutes later, her EPC evolved into a generalized tonic–clonic seizure. Urgent MRI brain and magnetic resonance venography (MRV) (Figure 1C-H) confirmed the SSS thrombosis and further defined filling defects in the superior bilateral frontal cortical veins. The MRV further revealed that the right transverse and sigmoid sinuses were hypoplastic (Figure 1H), which most likely reflects a less common (6%) albeit normal variant—in prior reports about 39% of transverse sinuses are symmetric and 39% of time the left sinus is hypoplastic.6
Figure 1.
Initial presentation of the patient with new severe headache, right-sided paresis, and epilepsia partialis continua involving the right foot. CT of the head with hyperdense signal in the SSS (A) and parasagittal left frontal IPH with surrounding edema (B). MRI of the brain GRE (C) and DWI (D) sequences reveal acute venous hemorrhagic infarct with multilobulated appearance. The coronal (E) and sagittal (F) FLAIR sequences demonstrate the extent of the vasogenic edema with frontal midline shift and mass effect. The post-contrast T1-MPRAGE sequence (G) shows the empty delta sign (arrow), where contrast outlines a triangular filling defect in the SSS. The MRV (H) confirms dural CVT with complete absence of signal in the thrombosed SSS and normal signal in the transverse and sigmoid sinuses. CT indicates computed tomography; CVT, cerebral vein thrombosis; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; GRE, gradient echo; IPH, intraparenchymal hemorrhage; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; SSS, superior sagittal sinus; T1-MPRAGE, T1-weighted magnetization-prepared rapid gradient echo.
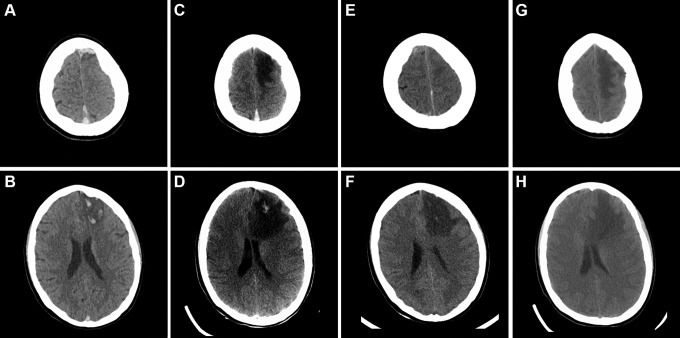
When the patient arrived to the accepting hospital, she was immediately evaluated by the neurological and neurosurgical teams, and after thorough discussion, it was decided for the time being to continue medical management as her examination and vitals were stable from transfer, and because her unfractionated heparin (UFH) anti-Xa activity level was subtherapeutic at 0.1 to 0.2 with partial thromboplastin time between 27.8 and 31.4 seconds. Thus, her heparin drip was augmented in an initial attempt to get it therapeutic before any neurointervention or neurosurgery. Her platelet count was 201 at time of diagnosis at the cancer center but had dropped to 126 at the accepting hospital. Over the next few hours, her UFH anti-Xa activity remained subtherapeutic despite repeated-dose augmentations and boluses, and her neurological status declined from NIHSS 9 to 20 as she became nonverbal and unable to follow commands. Repeat STAT CT (Figure 2C and D) showed increased edema around the venous infarct and increased midline shift to 1.1 cm. The cumulative duration of heparin drip at that time (including use by both hospitals) was 28 hours. Thus, the neurointerventional alert was activated and she underwent emergent angiography and endovascular aspiration thrombectomy with balloon venoplasty. This procedure led to immediate restoration of the patency of the SSS (Figure 3).
Figure 2.
Time course of the dural CVT and the associated venous hemorrhagic infarct. Side-by-side comparison of the initial CT of the head at time of presentation of the patient with new headache, EPC, and right-sided weakness (A, B) with imaging at time of clinical deterioration immediately prior to endovascular intervention (C, D), imaging on day 1 postintervention (E, F), and follow-up CT imaging on day 8 postintervention (G, H). Note the interval disease progression (C, D) when compared to initial presentation (A, B) and the resolution of the blood clot in the SSS (E) together with improvement of the hemorrhagic infarct and reduction of mass effect (F) on postprocedure day 1 and the interval evolution (G, H) on postprocedure day 8. CT indicates computed tomography; CVT, cerebral vein thrombosis; EPC, epilepsia partialis continua; SSS, superior sagittal sinus.
Figure 3.
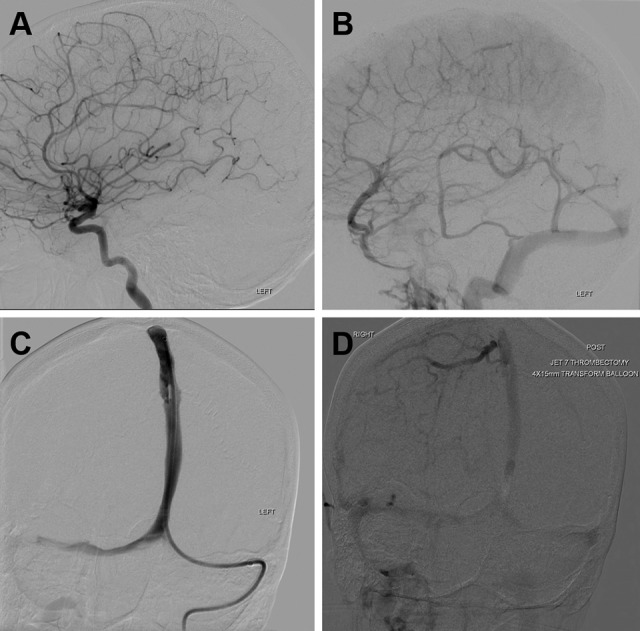
Endovascular intervention. The cerebral angiogram shows unremarkable arterial phase (A) while the venous phase (B) confirms complete SSS thrombosis. Direct injections after placement of the aspiration catheter through the left transverse sinus demonstrate gradual improvement in the patency of the SSS (C). Final right internal carotid artery injection shows partial opacification of the SSS (D), which is improved in comparison to the preaspiration images. SSS indicates superior sagittal sinus
The next day, off sedation, she demonstrated significant clinical neurologic improvement. She regained the ability to follow commands, comprehend and even was sitting up in bed using her cellphone to text her friends. Postprocedure day 1, her NIHSS improved to 6 for right arm and leg weakness and mild aphasia and clinically she continued to improve on a daily basis. CT head on day 1 postintervention showed resolution of the thrombus in the SSS (Figure 2E) together with interval improvement of the hemorrhagic infarct and reduction of mass effect (Figure 2F). Removal of the SSS thrombus essentially (partially) reversed the pathophysiology of the damage typically incurred by CVT,1-3 because the driving force for the venous IPH and vasogenic edema is removed after restoration of the cerebrospinal fluid outflow tract through the SSS. On day 4 postintervention, heparin was switched to therapeutic enoxaparin and she was transferred back to the cancer center where chemotherapy with daunorubicin and vincristine for ALL was resumed. In total, she experienced an ultra-short 5-day hiatus, which is remarkably minimal in such circumstances for which the predicted outcome would be very poor. CT head on postinterventional day 8 showed interval improvement of edema, hemorrhage, and ischemic infarct (Figure 2G and H). Some hypodense areas on CT do appear larger postintervention, and this observation is consistent with the evolution of venous infarctions and cytotoxic edema associated with CVT.
At 6-week follow-up, she continued to have a favorable outcome with only mild neurological deficits, corresponding to a modified Rankin Scale (mRS) of 2. Further use of PEG-asparaginase was absolutely excluded from the remainder of the patient’s induction chemotherapy cycle until day 43 of her subsequent intensification/consolidation chemotherapy cycle, which corresponds to postintervention day 78, and at that time she was receiving full-dose anticoagulation.
Discussion
Cerebral vein thrombosis is generally treated with the parenteral anticoagulant heparin to arrest the thrombotic process.7,8 Almost paradoxically, anticoagulation is even indicated for CVT with concomitant venous IPH: Per American Heart Association/American Stroke Association guidelines, the level of recommendation is Class IIa with level of evidence B, that is, recommendation in favor of treatment being effective with some conflicting evidence from nonrandomized studies.9 Per European Stroke Organization guidelines, the quality of evidence for anticoagulation is moderate with strength of recommendation being strong.10 A Cochrane review of randomized controlled trials suggests that anticoagulant treatment for cerebral venous sinus thrombosis appears to be safe and efficacious with an absolute reduction of risk of death or dependency by 13%.11 A multicenter, prospective observational study found that most patients (86.6%) experienced a good outcome (mRS 0-2) at a median follow-up of 16 months, while 2.9% had moderate impairment (mRS 3), 2.2% had severe impairment (mRS 4-5), and 8.6% died.12 The authors further identified IPH, malignancy, and coma as biomarkers for poor prognosis, death, and dependence. Hence, it is reasonable to target the highest risk subgroup of patients with bad outcomes despite medical treatment for more aggressive (and potentially more beneficial) neurointervention such as local thrombolysis,13 decompressive craniectomy, or, in our case, clot extraction. In patients who do not respond to medical therapy, the thrombosis can progress despite full-dose anticoagulation augmentation and repeat boluses, and will eventually lead to severely worsening edema, mass effect, herniation, and, eventually, death. This scenario is not always amenable to medical management and requires consideration of neurosurgical decompression and/or endovascular mechanical thrombectomy, which currently is emerging as a reasonable therapy in complicated CVT.14,15 Despite the potential upside for huge benefit such as our patient experienced, there are risks for venous thrombectomy and angioplasty in patients with CVT: vessel perforation, dissection, new or increased IPH, pseudoaneurysm, rethrombosis, catheter tip fracture, and ischemic stroke,14 as well as the well-known risks of catheterization of the common femoral artery and/or vein for access to the cerebral sinuses via the jugular vein. These procedural risks must always be weighed against the benefits in each patient’s specific case, taking into account their premorbid functional status and severity of the underlying condition associated with CVT. Given that ALL frequently occurs in children and young adults and survival in ALL has improved over time to 90%,16 the aforementioned risks associated with neurovascular intervention seem acceptable given the potential benefit. Furthermore, as we have shown, asparaginase can be restarted after stabilization from complications of venous thromboembolism, by utilizing full-dose anticoagulation, and moreover, all this can be accomplished without adversely impacting ALL prognosis.4 Randomized controlled studies are still lacking to more thoroughly evaluate the efficacy and safety of this concept.9,10,14,15
Cerebral vein thrombosis itself is associated with multiple risk factors such as young age, female sex, estrogen-based contraceptive use, procoagulant drugs, pregnancy (or the postpartum period), hematologic conditions, cancer, prothrombotic conditions (genetic or acquired), inflammatory disease, infections, obesity, immobility, anatomical variants (hypoplastic/aplastic transverse sinus), mechanical issues (head/neck trauma, lumbar puncture, central lines), and dehydration, among others.1,2,9 The identification of a single risk factor should not deter the search for additional determinants of increased risk.12 For example, venous thromboembolism is a well-established complication in asparaginase-treated patients with ALL.4,5 However, as demonstrated in the case of our 18-year-old woman with B-cell ALL, she was at even higher risk for venous thromboembolism due to the concurrent presence of 9 risk factors (age, sex, hypoplastic transverse sinus, estrogen-based contraceptive use, reduced mobility, lumbar puncture, central/peripherally inserted central catheter line, leukemia, and recent infections) besides the use of asparaginase. Oral contraceptive use carries a whopping odds ratio of 13 for development of CVT (odds ratio of 2 for the use of third-generation OCP over earlier generation OCP).17 Although it is impossible to establish a direct causation in a single case study such as ours, her multiple risk factors may have all in part contributed to the development of her CVT and its initial presentation. Moreover, with 4 biomarkers of poor prognosis (IPH, seizures, signs of intracranial hypertension, and rapid clinical progression), she required immediate and aggressive treatment: the first step being early transfer to a thrombectomy center and then neurointerventional clot extraction as described above.
In summary, aggressive management of CVT and its multiple secondary neurological sequelae together with immediate transfer of the patient from a Comprehensive Cancer Center to a Comprehensive Stroke Center with 24/7/365 neuroendovascular services enabled timely discussion of and planning for venous aspiration thrombectomy with balloon angioplasty of the SSS after medical management failed. This clearly resulted in a remarkable, unexpectedly favorable neurological outcome, which appeared unlikely given her multiple biomarkers for poor prognosis.
Ultimately, her dramatic clinical and radiographic improvement after this neurointerventional procedure led to early resumption of chemotherapy for ALL on day 16 postinduction after only an ultra-short 5-day therapy hiatus. While, for many patients, cancer-associated CVT can be safely managed at the cancer center if therapeutic anticoagulation can be safely achieved, patients with multiple CVT risk factors and biomarkers for poor prognosis from CVT may benefit from immediate transfer to a Comprehensive Stroke Center facility with neuroendovascular and neurosurgical capabilities, thereby preventing clinical deterioration due to escalating intracranial pressure with impending herniation, which may occur rapidly and unforgivingly on medical therapy alone.
Footnotes
Authors’ Note: The patient provided an informed consent for publication of this report.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Karl A. Kasischke, MD  https://orcid.org/0000-0003-0582-4319
https://orcid.org/0000-0003-0582-4319
References
- 1. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791–1798. [DOI] [PubMed] [Google Scholar]
- 2. DeVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345(6):417–423. [DOI] [PubMed] [Google Scholar]
- 3. Koenig M. Cerebral edema and elevated intracranial pressure. Continuum. 2018;24(6):1588–1602. [DOI] [PubMed] [Google Scholar]
- 4. Grace RF, Dahlberg SE, Neuberg D, et al. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goyal G, Vijaya RB. l-Asparaginase and venous thromboembolism in acute lymphocytic leukemia. Future Oncol. 2015;11(17):2459–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alper F, Kantarci M, Dane S, Gumustekin K, Onbas O, Durur I. Importance of anatomical asymmetries of transverse sinuses: an MR venographic study. Cerebrovasc Dis. 2004;18(3):236–239. [DOI] [PubMed] [Google Scholar]
- 7. Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597–600. [DOI] [PubMed] [Google Scholar]
- 8. Bruijn de SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484–488. [DOI] [PubMed] [Google Scholar]
- 9. Saposnik G, Barinagarrementeria F, Brown RD, Jr, et al. American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158–1192. [DOI] [PubMed] [Google Scholar]
- 10. Ferro JM, Bousser MG, Canhão P; et al. European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–1213. [DOI] [PubMed] [Google Scholar]
- 11. Coutinho J, de Bruijn S, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev. 2011;10(8):CD002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F. ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664–670. [DOI] [PubMed] [Google Scholar]
- 13. Smith AG, Cornblath WT, Deveikis JP. Local thrombolytic therapy in deep cerebral venous thrombosis. Neurology. 1997;48(6):1613–1619. [DOI] [PubMed] [Google Scholar]
- 14. Siddiqui FM, Dandapat S, Banerjee C, et al. Mechanical thrombectomy in cerebral venous thrombosis. Stroke. 2015;46(5):1263–1268. [DOI] [PubMed] [Google Scholar]
- 15. Ilyas A, Chen CJ, Raper DM, et al. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg. 2017;9(11):1086–1092. [DOI] [PubMed] [Google Scholar]
- 16. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brujin de SFTM, Stam J, Vandenbroucke JP. Cerebral venous sinus thrombosis study group. Increased risk of cerebral venous sinus thrombosis with third-generation oral contraceptives. Lancet. 1998;351(9113):1404. [DOI] [PubMed] [Google Scholar]