Abstract
Background and Purpose:
At present, stroke patients receiving intravenous thrombolysis (IVT) undergo monitoring of their neurological status and vital signs every 15 minutes for the first 2 hours, every 30 minutes for the next 6 hours, and every hour thereafter up to 24 hours post-IVT. The present study sought to prospectively evaluate whether post-IVT stroke patients with low risk for complications may safely be cared for utilizing a novel low-intensity monitoring protocol.
Methods:
In this pragmatic, prospective, single-center, open-label, single-arm safety study, we enrolled 35 post-IVT stroke patients. Adult patients were eligible if their NIH Stroke Scale (NIHSS) was less than 10 at the time of presentation, and if they had no critical care needs by the end of the IVT infusion. Patients underwent a low-intensity monitoring protocol during the first 24 hours after IVT. The primary outcome was need for a critical care intervention in the first 24 hours after IVT.
Results:
The median age was 54 years (range: 32-79), and the median pre-IVT NIHSS was 3 (interquartile range [IQR]: 1-6). None of the 35 patients required transfer to the intensive care unit or a critical care intervention in the first 24 hours after IVT. The median NIHSS at 24 hours after IVT was 1 (IQR: 0-3). Four (11.4%) patients were stroke mimics, and the vast majority was discharged to home (82.9%). At 90 days, the median NIHSS was 0 (IQR: 0-1), and the median modified Rankin Scale was 0 (range: 0-6).
Conclusion:
Post-IVT stroke patients may be safely monitored in the setting of a low-intensity protocol.
Keywords: thrombolysis, critical care needs, low-intensity monitoring, safety study
Introduction
Ischemic stroke is a leading cause of disability and mortality in the United States.1,2 Intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator is the cornerstone of acute stroke therapy, however, harbors the risk of systemic and intracranial hemorrhage as a potential life-threatening complication.3 Therefore, it is currently recommended that all patients undergoing IVT are closely monitored for at least 24 hours with frequent vital sign checks and neurological assessments.4 Not uncommonly, post-IVT care occurs in a critical care environment, such as an intensive care unit (ICU) or a stroke unit with ICU-like capabilities. The frequency of vital sign checks and neurological assessments is strictly protocolized as per consensus guidelines on post-IVT care, mandating checks every 15 minutes for the first 2 hours, every 30 minutes for the next 6 hours, and every hour thereafter as part of the current standard of care.5,6 Post-IVT monitoring is resource intensive and commonly requires one-to-one nursing, resulting in increased health-care costs. In resource-limited settings, post-IVT monitoring may tie-up valuable hospital resources, including critical care beds.
At present, it is unknown if high-intensity monitoring is necessary for all post-IVT patients. In a previous retrospective study, we have shown that complications and need for ICU care are exceedingly rare in post-IVT patients presenting with an NIH Stroke Scale (NIHSS) <10 and who are otherwise free of complications by the end of their IVT infusion.7
The present study sought to prospectively evaluate whether post-IVT stroke patients with low NIHSS who do not require critical care by the end of the IVT infusion may safely be cared for by utilizing a low-intensity monitoring protocol. We hypothesized that these patients are at low risk for complications and that monitoring these patients under a low-intensity monitoring protocol will be feasible and safe.
Methods
Study Design, Setting, and Participants
The Optimal Post Tpa-Iv Monitoring in Ischemic STroke trial is a pragmatic, prospective, single-center, open-label, single-arm safety study conducted at Johns Hopkins Hospital. The study site is a comprehensive stroke center certified by The Joint Commission, and IVT is administered in the emergency department (ED) according to American Heart Association guidelines.4 We enrolled 35 patients with acute stroke who received IVT between March 1, 2014, and March 31, 2018 (Supplemental Figure 1). Patients were eligible if they were between ages 18 and 80, if their NIHSS was less than 10 at the time of presentation and at the end of the IVT infusion, and if they had no critical care needs by the end of the IVT infusion. The practice at our institution has been that only patients with disabling symptoms are offered IVT. Patients who underwent endovascular therapy were excluded. This study was approved by the institutional review board of Johns Hopkins University School of Medicine. This study was registered at clinicaltrials.gov (NCT02039375).
Low-Intensity Monitoring Protocol
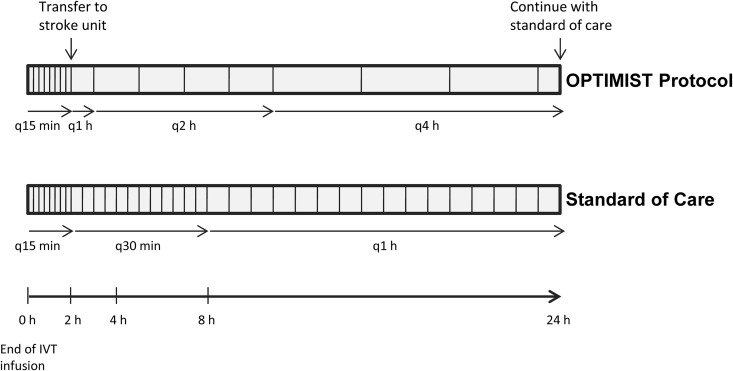
During the IVT infusion and in the first hour after IVT completion, patients underwent vital sign checks and neurological assessments every 15 minutes according to the current standard of care. Patients were then transferred to our stroke unit, a telemetry unit with a nurse-to-patient ratio of 1:3, but without critical/intermediate care capabilities. Our stroke unit is staffed by stroke-trained nurses; however, nurses do not have expertise, training, or experience in critical/intermediate care. Monitoring under the low-intensity protocol commenced at the time of the patients’ arrival in our stroke unit. The protocol is comprised of vital sign checks and neurological assessment on admission to the stroke unit, then 1 hour after admission, then every 2 hours for another 8 hours, followed by vital sign checks and neurological assessments every 4 hours until 24 hours post IVT are complete. We aimed to transfer all patients to our stroke unit from the ED by the end of the first hour after IVT completion. Patients who could not immediately be transferred to our stroke unit by 1 hour post IVT (ie, due to bed availability) were monitored in the ED under the current standard of care until the time of transfer. At completion of the protocol, 24 hours post IVT, patients were cared for according to the current standard of care. Figure 1 outlines the low-intensity monitoring schedule and juxtaposes the current standard of care post-IVT monitoring schedule for the first 24 hours after IVT.
Figure 1.
Diagram illustrating the frequency of vital sign checks and neurological assessments under the Optimal Post Tpa-Iv Monitoring in Ischemic Stroke (OPTIMIST) low-intensity monitoring protocol versus current standard of care.
Primary Outcome, Follow-Up, and Secondary Outcomes
The primary outcome was need for a critical care intervention in the first 24 hours after IVT, or perceived need to transfer the patient to the ICU even if no actual critical care intervention was performed. Secondary outcomes included NIHSS at 24 hours, NIHSS and modified Rankin Scale (mRS) at discharge, and NIHSS and mRS at 90 days.
Infarct Volume Measurements
Quantitative analysis of infarct volumes on post-IVT magnetic resonance imaging was performed by using a commercially available segmentation software integrated into Picture Archiving and Communication System (PACS) (Carestream Health, Rochester, New York) to obtain a semiautomated infarct segmentation. In this approach, the user measures the longest diameter of each lesion on the diffusion-weighted MR image, and then the software segments the lesion volumetrically. This is followed by visual inspection and manual correction by the user to obtain the final infarct volume.
Statistical Analysis
Baseline demographics, comorbidities, medication use, stroke severity measures, physiological/laboratory parameters, and outcome data were assessed using descriptive statistics. Frequencies are reported for categorical variables; continuous variables are presented as medians with interquartile range (IQR), means, or range. Statistical analysis was performed using Stata version 15 (Stata Statistical Software: Release 15, College Station, Texas).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Patient Characteristics
The median age was 54 years (range: 32-79; Table 1); 21 (60.0%) patients were male. The median pre-IVT NIHSS was 3 (IQR: 1-6; range 0-9), and the median admission systolic blood pressure was 157 mm Hg (IQR: 140-177 mm Hg). Twenty-three (65.7%) of 35 patients were treated within 3 hours from last known well. The median time from end of IVT infusion to transfer out of the ED was 109 minutes (IQR: 60-153 minutes). There were no intracranial large vessel occlusions; however, one patient had an extracranial occlusion of the internal carotid artery (ICA) in the setting of a dissection, and another had a symptomatic high-grade ICA stenosis. Four (11.4%) patients were stroke mimics, and the vast majority was discharged home (82.9%). There were no in-hospital deaths. Further characteristics of the study population are presented in Table 1.
Table 1.
Characteristics of the Study Population.a
| Characteristics | Value |
|---|---|
| Age, years: median (range) | 54 (32-79) |
| Male, n (%) | 21 (60.0) |
| Black race, n (%) | 22 (62.9) |
| Pre-IVT NIHSS: median (IQR) | 3 (1-6) |
| Pre IVT mRS: median (range) | 0 (0-4) |
| IVT window <3 hours, n (%) | 23 (65.7) |
| BP systolic, mm Hg: median (IQR) | 157 (140-177) |
| BP diastolic, mm Hg: median (IQR) | 86 (75-100) |
| Glucose, mg/dL: median (IQR) | 119 (94-138) |
| Hypertension, n (%) | 28 (80.0) |
| Hyperlipidemia, n (%) | 15 (42.9) |
| Diabetes mellitus, n (%) | 7 (20.0) |
| Atrial fibrillation, n (%) | 5 (14.3) |
| Smoking, n (%) | 10 (28.6) |
| Antiplatelets, n (%) | 14 (40.0) |
| Anticoagulation, n (%) | 1 (2.9) |
| Statin, n (%) | 13 (37.1) |
| Hemorrhagic transformation, n (%) | |
| Symptomatic | 0 (0) |
| Asymptomatic | 4 (11.4) |
| Infarct volume, mL: mean (range) | 3.4 (0-44.8) |
| Diagnosis, n (%) | |
| Stroke | 31 (88.6) |
| Conversion disorder | 3 (8.6) |
| Seizure | 1 (2.9) |
| Length of stay, days: median (IQR) | 2 (2-3) |
| Discharge destination, n (%) | |
| Home | 29 (82.9) |
| ACIR | 4 (11.4) |
| SA | 1 (2.9) |
| Prison | 1 (2.9) |
| In-hospital death, n (%) | 0 (0) |
Abbreviations: ACIR, acute inpatient rehab; BP, blood pressure; IQR, interquartile range; IVT, intravenous thrombolysis; mRS, modified Rankin Scale; NIHSS, NIH Stroke Scale; SA, subacute rehab.
an = 35.
Primary and Secondary Outcomes
Study outcomes are summarized in Table 2. None of the 35 patients required transfer to the ICU or a critical care intervention in the first 24 hours after IVT. Two patients required ICU care later in the hospital course: one for routine postoperative care after carotid endarterectomy, and one on hospital day 4 after hemorrhagic transformation in the setting of a heparin drip for carotid dissection.
Table 2.
Study Outcomes.
| Outcome | Value |
|---|---|
| Primary outcome | |
| ICU need by 24 hours, n (%) | 0 (0) |
| Secondary outcomes | |
| NIHSS at 24 hours: median (IQR) | 1 (0-3) |
| NIHSS at discharge: median (IQR) | 1 (0-2) |
| mRS at discharge: median (range) | 1 (0-3) |
| NIHSS at 90 days: median (IQR), n = 28 | 0 (0-1) |
| mRS at 90 days: median (range), n = 33 | 0 (0-6) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, NIH Stroke Scale.
The median NIHSS at 24 hours after IVT was 1 (IQR: 0-3; Table 2), and the median improvement in NIHSS at 24 hours from baseline was 2 (IQR: 0-4). The median NIHSS and mRS at discharge were 1 (IQR: 0-2) and 1 (range: 0-4), respectively. One patient died several weeks after discharge due to a cardiovascular event unrelated to his stroke. At 90 days, the median NIHSS was 0 (IQR: 0-1), and the median mRS was 0 (range: 0-6).
Discussion
In the present study, we show that it is feasible and safe to care for of a subset of post-IVT patients in a low-intensity monitoring environment. Our low-intensity monitoring protocol is identical to the standard of care protocol only in the first 2 hours after IVT bolus administration, and thereafter transitions to every 1-hour monitoring for 1 hour, every 2-hour monitoring for 8 hours, followed by every 4-hour monitoring until 24 hours post IVT are complete. Patient selection based on critical care needs at the end of the IVT infusion and NIHSS were informed and supported by prior data by us, and others suggesting that complications requiring ICU care are relatively uncommon in patients with NIHSS less than 10; and if complications occur, they are typically apparent by the end of the IVT infusion or shortly thereafter.7-9 Patients with large vessel occlusion nowadays routinely undergo mechanical thrombectomy in conjunction with IVT.10 At our institution, post-thrombectomy patients are routinely monitored in the ICU, and these patients were not included in our study. Therefore, we do not suggest that post-thrombectomy patients be monitored in a low-intensity monitoring environment, even if their NIHSS is less than 10.
The monitoring location of post-IVT patients under the current standard of care may vary by institutions, that is, post-IVT monitoring may occur in an ICU, an intermediate care unit, or a designated stroke unit. However, regardless of physical location, all patients are presently required to undergo high-intensity monitoring in order to comply with current guidelines.5,6 High frequency vital sign checks and neurological assessments as per the current standard of care typically require one-to-one nursing care and therefore dictate resource-intense monitoring regardless of physical patient location. Thus, in times of increasing financial constraints and limited resources, the implementation of our low-intensity protocol may free up nursing resources and may allow for reallocation of critical care resources in institutions where post-IVT patients are presently monitored in the ICU. The pragmatic approach of this trial was intended to facilitate swift and effortless implementation of our low-intensity monitoring protocol across other institutions.
The median NIHSS of our study population was relatively low; however, patients with low NIHSS comprise a significant proportion of all acute strokes eligible for IVT,11 and there is increasing evidence that IVT is efficacious in these patients.12,13 Similarly, stroke mimics constitute a not-insignificant and unavoidable subset of all IVT-treated patients, and IVT is generally considered safe in this patient population.14-17 Patients with low NIHSS and/or stroke mimics may therefore not require the same intensity of post-IVT monitoring as more severe strokes, that is, due to large-vessel occlusion. Since stroke mimics typically present with low NIHSS,14,17 the criteria for inclusion in our monitoring protocol may enrich for stroke mimics and strokes with low NIHSS to be monitored under low-intensity conditions. Our data may suggest that low intensity, and thus cost- and resource-effective, monitoring of a subpopulation of IVT patients is feasible and safe, and we propose to reserve high-intensity post-IVT care only for those patients deemed at high risk for post-IVT complications.
Our study is limited by the relatively small sample size. Although our results are generalizable to eligible post-IVT patients in other institutions, it is unknown whether high-intensity monitoring provides additional benefits beyond detection of complications and hemorrhages that may impact functional outcome. Although we did not have any in-hospital deaths and over 80% of patients were discharged to home, our study was not sufficiently powered to show that long-term functional outcomes under the low-intensity protocol are comparable to outcomes with standard-of-care monitoring. Lastly, our results are not generalizable to patients undergoing mechanical thrombectomy.
We propose that the current “one size fits all” approach of post-IVT monitoring may be unnecessary for a subset of post-IVT patients. Patients with an NIHSS of less than 10 and no critical care needs during the IVT infusion may be safely cared for in a low-intensity monitoring environment post IVT. This may allow for more cost-effective utilization of critical care and nursing resources, reduce the number of hand offs, and potentially shorten length of stay. Future studies with larger patient numbers will have to confirm the safety and efficacy of our protocol.
Supplemental Material
Supplemental Material, CONSORT_2010_Flow_Diagram for Safety Trial of Low-Intensity Monitoring After Thrombolysis: Optimal Post Tpa-Iv Monitoring in Ischemic STroke (OPTIMIST) by Roland Faigle, Jaime Butler, Juan R. Carhuapoma, Brenda Johnson, Elizabeth K. Zink, Tenise Shakes, Melissa Rosenblum, Mustapha Saheed and Victor C. Urrutia in The Neurohospitalist
Acknowledgments
The authors thank Dr Hans Adrian Puttgen for serving as the safety officer of this study.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Victor C. Urrutia has served as the principal investigator for the investigator-initiated trial Safety of Intravenous Thrombolytics in Stroke on Awakening (SAIL ON), funded by Genentech Inc.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Faigle was supported by an institutional KL2 grant from the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number KL2TR001077 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence ES, Coshall C, Dundas R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–1284. [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774. [DOI] [PubMed] [Google Scholar]
- 4. Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. [DOI] [PubMed] [Google Scholar]
- 5. Braimah J, Kongable G, Rapp K, et al. Nursing care of acute stroke patients after receiving rt-PA therapy. The NINDS rt-PA Stroke Study Group. J Neurosc Nurs. 1997;29(6):373–383. [DOI] [PubMed] [Google Scholar]
- 6. Summers D, Leonard A, Wentworth D, et al. Comprehensive overview of nursing and interdisciplinary care of the acute ischemic stroke patient: a scientific statement from the American Heart Association. Stroke. 2009;40(8):2911–2944. [DOI] [PubMed] [Google Scholar]
- 7. Faigle R, Sharrief A, Marsh EB, Llinas RH, Urrutia VC. Predictors of critical care needs after IV thrombolysis for acute ischemic stroke. PLoS One. 2014;9(2):e88652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernandez-Gotico H, Lightfoot T, Meighan M. Multicenter study of adverse events after intravenous tissue-type plasminogen activator treatment of acute ischemic stroke. J Neurosci Nurs. 2017;49(1):31–36. [DOI] [PubMed] [Google Scholar]
- 9. Strbian D, Meretoja A, Ahlhelm FJ, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology. 2012;78(6):427–432. [DOI] [PubMed] [Google Scholar]
- 10. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. [DOI] [PubMed] [Google Scholar]
- 11. Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56(8):1015–1020. [DOI] [PubMed] [Google Scholar]
- 12. Kohrmann M, Nowe T, Huttner HB, et al. Safety and outcome after thrombolysis in stroke patients with mild symptoms. Cerebrovasc Dis. 2009;27(2):160–166. [DOI] [PubMed] [Google Scholar]
- 13. Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from get with the guidelines-stroke. Stroke. 2011;42(11):3110–3115. [DOI] [PubMed] [Google Scholar]
- 14. Chernyshev OY, Martin-Schild S, Albright KC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology. 2010;74(17):1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faigle R, Marsh EB, Llinas RH, Urrutia VC. Critical care needs in patients with diffusion-weighted imaging negative MRI after tPA—does one size fit all? PLoS One. 2015;10(10):e0141204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Winkler DT, Fluri F, Fuhr P, et al. Thrombolysis in stroke mimics: frequency, clinical characteristics, and outcome. Stroke. 2009;40(4):1522–1525. [DOI] [PubMed] [Google Scholar]
- 17. Zinkstok SM, Engelter ST, Gensicke H, et al. Safety of thrombolysis in stroke mimics: results from a multicenter cohort study. Stroke. 2013;44(4):1080–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, CONSORT_2010_Flow_Diagram for Safety Trial of Low-Intensity Monitoring After Thrombolysis: Optimal Post Tpa-Iv Monitoring in Ischemic STroke (OPTIMIST) by Roland Faigle, Jaime Butler, Juan R. Carhuapoma, Brenda Johnson, Elizabeth K. Zink, Tenise Shakes, Melissa Rosenblum, Mustapha Saheed and Victor C. Urrutia in The Neurohospitalist
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.