Abstract
Background: The effects of musical training on the body in professional musicians remain an understudied area, particularly in reference to understanding and managing orthopedic/neuromuscular deviations and injuries in this population. The purpose of this study was to evaluate hand/finger fine motor function in musicians via physical examination as well as laboratory-based evaluations. Methods: Thirteen healthy noninjured young elite string musicians participated in this study. Performance of musicians was compared with healthy age-matched, sex-matched, and handedness-matched nonmusician controls. Results: Musicians exhibited decreased intrinsic muscle strength compared with controls; however, no change in extrinsic muscle strength was found between groups. No between-group differences in overall force control were found; however, Group × Hand (right vs left) interactions were found in force control. Conclusions: These data suggest that musicians are a unique population with respect to: (1) fine motor control of the hand; and (2) exhibit changes in differential hand use. This suggests cortical reorganization of string musicians, such that this population should be studied separately from typical healthy controls with respect to hand function.
Keywords: musicians, stringed instruments, fine motor control, handedness, biomechanics
Introduction
In recent years, the impact of music and musical training on the mind and body has been of interest. In most of these studies, researchers have learned more about how the brain reacts to listening to music, particularly classical music.2,26,35,39 Despite this interest, the effects of musical training on the body in professional musicians remain an understudied area, particularly in reference to understanding and managing orthopedic and neuromuscular deviations and injuries in this population. Given that professional musicians practice for anywhere between 2 and 8 hours a day, rehearse for up to 3 hours at a time, and play for about 2 hours in a given performance,18 it is likely that they have superior motor and force control of the hands. This is supported by reports that trained pianists have superior control over the timing and loudness of keystrokes compared with nonmusicians.10 Given the high workload of the upper extremities, it is also likely that professional musicians have a higher incidence of neurological and musculoskeletal alterations and or maladaptations in the upper extremities, supported by recent cross-sectional studies.23,33,34
To better understand this population and their potential neuromuscular alterations, it is important to measure basic features of motor function in professional musicians. Specifically, we are interested in measures of strength and force control in this population, as differences or imbalances in these measures in musicians may indicate the source of maladaptations associated with musical training. The majority of previous studies on professional musicians have focused on assessment of temporal tapping measures,1,10,25,30 not kinetic function and performance. Accordingly, the goal of this study is to assess the effects of professional musical instrument playing on measures of upper extremity strength and motor function via force production.
In addition to this goal, we are also interested in assessing kinetic performance differences between the 2 hands in professional musicians versus nonmusician controls. Our interest in between-hand differences stems from the differential use of the 2 hands in most musical instruments, particularly stringed instruments, such that the right hand is typically used to control instrument volume via dynamic movements whereas the left hand is primarily used to control sound tone and physical stability of the instrument.18,20 The separation of roles between the 2 hands in a single motor task, particularly in stringed instruments, is notable. This separation of hand roles appears to be in line with the dynamic dominance theory,27 such that one arm/limb acts as a stabilizer whereas the other arm/limb performs dynamic actions in bimanual tasks.
To investigate these phenomena, we assessed upper extremity strength using digital dynamometers and motor function via submaximal force production control. Specifically, we hypothesize that: (1) musicians will exhibit larger maximal force values for both intrinsic and extrinsic muscles of the hand; and (2) measures of force control (aka steadiness) will be better in musicians versus controls. No hypotheses regarding differences between hands were developed a priori, as exploration of between-hand differences in the musicians group was an exploratory aim of this study.
Materials and Methods
Participants
Thirteen healthy musicians (22 ± 3 years old; 6 men and 7 women) and 13 healthy nonmusicians (22 ± 3 years old; 6 men and 7 women) volunteered to participate (mean ± SD) in the current study. Twelve of the musicians identified as viola or violin players and 1 as a cello player. Each of the musicians identified as professional musicians. Nonmusician controls were matched for age, sex, and declared handedness (dominance). Nonmusician controls did not report a history of instrument or other musical training/playing. Eleven participants in each group declared right handedness, indicated by preferential use of that limb during writing, drawing, and eating, whereas 2 participants in each group declared left handedness. Participants with a history of any neurological disability (eg, cerebrovascular accident (stroke), multiple sclerosis, Parkinson disease, Huntington disease, myasthenia gravis, traumatic brain injury, and hereditary or acquired neuropathies) were excluded from the current study. Participants did not report current or previous injury to the upper extremities. All participants gave informed consent according to the procedures approved by the Institutional Review Board of Houston Methodist Hospital.
Maximal Force Production
Extrinsic and intrinsic muscle strength of each participant was evaluated for each hand. Extrinsic muscle evaluation consisted of power grip and precision pinch. Participants performed maximal grip strength (MGS) with all 5 fingers of their hand in a power grip. In addition, maximal precision pinch strength (MPS) was evaluated with the thumb and index finger of the hand. Maximal strengths were evaluated using Biometrics Grip and Pinch Dynamometers with a wireless DataLOG system (models G200 and P200, and DataLOG model MWX8, Biometrics Ltd., Newport, UK). There were a total of 3 trials per grip type (power grip and precision pinch) for the dominant hand, lasting approximately 5 seconds; data were collected at 100 Hz. The maximum MPS value generated output for the 3 trials per hand was used to calculate the force percentage used in the submaximal force production tasks for each hand. Intrinsic muscle strength was evaluated using the PRIME dynamometer device (OrthoIntrinsics, Houston, Texas). Maximal intrinsic muscle forces of the first dorsal interosseous, abductor digiti minimi, and the opponens pollicis were measured using PRIME.32,38
Submaximal Force Production
After maximal force evaluation, participants performed a series of submaximal force production tasks. Each task involved using the index finger and thumb in a precision pinch grip to produce a specified level of force, with feedback provided via a computer screen. Each participant performed 3 trials for each submaximal condition (15% and 40%), lasting 15 seconds each. Participants were asked to match and maintain precision pinch forces to the best of their ability on the screen. Forces presented and recorded were a sum of the normal forces produced on the object. All forces produced by the thumb and index finger were recorded simultaneously using 2 identical 6-component force—moment transducers (Nano-25 transducers; ATI Industrial Automation, Garner, North Carolina). The grip width of the object (defined as the distance between the contact surfaces of the Nano-25 sensors) was 0.07 m. The total mass of the object was 0.170 kg. The transducers were mounted to a fixed aluminum device throughout the entire testing session. Sandpaper (320-grit) was attached to the contact surfaces of each sensor to increase the friction between the digits and the transducers. The finger pad–sandpaper coefficient for static friction was approximately 0.96.8,28 Transducer signals were amplified and multiplexed using ATI hardware prior to being routed to an analog to digital converter (via cDAQ-9174 chassis and NI-9205 input modules, National Instruments, Austin, Texas). A customized Labview program (National Instruments, Austin, Texas) was used for data acquisition, and customized MATLAB (Mathworks Inc., Natick, Massachusetts) programs were written for data processing. The data sampling frequency was set at 100 Hz. The axes of the presented visual feedback remained the same across all study participants (0-60 N along the y-axis and 0-15 s across the x-axis).
Participants were instructed to sit in a chair facing a table with an upright posture. The transducer was situated 0.4 m near the front of the participant’s torso and approximately 0.15 m away from the midline of the body toward the dominant (right) hand. Neutral wrist position and hand orientation were maintained during testing. Participants were instructed to only produce pinch forces necessary to complete the task. Participants were not permitted to move the object (ie, lift the object) at any time and the forearm of the hand used was supported by the table at all times. The test object remained in the same position on the testing table at all times.
Linear Analysis of Force Variability
Submaximal force data were analyzed with respect to both linear and nonlinear measures. Each form of analysis was intended to provide complementary information regarding the variability profiles of the data collected in this study. At the most basic level, mean force produced and variability of force production were evaluated. Variability measures included root mean square error (RMSE) of the force output relative to the target and the coefficient of variation (CV). As variability in force production increases with mean force production value,31 CV may capture consistency in the signal to variability ratio, whereas RMSE may show differences among tasks. Both measures are used to evaluate accuracy of the forces produced with respect to the presented target values.15
Nonlinear Analysis of Force Variability
The structure of force output variability was quantified via approximate entropy (ApEn) and detrended fluctuation exponent analysis (DFAα).8 ApEn was calculated according to Pincus’s algorithm, using vector length (m) of 2 and tolerance (r) of 0.2.21,22,31 The ApEn measure indicates the irregularity (variability) in both short-term and long-term ranges of a signal in the time domain given the probability distribution of its components.21,22,31 The value of ApEn increases as the signal consists of more random components. The amount of time-dependent variability in the signal is also reflected by DFAα, such that long-term self-affinity in the signal is reflected by values greater than 0.5. DFAα values less than 0.5 indicate less systematic (more random) signal components.8,15
Statistics
The data are presented in the text and figures as means ± standard errors. Repeated measures analyses of variance (RM-ANOVAs) were performed to observe the effects of Group (musicians vs controls), Hand (left vs right), and Level (2 levels for submaximal force production: 15% and 40% MPS). Dependent measures included maximal strength (grip, pinch, first dorsal interosseous, abductor digiti minimi, and the opponens pollicis), RMSE, CV, ApEn, and DFAα. For all ANOVAs, the assumption of sphericity was verified using Mauchly’s sphericity test. If sphericity was violated, the degrees of freedom were adjusted as necessary using Greenhouse-Geisser corrections.
Results
Maximal Force Production
Extrinsic muscles
Strength evaluation of extrinsic hand muscles via measurement of maximal grip and pinch forces did not indicate strength differences between Groups. No between-hand (right vs left) differences were found in either measure of extrinsic muscle strength. Data can be found in Figures 1a and 1b.
Figure 1.
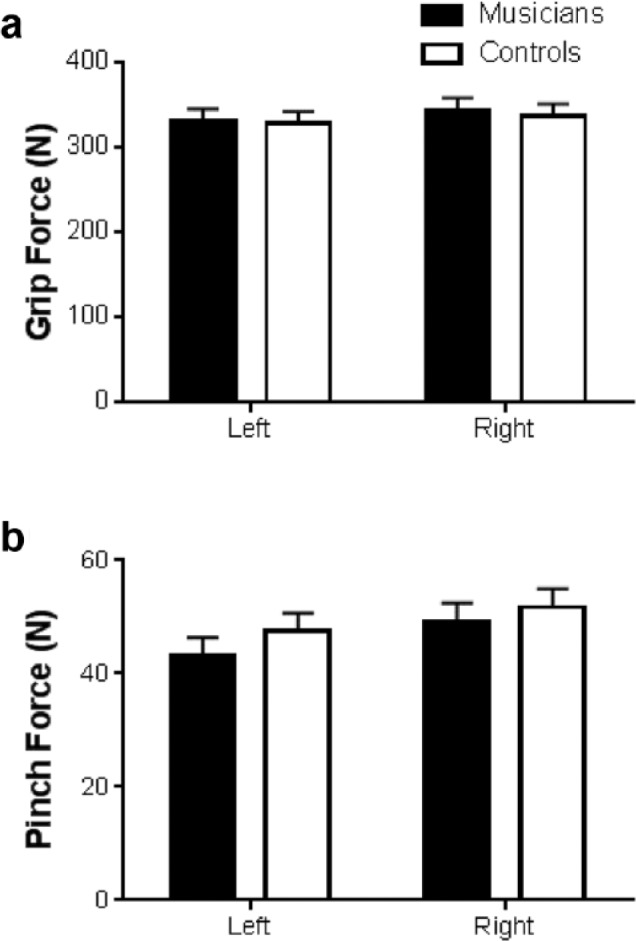
Mean and standard error values for maximal extrinsic muscle force production in the left and right hands for musicians versus healthy nonmusician controls: (a) maximal grip strength, (b) maximal pinch strength.
Intrinsic muscles
In contrast, strength evaluation of intrinsic hand muscles indicated significant differences between the Groups. Significant Group differences in strength of the first dorsal interosseous (F1,36 = 7.708, P < .01), abductor digiti minimi (F1,36 = 13.877, P < .001), and the opponens pollicis (F1,36 = 8.262, P < .01) indicated that healthy nonmusician controls possessed stronger intrinsic hand muscles as compared with musicians (Figures 2a-2c). No significant differences were found between hands (right vs left) for any measures of intrinsic muscle strength and no Group × Hand interactions were found.
Figure 2.
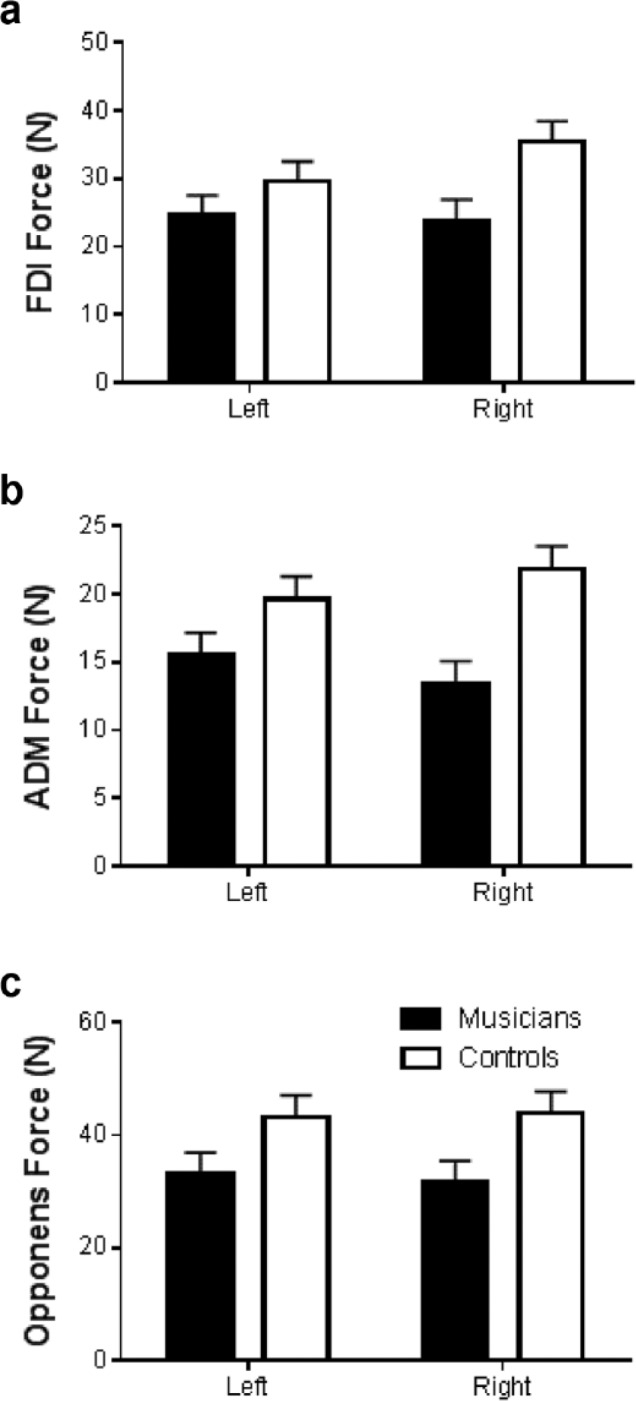
Mean and standard error values for maximal intrinsic muscle force production in the left and right hands for musicians versus healthy nonmusician controls: (a) maximal first dorsal interosseous (FDI) strength, (b) maximal abductor digiti minimi (ADM) strength, and (c) maximal opponens pollicis strength.
Submaximal Force Production
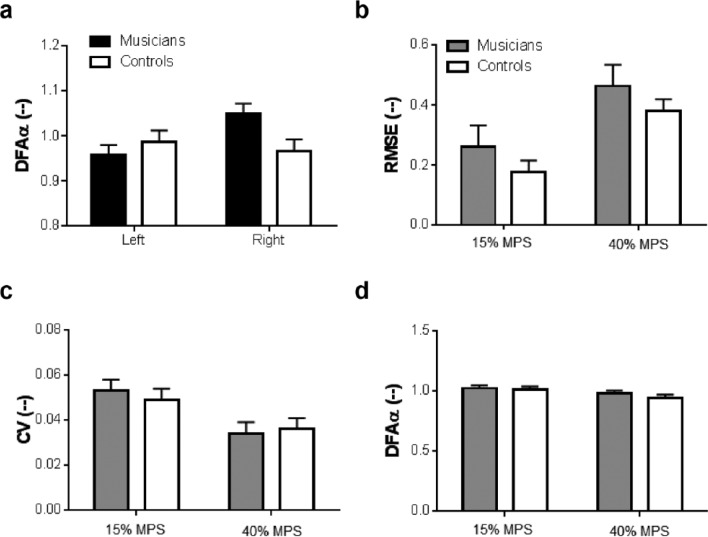
None of the measures of linear force variability indicated between-group or between-hand differences in this study. One nonlinear measure of force variability did indicate some between-group differences via an interaction term. DFAα exhibited a Group × Hand interaction (F1,48 = 4.987, P < .05), indicating similar DFAα values in musicians across hands, shown in Figure 3a.
Figure 3.
Mean and standard error of submaximal force production in musicians versus healthy nonmusician controls: (a) Between-hand differences in DFAα values, (b) RMSE differences between force output levels, (c) CV differences between force output levels, and (d) DFAα differences between force output levels.
Note. DFAα = detrended fluctuation exponent analysis; RMSE = root mean square error; FDI = first dorsal interosseus; CV = coefficient of variation; MPS = maximal precision pinch strength.
Despite the lack of group differences in the variability measures, the traditional Task-based differences did emerge in most of the variability measures (RMSE: F1,48 = 16.084, P < .001; CV: F1,48 = 12.566, P < .001; and DFAα: F1,48 = 5.277, P < .05), all shown in Figures 3b to 3d.
Discussion
The purpose of this study was to assess the effects of professional musical instrument playing on measures of upper extremity strength and motor function. We hypothesized that: (1) musicians will exhibit larger maximal force values for both intrinsic and extrinsic muscles of the hand; and (2) measures of force control (aka steadiness) will be better in musicians versus controls. No hypotheses regarding differences between hands were developed a priori, given the exploratory nature of this particular aim. Our findings do not support the primary hypotheses and suggest evidence of magnified between-hand performance differences in musicians versus controls. In the following paragraphs, we discuss our results in terms of the published literature as it relates muscle overuse, differential hand use in musicians, and cortical adaptation due to musician training.
Muscle Overuse in Musicians
In this study, we explored between-hand differences in indices of strength and force production control in professional string musicians as compared with nonmusician age-matched, sex-matched, and handedness-matched controls. None of the measures of extrinsic forearm muscle strength (maximal grip and pinch force) indicated between-group differences; however, measures of intrinsic hand muscle strength were significantly lower in the musicians group versus controls in the intrinsic hand muscles evaluated in this study. These results are in direct contrast with our first hypothesis. Despite this initially surprising result, it is possible that the reduced maximal force values in the musicians group may be due to both muscle overuse and prolonged use of unusual hand/finger postures induced by the physical demands of the profession.17,28,37 None of the musicians in this study presented with symptoms of neuromuscular or orthopedic disorders. Each study participant was assessed for such issues by an orthopedic surgeon with extensive experience in treating musicians; thus, we do not believe that the reduced maximal forces observed in this study were due to obvious neuromuscular or orthopedic problems. The increased demands on the upper extremities induced by constant repetition of hand/finger movements coupled with muscular fatigue, unusual joint positions, and cumulative microtrauma of the soft tissues of the upper extremity are well documented in string musicians.17,29,37 Left untreated or unaddressed, these microtraumas commonly develop into a myriad of chronic neuromuscular problems in this population, supported by reports of decreased movement quality associated with long-term instrument playing.7 Observations of decreased muscle strength along with increased fatigue have been shown to be associated with an increased risk of neuromuscular injury in other populations with high muscular and orthopedic demands (eg, military personnel, runners, etc.).9,36 Although measuring intrinsic muscle strength may be a clinical challenge, long-term monitoring of intrinsic hand strength may be a window into early identification of upper extremity neuromuscular and/or orthopedic diagnoses in this population; however, more research is warranted in this area.
Differential Hand Use in Musicians
In this study, we also explored between-hand differences in indices of force production and control. Notably, none of the measures of muscle strength (intrinsic nor extrinsic muscles) indicated between-hand differences in either group. On average, none of the measures of force steadiness (control) revealed overall group or hand effects, generally not supporting our second hypothesis; however, a difference in one measure of force control was notably different in musicians versus nonmusician controls between the hands. A Group × Hand interaction in one of the nonlinear measures of force control (DFAα, Figure 3a) indicated a significant discrepancy in value between the right and left hands of musicians, whereas this difference was absent in nonmusician controls. These unexpected results suggest that forces produced by the right hand of musicians were the result of a more consistent motor unit firing pattern as compared with the left hand, in which the force signal (and presumably motor unit firing patterns) consisted of more random frequency components. Given that the force signals were generated at the same percentage of maximal force (per each hand), this suggests a limb-specific organization of motor units and control of those units in musicians. This limb-specific control is most likely the result of long-term musical training in which the 2 hands are used for different, yet complimentary, components of a bimanual task,18,20 consistent with the dynamic dominance theory.27 Overcoming the symmetric nature of motor coordination in a bimanual task is not easy, indicated by self-reported difficulties in training the left hand by musicians.20 Although the origin of these control differences in musicians is speculative, we suggest that these hand-specific changes are central in nature, given well-documented cortical adaptations to long-term musical training in the evidence base.
Cortical Adaptations Due to Musical Training
In recent years, cortical adaptation to musical training has been reported within the literature. Increased amounts of white matter,4,12 structural changes,3,6,16 reduced cortical activity,1,5,11,13 and interhemispheric connectivity changes14,19,25 have all been reported in musicians. Notably, asymmetry in specific cortical areas in the motor and sensory regions suggests some evidence of hemispheric specialization.1,3,6,25 Interestingly, decreases in cortical activity in the primary motor and sensory function areas have been reported1,5,11,13 while reduced interhemispheric inhibition and increased interhemispheric connectivity14,19,25 seem to occur in professionally trained musicians. It is possible that all of these changes are complimentary in nature and in direct response to the bimanual nature of musical performance. It has also been noted that the strength of these changes appear to be directly linked to the number of years of study with an instrument and/or age at the start of musical training.1,4,6,12,24 One group has suggested that these changes allow for a smaller neural network to be used during motor activity, thereby solving Bernstein’s degrees of freedom problem by using less neuronal resources.13 However, the cortical changes observed do appear to be linked to the instrument type used, eg, stringed instruments (violin) versus keyboard instruments (piano).3,24 In some cases, researchers have suggested that these changes are generally maladaptive.7,12 This indicates that more research in this area is needed to better understand the relationship between observed between-hand differences in professional musicians, differential hand use based on the instrument type, and the resulting neuromuscular, orthopedic, and cortical adaptations.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: SL Gorniak  https://orcid.org/0000-0002-8192-850X
https://orcid.org/0000-0002-8192-850X
References
- 1. Amunts K, Schlaug G, Jäncke L, et al. Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp. 1997;5(3):206-215. [DOI] [PubMed] [Google Scholar]
- 2. Ball P. Science & music: facing the music. Nature. 2008;453(7192):160-162. [DOI] [PubMed] [Google Scholar]
- 3. Bangert M, Schlaug G. Specialization of the specialized in features of external human brain morphology. Eur J Neurosci. 2006;24(6):1832-1834. [DOI] [PubMed] [Google Scholar]
- 4. Bengtsson SL, Nagy Z, Skare S, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148-1150. [DOI] [PubMed] [Google Scholar]
- 5. Elbert T, Candia V, Altenmüller E, et al. Alteration of digital representations in somatosensory cortex in focal hand dystonia. NeuroReport. 1998;9(16):3571-3575. [DOI] [PubMed] [Google Scholar]
- 6. Elbert T, Pantev C, Wienbruch C, et al. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270(5234):305-307. [DOI] [PubMed] [Google Scholar]
- 7. Gentner R, Gorges S, Weise D, et al. Encoding of motor skill in the corticomuscular system of musicians. Curr Biol. 2010;20(20):1869-1874. [DOI] [PubMed] [Google Scholar]
- 8. Gorniak SL, Khan A, Ochoa N, et al. Detecting subtle fingertip sensory and motor dysfunction in adults with type II diabetes. Exp Brain Res. 2014;232(4):1283-1291. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman JR, Chapnik L, Shamis A, et al. The effect of leg strength on the incidence of lower extremity overuse injuries during military training. Mil Med. 1999;164(2):153-156. [PubMed] [Google Scholar]
- 10. Hosoda M, Furuya S. Shared somatosensory and motor functions in musicians. Sci Rep. 2016;6:37632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res. 1999;125(4):417-425. [DOI] [PubMed] [Google Scholar]
- 12. Imfeld A, Oechslin MS, Meyer M, et al. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. NeuroImage. 2009;46(3):600-607. [DOI] [PubMed] [Google Scholar]
- 13. Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res. 2000;10(1-2):177-183. [DOI] [PubMed] [Google Scholar]
- 14. Kang SY, Hallett M, Sohn YH. Exercise-induced strengthening of inter-digital connections in musicians. Clin Neurophysiol. 2013;124(8):1622-1627. [DOI] [PubMed] [Google Scholar]
- 15. Khan A, Gorniak SL. Effects of force requirements on pinch force production in healthy adults. Motor Control. 2016;20(3):299-315. [DOI] [PubMed] [Google Scholar]
- 16. Lee DJ, Chen Y, Schlaug G. Corpus callosum: musician and gender effects. NeuroReport. 2003;14(2):205-209. [DOI] [PubMed] [Google Scholar]
- 17. Lee H-S, Park HY, Yoon JO, et al. Musicians’ medicine: musculoskeletal problems in string players. Clin Orthop Surg. 2013;5(3):155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nolet R. Virtuoso hands. Clin Rheumatol. 2013;32(4):435-438. [DOI] [PubMed] [Google Scholar]
- 19. Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp Brain Res. 2002;144(3):336-342. [DOI] [PubMed] [Google Scholar]
- 20. Oldfield RC. Handedness in musicians. Br J Psychol. 1969;60(1):91-99. [DOI] [PubMed] [Google Scholar]
- 21. Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88(6):2297-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266(4, Pt. 2):H1643-H1656. [DOI] [PubMed] [Google Scholar]
- 23. Raymond DM, Romeo JH, Kumke KV. A pilot study of occupational injury and illness experienced by classical musicians. Workplace Health Saf. 2012;60(1):19-24. [DOI] [PubMed] [Google Scholar]
- 24. Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27(19):5200-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rüber T, Lindenberg R, Schlaug G. Differential adaptation of descending motor tracts in musicians. Cereb Cortex. 2015;25(6):1490-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacks O. Musical ability. Science. 1995;268(5211):621-622. [DOI] [PubMed] [Google Scholar]
- 27. Sainburg R. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142(2):241-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Savescu AV, Latash ML, Zatsiorsky VM. A technique to determine friction at the finger tips. J Appl Biomech. 2008;24(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savino E, Iannelli S, Forcella L, et al. Muscoloskeletal disorders and occupational stress of violinists. J Biol Regul Homeost Agents. 2013;27(3):853-859. [PubMed] [Google Scholar]
- 30. Schwenkreis P, El Tom S, Ragert P, et al. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26(11):3291-3302. [DOI] [PubMed] [Google Scholar]
- 31. Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol Hum Percept Perform. 1999;25(3):837-851. [DOI] [PubMed] [Google Scholar]
- 32. Staines KG, Gorniak S, Brooks F, et al. A pilot comparison of peak hand force in musicians using the Peg Retrained Intrinsic Muscle Evaluator (PRIME). J Hand Ther. 2016;29(3):383. [Google Scholar]
- 33. Steinmetz A, Möller H, Seidel W, Rigotti T. Playing-related musculoskeletal disorders in music students-associated musculoskeletal signs. Eur J Phys Rehabil Med. 2012;48(4):625-633. [PubMed] [Google Scholar]
- 34. Steinmetz A, Seidel W, Muche B. Impairment of postural stabilization systems in musicians with playing-related musculoskeletal disorders. J Manipulative Physiol Ther. 2010;33(8):603-611. [DOI] [PubMed] [Google Scholar]
- 35. Trainor L. Science & music: the neural roots of music. Nature. 2008;453(7195):598-599. [DOI] [PubMed] [Google Scholar]
- 36. Weist R, Eils E, Rosenbaum D. The influence of muscle fatigue on electromyogram and plantar pressure patterns as an explanation for the incidence of metatarsal stress fractures. Am J Sports Med. 2004;32(8):1893-1898. [DOI] [PubMed] [Google Scholar]
- 37. Winspur I. Advances in objective assessment of hand function and outcome assessment of the musician’s hand. Hand Clin. 2003;19(3):483-493. [DOI] [PubMed] [Google Scholar]
- 38. Xu S, Morse AM, Lacy B, et al. Peg restrained intrinsic muscle evaluator (PRIME): development, reliability, and normative values of a device to quantify intrinsic hand muscle strength in children. J Hand Surg. 2011;36(5):894-903. [DOI] [PubMed] [Google Scholar]
- 39. Zatorre R, McGill J. Music, the food of neuroscience? Nature. 2005;434(7031):312-315. [DOI] [PubMed] [Google Scholar]