Short abstract
LPS can induce an inflammatory immune response in the intestine, and long non-coding RNA (lncRNA) is involved in the process of inflammatory disease. However, the biological role of lncRNA in the intestinal inflammation of piglets remains unclear. In this study, the lncRNA expression profile of the ileal mucosa of piglets challenged by LPS was analysed using lncRNA sequencing. In total, 112 novel lncRNAs were predicted, of which 58 were up-regulated and 54 down-regulated following LPS challenge. Expression of 15 selected lncRNAs was validated by quantitative PCR. We further investigated the target genes of lncRNA that were enriched in the signalling pathways involved in the inflammatory immune response by utilising Gene Ontology and Kyoto Encyclopaedia of Genes and Genomes analysis, with cell adhesion molecules and mTOR signalling pathway identified. In addition, the co-expression networks between the differentially expressed lncRNAs and the target mRNAs were constructed, with seven core lncRNAs identified, which also demonstrated that the relationship between lncRNAs and the target genes was highly correlated. Our study offers important information about the lncRNAs of the mucosal immune system in piglets and provides new insights into the inflammatory mechanism of LPS challenge, which might serve as a novel target to control intestinal inflammation.
Keywords: Long non-coding RNA, lipopolysaccharide, intestinal inflammation, cam signalling pathway, mTOR signalling pathway
Introduction
Long non-coding RNAs (lncRNAs) are more than 200 nucleotides long, belong to the subgroup of non-coding RNAs and have the characteristic of no protein coding ability.1 Previous research has shown that lncRNAs have important biological functions. lncRNAs potentially influence the extracellular matrix and are involved in the metastasis of hepatocellular carcinoma.2 It has been documented that overexpression of lncRNA has an impact on proliferation, invasion and migration of OVCAR-3 tumor cells and attenuates apoptosis by activation of the PI3K/Akt/mTOR signalling pathway.3 Expression of lncRNA p21 is aberrantly up-regulated in human non-small-cell lung cancer and reduces apoptosis by down-regulating PUMA expression.4 In addition, lncRNA uc.173 regulates the growth of mouse intestinal mucosa and promotes intestinal epithelial renewal through attenuating expression of miRNA195.5 lncRNA H19 is related to mucosal regeneration, and is significantly up-regulated by IL-22 inflamed intestinal tissues and epithelial cells of mice induced by LPS.6 However, whether the changes in lncRNAs in the ileal mucosa are associated with inflammation in piglets has not been investigated.
LPS is an important component of the Gram-negative bacterial outer membrane.7 Previous research has reported that Helicobacter pylori LPS modulates pathogen-elicited host inflammatory immune responses, leading to chronic inflammation in the gastrointestinal tract.8 LPS induces host inflammation that enhances prostate cancer metastasis by NF-κB activation.9 Low-grade inflammation triggered by LPS can up-regulate expression of hypothalamic C-Jun N-terminal kinase, resulting in insulin resistance.10 It has been documented that LPS induces lncRNA changes in human endothelial cells that might be responsible for sepsis-induced endothelial dysfunction.11 Investigation of LPS-triggered expression profiles in the rodent central nervous system is crucial to exploration of the function of lncRNAs, which is related to the pathogenesis of sepsis-associated encephalopathy.12 Overexpression of lncRNA THRIL could promote LPS-stimulated osteoarthritis cell (ATDC5) inflammatory injury through decreasing miR-125b expression and activating the JAK1/STAT3 and NF-κB signalling pathways.13 So, we speculated that lncRNA might be involved in intestinal inflammation.
Whether lncRNA is involved in ileal mucosal inflammatory response to LPS has not been explored in vivo. In addition, the signalling pathways enriched by the target genes of the lncRNAs that were induced by LPS have not been fully understood. In this study, our objective was to investigate the lncRNAs that participated in the ileal mucosal inflammation stimulated by LPS. Our results suggested that lncRNA is involved in the regulation of host inflammatory immune response. Our study provides new insights into the inflammatory mechanism of LPS induction, which might serve as a novel target to control intestine inflammation.
Materials and methods
Ethics approval
This study was carried out in strict accordance with the recommendations of the China Regulations for the Administration of Affairs Concerning Experimental Animals 1988 and Hubei Regulations for the Administration of Affairs Concerning Experimental Animals 2005. The protocols were approved by China Hubei Province Science and Technology Department (permit number SYXK(ER) 2010-0029). All experimental animals were killed at the end of the experiments. All experiments were approved by Wuhan Polytechnic University guidelines and regulations.
Experimental design
Six 35-d-old naturally farrowed early-weaned piglets (Duroc×Landrace×Large White), weighing 9–10 kg, were purchased and used for in vivo experiments. The piglets were randomly divided into two groups. Group 1 was challenged i.p. with LPS (Sigma–Aldrich, St. Louis, MO) from Escherichia coli at 100 μg/kg. Group 2 was administered i.p. with the equivalent amount of 0.9% NaCl solution (Sinopharm, Beijing, PR China) as the control group. Three h after injection of LPS, all the piglets from both groups were killed. The ileal mucosa was collected, frozen in liquid nitrogen and stored at −80°C for sequencing analysis.
RNA extraction and quality control
Approximately 25 mg ileal mucosa was re-suspended in TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA was extracted according to the manufacturer’s protocols.14 The amount of total RNA was measured using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The quality of the total RNA was determined by an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA).
Construction of RNA-Sequencing libraries
The RNA-Sequencing (RNA-Seq) libraries were prepared using ileal mucosa, and 150 bp paired-end sequencing were carried out using the HiSeq platform (Illumina, San Diego, CA).15 The RNA-Seq libraries were constructed with 2 μg total RNA using the TruSeq Kit (Illumina), with some modifications. rRNA was removed by applying the Ribo-Zero rRNA Removal Kit (Illumina), which was instead of purifying of poly-A RNA by utilising the poly-dT primer beads. Other steps were determined according to the manufacturer’s instructions. Analysis of RNA-Seq libraries was performed for quality control, and the average length of inserts was 200–300 bp. The libraries were sequenced using the HiSeq platform (Illumina).
Prediction of lncRNA
Transcripts of FPKM=0 were deleted based on the results of assembly. The open reading frames of the transcripts were predicted using the tool of TransDecoder (https://transdecoder.github.io/), and transcripts > 300 nt or < 200 nt were removed.16 The non-coding potentials of lncRNAs were predicted by using the combination of four software platforms of Pfamscan (http://www.ebi.ac.uk/Tools/pfa/pfamscan), Coding Potential Calculator (CPC) (http://www.cpc.cbi.pku.edu.cn), Coding-Non-Coding Index (CNCI) (http://www.bioinfo.org/software/cnci/) and PhyloCSF (http://www.github.com/mlin/PhyloCSF/wiki) at the same time.17
Prediction and annotation of lncRNA targets
We identified the target genes 100 kb upstream and downstream of the lncRNAs, and the relationship between the target genes and lncRNAs was determined utilising the Bedtools programme.18 Prediction of the lncRNA target genes included the cis and trans target genes. Prediction of the cis target genes relied upon the lncRNA function being associated with the protein-coding genes that were adjacent to their coordinates. The prediction of the trans target genes was to screen the genes encoded by the nearest protein of the lncRNA. We thought that the screened protein-encoding gene could be a cis-regulated target gene that interacted with the lncRNA.
Gene Ontology and Kyoto Encyclopaedia of Genes and Genomes enrichment analysis
The analysis of Gene Ontology (GO; http://geneontology.org/) and Kyoto Encyclopaedia of Gene and Genomes (KEGG; http://www.kegg.jp/) enrichment was carried out to identify target genes of differentially expressed lncRNAs.19 All target genes were mapped to each term within the GO database. GO terms with corrected a P-value of ≤ 0.05 were considered as significantly enriched. The KEGG automatic annotation server (KASS) was utilised to carry out pathway annotation using the entire genome as the background. Pathways with a P-value of ≤ 0.05 were thought to be significantly enriched.
Co-expression analysis of lncRNA and mRNA
lncRNAs interact with mRNA and can regulate expression of the target genes. CytoScape software was utilised to screen the lncRNA target genes, as described previously.20 To construct a co-expression network, the expression levels derived from the total RNA-Seq data were screened to detect the similar expression patterns of DElncRNAs and their potential targets. The Pearson correlation coefficient and corresponding P-value was calculated, but only the strongest correlations (correlation coefficient > 0.9 or < –0.9, and P<0.05) were retained to make a visual representation of the co-expression network.
Quantitative real-time PCR
RNA was extracted from ileal mucosa using TRIzol reagent (Invitrogen). Following that, cDNA was reverse transcribed using reverse transcriptase (TaKaRa, Dalian, PR China) and was further quantified using a SYBR Green PCR Kit (TaKaRa) according to the manufacturer’s instructions. Three technical repeats were utilised for individual transcript in each sample, with GAPDH as the reference gene. The primer sequences used for the quantitative PCR are listed in online Supplemental Table S1.
Statistical analysis
The experimental data are expressed as mean ± SD. The difference between two groups was analysed by a two-tailed Student’s t-test. P < 0.05 was considered significant.
Results
lncRNA sequencing results
To explore the global characteristics of the changes in lncRNA in the ileal mucosa of piglets following challenge with LPS, we used the lncRNA sequencing in the HiSeq platform (Illumina). A total of 259,220,396 ± 2,590,095.8 raw reads were obtained from the ileal mucosa of the piglets challenged with LPS, which were aligned to the Sus scrofa genome compared to the control group of 259,816,902 ± 2,454,107.3 raw reads (Table 1). In addition, 515,005,900 clean reads were acquired by quality control, with the clean reads rate ranging from 99.14 to 99.28 (Table 1). Furthermore, 165,736,502 ± 1,232,067.5 and 169,911,087 ± 1,966,214 of uniquely mapped reads were gained from the LPS challenge and control groups, respectively (Table 1). The uniquely mapped reads were detected in the range 82.39–84.65 (Table 1), which indicated that the high-quality sequencing data could be serviced for the next analysis.
Table 1.
Statistics of clean reads in the ileum mucosa in piglets.
| Samples | Reads num. | Q30 reads (%) | Total clean reads | Clean reads (%) | Mapped reads | Mapping rate (%) | Uniquely mapped reads | Uniquely mapped reads (%) |
|---|---|---|---|---|---|---|---|---|
| S12 | 83,840,036 | 93.22 | 83,218,398 | 99.25 | 65,074,641 | 78.20 | 54,508,006 | 83.76 |
| S13 | 87,453,416 | 92.81 | 86,747,160 | 99.19 | 68,084,409 | 78.49 | 57,018,574 | 83.75 |
| S14 | 88,523,450 | 93.16 | 87,876,806 | 99.26 | 68,973,826 | 78.49 | 58,384,507 | 84.65 |
| S22 | 83,952,602 | 93.26 | 83,352,740 | 99.28 | 65,141,394 | 78.15 | 54,258,618 | 83.29 |
| S23 | 86,153,598 | 93.24 | 85,455,516 | 99.18 | 66,577,877 | 77.91 | 54,851,509 | 82.39 |
| S25 | 89,114,196 | 92.58 | 88,355,280 | 99.14 | 68,008,307 | 76.97 | 56,626,375 | 83.26 |
The control group: S12, S13, S14; Ileal mucosa of piglets challenged by LPS: S22, S23, S25.
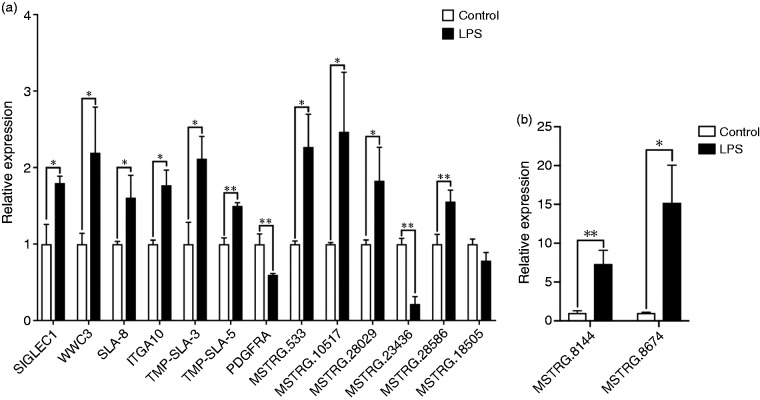
Our results showed that 112 lncRNAs were significantly differentially expressed in ileal mucosa of the piglets challenged with LPS. In addition, compared to the control group, 58 lncRNAs were up-regulated and 54 down-regulated following LPS challenge, with a fold change > 2 (P < 0.05; Figure 1). Furthermore, the top 10 expression level at up- and down-regulation are showed in Table 2. The most significant differential lncRNAs, as well as lncRNAs identified in the co-expression analysis, were selected as the candidates for further quantitative RT-PCR validation. Results showed that SIGLEC1, WWC3, SLA-8, ITGA10, TMP-SLA3, TMP-SLA-5, MSTRG.533, MSTRG.10517, MSTRG.28029, MSTRG.28586, MSTRG.8144 and MSTRG.8674 were significantly up-regulated in the LPS challenged ileal mucosa, while PDGFRA and MSTRG.23436 were significantly down-regulated (Figure 2).
Figure 1.
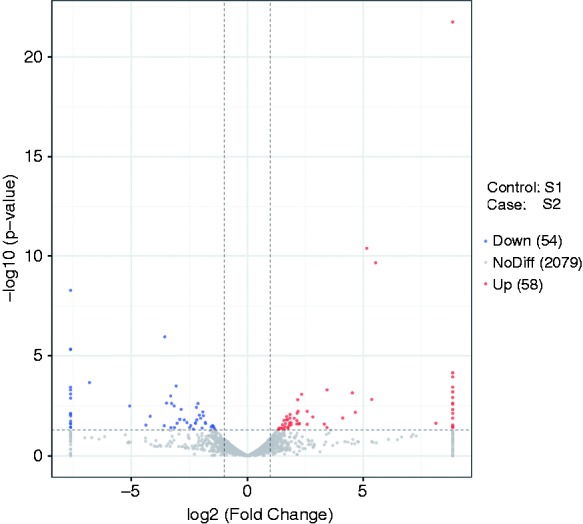
Volcano plots of the detected long non-coding RNAs (lncRNAs) in the ileal mucosa of piglets challenged with LPS. The control group (S1): S12, S13, S14; ileal mucosa of piglets challenged by LPS (S2): S22, S23, S25.
Table 2.
Top 10 expression levels at up- and down-regulation of LncRNAs.
| GeneID | log2 fold change | Up/down | P-value |
|---|---|---|---|
| ENSSSCG00000030767 | 8.125782453 | Up | 0.023431105 |
| MSTRG.533 | 5.532879028 | Up | 0.000000021 |
| MSTRG.10517 | 5.364472577 | Up | 0.001519058 |
| MSTRG.50977 | 5.152625922 | Up | 0.000000159 |
| MSTRG.28029 | 4.657895757 | Up | 0.006710455 |
| MSTRG.37166 | 4.532799202 | Up | 0.000707623 |
| MSTRG.34715 | 4.116648791 | Up | 0.012862051 |
| MSTRG.38432 | 3.440076305 | Up | 0.000500609 |
| MSTRG.27410 | 3.438033247 | Up | 0.038478878 |
| MSTRG.43434 | 3.325514473 | Up | 0.025942251 |
| MSTRG.20741 | –7.612131114 | Down | 0.000004622 |
| MSTRG.23436 | –6.797028078 | Down | 0.000216454 |
| MSTRG.30184 | –5.067784487 | Down | 0.003200392 |
| MSTRG.8674 | –4.37402212 | Down | 0.02892462 |
| MSTRG.37395 | –4.190284089 | Down | 0.010521 |
| MSTRG.41908 | –3.585430649 | Down | 0.030951158 |
| MSTRG.28586 | –3.561576869 | Down | 0.000001103 |
| MSTRG.8144 | –3.487897829 | Down | 0.002299782 |
| MSTRG.50678 | –3.30787314 | Down | 0.001018054 |
| MSTRG.19976 | –3.297246311 | Down | 0.038865818 |
Figure 2.
Quantitative PCR validation of selected lncRNA transcripts. (a) Relative expression of lncRNA SIGLEC1, WWC3, SLA-8, ITGA10, TMP-SLA3, TMP-SLA-5, PDGFRA, MSTRG.533, MSTRG.10517, MSTRG.28029, MSTRG.23436, MSTRG.28586 and MSTRG.18505. (b) Relative expression of lncRNA MSTRG.8144 and MSTRG.8674. *P < 0.01; **P < 0.01.
lncRNA functional prediction in the ileal mucosa of the piglets
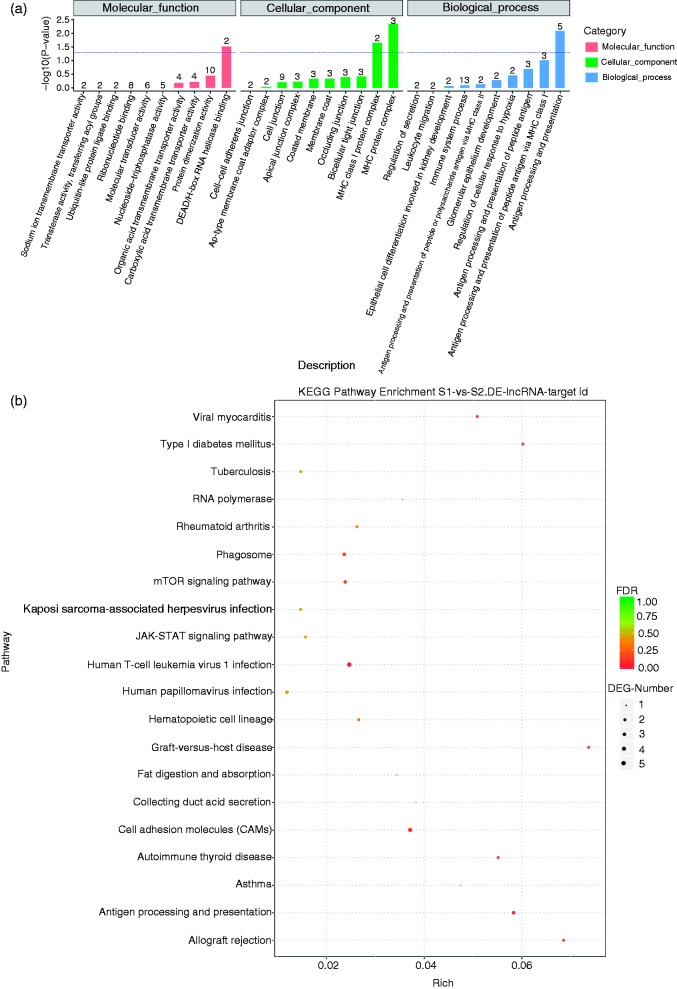
To determine the important biological functions of the lncRNA involved in the ileal mucosa of the piglets, the candidate target genes of the lncRNA were predicted by evaluating the cis functions. Cis function prediction analysis showed that 1945 target genes were screened for the lncRNAs (online Supplemental Table S2). Hence, the target genes of the lncRNAs were characterised by exploring the enrichment analysis by utilising the GO classification and KEGG pathway. GO enrichment analysis demonstrated that the target genes were attributed to three types, which were involved in biological process, molecular function and cellular components (Figure 3a). Further analysis indicated that DEAD/H-box RNA helicase binding, MHC protein complex and Ag processing and presentation were the most abundant with which the target genes of the lncRNAs were involved (Figure 3a).
Figure 3.
GO functional category analysis and KEGG pathway analysis of the target genes of the differentially expressed lncRNAs. (a) DEAD/H-box RNA helicase binding, MHC protein complex, and Ag processing and presentation were the most abundant in GO analysis. (b) Cell adhesion molecules (CAMs), mTOR signalling pathway, antigen processing and presentation, phagosomes, JAK/STAT signalling pathway, and other disease-related pathways were revealed by KEGG pathway analysis.
The target genes involved in the signalling pathways were explored by KEGG pathway analysis. The data demonstrated that the main signalling pathways were cell adhesion molecules (CAMs), mTOR signalling pathway, Ag processing and presentation, phagosomes, JAK/STAT signalling pathway and other disease-related pathways (Figure 3b).
Global gene changes of ileal mucosa in piglets following LPS challenge
To explore the transcriptional regulation in inflammatory immune responses induced by LPS further, gene expression profiling was determined using RNA-Seq. We identified 415 differentially expressed genes (DEGs), of which 228 were up-regulated and 187 down-regulated (Figure 4).
Figure 4.
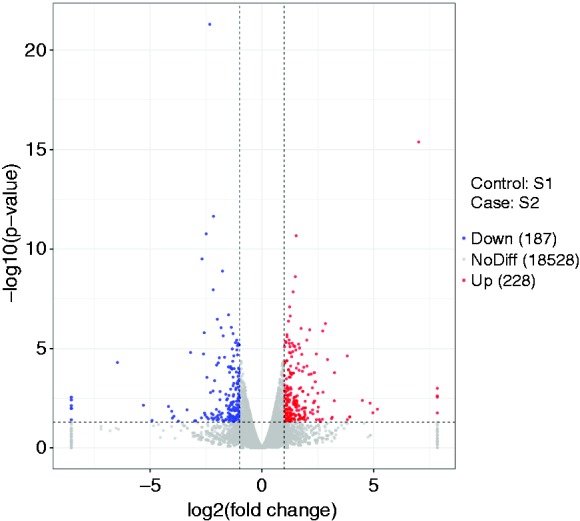
Volcano plots of the mRNAs detected by RNA-sequencing in ileal mucosa of piglets challenged with LPS. The control group (S1): S12, S13, S14; ileal mucosa of piglets challenged by LPS (S2): S22, S23, S25.
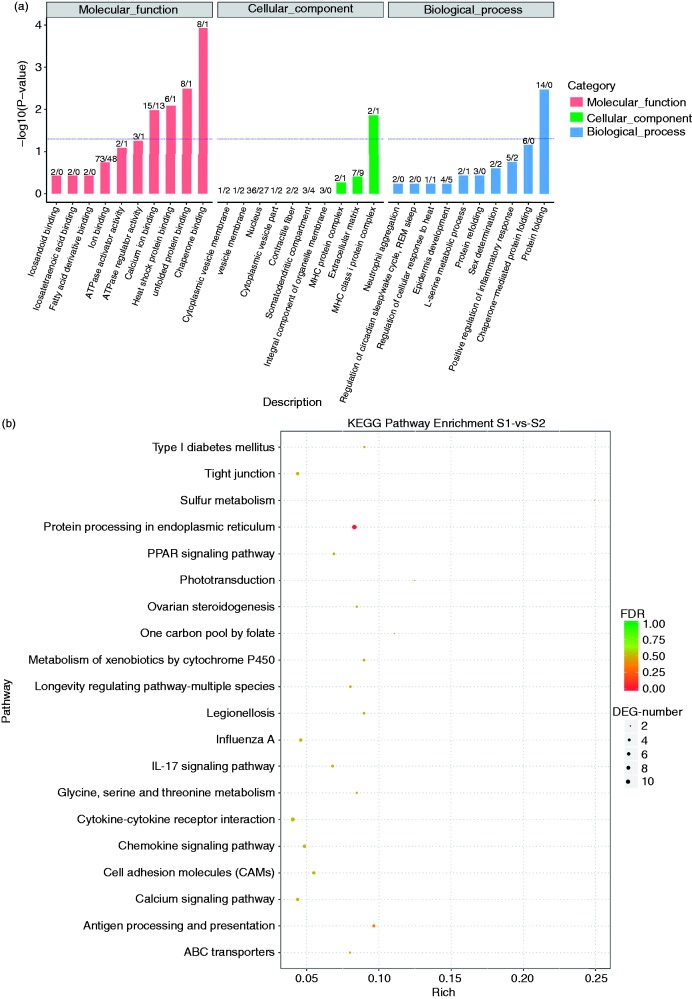
GO classes associated with DEGs were chaperone binding, MHC class I protein complex and protein folding. GO analysis related to molecular function, cellular component and biological process is shown in Figure 5a. In KEGG analysis, the significant signalling pathways in which DEGs were involved were protein processing in the endoplasmic reticulum, Ag processing and presentation, cytokine–cytokine receptor interaction, chemokine signalling pathway, cell adhesion molecules and tight junctions, which may be related to inflammatory immune responses (Figure 5b).
Figure 5.
Gene Ontology (GO) functional category analysis and Kyoto Encyclopaedia of Gene and Genomes (KEGG) pathway analysis of the differentially expressed genes. (a) Chaperone binding, MHC class protein complex and protein folding were the most abundant in GO analysis. (b) Protein processing in the endoplasmic reticulum, antigen processing and presentation, cytokine–cytokine receptor interaction, chemokine signalling pathway, cell adhesion molecules and tight junctions were revealed by KEGG pathway analysis.
KEGG analysis of CAMs and mTOR signalling pathway
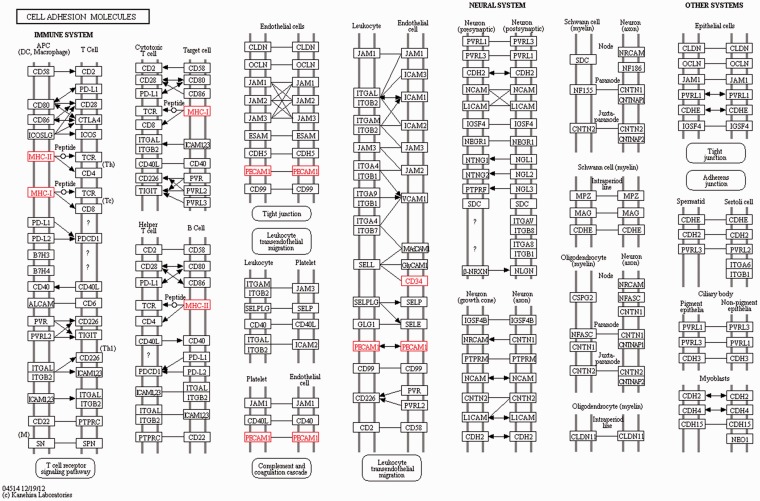
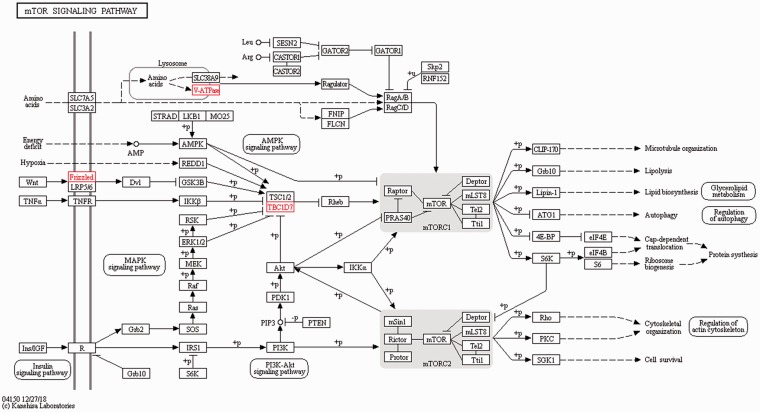
The target genes of the DElncRNA identified were utilised to explore the effects and network of the proteins that the genes encoded using KEGG analysis. The target genes that likely participated in the interesting signalling pathway and might have been associated with the inflammatory immune response or damage were screened by KEGG pathway analysis. The signalling pathway analysis demonstrated that compared to the control group, four target genes (MHC-I, MHC-II, PECAM1 and CD34) participated in the CAM signalling pathway, which were all up-regulated (Figure 6). In addition, another three up-regulated target genes (TBC1D7, v-ATPase and Frizzled) were involved in the mTOR signalling pathway (Figure 7). Activation of these important signalling pathways might be considered as key, resulting in an inflammatory immune reaction or damage induced by LPS.
Figure 6.
CAM signalling pathway identified by KEGG analysis of the target genes of the DElncRNA. MHC-I, MHC-II, PECAM1 and CD34 were up-regulated and participated in the CAM signalling pathway.
Figure 7.
mTOR signalling pathway identified by KEGG analysis of the target genes of the DElncRNA. TBC1D7, v-ATPase and Frizzled were up-regulated and participated in the mTOR signalling pathway.
Co-expression analysis of interaction between differentially expressed lncRNAs and target genes in the ileal mucosa of the piglets challenged by LPS
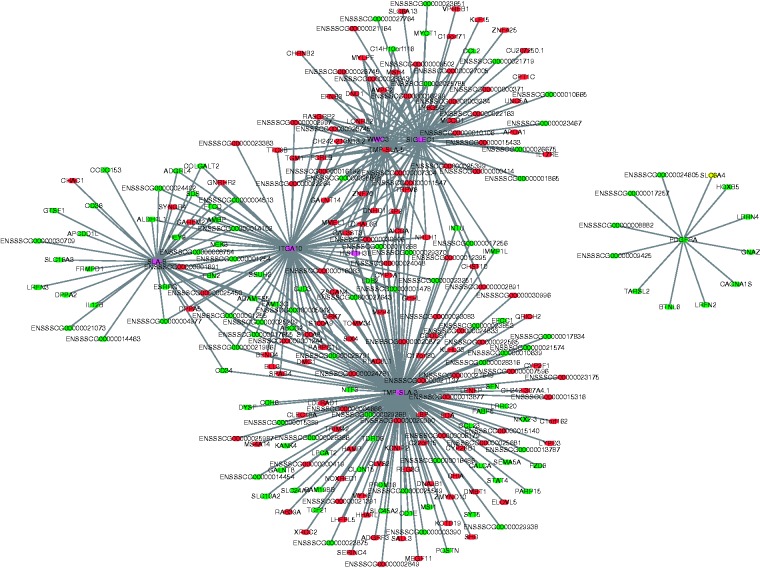
To explore the function of the identified lncRNAs, co-expression networks were constructed between differentially expressed lncRNA and mRNAs using CytoScape software. A total of 112 DElncRNAs and 415 DEGs were correlated to each other according to their similar expression pattern, and only the strong correlations (correlation > 0.9, P < 0.05) were utilised to construct the co-expression network (Figure 8), in which seven core lncRNAs (SLA-8, ITGA10, WWC3, SIGLEC1, TMP-SLA-5, TMP-SLA-3 and PDGFRA) and the corresponding targets were identified. This suggests that these interactions may have important effects on the process of inflammatory response in LPS challenge. Meanwhile, none of these core lncRNAs have homologs in humans or mice after searching the NONCODE database (http://www.noncode.org/index.php). Furthermore, the qPCR result showed that lncRNA SLA-8, ITGA10, WWC3, SIGLEC1, TMP-SLA-3 and TMP-SLA-5 were up-regulated in the LPS-challenged ileal mucosa, while PDGFRA was down-regulated (Figure 2).
Figure 8.
Construction of the co-expression network of lncRNAs and mRNAs. In the interaction network, circles and diamonds represent mRNA and lncRNA, respectively. Genes coloured red were up-regulated, genes coloured green were down-regulated, genes coloured purple were up-regulated or down-regulated or were unchanged for different transcripts.
Discussion
We explored the expression profile of lncRNAs and mRNAs through RNA-Seq in the ileal mucosa of piglets challenged by LPS. A total of 112 novel lncRNAs were identified to be differentially expressed between the LPS challenge and control groups, of which 58 were up-regulated and 54 down-regulated. We also analysed the mRNA changes, and we found that 228 DEGs were up-regulated and 187 were down-regulated. The changes in lncRNAs and mRNAs provided an important basis for understanding the inflammatory immune responses in the ileal mucosa challenged by LPS.
With the improvement of the sequencing technology, mammalian genomes have been shown to have many kinds of lncRNAs.21,22 lncRNAs have important biological functions involved in the mucosal immune responses.23 So far, many lncRNAs have been identified, but the interactions between lncRNAs and their target genes have not been explored in detail. Pathological damage of the ileal mucosa has been seen when piglets are challenged by LPS.24 The question is whether this damage is related to the effect of lncRNAs. The relationship between the change in lncRNA expression in the ileal mucosa resulting from LPS stimulation and the pathological damage needs to be further investigated.
In our study, a co-expression network composed with seven core lncRNAs and their corresponding targets were identified in the LPS challenged ileal mucosa. Quantitative RT-PCR showed that SLA-8, ITGA10, WWC3, SIGLEC1, TMP-SLA-3 and TMP-SLA-5 were up-regulated and PDGFRA was down-regulated. Among these targets, many genes participate in the process of inflammation responses. IL-17RE regulates mucosal immunity to infection, with intestinal pathogens as a receptor of IL-17C.25 CCL2 during inflammation provides a mechanism to limit and resolve acute inflammation.26 Overexpression of CCL23 might contribute to the recruitment of inflammatory cells, including monocytes and macrophages, and the amplification of local inflammation.27 Thus, we infer that these core lncRNAs may participate in the inflammatory immune responses by interacting with their target genes.
CAMs are glycoproteins expressed on the cell surface that play an important role in the inflammatory immune response.28 It has been documented that CAMs are involved in neuronal differentiation and may serve as an important target to improve nerve regeneration.29 Inflammatory cytokines could stimulate CAM expression in neutrophils and macrophages and recruit leukocytes, leading to the pathogenesis of vascular inflammatory diseases.30 CAMs also participate in the process of allergic inflammation.31 In this study, the lncRNA target genes MHC-I, MHC-II, PECAM1 and CD34 were all significantly up-regulated in the ileal mucosa induced by LPS, which participates in the CAM signalling pathway. It has been shown that CD34 is an important inflammatory biomarker in peri-implant soft tissues.32 Therefore, CAMs might be considered as a novel therapeutic target to control the ileal mucosal inflammation.
We also found that three target genes (TBC1D7, v-ATPase and Frizzled) were up-regulated after LPS stimulation and involved in the mTOR signalling pathway. Previous research has shown that autophagy and inflammation are regulated by lncRNA-FA2H-2-mixed lineage kinase domain-like protein (MLKL), which is essential via mTOR-dependent signalling pathway in atherosclerosis-related diseases.33 The regulation of airway remodeling of asthma is adjusted by miRNA-133a utilising the mTOR/PI3K/Akt signalling pathway, which targets insulin-like growth factor-1 receptor.34 Neutrophil infiltration in rheumatoid arthritis was attenuated by blocking of Yin Yang 1 (YY1) through the PI3K/Akt/mTOR signalling pathway.35 Decrease of lncRNA for nuclear enriched abundant transcript 1 (NEAT1) in a streptozotocin-induced diabetes model inhibited proliferation and fibrosis in diabetic nephropathy through activating the Akt/mTOR signalling pathway.36 lncRNA JPX inhibits cell proliferation, invasion and migration in human ovarian cancer cell lines via activating the PI3K/Akt/mTOR signalling pathway.37 We speculate that the mTOR signalling pathway is a key pathway involved in the inflammatory response of the ileal mucosa when stimulated by LPS, which might provide a novel pathological mechanism for ileal mucosal inflammation.
This is believed to be the first report of lncRNA and mRNA expression patterns in the ileal mucosa induced by LPS. Our study may provide some novel candidate units for exploration of lncRNAs and mRNAs related to ileal mucosal inflammation, which suggests new therapeutic targets to reduce inflammatory responses in the ileal mucosa.
Supplemental Material
Supplemental material, Supplemental Material1 for Long non-coding RNA profiling in LPS-induced intestinal inflammation model: New insight into pathogenesis by Ling Guo, Linna Li, Yang Zhang, Shulin Fu, Jing Zhang, Xiuying Wang, Huiling Zhu, Mu Qiao, Lingying Wu and Yulan Liu in Innate Immunity
Supplemental Material
Supplemental material, Supplemental Material2 for Long non-coding RNA profiling in LPS-induced intestinal inflammation model: New insight into pathogenesis by Ling Guo, Linna Li, Yang Zhang, Shulin Fu, Jing Zhang, Xiuying Wang, Huiling Zhu, Mu Qiao, Lingying Wu and Yulan Liu in Innate Immunity
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Project of the Hubei Provincial Department of Education (T201508), the National Natural Science Foundation of China (Grant No. 31601922) and the Project of Wuhan Science and Technology Bureau (2018020401011304).
Supplemental material
Supplemental material for this article is available online.
References
- 1.Dong J, Teng F, Guo W, et al. lncRNA SNHG8 promotes the tumorigenesis and metastasis by sponging miR-149-5p and predicts tumor recurrence in hepatocellular carcinoma. Cell Physiol Biochem 2018; 51: 2262–2274. [DOI] [PubMed] [Google Scholar]
- 2.Ji D, Chen GF, Liu X, et al. Identification of LINC01615 as potential metastasis-related long noncoding RNA in hepatocellular carcinoma. J Cell Physiol 2019; 234: 12964–12970. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Feng L, Tian C, et al. Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway. Eur Rev Med Pharmacol Sci 2018; 22: 8135–8144. [DOI] [PubMed] [Google Scholar]
- 4.Yang T, Zhang W, Wang L, et al. Long intergenic noncoding RNA-p21 inhibits apoptosis by decreasing PUMA expression in non-small cell lung cancer. J Int Med Res 2019; 47: 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L, Wu J, Wang JY, et al. Long noncoding RNA uc.173 promotes renewal of the intestinal mucosa by inducing degradation of microRNA 195. Gastroenterology 2018; 154: 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng H, Bu HF, Liu F, et al. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology 2018; 155: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leker K, Lozano-Pope I, Bandyopadhyay K, et al. Comparison of lipopolysaccharides composition of two different strains of Helicobacter pylori. BMC Microbiol 2017; 17: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slomiany BL, Slomiany A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin. Inflammopharmacology 2017; 25: 415–429. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Dash P, Minz AP, et al. Lipopolysaccharide (LPS) enhances prostate cancer metastasis potentially through NF‐κB activation and recurrent dexamethasone administration fails to suppress it in vivo. Prostate 2019; 79: 168–182. [DOI] [PubMed] [Google Scholar]
- 10.Rorato R, Borges BC, Uchoa ET, et al. LPS-induced low-grade inflammation increases hypothalamic JNK expression and causes central insulin resistance irrespective of body weight changes. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh KK, Matkar PN, Muhammad S, et al. Investigation of novel LPS-induced differentially expressed long non-coding RNAs in endothelial cells. Mol Cell Biochem 2016; 421: 157–168. [DOI] [PubMed] [Google Scholar]
- 12.Sun W, Pei L, Liang Z. mRNA and long non-coding RNA expression profiles in rats reveal inflammatory features in sepsis-associated encephalopathy. Neurochem Res 2017; 42: 3199–3219. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Wang Y, Zhang M, et al. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol 2019; 66: 354–361. [DOI] [PubMed] [Google Scholar]
- 14.Muret K, Klopp C, Wucher V, et al. Long noncoding RNA repertoire in chicken liver and adipose tissue. Genet Sel Evol 2017; 49: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Che T, Li D, Jin L, et al. Long non-coding RNAs and mRNAs profiling during spleen development in pig. PLoS One 2018; 13: e0193552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Zhang X, Han K, et al. Analysis of long noncoding RNA and mRNA using RNA sequencing during the differentiation of intramuscular preadipocytes in chicken. PLoS One 2017; 12: e0172389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong C, Chen Q, Zhao L, et al. Identification and characterization of long intergenic noncoding RNAs in bovine mammary glands. BMC Genomics 2017; 18: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menschaert G, Wang X, Jones AR, et al. The proBAM and proBed standard formats: enabling a seamless integration of genomics and proteomics data. Genome Biol 2018; 19: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HS, Tong HS, Zhao Y, et al. Differential expression pattern of exosome long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) in vascular endothelial cells under heat stroke. Med Sci Monit 2018; 24: 7965–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C, Yuan G, Hu Z. Bioinformatics analysis of the interactions among lncRNA, miRNA and mRNA expression, genetic mutations and epigenetic modifications in hepatocellular carcinoma. Mol Med Rep 2019; 19: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 21.Deng B, Cheng X, Li H, et al. Microarray expression profiling in the denervated hippocampus identifies long noncoding RNAs functionally involved in neurogenesis. BMC Mol Biol 2017; 18: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nojima T, Tellier M, Foxwell J, et al. Deregulated expression of mammalian lncRNA through loss of SPT6 induces R-loop formation, replication stress, and cellular senescence. Mol Cell 2018; 72: 970–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Wan J, Ye J, et al. Emerging role of lncRNAs in the normal and diseased intestinal barrier. Inflamm Res 2018; 67: 757–764. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Wang X, Wu H, et al. Glycine relieves intestinal injury by maintaining mTOR signaling and suppressing AMPK, TLR4, and NOD signaling in weaned piglets after lipopolysaccharide challenge. Int J Mol Sci 2018; 19: 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Zhu S, Shi P, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol 2011; 12: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 26.Barker CE, Thompson S, O’Boyle G, et al. CCL2 nitration is a negative regulator of chemokine-mediated inflammation. Sci Rep 2017; 7: 44384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poposki JA, Uzzaman A, Nagarkar DR, et al. Increased expression of the chemokine CCL23 in eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2011; 128: 73–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elangbam CS Qualls CW JrandDahlgren RR.. Cell adhesion molecules – update. Vet Pathol 1997; 34: 61–73. [DOI] [PubMed] [Google Scholar]
- 29.Chooi WH, Chew SY. Modulation of cell–cell interactions for neural tissue engineering: potential therapeutic applications of cell adhesion molecules in nerve regeneration. Biomaterials 2019; 197: 327–344. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Alcaide P, Liu L, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One 2011; 6: e14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bochner BS. Adhesion molecules as therapeutic targets. Immunol Allergy Clin North Am 2004; 24: 615–630. [DOI] [PubMed] [Google Scholar]
- 32.Lucarini G, Zizzi A, Rubini C, et al. VEGF, microvessel density, and CD44 as inflammation markers in peri-implant healthy mucosa, peri-implant mucositis, and peri-implantitis: impact of age, smoking, PPD, and obesity. Inflammation 2019; 42: 682–689. [DOI] [PubMed] [Google Scholar]
- 33.Guo FX, Wu Q, Li P, et al. The role of the LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ 2019; 26: 1670–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao Y, Chong L, Lin P, et al. MicroRNA-133a alleviates airway remodeling in asthtama through PI3K/AKT/mTOR signaling pathway by targeting IGF1R. J Cell Physiol 2019; 234: 4068–4080. [DOI] [PubMed] [Google Scholar]
- 35.Lin J, He Y, Wang B, et al. Blocking of YY1 reduce neutrophil infiltration by inhibiting IL-8 production via the PI3K-Akt-mTOR signaling pathway in rheumatoid arthritis. Clin Exp Immunol 2019; 195: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang S, Xu Y, Ge X, et al. Long noncoding RNA NEAT1 accelerates the proliferation and fibrosis in diabetic nephropathy through activating Akt/mTOR signaling pathway. J Cell Physiol 2019; 234: 11200–11207. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Feng L, Tian C, et al. Long noncoding RNA-JPX predicts the poor prognosis of ovarian cancer patients and promotes tumor cell proliferation, invasion and migration by the PI3K/Akt/mTOR signaling pathway. Eur Rev Medical Pharmacol Sci 2018; 22: 8135–8144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Long non-coding RNA profiling in LPS-induced intestinal inflammation model: New insight into pathogenesis by Ling Guo, Linna Li, Yang Zhang, Shulin Fu, Jing Zhang, Xiuying Wang, Huiling Zhu, Mu Qiao, Lingying Wu and Yulan Liu in Innate Immunity
Supplemental material, Supplemental Material2 for Long non-coding RNA profiling in LPS-induced intestinal inflammation model: New insight into pathogenesis by Ling Guo, Linna Li, Yang Zhang, Shulin Fu, Jing Zhang, Xiuying Wang, Huiling Zhu, Mu Qiao, Lingying Wu and Yulan Liu in Innate Immunity