Short abstract
LPS delays neutrophil apoptosis by a process generally assumed to involve cell-intrinsic TLR4 signaling. However, neutrophil survival responses to LPS have been reported to be monocyte-dependent, which would indicate more complexity than is currently appreciated. We compared the survival responses of conventionally purified vs highly purified neutrophils to confirm or refute the need for secondary cell-types and to identify the cellular or molecular mechanisms involved. Direct stimulation of TLR4 failed to extend the survival of highly purified neutrophils, but survival activity was retained in less pure neutrophil preparations containing low numbers of eosinophils, monocytes, platelets and CD3+ lymphocytes. Sequential depletions identified monocytes as the only cell type required. Transfer of culture supernatants after lipid A-conditioning revealed that purified monocytes were sufficient for production of nearly all of the survival activity observed in mixed populations. The survival factors secreted upon TLR4 stimulation remain unidentified, but were not correlated with IL-1β, IL-6 or TNF-α nor could survival activity be inhibited by Ab blockade of IL-8 or of several other candidate factors other than endogenously produced GM-CSF, which was responsible for about one-tenth of the survival activity present in conditioned supernatants. These observations confirm that ex vivo neutrophil survival responses to TLR4 agonists are not cell intrinsic and involve potentially novel factors secreted by TLR4-stimulated monocytes.
Keywords: Neutrophil, apoptosis, TLR4, IL-8, GM-CSF, cytokines, monocytes
Introduction
The lifespans of terminally differentiated granulocytes are prolonged by numerous microbial components, host-derived cytokines, chemokines and damage-associated molecular patterns (DAMPs) implicated in inflammatory disorders.1 For example, acute lung injury induced by LPS is correlated with prolonged neutrophil survival in mouse and rat models of airway disease.2,3 LPS survival effects have also been observed with human neutrophils.4–10 Delayed apoptosis of neutrophils both amplifies their anti-microbial activities and increases the risk of inflammatory damage.11 Factors that prolong neutrophil survival potentially delay resolution of inflammation, increasing the risk of counterproductive tissue damage as is reported to occur in endotoxemia or chronic infection.3,12,13 A detailed understanding of the survival factors and cellular networks that regulate neutrophil survival is thus needed in order to develop anti-inflammatory therapies that are effective without compromising host defense.
LPS has well-documented survival effects on neutrophils when administered in vivo or added to ex vivo cultures.4–7,9 The assumption that this anti-apoptotic signaling is a direct result of neutrophil intrinsic TLR4 stimulation was challenged over a decade ago by Sabroe et al.14,15 and Francois et al.8 who reported that LPS only transiently prolonged the survival of highly purified neutrophils unless PBMC were added. Both groups concluded that monocyte-derived cytokines were likely to be required,8,14,15 although no specific factors were identified or ruled out and no other groups have reproduced their results. Candidate survival factors include IL-8,16–20 IL-6,21–23 IL-1β,5,24,25 TNF-α,5,26,27 and IFN-γ,5,26,28 all of which were identified, however, using neutrophil preparations that retained other immune cell types that could mediate survival through secondary cytokine responses.
Identification of the proximal factor(s) responsible for survival effects on neutrophils would increase the precision with which neutrophil longevity could be manipulated for therapeutic purposes. Here, we tested the survival responses of highly purified and less-purified neutrophils after stimulation of TLR4, addition of exogenous cytokines, or neutralization of endogenous cytokines by Ab blockade to determine which factors or signaling pathways are cell-intrinsic regulators of neutrophil survival.
Material and methods
Materials
Chemically pure Escherichia coli lipid A (Compound 506, LA-15-PP29) and lipid IVa (both from Peptides International), were used to ensure TLR4 specificity and to avoid potential humoral reactivity with O-Ag epitopes found in LPS. Key findings from LA-15-PP experiments were confirmed with highly purified LPS from E. coli serotype O55:B5 (ALX-581-013-L001, Enzo Life Sciences).
Recombinant GM-CSF, IL-6, and IL-8 (Prospec); bacterial peptide fMLF (Sigma-Aldrich); fibrinogen and fibronectin (Millipore); ICAM-1 and VCAM-1 (Prospec); ERK Inhibitor II (FR180204) (Cayman Chemical); PE-conjugated anti-human CD62L Ab (clone DREG-56, BioLegend); neutralizing Ab against human IL-8 (cat. no. MAB208-100); GM-CSF (cat. no. AF-215-NA) and corresponding isotypes (R&D systems); control mouse (clone 11711); and goat IgG (AB-108-C), were used without modification. Tetrameric Ab complexes (TAC) were generated to perform selective cell depletions using EasySep™ Human ‘Do-It-Yourself’ Positive Selection Kit II (cat. no. 17699) and mouse IgG1 mAbs specific for human Siglec-8 (clone 7C9, BioLegend), CD3, CD36, CD41a, or CD56 (clones UCHT1, FA6-152, HIP8, or HCD56, StemCell Technologies). Mouse monoclonal IgG1 kappa (clone MOPC-21, StemCell Technologies) was used as isotype control for mock depletions.
Isolation of human neutrophils
Approximately 60 blood draws were performed for this study following procedures approved by the Institutional Review Board of the University of Louisville. Donors were aged 25–40 yr, 60% female and allowed to provide 50–450 ml blood up to once per month if healthy and not taking any medications. PMN were purified using plasma-Percoll gradients as described elsewhere.30 Cells were > 90–95% neutrophils and > 97% viable as evaluated by microscopy and will be referred to as ‘N90’ PMN hereafter. N90 populations were further enriched by negative selection to obtain highly pure neutrophil preparations (> 99%, ‘N99’) using Ab-mediated magnetic removal of all cells other than neutrophils with an EasySep™ Human Neutrophil Enrichment Kit (StemCell Technologies cat. no. 19257). Cell purity was assessed by staining with FITC-conjugated anti-CD66b (clone G10F5, BioLegend), and APC-conjugated anti-CD16 (clone CB16, eBioscience) Abs using an LSR II flow cytometer and FlowJo analysis software (Supplemental Figure 1A). Neutrophils were resuspended in complete RPMI-1640 medium supplemented with 5% human serum (type AB male, Sigma-Aldrich, cat. no. H4522).
Selective depletion of accessory immune cells
Residual immune cells were selectively depleted from N90 fractions by the same magnetic negative selection procedure used to generate N99 fractions, except that the Human Neutrophil Isolation Cocktail was replaced with Ab complexes specific for CD3, CD36, CD41a, CD56, or Siglec-8 to deplete T cells, monocytes and NK cells, platelets, NK cells, or eosinophils, respectively. Depletion of the intended cell type was confirmed by flow cytometric staining for CD14, CD16, CD19, CD66b plus Siglec-8, or for CD3, CD4, CD8, CD41a plus CD45.
Isolation of human monocytes
PBMCs were isolated using Histopaque-1077 (Sigma-Aldrich) gradients of whole blood of normal healthy donors. Monocytes were purified from the isolated PBMCs using an EasyEights™ Magnet and EasySep™ Human Monocyte Isolation Kit (cat. no. 19359, StemCell Technologies). Monocytes were resuspended in complete RPMI-1640 medium (Gibco) containing 2 mM L-glutamine, 50 units/ml penicillin, 50 mg/ml streptomycin, 1 mM sodium pyruvate, and 5% heat-inactivated human AB serum (Sigma-Aldrich). Purity, generally 70–90%, was assessed by simultaneously staining with APC-eFluor 780-conjugated anti-CD45 (clone HI30, eBioscience), and PE-conjugated anti-CD14 (clone 61D3, eBioscience) Abs, and then determining the percentage of CD45+CD14+ cells using a BD LSR II flow cytometer.
Culture conditions
For adherent culture conditions, neutrophils (5 × 105 cells/well) or monocytes (5 × 104 cells/well) were plated in a total volume of 200 µl in a 96-well tissue culture plate. In experiments with matrix protein coated plates, wells were coated with 100 µg fibrinogen or 10 µg fibronectin (Millipore) for 2 h at room temperature (RT) followed by gentle washing with HBSS (Gibco). Stationary plates containing cells were incubated at 37°C for 20–24 h.
Soluble factor quantification
IL-8, IL-6, IL-1β, TNF-α, and IFN-γ in cell-free supernatants collected from neutrophils after culture at 5 × 105 cells/well in 96-well tissue culture plates with E. coli lipid A or medium for 20 h were quantified by electrochemiluminescence assay using the V-PLEX Human Proinflammatory Panel (Meso Scale Discovery). GM-CSF was assayed by colorimetric ELISA (Human GM-CSF ELISA Ready-SET-Go kit, eBioscience; limit of detection 10 pg/ml).
Neutrophil survival assays
Highly purified N99 neutrophils were cultured with chemically pure E. coli lipid A, exogenous GM-CSF as a positive control, candidate survival factors, or culture medium alone as negative control for 24 h at 37°C prior to flow cytometric staining with APC-conjugated Annexin V (BD Biosciences) and 7-AAD (Biotium or Tonbo Biosciences). Neutrophils that were double negative for Annexin V and 7-AAD (see Supplemental Figure 1B) were recorded as viable. Culture supernatants containing secreted survival factors were obtained by exposing monocytes or N90 neutrophils to lipid A for 20 h at 37°C before harvesting as cell-free culture supernatants, which were frozen until use. Ab blockade of cytokines in cell-free culture supernatants was performed by incubation of Ab or isotype control for 30 min at RT before adding to N99 PMNs for 24 h culture. Abs were used at concentrations that we determined were sufficient to block survival activity of 100 ng/ml recombinant IL-8 or 100 pg/ml of recombinant GM-CSF (using 10 µg/ml anti-IL-8 or 5 µg/ml anti-GM-CSF Abs, respectively).
The effects of ERK inhibition on neutrophil survival were evaluated by pre-treating N99 PMN with 20 µM ERK Inhibitor II,31 or diluted vehicle control (DMSO), for 30 min at 37°C prior to 24 h stimulation with lipid A or culture supernatants. The effects of ERK inhibition on survival factor production were tested by pre-treating N90 PMN with ERK inhibitor or vehicle control prior to 24 h stimulation with lipid A and collection of culture supernatants.
Western blotting analysis
N99 PMNs (4 × 106) in polypropylene tubes or plated in 24-well polystyrene tissue culture dishes were exposed to medium or lipid A, 100 ng/ml, for 5, 15, 30, and 60 min at 37°C. At each time point, cells were lysed in 1X Laemmli buffer containing 5% β-mercaptoethanol at 95°C for 5 min. An equal volume of each, 30 µl, was resolved by 10% SDS–PAGE, transferred onto PVDF membranes (Millipore), blocked with 5% nonfat dry milk for 1 h and probed with primary Abs (Cell Signaling Technology): phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204, cat. no. 4370); total p44/42 MAPK (ERK1/2, cat. no. 4695); phospho-p38 MAPK (Thr180/Tyr182, cat. no. 9215); and total p38 MAPK (cat. no. 9212). Bands were visualized with HRP-conjugated secondary Abs (Jackson ImmunoResearch), Amersham Prime ECL substrate, and a Bio-Rad ChemiDoc Touch Imaging System.
Statistical analysis
Data were plotted using GraphPad Prism software. Statistical significance among three or more groups was evaluated with the same software. ANOVA using Dunnett’s multiple comparisons was conducted to determine significance in experiments with multiple conditions. Differences with P < 0.05 were considered as statistically significant (see P values in each figure legend).
Results
N99 PMNs lack survival response to TLR4 stimulation
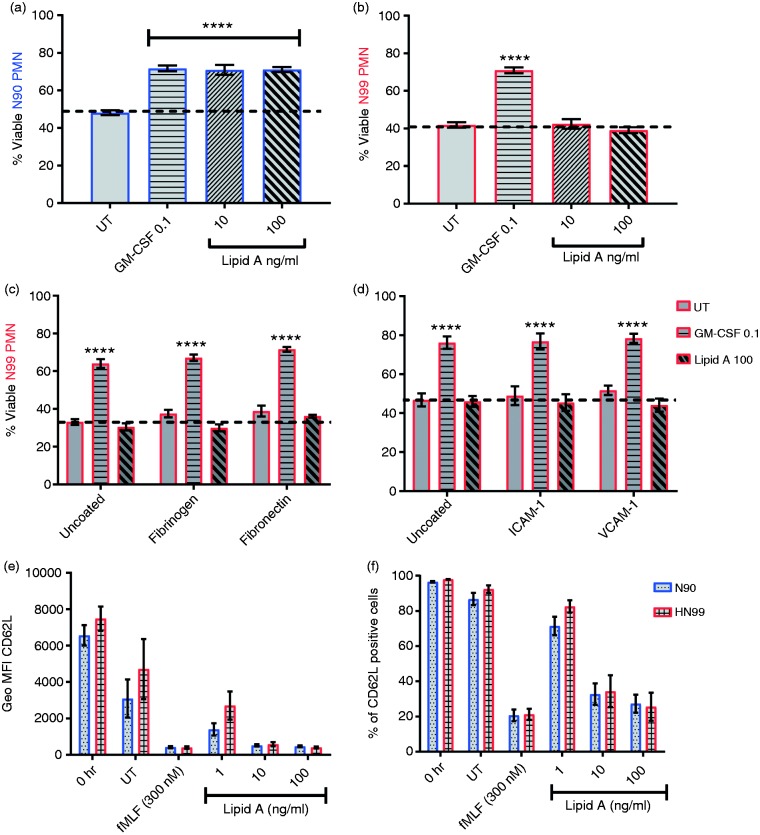
To evaluate neutrophil-intrinsic survival signaling downstream of TLR4, we compared the responses of neutrophils purified by plasma-percoll gradient centrifugation alone (> 90% purity, N90 PMN) or by further magnetic bead depletion of residual leukocytes (> 99% purity, N99 PMN). N90 and N99 PMN were cultured under conditions that favor adherence (flat-bottom 96-well polystyrene plates) in the presence or absence of lipid A, hexa-acylated E. coli chemotype, a reagent that stimulates TLR4 exclusively. After 24 h culture, 40–50% of unstimulated N90 and N99 PMNs remained viable (Figure 1a and b). Exogenously added GM-CSF, a neutrophil survival factor,32–34 increased the percentage of viable cells to about 70%. Accordingly, a 24 h culture time point was used thereafter to measure survival activity of exogenously added materials. With this assay, we found that chemically pure lipid A had a robust survival effect only on N90 PMN cell cultures (Figure 1a) and none on N99 cell populations (Figure 1b) from which other immune cell types had been depleted. Biological preparations of E. coli LPS also had no survival effect on N99 populations. These results suggested that highly purified human neutrophils lack intrinsic survival responses to TLR4 agonists.
Figure 1.
Lack of an intrinsic survival response to TLR4 stimulation by highly purified neutrophils. Neutrophils that were 90–95% (N90) or > 99% pure (N99) were incubated for 24 h in the absence (untreated, UT) or presence of control GM-CSF (0.1 ng/ml), or lipid A (10 and 100 ng/ml) under adherent culture conditions; cell viability was assessed by Annexin V-APC/7-AAD staining (a–d). Shown are percentages of viable (a) N90 or (b) N99 neutrophils in uncoated tissue culture plates; and of N99 neutrophils in wells coated with (c) fibrinogen (100 µg) or fibronectin (10 µg); (d) ICAM-1 (2 µg), or VCAM-1 (2 µg). (e–f) N99 and N90 neutrophils were stimulated for 1 h with control fMLF peptide or increasing doses of lipid A (1, 10, or 100 ng/ml) or with medium (UT) under adherent culture conditions. Neutrophil activation in response to TLR4 stimulation was measured as induced down-regulation of cell-surface CD62L. Bars show percent means ± SEM from (a and b) n = 14 or (c and d) n = 3 blood donors, (e) mean percentage of positive cells or (f) mean geometric MFI ± SEM from n = 4 donors. ****P ≤ 0.0001 when compared with untreated (a–d). PMN, Polymorphonuclear neutrophils. Each donor (n) represents an independent experiment.
Because adherence affects neutrophil survival,35 we tested whether the absence of a survival response to lipid A was due to the lack of a physiological substrate. Culture of N99 PMNs in wells pre-coated with matrix proteins (fibrinogen or fibronectin) or endothelial surface adhesion molecules (ICAM-1 or VCAM-1) did not restore survival responses to lipid A (Figure 1c and d). To determine whether TLR4 retained functionality in highly purified N99 PMNs, we measured their surface expression of the activation marker CD62L, which is released from cells in response to TLR4 stimulation.36 We found equivalent CD62L shedding from N99 as from N90 PMNs over a wide dose range of lipid A (1–100 ng/ml) as measured either by mean fluorescence intensity (MFI) per cell (Figure 1e) or by the proportion of cells that became CD62L-negative (Figure 1f). Hence, TLR4 was expressed and functional in highly purified neutrophil populations but appeared to lack survival signaling activity. This result is consistent with the fact that LPS is generally considered to be a survival factor for neutrophils,4,5,7,9,24 while also implicating residual leukocytes—which comprise 5–10% of neutrophil suspensions prepared by standard methods—as the actual mediators of TLR4 survival effects on neutrophils.
Soluble factors mediate TLR4 survival effects on N90 PMNs
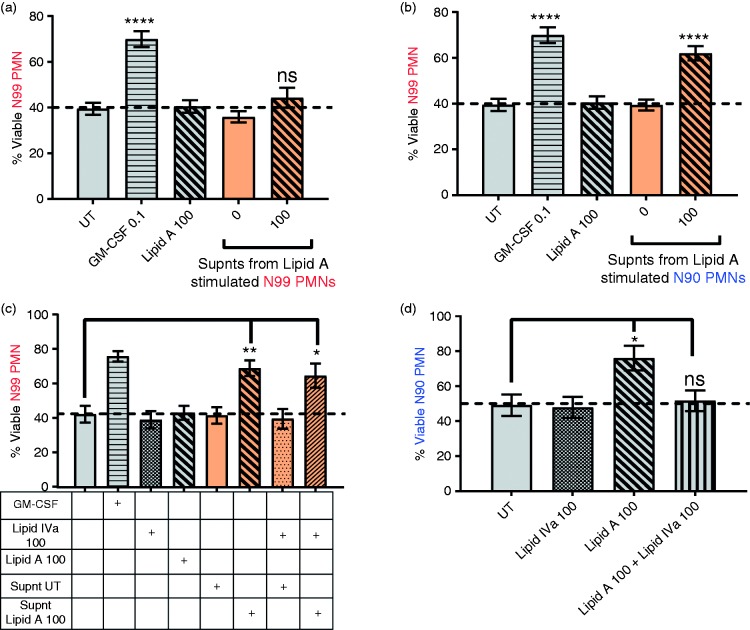
One explanation for TLR4 survival activity in conventionally purified N90 populations is that it is mediated by survival factors secreted by low numbers of other innate immune effector cells. To test this, we collected and froze culture supernatants from lipid A-stimulated N90 or N99 PMN and later added them to freshly prepared N99 PMNs for survival assay after 20 h culture. Culture supernatants from lipid A-stimulated N99 PMN contained no survival activity (Figure 2a) while those from lipid A-treated N90 PMN cell populations prolonged neutrophil survival to the same extent as exogenously added GM-CSF, 100 pg/ml (Figure 2b). As expected, survival activity in these conditioned supernatants was not due to carry-over of lipid A because it did not occur upon addition of more lipid A (Figure 2a–c) and was not inhibited by lipid IVa, a human TLR4 antagonist (Figure 2c; with lipid IVa demonstrated to be functional in Figure 2d). Lipid A-induced secretion of survival factors by N90 PMN required at least 8 h to be detectable, and reached a plateau by about 20 h (data not shown) which is suggestive of de novo gene expression. These results further support the hypothesis that the survival response of neutrophils to TLR4 agonists is mediated or regulated by other innate immune effector cells.
Figure 2.
Survival effects of TLR4 stimulation on neutrophils are mediated by soluble factors. Neutrophils were incubated for 24 h in the absence (untreated, UT) or presence of control GM-CSF (0.1 ng/ml), lipid A, lipid IVa, or supernatants (supnt) collected from heterologous neutrophils stimulated with lipid A at 100 ng/ml for 20 h. Cell viability was analyzed by Annexin V-APC/7-AAD staining and flow cytometry. Percentages of viable (a and b) N99 PMNs after culture with lipid A at 100 ng/ml or supernatants from (a) N99 PMNs or (b) N90 PMNs. Percentage of viable (c) N99 or (D) N90 PMNs after culture with lipid A at 100 ng/ml or supernatants from N90 PMN in the presence or absence of TLR4 inhibitor lipid IVa. Bars show percent means ± SEM from (a and b) n ≥ 6 and (c and b) n = 3 blood donors. ****P ≤ 0.0001, **P ≤ 0.01, *P ≤ 0.05, and ns P > 0.05 when compared with untreated neutrophils. Each donor (n) represents an independent experiment.
Potential soluble survival factors in the N90 culture supernatants
In an attempt to identify TLR4-induced survival factors in N90 cultures, we measured a panel of factors reported by others to have anti-apoptotic effects on neutrophils, including IL-8,16–20 IL-6,21–23 IL-1β,5,24,25 TNF-α,5,26,27 and IFN-γ.5,26,28 Multiplex analysis was performed on supernatants harvested from lipid-A-stimulated N90 and N99 PMNs from six healthy blood donors (Supplemental Figure 2A-D). IL-8 was on average 10-fold more abundant in the supernatants of N90 than N99 cultures, after culture with lipid A. A moderate amount of IL-6, very little IL-1β and TNF-α, and no IFN-γ were detected in the supernatants of N90 PMNs. None of these latter factors were present in the supernatants from N99 culture (Supplemental Figure 2C and 2D). We also tested supernatants from N90 PMNs for the presence of GM-CSF by ELISA but did not detect any above the assay limit of detection (10 pg/ml).
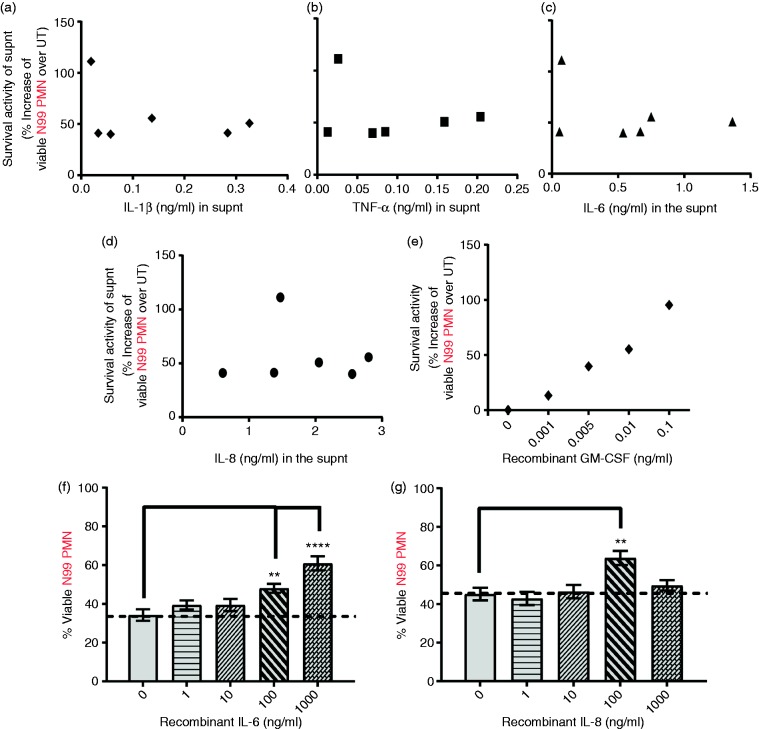
We compared the abundances of IL-8, IL-6, TNF-α, and IL-1β to survival activities of the individual supernatants in which they were measured to determine if they were correlated. Less abundant IL-1β (Figure 3a) and TNF-α (Figure 3b) showed no correlation with the magnitude of the survival activities of the supernatants. IL-8 and IL-6, with their relatively higher abundance in the supernatants, seemed the most likely candidate factors; surprisingly, however, neither IL-6 (Figure 3c) nor IL-8 (Figure 3d) were positively correlated with N99 PMN viability, suggesting no role or either factor. Recombinant GM-CSF, added exogenously as a positive control, showed a linear correlation of dose to PMN viability, as expected (Figure 3e). Exogenously added IL-6 and IL-8 also prolonged neutrophil survival, but only at doses ≥ 100 ng/ml, much higher than was detected in any of the culture supernatants (Figure 3f and g), once again suggesting no plausible role of either of these factors in the survival activity of the supernatants.
Figure 3.
Abundance of candidate factors does not correlate with the survival activities of supernatants from TLR4 stimulated N90 PMN culture. N99 PMNs were cultured for 24 h with supernatants from lipid A (100 ng/ml) stimulated N90 PMNs (Supnt donors, n = 6), or directly with exogenous GM-CSF, IL-6, or IL-8. Cell viability was analyzed by Annexin V-APC/7-AAD staining and flow cytometry. Survival activities of (a–d) supernatants or (e) GM-CSF were plotted against the amount of (a) IL-1β, (b) TNF-α, (c) IL-6, or (d) IL-8 present in the supnts (Supplemental Figure S2), or (c) with indicated concentrations of exogenous GM-CSF. Percentage of viable N99 PMNs after culture with increasing doses of (d) IL-6 or (e) IL-8. Each dot represents (a–d) a supnt from N90 prepared from one of n = 6 donors, or (e) the mean survival response of N99 cells from n = 3 donors to the indicated doses of recombinant GM-CSF. Bars (f and g) show mean viability ± SEM of N99 from n = 3 donors after exposure to the indicated doses of IL-6 and IL-8. ***P ≤ 0.001 and **P ≤ 0.01 when compared with untreated neutrophils.
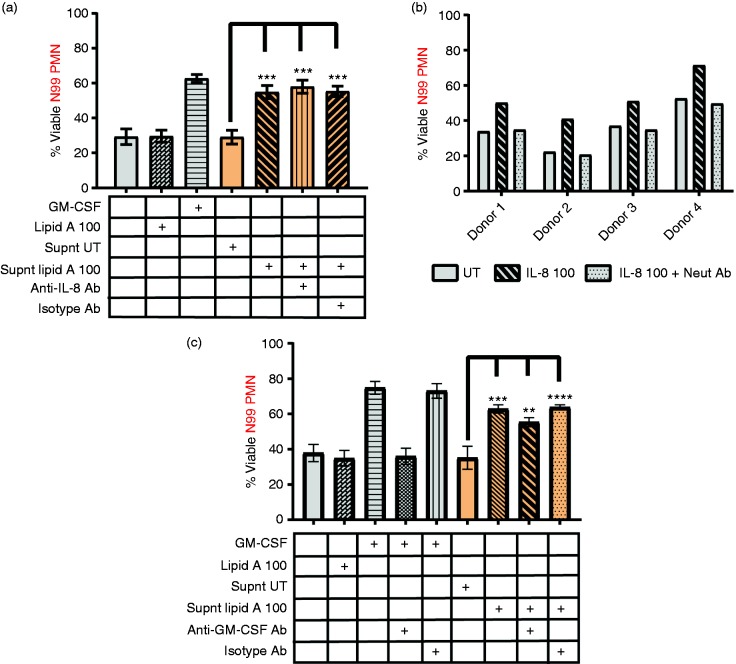
We further tested a role for IL-8 in mediating TLR4 survival effects by measuring survival activity of multiple supernatants in the presence of 10 µg/ml IL-8 neutralizing Ab, an amount we confirmed was sufficient to completely block the survival effect of 100 ng/ml exogenous IL-8 (Figure 4b). The IL-8 neutralizing Ab had no effect on lipid-A-induced survival activity when added to N90 culture supernatants, despite being sufficient for blockade of at least 30-fold more IL-8 than was present in the culture supernatants (Figure 4a). The TLR4-induced survival factor is therefore highly unlikely to be IL-8.
Figure 4.
Neutrophil survival after Ab blockade of GM-CSF or IL-8 in lipid-A-conditioned culture supernatants. Lipid-A-conditioned supernatants were prepared from N90 cell cultures and tested for survival activity in the presence or absence of neutralizing Abs. (a) IL-8 blockade. Shown are the percentages of viable N99 PMNs after 24 h culture with lipid-A-conditioned supernatants in the absence or presence of IL-8 neutralizing Ab or goat IgG control. Also shown is a negative control for lack of survival response to lipid A (100 ng/ml) and positive control GM-CSF (0.1 ng/ml). (b) Confirmation of IL-8 blockade. Percentages of viable N99 PMNs after culture with IL-8 at 100 ng/ml in the absence or presence of neutralizing goat pAb specific for IL-8 (n = 4 blood donors). (c) GM-CSF blockade. Percentages of viable N99 PMNs cultured with GM-CSF at 100 pg/ml or with supernatants of untreated (Supnt UT) or 100 ng/ml lipid A-treated (Supnt lipid A 100) N90 cultures from n = 6 blood donors in the absence or presence of neutralizing goat pAb specific for GM-CSF or goat IgG as control. (a and c) Bars show mean viability from (a) n = 3 and (c) n = 6 individuals ****P ≤ 0.0001, ***P ≤ 0.001, **P ≤ 0.01, and ns P > 0.05 when compared with neutrophils cultured with Supnt UT. Each donor whose blood was used to prepare highly purified neutrophils represents an independent experiment.
Exogenous, recombinant GM-CSF (100 pg/ml) was notably potent as a neutrophil survival factor (Figure 3e) and hence could not be excluded despite being undetectable by ELISA. To test for endogenous GM-CSF survival activity, we incubated culture supernatants with 5 µg/ml GM-CSF neutralizing Ab, an amount sufficient to abrogate the survival activity of at least 100 pg/ml of recombinant GM-CSF. The survival activity of supernatants incubated with the GM-CSF neutralizing Ab was reduced by about 10% relative to those of supernatants incubated with goat polyclonal IgG control as negative control (Figure 4c). Hence, GM-CSF appears to play a minor but consistent role in mediating the TLR4 survival effects of accessory cells, indicating that other TLR4-induced survival factors for human neutrophils remain to be identified.
ERK activity contributes to the production of soluble factors in the presence of accessory immune cells
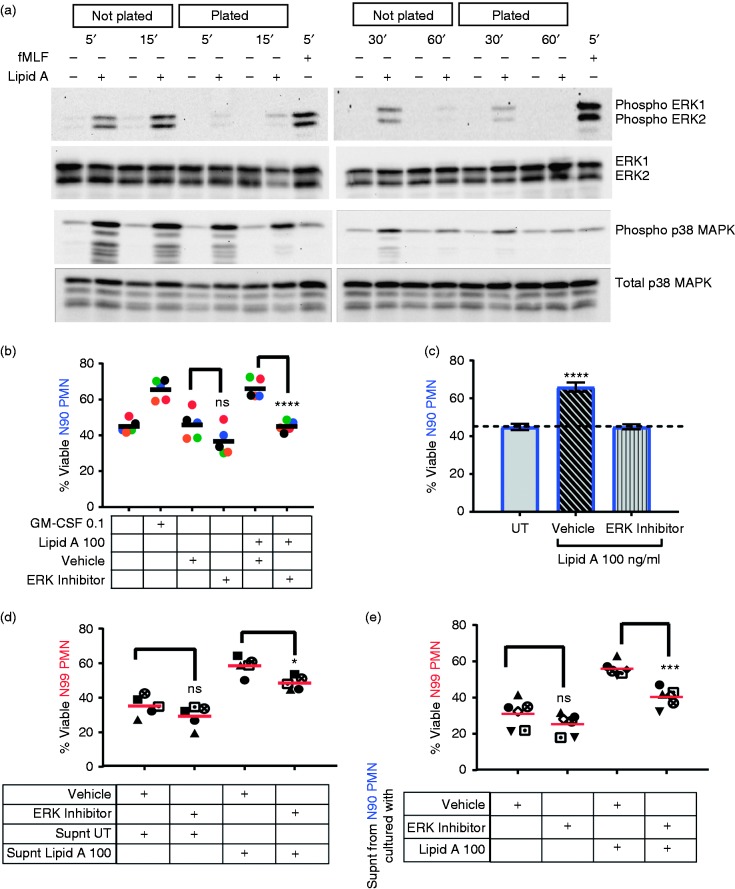
ERK, a MAPK, is involved in TLR4 survival effects on neutrophils purified by standard methods,4,31 which we hypothesized would be lost in highly purified neutrophil preparations. To test this, ERK phosphorylation was measured by immunoblot 5–60 min after addition of lipid A to highly purified neutrophils plated in polystyrene culture dishes (Figure 5a). Lipid-A-induced ERK phosphorylation was detectable but weak relative to positive control fMLF, reaching a peak at about 30 min. Interestingly, ERK was activated more strongly and quickly when the neutrophils were held in polypropylene tubes (‘Not plated’) than when seeded in polystyrene culture dishes (‘Plated’). The adherence inhibition was specific to ERK1/2 because activation of p38 MAPK was robust under both conditions (Figure 5a). Hence, the absence of intrinsic survival responses in adherent N99 cultures was correlated with loss of an ERK1/2 activation response immediately following TLR4 stimulation.
Figure 5.
ERK is involved in production and activity of soluble survival factor(s). (a) Plated or unplated N99 PMNs were stimulated with 100 ng/ml lipid A for the indicated times (5 min through 60 min); or with fMLF (300 nM, 5 min) as positive control for plated N99. Cell lysates were analyzed by immunoblot using Abs specific for phosphorylated or total ERK1/2 and p38 MAPK. Representative blots are shown; see Supplemental Figure S3 for quantitation of all blots. (b and c) Plated N90 PMNs were incubated with 20 μM ERK inhibitor or diluted vehicle (DMSO) for 30 min before they were cultured with 100 ng/ml lipid A for 24 h. GM-CSF was used as positive control. (d) N99 PMNs pre-treated with ERK inhibitor or vehicle for 30 min were cultured with supernatants from lipid A-stimulated (Supnt Lipid A 100) or untreated (Supnt UT) N90 PMNs for 24 h under adherent culture conditions. (e) N99 PMNs were cultured with supernatants collected from ERK inhibitor or vehicle pre-treated N90 PMNs that were subsequently stimulated with 100 ng/ml of lipid A. Cell viability was analyzed by Annexin V-APC/7-AAD staining and flow cytometry. Each symbol represents one donor; means are shown as black (b) or red (d and e) bars. ****P ≤ 0.0001, ***P ≤ 0.001, *P ≤ 0.05, and ns P > 0.05 when compared with the corresponding vehicle group. (c) Bars show mean percentage of viability ± SEM from five individuals; ****P ≤ 0.0001 when compared with untreated neutrophils. Each donor (n) represents an independent experiment.
To test whether ERK activation is functionally required for survival signaling, we pre-treated N90 PMNs with a chemical inhibitor of ERK, and then added lipid A for 24 h before measuring viability. Chemical inhibition of ERK completely blocked the survival activity of lipid A when added to N90 PMN cultures with lipid A (Figure 5b and c), consistent with previous reports.31 We next tested whether ERK was required for (i) responsiveness to survival factors or (ii) for production of those factors, or both, in N90 PMN cell preparations. First, the survival response was assessed by pre-treating N99 PMN with ERK inhibitor for 30 min before adding lipid-A-conditioned culture supernatants containing survival factors. The result was a partial reduction of survival responsiveness (Figure 5d), indicating that ERK contributes but is not solely responsible. Second, we measured the effect of ERK inhibition on production of survival factors by pre-treating N90 cultures with ERK inhibitor for 30 min before adding lipid A for 20 h to stimulate factor secretion. There was again a partial reduction, in this case of factor production (Figure 5e), which in this context indicated partial dependence on ERK activity for production of the survival factors by accessory immune cells. Neither effect was as complete as that of ERK inhibition in blocking TLR4 survival effects directly in N90 PMN cultures, where the factors are both produced and active, which may mean that complete inhibition reflects a cumulative effect on both production and activity of the secreted factors.
Monocytes are the primary sources of soluble survival factors in N90 PMN cultures
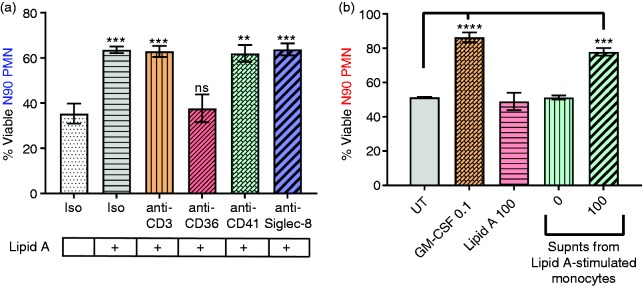
To identify the cell subtypes responsible for neutrophil survival in response to lipid A, we selectively depleted potential other immune cell lineages present in the N90 population (Supplemental Figure S1). Depletion of CD3+ T lymphocytes and Siglec-8+ eosinophils had no effect on neutrophil survival (Figure 6a). Platelets, which can interact with neutrophils and induce their activation in response to TLR4 agonists,37 similarly were of no consequence when depleted prior to lipid A stimulation. In contrast, selective depletion of CD36+ cells completely abolished survival activity (Figure 6a). Depletion of CD56+ NK cells, which also express CD36, did not impact neutrophil viability (data not shown), indicating CD36+ monocytes alone were required to mediate neutrophil survival responses to TLR4 agonists.
Figure 6.
Monocytes are required for TLR4 survival effects. (a) The indicated cell subsets were depleted from N90 neutrophil preparations prior to culture with 100 ng/ml lipid A, after which neutrophil viability was analyzed by Annexin V-APC/7-AAD staining and flow cytometry. (b) N99 PMNs viability after culture with supernatants from purified monocytes exposed to 0 or 100 ng/ml lipid A for 20 h. Bars show mean percentage of viability ± SEM from (a) n = 3–5 (***P ≤ 0.001, **P ≤ 0.01, and ns P > 0.05 when compared with N90 neutrophils subjected to mock depletion and cultured in the absence of lipid A (A) or (b) n = 3 blood donors (****P ≤ 0.0001, ***P ≤ 0.001 when compared with N99 neutrophils cultured in the absence of lipid A). Each donor (n) represents an independent experiment.
To determine if purified monocytes are sufficient for production of the unidentified neutrophil survival factor(s), we isolated CD14+CD16– monocytes from PBMC by negative selection of other cell types with magnetic particles and plated at 5 × 104 cells/well of a 96-well plate. After 20 h culture with lipid A (100 ng/ml), the monocyte cell supernatants were collected, stored frozen, thawed, and then added to freshly prepared N99 PMN to assess 24 h survival activity. As shown in Figure 6b, supernatants from lipid-A-stimulated monocytes were capable of extending neutrophil survival, whereas culture supernatants from unstimulated monocytes had no activity. These results, together with those of the cell subset depletion experiments, indicate that, in less purified neutrophil preparations, CD36+ monocytes are the primary source of TLR4-stimulated survival factors for co-cultured neutrophils.
Discussion
Neutrophil longevity is reported to be prolonged by numerous inflammatory cytokines and chemokines, DAMPs, and PAMPs.1,5 The majority of studies that have evaluated survival activities of these various factors used neutrophil preparations containing up to 5–10% of other immune cell types. Understanding whether survival responses to these factors is neutrophil intrinsic, or might be regulated by accessory immune cells during infection and inflammation, is important for the design of interventional therapeutics. Here, we compared the pro-survival effects of TLR4 stimulation in highly purified neutrophil fractions (> 99%) versus more commonly used less purified neutrophil populations (> 90%). We found that neutrophil survival responses to TLR4 agonists depends on soluble factors released primarily by CD36+ monocytes. Additionally, we determined that ERK is involved in both the production and the activity of the survival factor(s), which remain unidentified despite our efforts to evaluate several factors reported in the literature as likely candidates.
We first observed that TLR4 stimulation has dramatically different effects on N90 than on N99 cultures in the course of studying the survival and functional responses of neutrophils to structural variants of LPS synthesized by Pseudomonas aeruginosa in patients with cystic fibrosis.38 This effect, shown in Figure 2b, is an independent confirmation of early reports by Sabroe et al.14 that E. coli LPS lacks survival effects on monocyte-depleted neutrophils after 22 h culture unless supplemented with as few as 5% unfractionated PBMC. The method they and we used to purify neutrophils, i.e., depletion of contaminating cell-types with specific Abs and magnetic particles, could theoretically impair neutrophil responses to TLR4 stimulation. However, intrinsic TLR4 functionality was evidently not impaired by this procedure because lipid-A-induced CD62L shedding was similar in both N99 and N90 PMNs (Figure 1e and f) and ‘mock’ depletions with isotype control Ab had no effect on survival responses (Figure 6a).
Terminally differentiated neutrophils exhibit a high degree of functional specialization. Cellular specialization has resulted in the evolution of accessory cells that provide essential functions for client cells that the client cells themselves cannot perform. This division of labor makes cellular specialization and regulation possible, as exemplified by the dependent relationships between neurons and Schwann cells or spermatogonia and Sertoli cells.39 Similar relationships may exist between highly specialized neutrophils and monocytes as a means of regulating when and where neutrophils survive versus become apoptotic in order to be cleared through efferocytosis. Cross-talk between neutrophils and monocytes/macrophages or lymphocytes including T cells, NK cells, and NKT cells has been implicated in several inflammation-associated pathologies.40,41 Costantini et al.34 showed that activated NK cells potentiate neutrophil survival through soluble factors in vitro. Pelletier et al.42 also reported pro-survival effects of soluble factors released from activated CD4+ and CD8+ T cells on neutrophils. We investigated individual accessory cell types through selective depletion, and showed that prolonged neutrophil survival by factors in lipid-A-conditioned supernatants did not require eosinophils, T lymphocytes, NK cells or platelets. Only monocyte depletion rendered neutrophils unresponsive to TLR4 survival effects, which is consistent with, and extends, published results.14 We further found that purified monocytes were sufficient for robust survival factor production (Figure 6b), identifying monocytes as the primary source of survival factors for neutrophils purified by standard methods. Because monocytes are known to migrate to sites of inflammation as a second wave of immune cell recruitment following neutrophils, it will be important to understand in detail how they may regulate neutrophil survival in vivo.
We found no evidence that IL-8 plays a role in neutrophil survival, at least in our culture system (Figure 4a). Others have shown IL-8 is a survival factor for neutrophils,16–19 although most of these studies were performed with less purified preparations similar to ours. Cowburn et al.18 reported that an IL-8 autocrine effect mediates the late survival activity of TNF-α but not that of GM-CSF, although both TNF-α and GM-CSF induce neutrophil IL-8 production, suggesting that any survival effect of IL-8 is context dependent. Very high amounts of IL-8 produced by neutrophil ‘swarms’, for example, could exert survival effects without monocytic cell participation. In our experiments, highly purified N99 neutrophils responded to the survival activity of recombinant IL-8, but only at a concentration (100 ng/ml) 50 times higher than the average amount present in conditioned supernatants from N90 PMN. Using neutralizing Ab at a concentration sufficient to abrogate the survival effect of exogenously added IL-8 on N99 PMNs, we found no change in the survival activity of the supernatant. This confirmed that IL-8 is dispensable in the context of TLR4 stimulated survival effects on neutrophils in ex vivo culture.
GM-CSF appeared to play a consistent, but minor, role in supporting neutrophil survival in our culture system. No GM-CSF was detected in lipid A-stimulated N90 culture supernatants, as determined by ELISA with an assay limit of detection around 10 pg/ml. However, addition of GM-CSF neutralizing Ab to lipid-A-conditioned culture supernatants consistently reduced their survival activity by about 10% (Figure 4c). GM-CSF is a notably potent survival factor for neutrophils, as it is effective at concentrations as low as 1–5 pg/ml,34 and Figure 3e. This potency likely explains why GM-CSF activity could be revealed by Ab blockade in culture supernatants in which no GM-CSF was detectable by a standard ELISA. Additional studies are needed to identify the other, dominant survival factor(s) that act directly on neutrophils, as opposed to those that act indirectly through accessory immune cells. Our ongoing efforts using neutralizing Abs for several other cytokines (IL-4, IL-6, IL-12, IL-15, IL-17A/F, IL-24, IL-27, and IL-36G) have so far found no evidence that any of them are involved, at least in this culture system.
ERK activity is required for the neutrophil survival activity of lipid A in N90 cultures (Figure 5c) which is in agreement with published results.4,31 We further showed that ERK activity is involved in both survival factor production as well as function using ERK inhibitor pre-treatment of N90 and N99 cells before collecting or adding conditioned supernatants, respectively. Future studies will address other contributing signaling pathways in the indirect survival effects of TLR4 on neutrophils. We found more robust ERK phosphorylation when N99 neutrophils were stimulated with lipid A in suspension compared with adherent conditions. This apparently contrasts the published positive correlation between neutrophil adhesion and ERK activation,43,44 but once again suggests a cell autonomous effect similar to survival that needs further investigation.
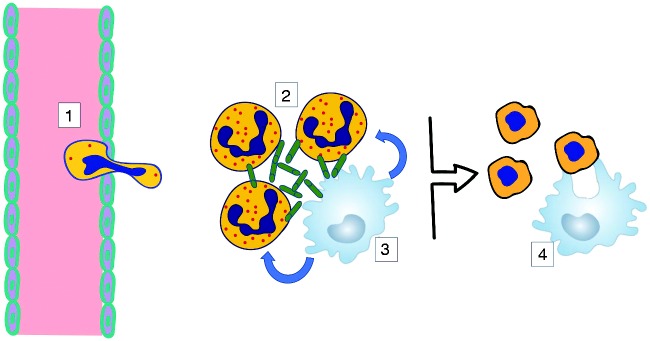
Neutrophils are highly specialized cells whose survival or apoptotic death is a critically important determinant of the transition from innate immune defense to resolution of an inflammatory event. In the context of TLR4 signaling, a requirement for monocyte-macrophages may serve as a checkpoint for neutrophils that prevents them from abnormally self-sustained survival once activated at sites of infection by Gram-negative bacteria or from dying prematurely before efferocytosis can occur (Figure 7). A detailed understanding of the factors and pathways involved is needed to advance the goal of identifying new targets of therapeutic intervention in the many inflammatory conditions associated with neutrophilia.
Figure 7.
A proposed model for monocyte/macrophage-dependent survival of neutrophils. 1. Neutrophil recruitment from blood to a site of Gram-negative bacterial infection involves adherence to extracellular matrix proteins during and after extravasation. 2. TLR4 signaling in adherent neutrophils is uncoupled from ERK activation and anti-apoptotic outcomes. 3. Neutrophil survival becomes dependent on survival factors secreted by TLR4-stimulated monocyte/macrophages. 4. Upon clearance of infection monocyte/macrophages stop secreting survival factors for proximal neutrophils, which are cleared through efferocytosis to initiate inflammation resolution.
Supplemental Material
Supplemental Material for Human neutrophils depend on extrinsic factors produced by monocytes for their survival response to TLR4 stimulation by Shuvasree SenGupta, Madhavi J Rane, Silvia M Uriarte, Cassandra Woolley and Thomas C Mitchell in Innate Immunity
Acknowledgements
The authors are indebted to Ms. Terri Manning for phlebotomy and preparation of plasma-percoll-purified neutrophils from blood donors for this study.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) award R01AI127970, the Kentucky Research Challenge Trust and the Barnstable-Brown Foundation, and an American Association of Immunologists Careers in Immunology Fellowship awarded to SS.
References
- 1.Geering B, et al. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol 2013; 34(8): 398–409. [DOI] [PubMed] [Google Scholar]
- 2.Kupfner JG, et al. Role of NF-kappaB in endotoxemia-induced alterations of lung neutrophil apoptosis. J Immunol 2001; 167(12): 7044–7051. [DOI] [PubMed] [Google Scholar]
- 3.Sunil VR, et al. Activation of adherent vascular neutrophils in the lung during acute endotoxemia. Respir Res 2002; 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein JB, et al. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell Signal 2001; 13(5): 335–343. [DOI] [PubMed] [Google Scholar]
- 5.Colotta F., et al. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 1992; 80(8): 2012–2020. [PubMed] [Google Scholar]
- 6.Nolan B, et al. Mitogen-activated protein kinases signal inhibition of apoptosis in lipopolysaccharide-stimulated neutrophils. Surgery 1999; 126(2): 406–412. [PubMed] [Google Scholar]
- 7.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol 1993; 54(4): 283–288. [PubMed] [Google Scholar]
- 8.Francois S, et al. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol 2005; 174(6): 3633–3642. [DOI] [PubMed] [Google Scholar]
- 9.Ward C, et al. Interleukin-10 inhibits lipopolysaccharide-induced survival and extracellular signal-regulated kinase activation in human neutrophils. Eur J Immunol 2005; 35(9): 2728–2737. [DOI] [PubMed] [Google Scholar]
- 10.de Kleijn S., et al. Transcriptome kinetics of circulating neutrophils during human experimental endotoxemia. PLoS One 2012; 7(6): e38255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox S, et al. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun 2010; 2(3): 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsey MV, et al. Neutrophil apoptosis in the lung after hemorrhage or endotoxemia: apoptosis and migration are independent of IL-1beta. Clin Immunol 1999; 91(2): 219–225. [DOI] [PubMed] [Google Scholar]
- 13.Lakschevitz FS Aboodi GM, andGlogauer M.. Oral neutrophil transcriptome changes result in a pro-survival phenotype in periodontal diseases. PLoS One 2013; 8(7): e68983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabroe I, et al. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 2002. 168(9): 4701–4710. [DOI] [PubMed] [Google Scholar]
- 15.Sabroe I, et al. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 2003; 170(10): 5268–5275. [DOI] [PubMed] [Google Scholar]
- 16.Kettritz R, et al. Interleukin-8 delays spontaneous and tumor necrosis factor-alpha-mediated apoptosis of human neutrophils. Kidney Int 1998; 53(1): 84–91. [DOI] [PubMed] [Google Scholar]
- 17.Leuenroth S., et al. Interleukin-8-induced suppression of polymorphonuclear leukocyte apoptosis is mediated by suppressing CD95 (Fas/Apo-1) Fas-1 interactions. Surgery 1998; 124(2): 409–417. [PubMed] [Google Scholar]
- 18.Cowburn AS, et al. The survival effect of TNF-alpha in human neutrophils is mediated via NF-kappa B-dependent IL-8 release. Eur J Immunol 2004; 34(6): 1733–1743. [DOI] [PubMed] [Google Scholar]
- 19.Klein JB, et al. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol 2000; 164(8): 4286–4291. [DOI] [PubMed] [Google Scholar]
- 20.Dunican A, et al. Neutrophils regulate their own apoptosis via preservation of CXC receptors. J Surg Res 2000; 90(1): 32–38. [DOI] [PubMed] [Google Scholar]
- 21.Biffl WL, et al. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma 1996; 40(4): 575–578; discussion 578–579. [DOI] [PubMed] [Google Scholar]
- 22.Biffl WL, et al. Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent. J Leukoc Biol 1995; 58(5): 582–584. [DOI] [PubMed] [Google Scholar]
- 23.Ottonello L, et al. Differential regulation of spontaneous and immune complex-induced neutrophil apoptosis by proinflammatory cytokines. Role of oxidants, Bax and caspase-3. J Leukoc Biol 2002; 72(1): 125–132. [PubMed] [Google Scholar]
- 24.Moulding DA, et al. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood 1998; 92(7): 2495–2502. [PubMed] [Google Scholar]
- 25.Prince LR, et al. The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival. Am J Pathol 2004; 165(5): 1819–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg JM, et al. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol 2001; 69(3): 467–473. [PubMed] [Google Scholar]
- 27.Walmsley SR, et al. Characterization of the survival effect of tumour necrosis factor-alpha in human neutrophils. Biochem Soc Trans 2004; 32(Pt3): 456–460. [DOI] [PubMed] [Google Scholar]
- 28.Dibbert B, et al. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA 1999; 96(23): 13330–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanegasaki S, et al. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J Biochem 1986; 99(4): 1203–1210. [DOI] [PubMed] [Google Scholar]
- 30.Haslett C., et al. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol 1985; 119(1): 101–110. [PMC free article] [PubMed] [Google Scholar]
- 31.Simard FA, et al. MEK-independent ERK activation in human neutrophils and its impact on functional responses. J Leukoc Biol 2015; 98(4): 565–573. [DOI] [PubMed] [Google Scholar]
- 32.Brach MA, et al. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood 1992; 80(11): 2920–2924. [PubMed] [Google Scholar]
- 33.Kobayashi SD, et al. Spontaneous neutrophil apoptosis and regulation of cell survival by granulocyte macrophage-colony stimulating factor. J Leukoc Biol 2005; 78(6): 1408–1418. [DOI] [PubMed] [Google Scholar]
- 34.Costantini C, et al. Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol 2010; 22(10): 827–838. [DOI] [PubMed] [Google Scholar]
- 35.Mayadas TN, Cullere X. Neutrophil beta2 integrins: moderators of life or death decisions. Trends Immunol 2005; 26(7): 388–395. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood 2003; 102(7): 2660–2669. [DOI] [PubMed] [Google Scholar]
- 37.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13(4): 463–469. [DOI] [PubMed] [Google Scholar]
- 38.SenGupta S, et al. A Pseudomonas aeruginosa hepta-acylated lipid A variant associated with cystic fibrosis selectively activates human neutrophils. J Leukoc Biol 2016; 100(5): 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol 2016; 17(1): 9–17. [DOI] [PubMed] [Google Scholar]
- 40.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood 2014; 124(5): 710–719. [DOI] [PubMed] [Google Scholar]
- 41.Kumar V, Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 2010; 10(11): 1325–1334. [DOI] [PubMed] [Google Scholar]
- 42.Pelletier M Micheletti A, andCassatella MA.. Modulation of human neutrophil survival and antigen expression by activated CD4+ and CD8+ T cells. J Leukoc Biol 2010; 88(6): 1163–1170. [DOI] [PubMed] [Google Scholar]
- 43.Zouki C, et al. Peroxynitrite induces integrin-dependent adhesion of human neutrophils to endothelial cells via activation of the Raf-1/MEK/Erk pathway. FASEB J 2001; 15(1): 25–27. [DOI] [PubMed] [Google Scholar]
- 44.Rafiee P, et al. TNF-alpha induces tyrosine phosphorylation of mitogen-activated protein kinase in adherent human neutrophils. J Immunol 1995; 154(9): 4785–4792. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Human neutrophils depend on extrinsic factors produced by monocytes for their survival response to TLR4 stimulation by Shuvasree SenGupta, Madhavi J Rane, Silvia M Uriarte, Cassandra Woolley and Thomas C Mitchell in Innate Immunity