Abstract
Factor XI (FXI) deficiency is an uncommon autosomal disorder with variable bleeding phenotype, making peripartum management challenging. We describe our experience in pregnant women with FXI deficiency and identify strategies to minimize the use of hemostatic agents and increase utilization of neuraxial anesthesia. Electronic records of 28 pregnant women with FXI deficiency seen by a hematology service in an academic medical center from January 2006 to August 2018 were reviewed. Data on bleeding, obstetric history, peripartum management, and FXI activity were collected. Partial FXI deficiency was defined as >20 IU/dL and severe <20 IU/dL. Median FXI activity was 42 IU/dL (range <1-73 IU/dL), and median activated partial thromboplastin time was 32.2 seconds (range: 27.8-75 seconds). There were 64 pregnancies: 53 (83%) live births and 11 (17%) pregnancy losses. Postpartum hemorrhage occurred in 9 (17%) pregnancies. Antifibrinolytic agents and fresh frozen plasma were used only in women with severe deficiency (42% with bleeding and 17% with no bleeding phenotype, respectively). Neuraxial anesthesia was successfully administered in 32 (59%) deliveries. Most women with FXI deficiency have uncomplicated pregnancies and deliveries with minimal hemostatic support. Neuraxial anesthesia can be safely administered in most women.
Keywords: pregnancy, factor XI deficiency, outcomes, hemorrhage, neuraxial anesthesia, hemostatic agents
Introduction
Factor XI (FXI) plays an important role in the amplification phase of coagulation. Upon activation by factor XIIa, thrombin (generated by tissue factor–FVIIa complex), or autocatalysis, FXI is activated and contributes to hemostasis through activation of factor IX, which leads to downstream amplification of thrombin generation.1–3 Polyphosphates released from platelet-dense granules upon activation enhance FXI activation by thrombin several thousand-fold.4 An important function of FXI is indirect inhibition of fibrinolysis by promoting production of thrombin-activatable fibrinolytic inhibitor.5,6
Deficiency of FXI is an uncommon autosomal inherited bleeding disorder estimated at a prevalence of 1 per million in the general population but occurs more commonly in individuals of Ashkenazi Jewish ancestry with approximately 1 in 8 persons heterozygous and 1 in 450 homozygous for mutations in the FXI gene.7,8 Severe deficiency (SD) in homozygous or compound heterozygous carriers is classified as FXI activity <20 IU/dL and partial deficiency (PD) in heterozygous carriers with levels between 20 and 65 IU/dL.
The major manifestation of FXI deficiency is bleeding in areas of high fibrinolysis, including the oral cavity and genitourinary tract, and after trauma or surgical intervention.5–7 The bleeding tendency is unpredictable and not correlated with FXI activity. Individuals with the same FXI level may have different bleeding severities, and some individuals with PD have a bleeding phenotype.9 The strongest predictor of bleeding is a positive history of bleeding, regardless of the FXI level. The variability in clinical presentation of FXI deficiency makes assessment of bleeding risk and prophylactic management challenging, especially important in the peripartum period. Women with FXI deficiency have an unpredictable bleeding risk at delivery, that is, fresh frozen plasma (FFP) and or antifibrinolytic agents at the time of delivery and when it is safe to administer neuraxial anesthesia.
The level of FXI activity does not change during pregnancy.10 Postpartum hemorrhage (PPH) appears to be related to history of bleeding. There is a paucity of studies describing management of pregnant women with FXI deficiency, and therefore guidelines are not evidence based.10–16 Standard management of pregnant women with severe FXI deficiency is administration of FFP or FXI concentrate where available at the time of delivery, if women have a bleeding phenotype. Antifibrinolytics such as tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) are also commonly used to decrease the risk of PPH in women with bleeding phenotype, regardless of partial or severe FXI deficiency.15
Neuraxial anesthesia (epidural or spinal) in pregnant women with FXI deficiency is often avoided due to concern for spinal hematoma.10,15 Several small retrospective studies have reported using neuraxial anesthesia during labor and delivery with no cases of spinal hematomas.10,11,17–21 However, the administration of neuraxial anesthesia in pregnant women with FXI deficiency remains unsettled in clinical practice. The objective of this study was to characterize the experience in pregnant women with FXI deficiency at Weill Cornell Medicine/New York Presbyterian Hospital and identify strategies to increase utilization of neuraxial anesthesia while minimizing bleeding and avoid the unnecessary use of FFP.
Materials and Methods
Patient Population and Data Collection
We retrospectively evaluated the electronic medical records (EMRs) of pregnant women with FXI deficiency referred to the hematology clinic at New York Presbyterian Weill Cornell Medical Center in New York City between January 2006 and August 2018. Every time that an individual with an FXI deficiency is identified, their EMR information is placed in a secure data set. Subsequently, a database of consecutive pregnant women with a definitive diagnosis of FXI deficiency was assembled.
We collected the following data: demographics (age and ethnicity), reason for testing, bleeding history, family history, type of conception (natural or with assisted reproductive techniques [ART]), mode of delivery (vaginal or cesarean), type of anesthesia (neuraxial [epidural or spinal] or general), and peripartum outcomes. Women had an FXI activity (SD < 20 IU/dL and PD > 20 IU/dL) and activated partial thromboplastin time (aPTT) measured at the time of initial hematology consultation and at approximately 36 weeks of gestation.
The criteria to diagnose factor XI deficiency in our patients included either (1) a prolonged aPTT that corrected with mixing studies with a demonstration of a low FXI activity (below the lower limit of the normal range in our laboratory 65-150 IU/dL) or (2) the identification of a mutation in the FXI gene.
When available, we recorded blood type, factor VIII activity, von Willebrand factor (vWF) antigen, and vWF ristocetin cofactor in the third trimester closest to the time of delivery. We monitored hemoglobin and ferritin levels during pregnancy. We documented either oral or intravenous iron supplementation during pregnancy, administration of FFP or other blood products during delivery and the postpartum period, and use of antifibrinolytics (TXA or EACA). If delivery occurred at a different hospital, the outcomes from that delivery and peripartum management were collected based on patient report at postpartum follow-up in the hematology clinic. We excluded patients who delivered at a different hospital and did not follow-up in the postpartum period.
Defining Bleeding History and Bleeding Events During Pregnancy, Delivery, and Postpartum Periods
To determine a bleeding phenotype, we assessed 6 variables at the time of initial hematology consultation: (1) heavy menstrual bleeding characterized as lasting longer than 1 week, requiring changing pads or tampons every 2 hours or less, having quarter-size clots, interfering with ability to attend school or work, or necessitating hormonal contraception; (2) bleeding with dental procedures requiring intervention; (3) unexplained easy bruising; (4) epistaxis requiring medical intervention; (5) obstetrical bleeding; and (6) postoperative bleeding requiring intervention. Bleeding phenotype was defined as having 2 or more of the abovementioned criteria.22
Postpartum hemorrhage was defined as bleeding >500 mL following vaginal delivery or >1000 mL following cesarean delivery. Early PPH occurred within 24 hours of delivery and late PPH greater than 24 hours after delivery.23,24 Antenatal vaginal bleeding was described as bleeding occurring after 20 weeks of gestation and prior to delivery. The study was granted a waiver of informed consent and approved by our institutional review board.
Results
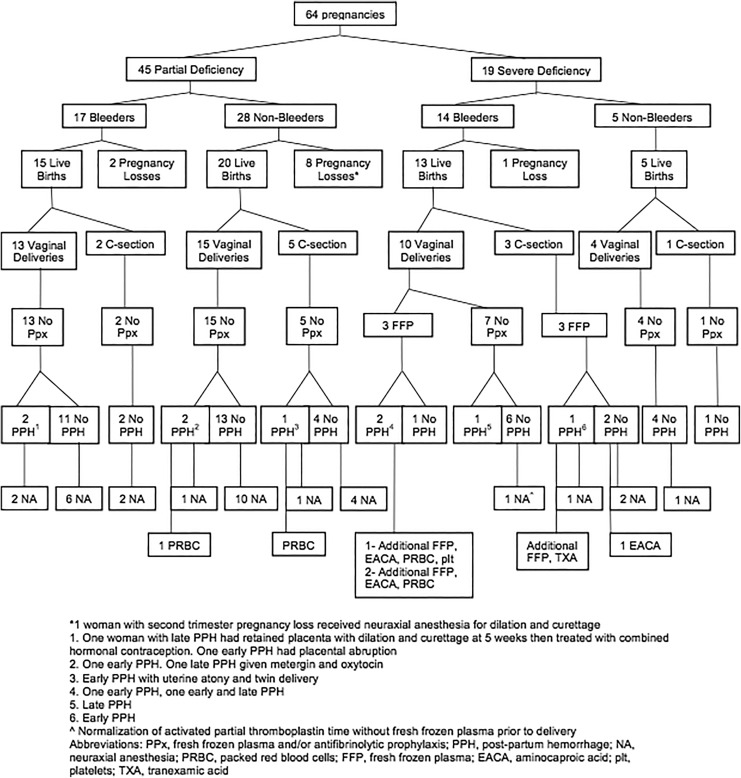
The study comprised 28 women with FXI deficiency (20 with PD and 8 with SD) who had 64 pregnancies (Figure 1). Twenty-four (86%) of 28 women were Ashkenazi Jewish (Table 1). Median FXI was 42 IU/dL (range: <1-73 IU/dL) and median aPTT = 32.2 seconds (range: 27.8-75 seconds), normal range = 25.9 to 35.1 seconds. Median FXI, aPTT, factor VIII, vWF antigen, and vWF ristocetin in the third trimester (when available) are shown in Table 2.
Figure 1.
Pregnancies in Women with factor XI (FXI) deficiency.
Table 1.
Baseline Patient Characteristics.
| All patients | 28 |
|---|---|
| Median age at delivery, years | 31 (19-39) |
| Ashkenazi Jewish | 24 |
| Non-Ashkenazi Jewisha | 4 |
| FXI PD | 20 |
| FXI SD | 8 |
| Median FXI activity, IU/dLb
Normal FXI activity (65-150 IU/dL) |
42 (≤1-73) |
| Median aPTT, secondsb
Normal aPTT, 25.9-35.1 seconds |
32.2 (27.8-75) |
| Diagnosis of FXI deficiency | |
| Positive family history | 7 |
| Bleeding history | 8 |
| Incidental prolonged aPTT | 4 |
| Genetic screening | 9 |
Abbreviations: aPTT, activated partial thromboplastin time; FXI, factor XI; PD, partial deficiency; SD, severe deficiency.
a1 Italian, 1 Northern Indian, 1 Afghani, 1 Chinese
bFXI Activity was obtained at the time of initial hematology evaluation.
Table 2.
Pregnancy, Delivery, and Anesthesia Characteristics.
| Pregnancy Characteristic | All Pregnancies | Partial Deficiency | Severe Deficiency |
|---|---|---|---|
| Number of pregnancies | 64 | 45/64 (70%) | 19/64 (30%) |
| Median pregnancies per patient | 2 (1-8) | 1.5 (1-8) | 2 (1-7) |
| Type of conception | |||
| Natural conception | 50/64 (78%) | 35/45 (78%) | 15/19 (79%) |
| Assisted reproductive techniques | 14/64 (22%) | 10/45 (22%) | 4/19 (21%) |
| Mode of delivery | |||
| Vaginal delivery | 42/53 (79%) | 28/35 (80%) | 14/18 (78%) |
| Cesarean delivery | 11/53 (21%) | 7/35 (20%) | 4/18 (22%) |
| Diagnosis known at delivery | 49/64 (77%) | 31/45 (69%) | 18/19 (95%) |
| Third-trimester laboratory evaluation (n = 53 deliveries) | |||
| Median FXI activity, IU/dL (35 deliveries) | 42 (<1-69) | 48 (24-69) | 4 (<1-15) |
| Median aPTT, seconds (36 deliveries) | 32.2 (27-77.2) | 30.3 (27-34) | 43.1 (34.9-77.2) |
| Median FVIII activity, IU/dL (28 deliveries) | 162 (87-339) | 179.5 (87-329) | 158 (98-339) |
| Median vWF antigen (25 deliveries) | 222 (83-509) | 222 (83-509) | 204.5 (122-324) |
| Median vWF ristocetin (25 deliveries) | 208 (66-417) | 208 (66-417) | 181 (102-260) |
| Plasma | 6 | - | 6 |
| Antifibrinolytic agenta (tranexamic acid/aminocaproic acid) | 4 | - | 4 |
| Iron (intravenous/oral) | 7 | 5 | 2 |
| Outcomes | |||
| Live birth | 53/64 (83%) | 35/45 (78%) | 18/19 (95%) |
| Pregnancy lossb | 11/64 (17%) | 10/45 (22%) | 1/19 (5%) |
| Postpartum hemorrhage | 9/53 (17%) | 5/35 (14%)c | 4/18 (22%) |
| Early pregnancy loss with >2 g/dL hemoglobin fall | 1/64 (2%) | 1/45 (2%) | - |
| Antepartum bleeding | 3/64 (5%) | 2/45 (4%)c | 1/19 (5%) |
| Lochia >6 weeks | 2/53 (4%) | 2/35 (6%) | - |
| Type of anesthesia used | |||
| Any neuraxial anesthesia | 32/54 (59%)d | 27/36 (75%)d | 5/18 (28%) |
| Epidural anesthesia | 25 | 22 | 3 |
| Spinal anesthesia | 6 | 4 | 2 |
| Combined spinal and epidural anesthesia | 1 | 1 | 0 |
Abbreviations: aPTT, activated partial thromboplastin time; EACA, epsilon aminocaproic acid; FXI, factor XI; FVIII, factor VIII; vWF, von Willebrand Factor; PPH, postpartum hemorrhage.
a Antifibrinolytic agents: aminocaproic acid (EACA); tranexamic acid (TXA). EACA was used in 3 deliveries and TXA in 1. All 4 women who received antifibrinolytics also received plasma.
b Pregnancy loss includes both early and late pregnancy losses.
c Two pregnancies in women with partial deficiency were complicated by both PPH and antepartum bleeding.
d Total includes all pregnancies >24 weeks.
Most women were diagnosed with FXI deficiency by genetic screening during preconception or early pregnancy (Table 1). Of the 64 pregnancies, 45 (70%) and 19 (30%) occurred in women with PD and SD, respectively. Of the 28 women, 13 had a bleeding phenotype (8 with PD and 5 with SD).
The majority (79%) of women had natural conception (Table 2). Only 1 woman who conceived with ART had a bleeding phenotype and did not experience any bleeding complications.
Fifty-three (83%) pregnancies resulted in live births: 35 (78%) and 18 (95%) in women with PD and SD, respectively. Forty-two (79%) women had vaginal delivery and 11 (21%) cesarean delivery, 4 in women with SD (6 planned, 1 repeat cesarean delivery, and 5 due to failed progression of labor).
Ten (17%) of 11 pregnancy losses (10 in women with PD and 1 in a woman with SD) occurred in the first trimester. No abnormal embryo karyotypes were identified in the spontaneous pregnancy losses that were evaluated. Two of the pregnancy losses were complicated by bleeding (one in the first trimester and one in the second trimester). Neither required blood products or hemostatic agents.
Pregnancy Postpartum Bleeding and Management
Antenatal bleeding occurred in 3 pregnancies. Two women had subchorionic hematomas, one resulting in a second-trimester pregnancy loss, and the third had third-trimester vaginal spotting.
Nine (17%) deliveries were complicated by PPH, 5 in women with PD and 4 in women with SD (Table 2). Two occurred after cesarean delivery and 7 after vaginal delivery. All the women with SD who experienced PPH had a bleeding phenotype, while only 2 of the ones with PD had PPH. There were additional obstetrical–gynecological reasons for PPH in 6 (66%) of the 9 deliveries complicated with PPH, including perineal laceration in 4, retained placenta in 1, and uterine atony after delivery of a twin pregnancy in another. None of the women with PD who experienced PPH received prophylaxis with antifibrinolytics or FFP; however, one required transfusion of 2U packed red blood cells, cryoprecipitate, and administration of uterotonics. Estimated blood loss for early PPH ranged from 750 to 2150 mL for vaginal deliveries and more than 1000 to 2000 mL for cesarean delivery. Postpartum hemorrhage occurred most frequently in women with blood group A, 5 (71%) of the 7 women, in 1 woman with blood type O, and another with blood type B.
Antifibrinolytic agents and FFP were used only in patients with SD. Fresh frozen plasma was prophylactically administered in 6 deliveries (3 vaginal and 3 cesarean delivery) among 4 women (Figure 1). Two women with SD did not receive either antifibrinolytics or FFP: One had normalization of the aPTT at the end of pregnancy, despite a baseline FXI of 18% and the other a precipitous delivery. Four of the 6 women received FFP at a dose of 15 to 20 mL/kg, 3 for early PPH. One received 30 mL/kg of FFP (20 mL/kg before and during cesarean delivery and 10 mL/kg post cesarean delivery for early PPH) without complications. All women who received FFP had SD, prolonged aPTT at onset of labor or planned cesarean delivery, and a bleeding phenotype. Except for one minor allergic reaction to administration of FFP, there were no associated complications.
Antifibrinolytic agents (TXA in 1 and EACA in 3) were given during 4 deliveries in 2 women. In 2 deliveries, prophylactic administration resulted in no bleeding events. Intravenous iron sucrose was administered in 7 (11%) pregnancies during the third trimester (Table 2).
Neuraxial Anesthesia
Neuraxial anesthesia was provided in 32 (59%) of the 54 deliveries: 27 (75%) in women with PD and 5 (28%) with SD (Figure 1; Tables 2 and 3). In 7 (22%) of 32 deliveries in which neuraxial anesthesia was administered, there was concomitant PPH (5 in PD and 2 SD) without any bleeding complications related to anesthesia.
Table 3.
Factor XI Deficient Women Receiving Neuraxial Anesthesia.
| ID | Severity | Pregnancy | FXIa | aPTTa | Anesthesia | Plasma and/or Antifibrinolytic |
|---|---|---|---|---|---|---|
| 1 | Partial | 1/1 | 69 | – | Spinal | n |
| 2 | Severe | 1/2 | – | – | Epidural | n |
| 3 | Partial | 1/5 | – | – | Epidural | n |
| 2/5 | – | – | Epidural | n | ||
| 3/5 | – | – | Epidural | n | ||
| 4/5 | 42 | 32 | Epidural | n | ||
| 5/5 | 45 | 30.4 | Epidural | n | ||
| 4 | Severe | 1/2 | <1 | 60 | Spinal | 2200cc (30 mL/kg) plasma, TXA |
| 2/2 | 2 | 61.4 | Spinal | 1100cc (14.5 mL/kg) plasma, EACA | ||
| 5 | Partial | 1/3 | – | – | Epidural | n |
| 2/3 | 52 | 31.7 | Epidural | n | ||
| 6 | Partial | 1/1 | 40 | 31.1 | Spinal | n |
| 7 | Partial | 1/2 | – | – | Epidural | n |
| 2/2 | 36 | 32.3 | Epidural | n | ||
| 8 | Partial | 2/2 | 50 | 32 | Epidural | n |
| 9 | Partial | 4/8 | – | – | Epidural | n |
| 10 | Partial | 1/1 | 48 | 28 | Epidural | n |
| 11 | Partial | 1/4 | – | – | Epidural | n |
| 12 | Partial | 4/4 | 44 | 33.9 | Spinal | n |
| 13 | Severe | 1/1 | 7 | 39.9 | Epidural | 1320cc (17.1 mL/kg) plasma |
| 14 | Partial | 1/1 | 46 | 29.7 | Spinal | N |
| 15 | Partial | 1/3 | – | 27.7 | Epidural | n |
| 3/3 | 48 | 27 | Spinal + Epidural | n | ||
| 16 | Partial | 1/1 | 59 | 27.7 | Epidural | n |
| 17 | Partial | 2/2 | 63 | 28.3 | Epidural | n |
| 18 | Partial | 2/2 | 48 | 30.7 | Epidural | n |
| 19 | Partial | 1/1 | 50 | 32 | Epidural | n |
| 20 | Partial | 1/1 | 49 | 27.1 | Epidural | n |
| 21 | Partial | 1/1 | 42 | 29.9 | Epidural | n |
| 22 | Severe | 2/2 | 15 | 34.9 | Epidural | n |
| 23 | Partial | 1/1 | 55 | 29 | Epidural | n |
| 24 | Partial | 1/1 | 44 | 30.2 | Epidural | n |
Abbreviations: aPTT, activated partial thromboplastin time (seconds); EACA, aminocaproic acid; FXI, Factor XI Activity (IU/dL); TXA, tranexamic acid.
a Third trimester value.
Two women with SD received FFP prior to neuraxial anesthesia in 3 total deliveries. Two women with SD had uncomplicated epidural anesthesia without administration of FFP. One of these women with FXI of 18% and mild bleeding phenotype experienced normalization of the aPTT without FFP prior to delivery. Neuraxial anesthesia was not offered in 5 deliveries in women with SD. These women received either general anesthesia in the case of cesarean delivery or remifentanil (a short-acting synthetic opioid analgesic) or patient-controlled anesthesia with fentanyl for vaginal deliveries, all without complications.
Discussion
In this study of 64 pregnancies in 28 women with FXI deficiency, similar rates of spontaneous pregnancy losses and antenatal vaginal bleeding but higher rates of PPH were seen than in the general obstetric population. The use of neuraxial anesthesia was nearly double that of prior reports, despite less administration of prophylactic hemostatic agents.10,16
The 16% rate of spontaneous pregnancy loss (majority in the first trimester) is within the range of 3% to 22% noted in 2 studies of pregnant women with FXI deficiency11,16 and similar to the 15% rate in the general obstetric population.25
Limited data exist on antepartum hemorrhage from 20 weeks of gestation to delivery in women with FXI deficiency. The 5% rate of antepartum hemorrhage is similar to the 2% to 5% rate of the general obstetric population26 and to the 4% to 5% rate in 2 prior studies of women with FXI deficiency.16,20
Our PPH rate of 17% in women with FXI deficiency is comparable to the 17% noted in 490 deliveries in 250 women,10 19% in study of 67 pregnancies in 25 women,16 and 18% rate reported in a systematic review of 27 studies involving 372 pregnancies.11 In all these studies, there was a higher administration of prophylactic hemostatic agents and much less neuraxial anesthesia.
Early and late PPH is increased in women with FXI deficiency compared to the general obstetric population, where the incidence of primary PPH is 2% to 6%, affecting 3% of deliveries in the United States.23,27 In our study, PPH was higher in women with SD (22%) than in women with PD (14%). Postpartum hemorrhage occurred despite administration of prophylactic FFP in one-third of the deliveries in women with SD and bleeding phenotype, suggesting that antifibrinolytic agents may needed to be added to prevent PPH in women with FXI deficiency and bleeding phenotype. Importantly, one major contributor to PPH is concomitant genital lacerations, retained placenta, and uterine atony as also reported in a previous study.28 This observation emphasizes that an experienced obstetrical team should perform delivery of these women.
In a review of 7 studies comprising 490 deliveries (276 women; 113 with SD) prophylaxis was provided in 20% of the deliveries which is twice the 11% in our study.10 Prophylaxis included FFP (28), FXI concentrate (20), TXA (46), and recombinant VIIa (4). In a single-center study (25 women, 67 pregnancies), the indications for administration of FFP included FXI <40 IU/dL and bleeding history, operative delivery, or neuraxial anesthesia. In the latter indication, a documentation of an FXI >40 IU/dL was required prior to neuraxial anesthesia. Tranexamic acid was given to women with FXI of 60 to 70 IU/dL.16 Despite the higher thresholds, both studies had similar rates of PPH to our study.
Most of our pregnancies were delivered under neuraxial anesthesia; 85% had neuraxial anesthesia with at least 1 pregnancy. This rate of neuroaxial anesthesia use is almost twice the rate of 31% (73/236 deliveries) reported in 6 studies of pregnant women with FXI deficiency. Another study reported a rate of 24% where neuraxial anesthesia was given only after administration of FFP with a FXI target of >40 IU/dL and in women with no significant bleeding history.10,16 While most women who received neuraxial anesthesia in our study had PD, 5 women with SD also did. In all women with SD, the aPTT correction was achieved with FFP administration, except for one in whom due to a substantial increase in FVIII; her aPTT corrected at term. Of note, during pregnancy, there is an increase in factor VIII level, which becomes more pronounced in the last trimester of pregnancy. This offers an advantage to women with borderline FXI levels <20 IU/mL because, as a consequence of an increase in FVIII, the aPTT will normalize avoiding unnecessary use of FFP. In a retrospective review of 13 pregnant women with FXI deficiency who presented for delivery, 9 were managed with neuraxial anesthesia. Baseline factor levels ranged from <15 IU/dL to 50 IU/dL. Fresh frozen plasma was administered to correct the aPTT in most, but not all, cases. Hematology consultation was obtained for all patients, and no hematological or anesthetic complications were noted.19
Among the 32 deliveries where neuraxial anesthesia was instituted, there was not one occurrence of spinal hematoma, suggesting this procedure is safe in selected women with FXI deficiency. This includes women with PD and no bleeding phenotype, women with PD and bleeding phenotype after administration of prophylactic antifibrinolytics, women with SD with and without bleeding phenotype after FFP administration (with correction of the aPTT), and additional antifibrinolytics in SD women with bleeding phenotype. The overall higher rate of neuraxial anesthesia in our study may reflect the higher rate of neuraxial anesthesia use in the United States when compared to other countries but also our approach to which patients with FXI deficiency can safely receive it.29
The use of Viscoelastic testing with either rotational thromboelastogram (ROTEM) or thromboelastogram is not part of the standard of care for pregnant patients with FXI deficiency to predict bleeding at the time of delivery. In a study comparing ROTEM among FXI pregnant women, pregnant women both in the third trimester and healthy nonpregnant controls there was strong correlation between the clotting time and clot formation time but not the maximum clot firmness and the bleeding score. Despite lack of correlation between ROTEM parameters and estimated blood loss, and the inability to determine whether using ROTEM analysis had any effect on the overall rate of PPH, there was a lower proportion of patients receiving FXI concentrate or recombinant factor VIIa during delivery and regional anesthesia in the group managed with ROTEM.28 Since ROTEM is becoming increasingly used, it may be an important tool in addition to bleeding phenotype and FXI level to prevent unnecessary exposure to blood products in these women at the time of delivery.
One study demonstrated that higher thrombin generation in the presence of platelets and absence of contact activation correlated well with less bleeding history in nonpregnant patients with FXI deficiency.30 Another study using a thrombin generation assay performed in nonpregnant patients with severe FXI deficiency showed that the bleeders exhibited lower fibrin network density and lower clot stability in the presence of tissue plasminogen activator compared to the nonbleeders.31 Findings of these studies may guide to design a prospect study in pregnant women with FXI deficiency to optimize their management at the time of delivery.
Our study has several important limitations including the retrospective design, the relatively small sample size, and the lack of standardization of bleeding history in patients with FXI deficiency. To some extent, these limitations were minimized by location at a single center under direction of a multidisciplinary team composed of a hematologist, high-risk obstetricians, and obstetric anesthesiologists sharing a single management plan. The blood loss to define PPH was estimated and not objectively quantified; however, many causes of PPH are obstetric in nature, and the occurrence of PPH despite prophylactic FFP administration is evidence of this in our cases. In the future, addition of ROTEM or thrombin-generation assays performed at the time of labor may guide administration of FFP and/or antifibrinolytics in the peripartum period.32
In conclusion, we demonstrated that the majority of women with FXI deficiency have uncomplicated pregnancies and deliveries with minimal use of FFP. The use of antifibrinolytics in these patients appears effective and safe. In our experience, neuraxial anesthesia can be administered in most women upon an individual assessment of the bleeding risk, correction of the aPTT, and multidisciplinary discussion among high-risk obstetrician, obstetric anesthesiologist, and hematologist.
Acknowledgments
The authors would like to acknowledge Drs James Bussel and Dr Stephen M. Pastores for their thorough comments and review of the manuscript.
Authors’ Note: This article was presented in part as an oral abstract at the International Society of Thrombosis and Hemostasis Congress, June 20-25, 2015, Toronto, Canada. Dr Gloria F. Gerber collected and analyzed the data and wrote the first draft of the manuscript. Dr Kelsey A. Klute collected and analyzed part of the data. Dr John Chapin contributed to the design of the study and reviewed the manuscript. Dr James Bussel contributed to write the final manuscript. Dr Maria T. DeSancho designed the study, analyzed the data, and wrote the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maria T. DeSancho  https://orcid.org/0000-0001-9239-5131
https://orcid.org/0000-0001-9239-5131
References
- 1. Gomez K, Bolton-Maggs P. Factor XI deficiency. Haemophilia. 2008;14(6):1183–1189. [DOI] [PubMed] [Google Scholar]
- 2. Butenas S, Dee JD, Mann KG. The function of factor XI in tissue factor-initiated thrombin generation. J Thromb Haemost. 2003;1(10):2103–2111. [DOI] [PubMed] [Google Scholar]
- 3. Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;115(13):2569–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wheeler AP, Gailani D. Why factor XI deficiency is a clinical concern. Expert Rev Hematol. 2016;9(7):629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolton-Maggs PHB. Factor XI deficiency-resolving the enigma? Hematology. 2009;1:97–105. [DOI] [PubMed] [Google Scholar]
- 6. Duga S, Salomon O. Congenital factor XI deficiency: an update. Semin Thromb Hemost. 2013;39(6):621–631. [DOI] [PubMed] [Google Scholar]
- 7. Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325(3):153–158. [DOI] [PubMed] [Google Scholar]
- 8. Shpilberg O, Peretz H, Zivelin A, et al. One of the two common mutations causing factor XI deficiency in Ashkenazi Jews (type II) is also prevalent in Iraqi Jews, who represent the ancient gene pool of Jews. Blood. 1995;85(2):429–432. [PubMed] [Google Scholar]
- 9. Santoro C, Di Mauro R, Baldacci E, et al. Bleeding phenotype and correlation with factor XI (FXI) activity in congenital FXI deficiency: results of a retrospective study from a single centre. Haemophilia. 2015;21(4):496–501. [DOI] [PubMed] [Google Scholar]
- 10. Davies J, Kadir R. The management of factor XI deficiency in pregnancy. Semin Thromb Hemost. 2016;42(7):732–740. [DOI] [PubMed] [Google Scholar]
- 11. Wiewel-Verschueren S, Arendz IJ, M Knol H, Meijer K. Gynaecological and obstetrical bleeding in women with factor XI deficiency—a systematic review. Haemophilia. 2016;22(2):188–195. [DOI] [PubMed] [Google Scholar]
- 12. Salomon O, Steinberg DM, Tamarin I, Zivelin A, Seligsohn U. Plasma replacement therapy during labor is not mandatory for women with severe factor XI deficiency. Blood Coagul Fibrinolysis. 2005;16(1):37–41. [DOI] [PubMed] [Google Scholar]
- 13. Mumford AD, Ackroyd S, Alikhan R, et al. Guideline for the diagnosis and management of the rare coagulation disorders. Br J Haematol. 2014;167(3):304–326. [DOI] [PubMed] [Google Scholar]
- 14. Lee CA, Chi C, Pavord SR, et al. The obstetric and gynaecological management of women with inherited bleeding disorders—review with guidelines produced by a taskforce of UK haemophilia centre doctors’ organization. Haemophilia. 2006;12(4):301–336. [DOI] [PubMed] [Google Scholar]
- 15. Management of inherited bleeding disorders in pregnancy: Green-top Guideline No. 71 (joint with UKHCDO). BJOG. 2017;124(8):e193–e263. [DOI] [PubMed] [Google Scholar]
- 16. Verghese L, Tingi E, Thachil J, Hay C, Byrd L. Management of parturients with factor XI deficiency-10 year case series and review of literature. Eur J Obstet Gynecol Reprod Biol. 2017;215:85–92. [DOI] [PubMed] [Google Scholar]
- 17. Reuveni A, Orbach-Zinger S, Eidelman LA, Ginosar Y, Ioscovich A. Peripartum anesthetic management of patients with factor XI deficiency. J Perinat Med. 2014;42(3):295–300. [DOI] [PubMed] [Google Scholar]
- 18. Setty S, Reddell A, England A, Gomez K, Kadir R. The role of recombinant factor VIIa for obstetric block in women with severe factor XI deficiency. Haemophilia. 2011;17(6):906–909. [DOI] [PubMed] [Google Scholar]
- 19. Singh A, Harnett MJ, Connors JM, Camann WR. Factor XI deficiency and obstetrical anesthesia. Anesth Analg. 2009;108(6):1882–1885. [DOI] [PubMed] [Google Scholar]
- 20. Chi C, Kulkarni A, Lee CA, Kadir RA. The obstetric experience of women with factor XI deficiency. Acta Obstet Gynecol Scand. 2009;88(10):1095–1100. [DOI] [PubMed] [Google Scholar]
- 21. Chi C, Lee CA, England A, Hingorani J, Paintsil J, Kadir RA. Obstetric analgesia and anaesthesia in women with inherited bleeding disorders. Thromb Haemost. 2009;101(6):1104–1111. [PubMed] [Google Scholar]
- 22. Bolton Maggs PHB, Patterson D, Wensley R, Tuddenham E. Definition of the bleeding tendency in factor XI-deficient kindreds—a clinical and laboratory study. Thromb Haemost. 1995;73(2):194–202. [PubMed] [Google Scholar]
- 23. Rath WH. Postpartum hemorrhage—update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand. 2011;90(5):421–428. [DOI] [PubMed] [Google Scholar]
- 24. Borovac-Pinheiro A, Pacagnella RC, Cecatti JG, et al. Postpartum hemorrhage: new insights for definition and diagnosis. Am J Obstet Gynecol. 2018;219(2):162–168. [DOI] [PubMed] [Google Scholar]
- 25. Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–611. [DOI] [PubMed] [Google Scholar]
- 26. Sinha P, Kuruba N. Ante-partum haemorrhage: an update. J Obstet Gynaecol 2008;28:377–381. [DOI] [PubMed] [Google Scholar]
- 27. Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the international postpartum hemorrhage collaborative group. BMC Pregnancy Childbirth. 2009;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davies J, Harper A, Kadir RA. The role of rotational thromboelastometry in assessment of haemostasis during pregnancy in women with factor XI deficiency. Haemophilia. 2016;22(2):276–284. [DOI] [PubMed] [Google Scholar]
- 29. Osterman MJ, Martin JA. Epidural and spinal anesthesia use during labor: 27-state reporting area, 2008. Natl Vital Stat Rep. 2011;59(5):1–13. [PubMed] [Google Scholar]
- 30. Pike GN, Cumming AM, Hay CRM, Bolton-Maggs PHB, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;126(3):397–405. [DOI] [PubMed] [Google Scholar]
- 31. Zucker M, Seligsohn U, Salomon O, Wolberg A. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12(7):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. James P, Salomon O, Mikovic D, Peyvandi F. Rare bleeding disorders—bleeding assessment tools, laboratory aspects and phenotype and therapy of FXI deficiency. Haemophilia. 2014;20(suppl 4):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]