Short abstract
The aim of the present study was to determine whether IL-6 polymorphisms correlate with sepsis. According to the inclusion criteria, the association of IL-6 polymorphisms with sepsis was searched in databases and analysed using comprehensive meta-analysis software. A total of 16 studies were included in this meta-analysis. There was no significant association between the IL-6-174G/C polymorphism and sepsis risk in the total population (C vs. G: OR = 1.04, 95% CI = 0.79–1.38; CC vs. GG: OR = 0.86, 95% CI = 0.53–1.41; CG vs. GG: OR = 0.99, 95% CI = 0.79–1.24; dominant model: OR = 0.97, 95% CI = 0.74–1.29; recessive model: OR = 0.92, 95% CI = 0.61–1.39). When patients were stratified according to ethnicity, a statistically significant association was observed in Asians and Africans. As for the -572G/C polymorphism, the results showed that the IL-6-572C/G polymorphism was not associated with sepsis susceptibility (G vs. C: OR = 0.98, 95% CI = 0.79–1.22; GG vs. CC: OR = 1.46, 95% CI = 0.53–4.03; GC vs. CC: OR = 0.82, 95% CI = 0.54–1.27; dominant model: OR = 0.88, 95% CI = 0.55–1.41; recessive model: OR = 1.55, 95% CI = 0.82–2.92). The data indicated that the IL-6-174G/C polymorphism may contribute to sepsis risk, especially in Africans and Asians. No significant association was observed between the IL-6-572G/C polymorphism and sepsis risk.
Keywords: Interleukin-6, sepsis, meta-analysis
Introduction
Sepsis is considered to be a complicated clinical syndrome arising from systemic inflammatory reactions to bacteria or bacterial products.1 Although there has been great improvement in antibiotic therapy and life support, the global death rate of sepsis patients remains up to 30–60%.2 Therefore, predictive markers to identify high-risk populations are urgently needed for early detection and preventive care. Cytokines are critically involved in modulation of host immune response, while changed cytokine levels have been shown to participate in sepsis development.3 Delineating the variation in gene expression and associated differences in responses to sepsis might facilitate in exploiting new genetically tailored diagnostic and therapeutic interventions, which could improve outcome in patients with sepsis susceptibility.
IL-6 is a vital inflammatory cytokine generated by various cells, such as leucocytes, endothelial cells, myocytes, adipocytes and fibroblasts.4 IL-6 plays a significant role in the immune response and regulation of inflammation reactions. High IL-6 levels have been demonstrated to be related to the elevated risk and death rate of severe sepsis.5 The IL-6 gene, located on 7p21 and spanning 5 kb, contains four introns and five exons. There have been several identified polymorphisms in the IL-6 gene promoter region. Among these, the common -174G/C polymorphism (rs1800795) contains a DNA-binding site for nuclear factor IL-6, a transcription factor which can interact with estradiol receptor complexes to regulate IL-6 gene expression.6 The -572G/C polymorphism (rs1800796) does not contain any potent homology to any identified transcription factor-binding site, with a close location to glucocorticoid response elements.7 To date, there have been much research evaluating the association of IL-6 polymorphisms with sepsis, albeit with inconsistent findings.
Meta-analysis is a useful method to summarise the different articles. It not only overcomes the drawbacks of small sample size and inadequate statistical power of genetic studies with complicated characteristics, but also provides more convincing conclusions compared to a single study.8 Thus, in this research study, a meta-analysis was conducted by enrolling eligible studies to assess the relationship between IL-6-174G/C and -572G/C polymorphisms and sepsis.
Materials and methods
Literature search
We thoroughly searched the online electronic databases (PubMed, EMBASE and CNKI) using the following search terms: (interleukin-6 or IL-6) and (polymorphism or variant or variation) and (‘sepsis’). In addition, we manually searched the references of original studies and reviews on this topic. There was no restriction on language.
Criteria for inclusion and exclusion
Eligible studies were enrolled if they met the following criteria: (a) case-control studies, (b) only cases with definitive diagnosis of sepsis and (c) evaluation of IL-6 polymorphisms and sepsis. Exclusion criteria were: (a) abstracts, reviews and duplication of studies; (b) not case-control studies; and (c) absence of genotype frequency.
Data extraction
The following information was extracted from every article by two independent authors: year of publication, first author, ethnicity, numbers as well as genotype numbers of cases and controls, with evidence of Hardy–Weinberg equilibrium (HWE) in controls. Discrepancies were resolved by discussion.
Statistical analysis
Stata v12.0 (Stata Corp., College Station, TX) was used for statistical analysis. ORs with corresponding 95% CIs was used to assess the correlation of IL-6 gene polymorphisms (-174G/C and -572G/C) with sepsis under A versus a, homozygote (AA vs. aa), heterozygote comparison (AA vs. Aa) and dominant model and recessive model between groups. To be specific, the dominant model referred to as Aa + aa versus AA, where ‘A’ and ‘a’ represented major and minor alleles, respectively, and the recessive model was aa versus AA + Aa.9 Moreover, the variation caused by heterogeneity was estimated by calculating the inconsistency index I2, with I2 values of 25%, 25–75% and 75% representing low, moderate or high degrees of inconsistency, respectively.10 When the heterogeneity was absent (I2 < 50%), a fixed-effects model was used to calculate the combined OR estimates for every study; otherwise, a random-effects model was applied. Additionally, sensitivity analysis was conducted by limiting the meta-analysis to research conforming to HWE. Finally, possible publication bias was visually determined by Begg’s funnel plots.11
Results
Characteristics of included studies
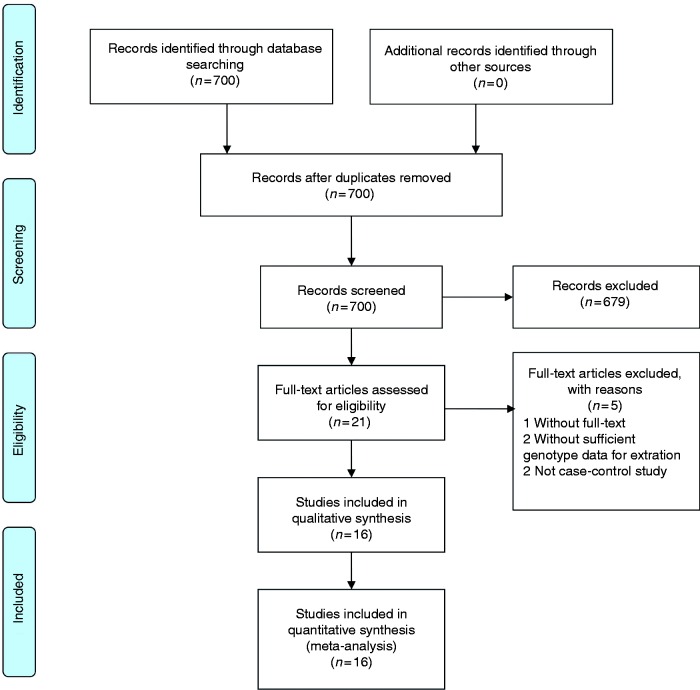
The study selection process is shown in Figure 1. According to the inclusion criteria, 16 qualified case-control studies were finally enrolled,12–27 all of which assessed the correlation of IL-6 polymorphisms with sepsis risk. All studies were published in English. Fifteen articles studied the -174G/C polymorphism and four studied the -572C/G polymorphism. For -174G/C, there were 11 studies with Caucasian subjects, two with Asian populations, two with African populations and one with a mixed population. Eight studies were performed in adults, six in neonates and two in paediatric populations. For -572C/G, there were two studies with Caucasian subjects and two with Asian populations. Three studies were conducted in adults and one in paediatric populations. The major features of enrolled studies are summarised in Table 1.
Figure 1.
Flow diagram for selection of publication.
Table 1.
Study selection and subject characteristics of included studies in meta-analysis.
| Author | Yr | Ethnicity | Age | Cases | Controls |
Genotypes for cases |
Genotypes for controls |
Genotypes for cases |
Genotypes for controls |
P for HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | C | G | C | GG | GC | CC | GG | GC | CC | |||||||
| -174G/C | ||||||||||||||||
| Schluter | 2002 | Caucasian | Adult | 50 | 276 | 51 | 49 | 313 | 239 | 13 | 25 | 12 | 91 | 131 | 54 | 0.58 |
| Treszl | 2003 | Caucasian | Neonatal | 33 | 70 | 49 | 17 | 97 | 43 | 18 | 13 | 2 | 34 | 29 | 7 | 0.82 |
| Balding | 2003 | Caucasian | Adult | 183 | 389 | 215 | 151 | 371 | 241 | 59 | 97 | 27 | 97 | 177 | 32 | 0.00 |
| Ahrens | 2004 | Caucasian | Neonatal | 50 | 306 | 69 | 31 | 57 | 309 | 24 | 21 | 5 | 3 | 51 | 129 | 0.42 |
| Sipahi | 2006 | Caucasian | Pediatric | 44 | 77 | 66 | 22 | 123 | 31 | 26 | 14 | 4 | 52 | 19 | 6 | 0.04 |
| Gopel | 2006 | Caucasian | Neonatal | 97 | 320 | 108 | 86 | 399 | 241 | 29 | 50 | 18 | 128 | 143 | 49 | 0.39 |
| Baier #1 | 2006 | African | Neonatal | 114 | 119 | 206 | 22 | 229 | 9 | 94 | 18 | 2 | 110 | 9 | 0 | 0.67 |
| Baier #2 | 2006 | Caucasian | Neonatal | 31 | 26 | 40 | 22 | 30 | 22 | 12 | 16 | 3 | 7 | 16 | 3 | 0.01 |
| Michalek | 2007 | Caucasian | Pediatric | 409 | 644 | 477 | 341 | 665 | 623 | 127 | 223 | 59 | 160 | 345 | 139 | 0.07 |
| Abdel-Hady | 2009 | African | Neonatal | 54 | 71 | 60 | 48 | 88 | 54 | 17 | 26 | 11 | 28 | 32 | 11 | 0.71 |
| Davis | 2010 | Caucasian | Adult | 23 | 52 | 31 | 15 | 64 | 40 | 10 | 11 | 2 | 21 | 22 | 9 | 0.44 |
| Carregaro | 2010 | Mix | Adult | 97 | 207 | 137 | 57 | 284 | 130 | 49 | 39 | 9 | 94 | 96 | 17 | 0.27 |
| Shimada | 2011 | Asian | Adult | 123 | 102 | 246 | 0 | 204 | 0 | 123 | 0 | 0 | 102 | 0 | 0 | 0.00 |
| Martin-Loeches | 2012 | Caucasian | Adult | 1227 | 953 | 1678 | 776 | 1289 | 617 | 581 | 516 | 130 | 438 | 413 | 102 | 0.75 |
| Panayides | 2015 | Caucasian | Adult | 185 | 108 | 303 | 67 | 184 | 32 | 123 | 57 | 5 | 79 | 26 | 3 | 0.63 |
| Mao | 2016 | Asian | Adult | 188 | 162 | 149 | 227 | 182 | 142 | 56 | 37 | 95 | 68 | 46 | 48 | 0.00 |
| -572C/G | ||||||||||||||||
| Michalek | 2007 | Caucasian | Pediatric | 409 | 644 | 51 | 767 | 56 | 1228 | 7 | 37 | 365 | 2 | 52 | 588 | 0.46 |
| Gu | 2008 | Asian | Adult | 54 | 321 | 26 | 82 | 153 | 489 | 5 | 16 | 33 | 16 | 121 | 184 | 0.49 |
| Shimada | 2011 | Asian | Adult | 123 | 102 | 53 | 191 | 41 | 163 | 3 | 47 | 72 | 2 | 37 | 63 | 0.19 |
| Panayides | 2015 | Caucasian | Adult | 198 | 115 | 49 | 347 | 49 | 181 | 6 | 37 | 155 | 6 | 37 | 72 | 0.66 |
Meta-analysis results
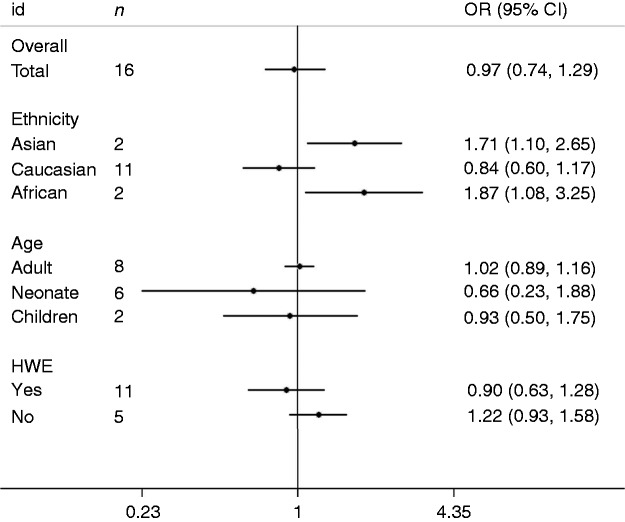
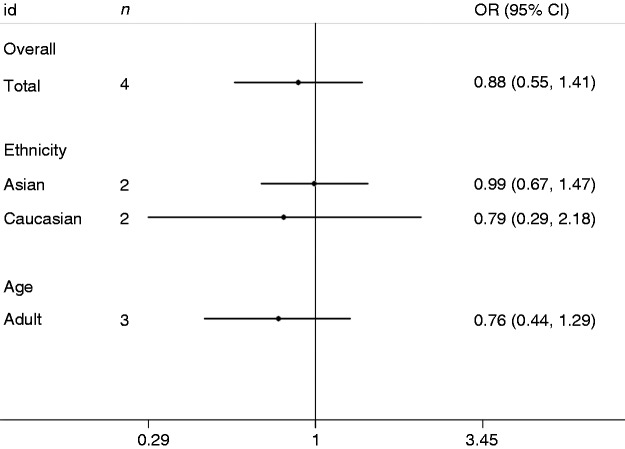
The major outcomes of the meta-analysis of the IL-6-174G/C polymorphism and sepsis risk are presented in Figure 2 and Table 2. Overall, the -174G/C polymorphism was not significantly associated with sepsis risk (C vs. G: OR = 1.04, 95% CI = 0.79–1.38; CC vs. GG: OR = 0.86, 95% CI = 0.53–1.41; CG vs. GG: OR = 0.99, 95% CI = 0.79–1.24; dominant model: OR = 0.97, 95% CI = 0.74–1.29; recessive model: OR = 0.92, 95% CI = 0.61–1.39). In subgroup analysis stratified by ethnicity, a significant association was detected in Asians and Africans but not in Caucasians. In subgroup analysis by age,-174G/C was insignificantly related to sepsis risk in adults, neonates and paediatric populations. On subgroup analysis stratified by HWE, eight papers in Caucasians were included with HWE studies, and the analyses on their cumulative data indicate that the IL-6-174G/C polymorphism is not associated with sepsis risk (C vs. G: OR = 1.35, 95% CI = 0.89–2.03; CC vs. GG: OR = 0.53, 95% CI = 0.25–1.12; CG vs. GG: OR = 0.93, 95% CI = 0.65–1.31; dominant model: OR = 0.77, 95% CI = 0.50–1.18; recessive model: OR = 0.62, 95% CI = 0.35–1.09). In addition, sensitivity analysis was conducted through omitting HWE studies. Non-HWE studies changed the final results, implicating the existence of publication bias in results due to non-HWE studies. The major findings of the IL-6-572C/G polymorphism and sepsis risk are listed in Figure 3 and Table 3. Combined results revealed that the IL-6-572C/G polymorphism was not related to sepsis susceptibility (G vs. C: OR = 0.98, 95% CI = 0.79–1.22; GG vs. CC: OR = 1.46, 95% CI = 0.53–4.03; GC vs. CC: OR = 0.82, 95% CI = 0.54–1.27; dominant model: OR = 0.88, 95% CI = 0.55–1.41; recessive model: OR = 1.55, 95% CI = 0.82–2.92). On subgroup analysis stratified by ethnicity, the IL-6-572C/G polymorphism was not statistically associated with sepsis risk in Asians or other ethnic groups. When patients were stratified according to age, this polymorphism was not related to sepsis risk in adults.
Figure 2.
Forest plot of OR for association between the IL-6-174G/C polymorphism and sepsis under the dominant model.
Table 2.
Summary ORs and 95% CIs of IL-6-174G/C polymorphism and sepsis risk.
|
C vs. G |
CC vs. GG |
GC vs. GG |
Dominant model |
Recessive model |
||
|---|---|---|---|---|---|---|
| n | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Total | 16 | 1.04 (0.79–1.38) | 0.86 (0.53–1.41) | 0.99 (0.79–1.24) | 0.97 (0.74–1.29) | 0.92 (0.61–1.39) |
| Ethnicity | ||||||
| Asian | 2 | 0.51 (0.38–0.69) | 2.40 (1.46–3.94) | 0.94 (0.72–1.24) | 1.71 (1.10–2.65) | 2.43 (1.56–3.77) |
| Caucasian | 11 | 1.22 (0.91–1.63) | 0.66 (0.37–1.19) | 0.97 (0.52–1.80) | 0.84 (0.60–1.17) | 0.74 (0.46–1.18) |
| African | 2 | 0.61 (0.40–0.93) | 1.96 (0.76–5.10) | 1.75 (0.98–3.11) | 1.87 (1.08–3.25) | 1.63 (0.69–3.87) |
| Age | ||||||
| Adult | 8 | 0.87 (0.69–1.09) | 1.21 (0.98–1.50) | 0.97 (0.84–1.11) | 1.02 (0.89–1.16) | 1.31 (0.91–1.88) |
| Neonate | 6 | 1.29 (0.48–3.46) | 0.50 (0.08–3.04) | 0.79 (0.35–1.80) | 0.66 (0.23–1.88) | 0.66 (0.18–2.51) |
| Children | 2 | 1.08 (0.65–1.81) | 0.57 (0.40–0.83) | 0.87 (0.66–1.14) | 0.93 (0.50–1.75) | 0.64 (0.46–0.88) |
| HWE | ||||||
| Yes | 11 | 1.15 (0.82–1.61) | 0.70 (0.38–1.28) | 1.00 (0.75–1.34) | 0.90 (0.63–1.28) | 0.75 (0.46–1.21) |
| No | 5 | 0.78 (0.52–1.17) | 1.79 (1.25–2.57) | 0.95 (0.71–1.28) | 1.22 (0.93–1.58) | 1.88 (1.36–2.60) |
| Caucasian (yes) | 8 | 1.35 (0.89–2.03) | 0.53 (0.25–1.12) | 0.93 (0.65–1.31) | 0.77 (0.50–1.18) | 0.62 (0.35–1.09) |
| Caucasian (no) | 3 | 0.93 (0.74–1.17) | 1.28 (0.76–2.18) | 0.94 (0.67–1.34) | 1.00 (0.72–1.40) | 1.38 (0.85–2.24) |
HWE: Hardy–Weinberg equilibrium.
Figure 3.
Forest plot of OR for association between the IL-6-572G/C polymorphism and sepsis under the dominant model.
Table 3.
Summary ORs and 95% CIs of IL-6-572C/G polymorphism and sepsis risk.
|
G vs. C |
GG vs. CC |
GC vs. CC |
Dominant model |
Recessive model |
||
|---|---|---|---|---|---|---|
| n | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Total | 4 | 0.98 (0.79–1.22) | 1.46 (0.53–4.03) | 0.82 (0.54–1.27) | 0.88 (0.55–1.41) | 1.55 (0.82–2.92) |
| Ethnicity | ||||||
| Asian | 2 | 1.06 (0.76–1.47) | 1.61 (0.63–4.08) | 0.93 (0.62–1.41) | 0.99 (0.67–1.47) | 1.71 (0.68–4.32) |
| Caucasian | 2 | 0.88 (0.32–2.40) | 1.53 (0.13–17.95) | 0.74 (0.31–1.79) | 0.79 (0.29–2.18) | 1.67 (0.18–15.81) |
| Age | ||||||
| Adult | 3 | 0.83 (0.52–1.34) | 0.98 (0.47–2.06) | 0.72 (0.43–1.22) | 0.76 (0.44–1.29) | 1.10 (0.53–2.28) |
| Paediatric | 1 | / | / | / | / | / |
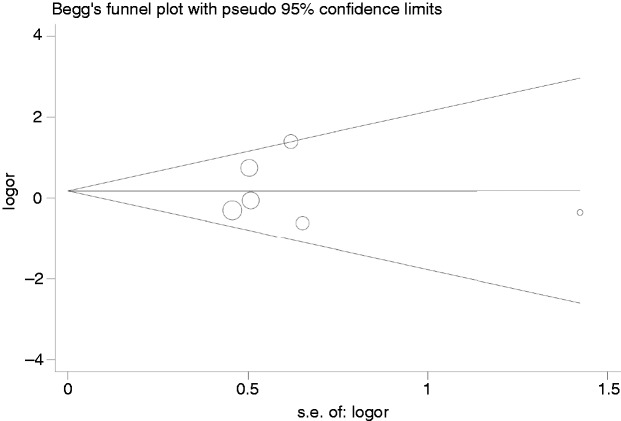
Publication bias
Begg’s funnel plots were conducted to evaluate the publication bias of enrolled articles. There was no obvious asymmetry in the allele model in consideration of the shapes of funnel plots in all genetic models, implying the low publication bias in our meta-analysis (Figures 4 and 5).
Figure 4.
Begg’s funnel plot for association between the IL-6-174G/C polymorphism and sepsis.
Figure 5.
Begg’s funnel plot for association between the IL-6-572G/C polymorphism and sepsis.
Discussion
Sepsis is characterised as a systemic inflammatory response due to infection. A central event in the pathophysiological cascade of sepsis is excessive systemic release of pro-inflammatory cytokines such as IL-6 in response to microbial invasion. IL-6 is a multifunctional cytokine regulating immune reactions and is regarded as an endogenous pyrogen that induces fever in patients with infections. The -174G/C and -572C/G polymorphisms in the IL-6 gene have been found to be related to IL-6 promoter activity, resulting in interindividual alterations in cytokine levels.28 In various genetic studies, IL-6 polymorphisms in the promoter region have been shown to increase the risk for sepsis, albeit with inconsistent results. Different ethnicities, insufficient power and the small effect of the polymorphism on sepsis risk make it even more complicated to interpret these studies. In consideration of the elevated numbers of studies in recent years, there is a need to reconcile these data.
In this meta-analysis, we systemically examined the correlation of IL-6 gene polymorphisms (-174G/C and -572C/G) with sepsis susceptibility. As a result, the IL-6 gene -174G/C polymorphism was not statistically associated with sepsis risk. In subgroup analysis stratified by nationality, this polymorphism was related to sepsis in Asians and Africans but not in Caucasians. On the one hand, only two articles on Asians and Africans were analysed, and the number of studies and samples were relatively small. On the other hand, two included studies for Asian ethnicity were not in HWE equilibrium. However, the included studies report very limited information to provide any detailed insight into these potential problems. Well-designed studies with more subjects will be required for further validation of these results. In subgroup analysis stratified by age, this polymorphism was not significantly correlated to sepsis risk in adult, neonatal or paediatric populations. Sensitivity analysis was conducted by omitting HWE studies for the total population and Caucasian ethnicity. Non-HWE studies changed the outcomes, implicating that our results had a publication bias due to non-HWE studies. For the IL-6-572C/G polymorphism, there was no significant relationship of this genotype with sepsis risk, which was still insignificant in subsequent subgroup analyses stratified by ethnicity or age. As with other diseases, the pathogenesis of sepsis is caused by synergistic interaction of several genes and gene environments, and these interactions require further investigation in future studies.
There are certain limitations in the study. First, the majority of included research only studied the correlation of IL-6 polymorphisms with sepsis risk, while there is inaccessibility of more accurate adjusted ORs for other covariates (including family history, age, environmental factors and infectious condition). Second, the incomplete raw data and publication limitations led to a failure to enrol all relevant studies in the present meta-analysis. Third, we failed to investigate the genetic–environmental interplays due to multiple testing and inadequate studies. Indeed, it is probable that certain lifestyle and environmental factors (such as smoking) could affect the relationship of gene polymorphisms with sepsis risk.
Taken together, we demonstrated that the IL-6-174G/C polymorphism may contribute to sepsis risk in Africans and Asians. No significant association was observed between the IL-6-572G/C polymorphism and sepsis risk. Further evaluation of the influence of gene polymorphisms on sepsis will require well-designed studies with large sample sizes.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Zhejiang Medical and Health Science and Technology Project (2019KY248).
References
- 1.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008; 8: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev 2013; 93: 1247–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grealy R, White M, Stordeur P, et al. Characterising cytokine gene expression signatures in patients with severe sepsis. Mediators Inflamm 2013; 2013: 164246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Snick J. . Interleukin-6: an overview. Annu Rev Immunol 1990; 8: 253–278. [DOI] [PubMed] [Google Scholar]
- 5.Palmiere C. Markers for sepsis diagnosis in the forensic setting: state of the art. Croat Med J 2014; 55: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 1998; 102: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem 2000; 275: 18138–18144. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Feng J, Li X. Association between the -174G/C polymorphism of the interleukin-6 gene and myocardial infarction risk: a meta-analysis. Genet Mol Res 2016; 15. [DOI] [PubMed] [Google Scholar]
- 9.Lu YM, Cao LF, Li YQ, et al. RANTES gene polymorphisms and risk of pediatric asthma: a meta-analysis. Exp Ther Med 2012; 4: 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlüter B, Raufhake C, Erren M, et al. Effect of the interleukin-6 promoter polymorphism (-174G/C) on the incidence and outcome of sepsis. Crit Care Med 2002; 30: 32–37. [DOI] [PubMed] [Google Scholar]
- 13.Treszl A, Kocsis I, Szathmári M, et al. Genetic variants of TNF-alpha, IL-1beta, IL-4 receptor alpha-chain, IL-6 and IL-10 genes are not risk factors for sepsis in low-birthweight infants. Biol Neonate 2003; 83: 241–245. [DOI] [PubMed] [Google Scholar]
- 14.Balding J, Healy CM, Livingstone WJ, et al. Genomic polymorphic profiles in an Irish population with meningococcaemia: is it possible to predict severity and outcome of disease. Genes Immun 2003; 4: 533–540. [DOI] [PubMed] [Google Scholar]
- 15.Ahrens P, Kattner E, Köhler B, et al. Mutations of genes involved in the innate immune system as predictors of sepsis in very low birth weight infants. Pediatr Res 2004; 55: 652–656. [DOI] [PubMed] [Google Scholar]
- 16.Sipahi T, Pocan H, Akar N. Effect of various genetic polymorphisms on the incidence and outcome of severe sepsis. Clin Appl Thromb Hemost 2006; 12: 47–54. [DOI] [PubMed] [Google Scholar]
- 17.Göpel W, Härtel C, Ahrens P, et al. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes Immun 2006: 7: 65–68. [DOI] [PubMed] [Google Scholar]
- 18.Baier RJ, Loggins J, Yanamandra K. IL-10, IL-6 and CD14 polymorphisms and sepsis outcome in ventilated very low birth weight infants. BMC Med 2006; 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalek J, Svetlikova P, Fedora M, et al. Interleukin-6 gene variants and the risk of sepsis development in children. Hum Immunol 2007; 68: 756–760. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Hady H, El-Naggar M, El-Nady G, et al. Genetic polymorphisms of IL-6-174 and IL-10-1082 in full term neonates with late onset blood stream infections. J Pediatr Infect Dis 2009; 4: 357–365. [Google Scholar]
- 21.Davis SM, Clark EA, Nelson LT, et al. The association of innate immune response gene polymorphisms and puerperal group A streptococcal sepsis. Am J Obstet Gynecol 2010; 202: 308.e1–8. [DOI] [PubMed] [Google Scholar]
- 22.Carregaro F, Carta A, Cordeiro JA, et al. Polymorphisms IL10–819 and TLR-2 are potentially associated with sepsis in Brazilian patients. Mem Inst Oswaldo Cruz 2010; 105: 649–656. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Oda S, Sadahiro T, et al. Outcome prediction in sepsis combined use of genetic polymorphisms – a study in Japanese population. Cytokine 2011; 54: 79–84. [DOI] [PubMed] [Google Scholar]
- 24.Martín-Loeches I, Solé-Violán J, Rodríguez de Castro F, et al. Variants at the promoter of the interleukin-6 gene are associated with severity and outcome of pneumococcal community-acquired pneumonia. Intensive Care Med 2012; 38: 256–262. [DOI] [PubMed] [Google Scholar]
- 25.Panayides A, Ioakeimidou A, Karamouzos V, et al. -572G/C single nucleotide polymorphism of interleukin-6 and sepsis predisposition in chronic renal disease. Eur J Clin Microbiol Infect Dis 2015; 34: 2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao ZR, Zhang SL, Feng B. Association of IL-10 (-819T/C,-592A/C and -1082A/G) and IL-6-174G/C gene polymorphism and the risk of pneumonia-induced sepsis. Biomarkers 2017; 22: 106–112. [DOI] [PubMed] [Google Scholar]
- 27.Gu W, Du DY, Huang J, et al. Identification of interleukin-6 promoter polymorphisms in the Chinese Han population and their functional significance. Crit Care Med 2008; 36: 1437–1443. [DOI] [PubMed] [Google Scholar]
- 28.Chérel M, Campion L, Bézieau S, et al. Molecular screening of interleukin-6 gene promoter and influence of -174G/C polymorphism on breast cancer. Cytokine 2009; 47: 214–223. [DOI] [PubMed] [Google Scholar]