Short abstract
Oxidative stress acts as an essential culprit factor in the development of stroke and Alzheimer’s disease. Norcepharadione B possesses various pharmacologic features as an extract obtained from Houttuynia cordata. Nevertheless, the anti-apoptotic and neuroprotective characteristics of norcepharadione B remain unclear. In this study, the neuronal protection effect provided by norcepharadione B against injury caused by hydrogen peroxide (H2O2) in HT22 cell as well as the fundamental mechanism was systematically explored. The neurotoxicity assays of hippocampal cells, which were co-cultured with H2O2, showed that norcepharadione B had the ability to insulate the toxicity induced by H2O2 with significant reduced cell apoptosis. Besides, norcepharadione B potentiated the activity of superoxide dismutase (SOD), increased the level of glutathione (GSH), and decreased malondialdehyde content. The H2O2-induced apoptotic protein Bax was suppressed, and the anti-apoptotic protein Bcl-2 was boosted by norcepharadione B. Norcepharadione B promoted Akt phosphorylation and further upregulated heme oxygenase (HO-1) in cells exposed to oxidative stress. However, the inductive effect of HO-1 by norcepharadione B was shut off via the PI3K/Akt inhibitor LY294002. Furthermore, 2-h incubation with H2O2 substantially increased cell volume in HT22 cells, while norcepharadione B effectively alleviated such effect by interrupting the activation of VSOR Cl− channel. Collectively, our data revealed protective properties of norcepharadione B in resisting oxidative stress induced by H2O2 through elevation of HO-1 in the dependence of PI3K/Akt and in inhibiting H2O2-induced cell swelling by VSOR Cl− channel obstruction in HT22 cells.
Impact statement
Norcepharadione B is an aporphine alkaloid compound extracted from Chinese herb Houttuynia cordata. It was well known for its anti-inflammatory, anti-cancer, and anti-platelet aggregation outcomes. Our study demonstrated that Norcepharadione B protected hippocampal neurons against oxidative stress and the resultant cell apoptosis upon H2O2 exposure. Meanwhile, Norcepharadione B also substantially reduced cell swelling induced by H2O2 via inhibiting VSOR Cl− channel in neurons. These findings uncovered the potential mechanisms of Norcepharadione B in protecting neuron apoptosis under oxidative stress and propose that Norcepharadione B may serve as a favorable herb medicine for restoring neuronal injury in the pathogenesis of stroke together with other neurodegenerative diseases.
Keywords: Norcepharadione B, oxidative stress, cell volume, VSOR Cl−channel, PI3K/Akt, HO-1
Introduction
Oxidative stress triggers cell damage and contributes to the pathophysiological processes in many diseases. Appearance of oxidative stress usually denotes loss of balance between oxidants and antioxidants, in turn leading to excessive production of free radicals along with diminished dynamic capacity of detoxification for reactive intermediates. Mammalian brain is characterized by high oxygen demand with relatively less antioxidants, making it particularly vulnerable to oxidative damages.1 Accumulation of free radicals prompts oxidative stress to affect both structure and function of neuronal cells via inducing cell swelling and cell apoptosis, contributing to an array of neurological diseases, including Parkinson’s disease (PD), cerebral ischemia/reperfusion injury, and Alzheimer’s disease (AD). To this end, antagonizing oxidative stress may serve as a possible target for the management of neuronal injury in stroke and other neurodegenerative diseases.
Oxidative stress is closely related to cell apoptosis and it can induce apoptosis through mitochondria, death receptor, endoplasmic reticulum stress, and other ways.2 Cell swelling is a characteristic for severe oxidative stress and cell death by necrosis. It is showed that lower dose of H2O2 causes cell membrane lipid peroxidation and membrane permeability decrease as well as cell organelles injury at early stage after the stimulation, which may contribute to the impairment of the volume-regulating ion channels and the subsequent cell swelling.3 In the condition of persistent oxidative stress, the insulted cells are subjected to resultant apoptosis or necrosis.4 However, cell shrinkage, also termed as apoptotic volume decrease, occurs when the programmed cell death is initiated. The neuronal injury or cell apoptosis may be caused by the resultant cell enlargement or shrinkage and disturbed cell volume control.5 Therefore, interfering with cell swelling and apoptosis could alleviate oxidative stress-induced neuronal injury.
Volume-sensitive outwardly rectifying (VSOR) anion channels, also as volume-regulated anion channels (VRACs), exert pivotal function in controlling the cell size in the condition of osmotic swelling and serve as an essential mechanism to maintain cytosolic homeostasis of cells.6,7 The identification of VSOR components has been disputed for over 20 years. LRRC8A, as a major component of VSOR containing a leucine-rich repeat (LRR) domain and four transmembrane domains at the carboxy-terminus, has been proved in various cell types recently.8,9 VSOR channels are present in majority of mammalian cells, including neuron cell.10 In addition to cell volume regulation, channels of VSOR also participate in balancing metabolic stress, apoptosis, and cell cycle in cells.11 It has been shown that H2O2 acts as an intracellular signaling molecule for VSOR activation.12 In multiple cell types, NADPH oxidase activation generated H2O2 was a major factor in VSOR Cl− channel activation, which was demonstrated by Varela et al.13 and Browe and Baumgarten.14 PI3K/Akt is a classical cascade for cell survival, and numerous studies have elucidated that activated PI3K/Akt cascade mediates the neuroprotection against neuronal damage caused by oxidative stress.15,16 Our earlier finding noted that staurosporine-activated VSOR Cl− channel may be blocked by 4,4-diisothiocya nostilbene-2,2ʹ disulfonic acid (DIDS) and 4–(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxybutyric acid (DCPIB) via PI3K/Akt-dependent mechanism.17 In recent years, emerging evidence have demonstrated that blocking VSOR Cl− channel efficiently improves cell survival in the pathogenesis of neurological diseases.18,19 It is found that VSOR has a relationship with the onset and the happening of plenty of cardiovascular diseases like arrhythmia, myocardial ischemia, and heart failure.20,21 VSOR also mediates ischemia-induced brain damage by regulating excitatory amino acid release from glial cells.22 Thus, VSOR inhibitor could be a potential tool for the treatment of osmotic swelling-relative diseases, for instance cerebrovascular and neurodegenerative diseases.
Norcepharadione B (NB) is an aporphine alkaloid composite which was concentrated from Chinese herb Houttuynia cordata. Previous data have identified the antiviral, anti-inflammatory, and anti-platelet aggregation effects of NB.23,24 Growing studies have demonstrated that active components of Houttuynia cordate extract are beneficial for the prevention and management of dementia, Parkinson’s disease and epilepsy.25–27 These observations have depicted the neuroprotective properties of NB, warranting further exploration of the underlying mechanisms. Our group has focused on chloride channels over the past years.28–30 In our previous study, high-throughput screening technology was used to identify natural monomer compound displaying chloride channel blocking effect from more than 6800 compounds. Among them, NB possesses an optimal chloride channel blocking effect in HeLa cell line.31 However, whether NB blocks H2O2-induced chloride channel activity in neuronal cells remains unknown. Given that the highly reactive oxygen species (ROS) H2O2 provokes oxidative neuronal damage and is regularly utilized to simulate oxidative stress in different cell lines in vitro,32 this study was performed to examine whether NB had neuroprotective effect through combating oxidation and blockade of chlorine channels on H2O2-induced nerve cell damage in hippocampus.
Materials and methods
Reagents
NB (C18H13NO4, molecular weight: 307.3, purity >98%) was gotten from the ChemFaces Biotechnology (Wuhan, China); Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were acquired from Corning Technology (Corning, NY, USA); 4–(2-butyl-6,7-dichlor-2-cyclopentylindan-1-on-5-yl) oxybutyric acid (DCPIB) and Dimethylsufoxide (DMSO) were purchased from Sigma-Aldrich China (Shanghai, China). Cell Counting kit-8 (CCK-8) was obtained from Med Chem Express (NJ. USA). From Cell Signaling Technology (Boston, USA), antibodies against p-Akt, Akt, β-actin, Bcl-2, and Bax were obtained. From Abcam (Cambridge, UK), antibody of Heme oxygenase 1 (HO-1) was acquired. From the companies of Beyotime Biotechnology (Shanghai, China) and Sinopharm Chemical Reagent Ltd (Shaanxi, China), PI3K inhibitor LY294002, N-acetyl-L-cysteine, (NAC) and 30% hydrogen peroxide (H2O2) were obtained. Nanjing Jiancheng Bioengineering Institute (Nanjing, China) supplied kits for the measurement of LDH, MDA, SOD, GSH, and whole protein contents. Sangon Biotech Co. Ltd. (Shanghai, China) provided primers sequences synthesis. In addition, RNAiso Plus reagent was obtained from TAKARA (Tokyo, Japan).
Cell culture and treatments
The Department of Neurosurgery of our hospital supplied HT22 cell line for free. HT22 is a subclone of HT4 that is originated from the mouse hippocampus. Cells were cultured in DMEM medium with 10% in volume of FBS, 100 units/mL penicillin, and 100 µg/mL streptomycin in a 37 °C humid incubator supplied with 5% CO2.
The stock solution of NB in DMSO was prepared at the concentration of 50 mmol/L, which was then diluted into sequential concentrations with DMEM before being used. For the detection of the effect of NB on HT22 cell viability, cells were incubated 24 h with escalating doses of NB at 10, 50, 100, and 200 µmol/L. In addition, the cytotoxic effect of H2O2 at different concentrations (10, 50, 100, 300, 500 and 1000 µmol/L) was determined using CCK8 assay after 24 h of culture in HT22 cells; 300 µmol/L H2O2 was used to stimulate oxidative damage in HT22 cells in the remaining experiments. For the following experiment, except for estimation of biochemical parameters (the cell were co-cultured with H2O2 for 6 h), HT22 cells were pre-conditioned with 100 µmol/L NB for 2 h, and then incubated with H2O2 (300 µmol/L) for 24 h; 5 mmol/L of NAC was used as positive control for its well-established anti-oxidative effect. To investigate the neuroprotective mechanism of NB, HT22 cells were previously incubated with LY294002 for 30 min before incubation with NB and H2O2 for 24 h. In our study, levels of SOD, GSH and MDA were detected 6 h after H2O2 treatment, while the outcomes of H2O2 affecting on cell viability and apoptosis were measured 24 h following exposure. We believed that changes in the levels of pro-apoptotic and anti-apoptotic factors preceded cell apoptosis, and neuronal death. Each parameter should be detected at an optimal point or timeframe. Based on our pre-experiment results on the time-effect of H2O2, those biochemical parameters reached an ideal level 6 h after the beginning of the incubation with H2O2. Therefore, we measured GSH, SOD, and MDA at 6 h of H2O2 exposure.
Cell viability determination
The CCK-8 kit was introduced to quantify the cell viability after treatment; 3–5 × 103 cells/well were plated in a 96-well plate. Following treatment, 100 µL of culture medium with 10% (v/v) CCK-8 reagent were added into the plate before further culture at the temperature of 37 °C for additional 1–2 h. The optical absorption (450 nm) was quantified using a microplate reader after shaking the plate thoroughly for 60 s.
LDH release assay
To determine neuronal damage by H2O2, LDH content in the supernatant of cell culture was quantified via an LDH kit based on the producer's recommendation. HT22 cells were seeded in a 24-well plate at 2–3 × 104 cells per well. Following co-cultured with H2O2 for 24 h, the released LDH was quantified by collecting the supernatant from each culture.
Detection of lipid peroxidation products and antioxidant enzyme activity
HT22 cells were plated in a six-well plate with 1 × 105 cells/well. After 6-h drug treatments, PBS was used for cells rinsing for three times, and digested with 0.25% trypsin and then pipetted to a 1.5 mL EP tube. Such tubes with cells were lysed on ice for 20 min followed by centrifuging for 10 min at 4 °C at the speed of 12,000 r/min to obtain 200 μL supernatant in pre-cooled centrifuging tube for further characterization. MDA, SOD, and GSH levels were examined using their corresponding kits based on the manufacturer’s protocols. SOD was measured based on a WST-1 method, which is presented for 2-(-4-iodophenyl)-3–(4-nitrophenyl)-5–(2,4-disulfophenyl)-2H-tetrazolium to quantify the superoxide anion radicals consumption and formation of WST-1 formazan. A volume of 3 mL reaction system contained 0.2 units xanthine oxidase and the total of 50.225 mM of WST-1, xanthine, EDTA, and Na2CO3 with a molar ratio of 1:4:4:2000 (pH 10.2), were kept at 37 °C for one-third hour. The optical absorption at 450 nm was identified. GSH level was characterized via a colorimetric assay which was established on the principle as described previously.33 Briefly, it can be reacted with dithiodinitrobenzoic acid (DTNB) to form a yellow compound, and a plate reader was introduced to quantify the optical absorption at 405 nm. With regard to the measurement of MDA, the prepared samples or standard were added with thiobarbituric acid (TBA) and trichloroacetic acid, and then were boiled for 1 h. The cell culture-derived MDA reacted with TBA to generate a TBA reactive substance. Afterwards, the generation of the TBA reactive substance was quenched through incubating the reactants on the ice for 10 min. The absorption at 530 nm of the supernatant, which was separated from the sample via centrifuging, was recorded. A BCA protein assay kit was employed for the quantification of the protein content from the supernatant. The MDA and GSH contents were expressed as nmol/mg proteins and SOD activity was presented as U/mg proteins.
Flow cytometric analysis
The 6-well plates were plated with 1 × 105 HT22 cells/well, and the cells were then treated 24 h with H2O2 and NB. Afterwards, the cells were collected and rinsed via centrifuging (1000 rpm/min, 5 min) and ice-cold PBS for three times; 1 × 106 cells/mL of rinsed cells were re-dispersed in 100 µL of the binding buffer, which were further treated with and 2 µL of propidium iodide (PI) and 5 µL of fluorescein5-isothiocyanate (FITC)-labeled anti-annexin-V antibody for 15 min in a light-sealed condition. The apoptotic cells were then quantified using flow cytometry. The late necrotic cells were double stained with Annexin V and PI (Annexin V+/PI+) cells, and cells only stained with Annexin V (Annexin V+/PI−) were early apoptotic cells. The ratio of apoptotic cells was identified by the total percentage of both early apoptotic and late necrotic cells.
Besides, flow cytometry was performed to discover the HT22 cell volume expansion which was caused by H2O2 treatment with or without NB. Cell volume was measured every 2 h within 8 h and 12 h as well as 24 h after the H2O2 treatment. Cells were then harvested and rinsed via centrifuging (1000 r/min, 5 min) and freezing PBS. Cell volume was measured in a flow cytometer. A cluster of cells were gated using WinMDI2.9 software and cell size was automatically calculated by the forward scatter (FSC) value.
Whole-cell patch-clamp experiments
VSOR Cl− currents in response to NB treatment in H2O2-insulted HT22 cells were recorded at 23–25°C with an Axon patch-clamp system using the configuration for whole-cell test (Amplifier: Multiclamp 700B, Digidata 1322 A, Axon Instruments, Foster, CA, USA). A pClamp10 software was used to execute voltage clamp protocols and data acquisition. The borosilicate glass capillaries were used to stretch pipettes with tip resistances approximately 3–5 MΩ when filling with internal solution. To calculate liquid junction potentials, pClamp10 program in the JPCalc mode was applied, and further adjusted online. During the experiment, the capacitive transients and access resistance could be maximally compensated. To selectively record Cl− current in cell, the pH7.4 pipette solution, containing 103 mM CsOH, 103 mM aspartic acid, 25 mM CsCl, 5 mM Mg-ATP, 0.3 mM Na3-GTP, 5 mM EGTA, 10 mM HEPES, and 30 mM mannitol, was altered using CsOH, 295 mosmol/Kg H2O. The pH7.4 isotonic bathing solution, containing 85 mM N-methyl-D-glucamine (NMDG), 85 mM HCl, 10 mM NaCl, 2 mM 4-aminopyridine (4-AP), 2.5 mM BaCl2, 0.33 mM NaH2PO4, 4 mM MgCl2, 5 mM Tetraethylammonium Cl (TEA-Cl), 10 mM HEPES, 5.5 mM glucose and 85 mM mannitol, was fixed with NMDG-OH, 305 mosmol/Kg H2O. To avoid the interference of Na+ and L-type Ca2+ currents, tetrodotoxin (TTX) at a final concentration of 8 µM and nifidipine at 5 µM was added into the bath solutions to block these channels, respectively. A freezing-point depression osmometer (OM802, Vogel, Giessen, Germany) was used for monitoring osmolality.
Western blot analysis
Expressions of targeted proteins in hippocampal neurons were assessed using Western blotting. Protein levels were determined using Bradford assay. SDS-PAGE on 10% gels was applied to separate the 20-µg harvested whole protein from samples, which was further electrically transferred to PVDF membranes. To block such membrane, 1× Tris-buffered saline containing 5% skim milk and Tween 20 (TBST) was used to immerse the membrane for 1 h. The primary antibody was applied on the block membrane overnight 4 °C. The primary antibody was cleaned off with TBST followed by horseradish peroxidase-conjugated secondary antibodies conjugation for additional 60 min. With further TBST cleaning off, an enhanced chemiluminescence detection kit was used for observing protein bands.
Real-time quantitative PCR
The mRNA level of HO-1 in HT22 cells after exposure to NB and H2O2, alone or in combination was assessed using RT-qPCR method. To extract total RNA from the treated neurons, RNAiso Plus was employed, and was used to prepare complementary DNA (cDNA) with the PrimeScript RT reagent kit. A total of 1 µg template cDNA was used for the amplification reaction. The RT-qPCR was run in triplicates using StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, USA) and the cycling program was set to be: pre-denaturation (95 °C, 10 min) and subsequent 40 cycles (95 °C, 15 s; 60 °C, 60 s; 95 °C, 15 s; 60 °C, 60 s). HO-1: 5ʹ-ACC GCC TTC CTG CTC AAC ATT G-3ʹ (forward), 5ʹ-CTC TGA CGA AGT G AC GCC ATC TG-3ʹ (reverse); GAPDH: 5ʹ-ACC ACA GTC CAT GCC ATC AC-3ʹ (forward), 5ʹ-TCC ACC ACC CTG TTG CTG TA-3ʹ (reverse) were used as the primer sequences.
Statistical analyses
Values were presented in the format of means± SD, and multiple comparisons were performed using one-way analysis of variance (ANOVA) combined with Tukey’s test. The P value smaller than 0.05 was considered statistically significant difference. The statistical analyses were performed with SPSS 19.0 software.
Results
NB alleviated neurotoxicity injury induced by H2O2 in HT22 cells
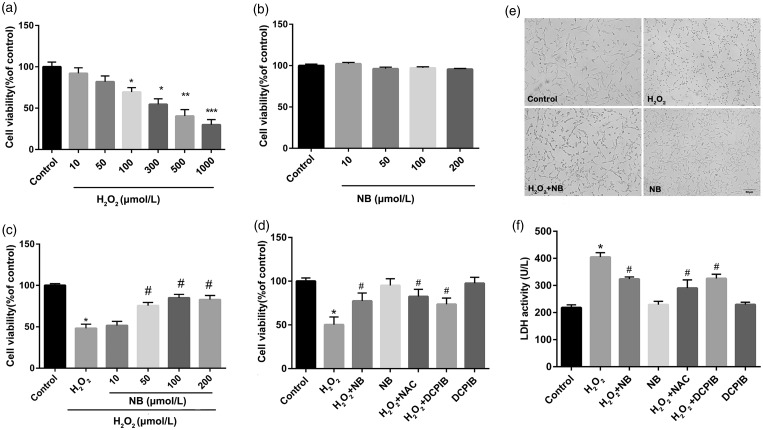
The neurotoxicity of H2O2 (ranging from10 µmol/L to 1000 µmol/L, co-incubated for 24 h) to HT22 cell line was systematically evaluated. Our result revealed that H2O2 dose-dependently suppressed cell viability of HT22 cells. The HT22 cells suffered approximately 50% reduction in cell viability with H2O2 treatment at a concentration of 300 µmol/L, and this dose of H2O2 was used for the remaining experiments (Figure 1(a)). In the contrast to control group, no change on cell viability was observed with the concentration of NB below 200 µmol/L, indicating that the drug had no detectable cytotoxicity (Figure 1(b)). In the context of oxidative damage by H2O2, pre-treatment of neurons with NB at levels > 50 µmol/L significantly increased cell viability, suggesting that NB protected HT22 cells against H2O2-induced neurotoxicity (Figure 1(c)). At the concentration of 100 µmol/L, NB achieved optimal protective effect against H2O2, in a manner comparable with the known anti-oxidant NAC (Figure 1(d)). Therefore, 100 µmol/L was chosen for NB concentration for subsequent experiments. Besides, the naive HT22 cells exhibited multiple mesh-like, dendritic protrusions under light microscope, while H2O2-insulted cells displayed rounded appearance with less protrusions, which was rescued by NB treatment (Figure 1(e)). Moreover, H2O2 challenge overtly promoted LDH release, the effect of which was diminished following NB treatment at 100 µmol/L (Figure 1(f)). In our study, we supposed that VSOR Cl− channel was involved in the neuroprotection of NB against H2O2 stimulation. The boosting of cell viability and shirking release of LDH was affected by DCPIB, an antagonist of VSOR Cl− channel, while sole DCPIB treatment failed to change the HT22 cell viability (Figure 1(d) and (f)).
Figure 1.
Effect of NB on H2O2-induced neuronal injury. (a) HT22 neuron cells were incubated with different concentrations H2O2 for 24 h, and cell viability was measured using CCK8 kit; (b) HT22 neuron cells were treated with different concentrations NB for 24 h and the cell viability was measured; (c) Cells were pretreated with different concentrations of NB or vehicle alone for 2 h and were then treated with 300 µmol/L H2O2 for 24 h before cell viability detection. (d) Cells were pretreated with 100 µmol/L NB or 10 µmol/L DCPIB for 2 h followed by the further incubation of 300 µmol/L H2O2 for 24 h. N-acetyl-L-cysteine (NAC) at 100 µmol/L was used as a positive control for indicating the anti-oxidative activity. (e) Morphology of cells in response to treatment of NB and H2O2. Bar = 50 µm. (f) LDH release in the supernatant of HT22 cells treated with NB or DCPIB followed by H2O2. *P < 0.05, **P < 0.01, ***P < 0.001 vs. the control group; #P < 0.05 vs. the H2O2 group.
NB inhibited cell volume alteration and apoptosis induced by H2O2 in HT22 cells
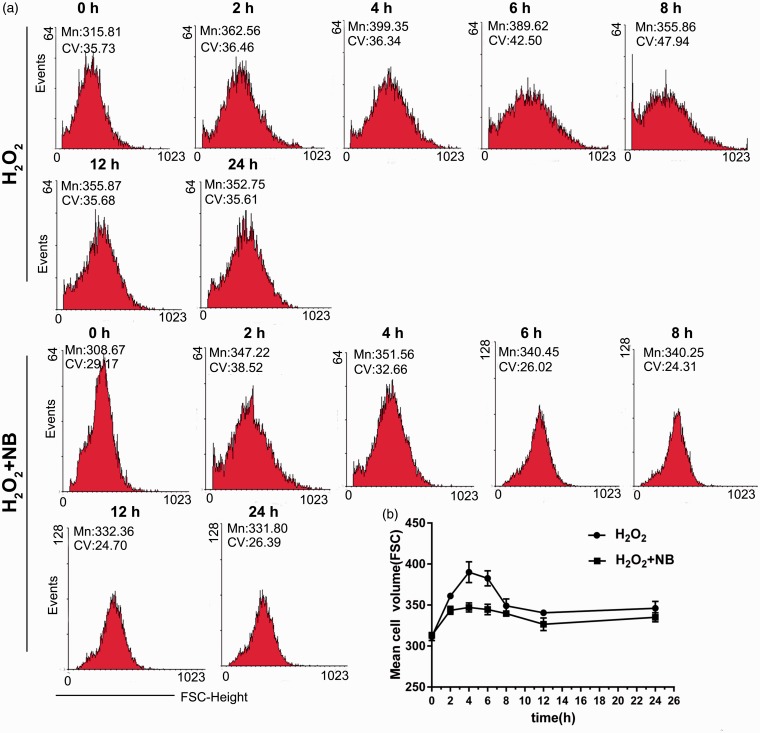
Studies have found that oxidative stress is closely related to cell swelling and apoptosis.34 Flow cytometry was performed to determine cell volume in H2O2-challenged cells with or without NB treatment at different time points. The volume of HT22 cells was gradually enlarged after the exposure of H2O2 approximately from 2 h of stimulation, and the volume significantly increased at 4–6 h. NB treatment notably suppressed the swelling of HT22 cells challenged with H2O2, especially at 4 h and 6 h of treatment (Figure 2(a) and (b)), suggesting that NB had an inhibitory effect on H2O2-induced volume increase of HT22 cells.
Figure 2.
Effects of NB on H2O2-induced swelling in neuronal cells. (a) HT22 cells were treated with 300 µmol/L H2O2 or H2O2 + NB (100 µmol/L), then cell volume changes were detected at 2 h, 4 h, 6 h, 8 h,12 h, and 24 h, separately. The cell volume changes were detected by flow cytometry using FSC-height and SSC-height; (b) Quantitative analysis of cell volume changes at different time points. (A color version of this figure is available in the online journal.)
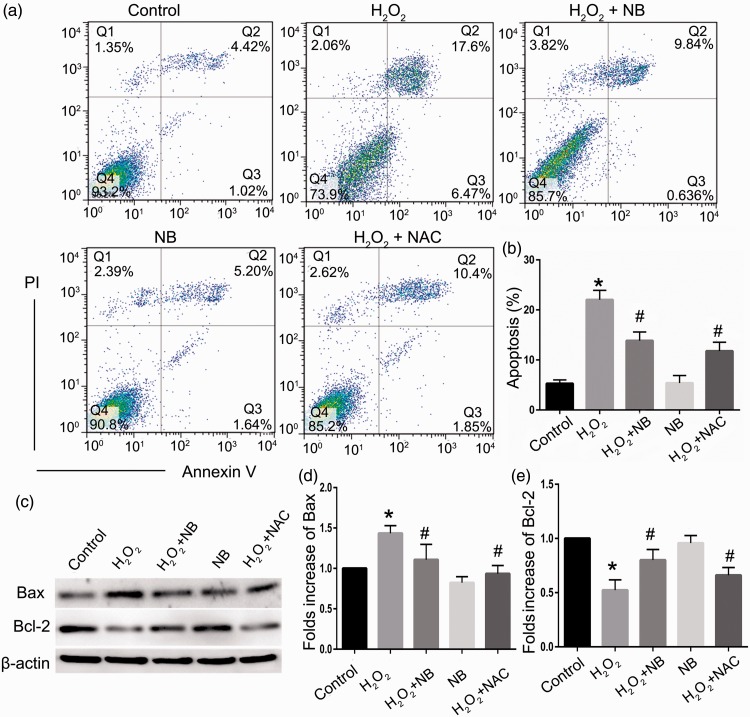
Assessment of apoptosis revealed that 4.68% of HT22 cells underwent apoptosis in normal condition, while H2O2 exposure significantly elevated apoptosis to 24.43% (P < 0.05). Administration of NB significantly reversed apoptosis in HT22 cells challenged with H2O2 (Figure 3(a) and (b)). Further evaluation of apoptotic proteins Bcl-2 and Bax which exerts anti- and pro-apoptotic properties, respectively, indicated that NB elevated the expression level of Bcl-2 and reduced the expression level of Bax compared with the H2O2 treatment group, in a manner reminiscent of NAC (Figure 3(c) and (e)).
Figure 3.
Effects of NB on H2O2-induced apoptosis in neuronal cells. (a) Representative images of flow cytometry analysis of cell apoptosis in HT22 cells treated with NB (100 µmol/L) and H2O2 (300 µmol/L); (b) The bar graph depicting the percentage of apoptotic cells. The data represent the mean ± SD (n = 3). *P < 0.05 vs. control group; # P < 0.05 vs. H2O2 group; (c–e) Western blot analysis and quantification of Bax and Bcl-2 expression. *P < 0.05 vs. control group; #P < 0.05 vs. H2O2 group. (A color version of this figure is available in the online journal.)
NB protected hippocampal neurons against oxidative stress
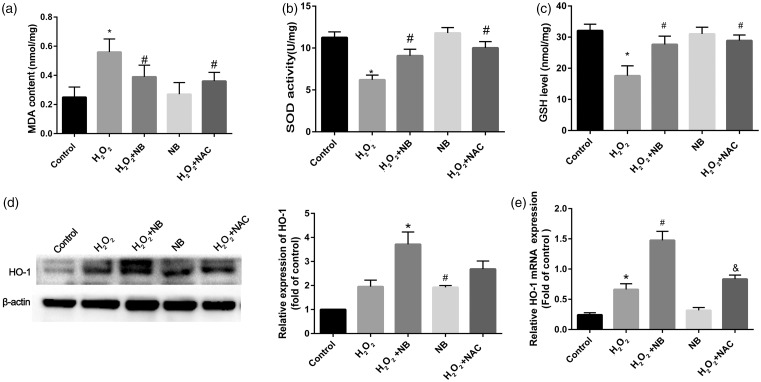
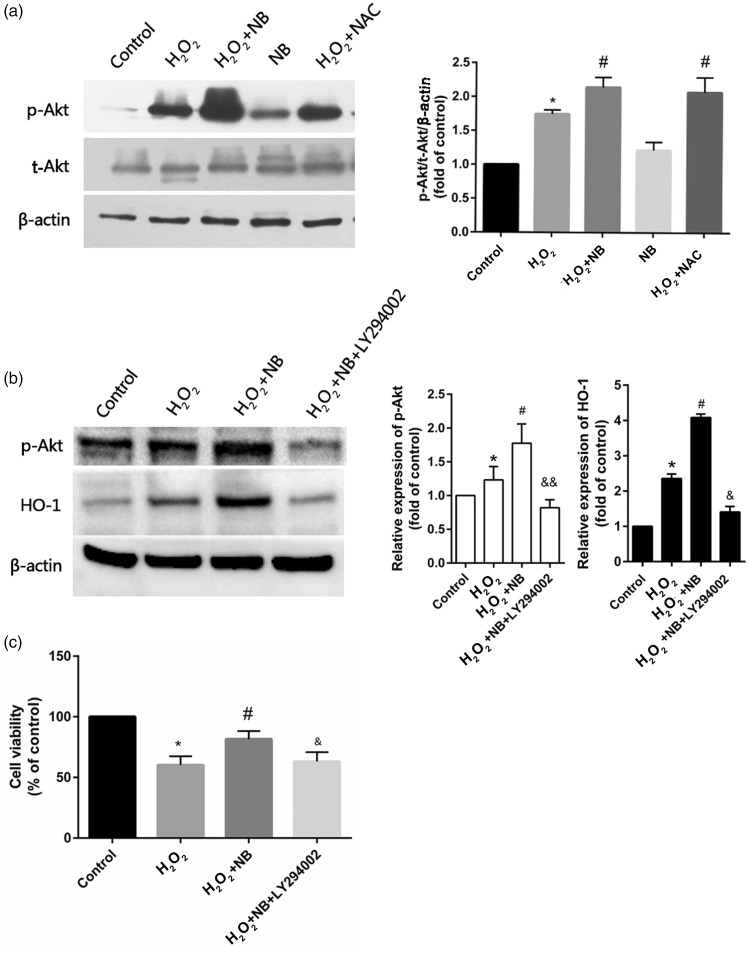
To discern how NB affects oxidative stress introduced by H2O2, levels of lipid peroxidation species and antioxidant were quantified in HT22 cells. As shown in Figure 4(a) to (c), suppressed SOD activities and decreasing of GSH level were caused by H2O2, while boosting the level of MDA and NB treatment significantly reduced MDA as well as increased SOD and GSH in the face of H2O2 challenge. The HO-1 mRNA and its protein expression were upregulated by H2O2, such effect was profoundly potentiated with the addition of NB. In the context of H2O2 exposure, the inductive effect of NB on HO-1 seemed to be superior to that of NAC. Interestingly, compared with the control, HO-1 expression upregulation was also found in the group of treating with NB alone (Figure 4(d)). Likewise, mRNA levels of HO-1 were altered in a manner comparable to that of the protein level in response to NB or H2O2, alone or in combination (Figure 4(e)).
Figure 4.
Effects of NB on oxidative stress in HT22 cells that exposed to H2O2. (a–c) At the termination of the treatment, the cells were lysed and 200 mL of the supernatant were collected for further spectrophotometric analysis of the reaction products of SOD, GSH, and MDA. SOD was detected based on a WST-1 tetrozolium method; GSH levels were indicated based on the reduction of reaction product and MDA was measured based on thiobarbituric acid methods using corresponding commercial kits; (d) HO-1 expression was determined and quantified using Western blot; (e) RT-PCR analysis of HO-1 mRNA. *P < 0.05 vs. control group; #P < 0.05 vs. H2O2 group.
NB elevated the expression of HO-1 caused by PI3K/Akt pathway opening
For examining the potential mechanism of NB in preventing oxidative stress, PI3K/Akt cascades activation was detected within HT22 cells using Western blot. Our results showed that PI3K/Akt pathway was activated as reflected by increased phosphorylation of target proteins in response to H2O2 challenge. NB pretreatment further increased the level of p-Akt compared with H2O2 treatment (Figure 5(a)). However, expressions of p-Akt and HO-1 were held back by LY294002, the PI3K/AKT inhibitor, compared with cells exposed to H2O2+NB (Figure 5(b)). Furthermore, we found that after treatment with LY294002, the neuroprotection offered by NB was abolished in H2O2-challenged cells (Figure 5(c)). Therefore, we considered that PI3K/Akt pathway may be a potential NB target.
Figure 5.
Neuroprotective mechanism of NB. (a) HT22 cells were pretreated with NB for 2 h, and added with 300 µmol/L H2O2 for 24 h. The expression of p-Akt and Akt was detected by Western blot; Quantitative data of the detected proteins; (b) Effect of PI3K inhibition using LY294002 on the expression of p-Akt and HO-1 in NB and H2O2-treated cells; Quantitative analysis of p-Akt and HO-1. (c) Effect of PI3K inhibition using LY294002 on cell viability. Data are shown as mean ± SD (n = 3). *P < 0.05 vs. Control group, #P < 0.05 vs. H2O2 group, &P < 0.05, &&P < 0.01 vs. H2O2 +NB group.
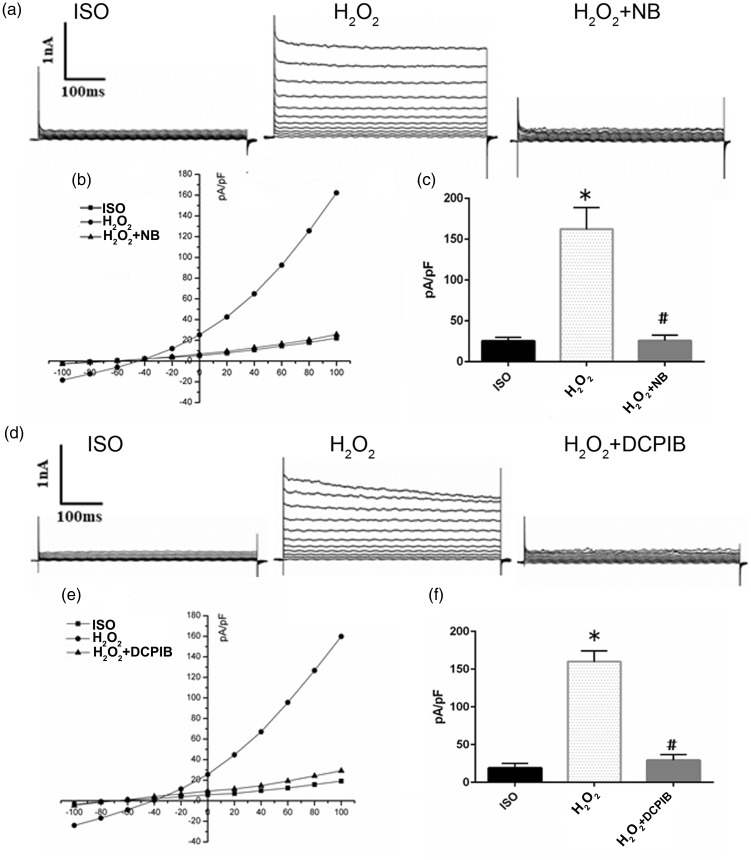
NB suppressed cell volume change by decreasing the outward Cl− currents of VSOR channel
The volume controlling of swollen cortical neurons was related with activated VSOR Cl− channel.10 Given that NB retarded H2O2-induced cell volume change, the insight of the relationship between VSOR Cl− channel and NB-involved regulation of cell volume was further inspected. VSOR Cl− currents were monitored using whole-cell configuration. VSOR Cl− currents were energetic in the response to H2O2 (500 µmol/L, 10–15 min) challenge (Figure 6(a)). Compared with control, H2O2 stimulation rapidly increased the VSOR Cl− currents, and the effect of which was markedly inhibited by NB (Figure 6(b)). The change of VSOR Cl− currents caused by NB (500 µmol/L) was further quantified (amplitude at −100 mV); 500 μmol/L of NB significantly inhibited H2O2-induced VSOR Cl− currents by > 80% (5–6 min, 83.05 ± 3.87%, N = 5, P < 0.05), exhibiting the highest efficacy for VSOR Cl− channel blockade (with a mean inhibition > 85%) (Figure 6(c)). Inhibitory effect of NB on VSOR Cl− currents was comparable with that of the reported selective VSOR Cl− channel blocker, DCPIB. It was demonstrated that DCPIB at 10 μmol/L significantly attenuated Cl− currents shown in either the time course of VSOR Cl− or conductance recorded at −100 mV. These findings indicate that higher dose of NB exhibited comparable effects with typical VSOR Cl− channel agonists in inhibiting Cl− current and cell swelling (Figure 6(d) to (f)).
Figure 6.
Effects of NB on VSOR Cl− currents in HT22 cells. (a) Cl− currents recorded under isosmotic solution (Ctrl), H2O2 (500 µmol/L) treatment, and H2O2 plus NB (500 µmol/L) treatment, n = 5. (b) Corresponding current voltage (I–V) relationship for the mean current densities of cells subjected to indicated treatments. (c) Current densities at +100 mV from panel b, n = 5. (d) Negligible background Cl− currents recorded under isosmotic solution (Ctrl). H2O2 (500 µM)-induced Cl− currents exhibiting representative properties of VSOR Cl− currents. H2O2-induced VSOR Cl− currents were inhibited by adding DCPIB (10 µmol/L); n =5. (E) Corresponding current–voltage (I–V) relationship for the mean current densities of cells subjected to indicated treatments. (f) Current densities at +100 mV from panel e. The data are presented as the mean ± SD of three experiments. *P < 0.05 vs. Control group. #P < 0.05 vs. H2O2 group.
Discussion
The neuroprotective property of NB is possibly related to its inhibitory role on VSOR chloride channel and oxidation to prevent cell apoptosis, which is the major result from this study. Furthermore, our study also provided evidence that NB protected against oxidative stress through activating the PI3K/AKT signaling cascade.
Houttuynia cordatas, a tradition Chinese herb, is a kind of plant resource with wide biological activity and high edible value, with great potential in clinical practice. NB is an aporphine alkaloid extracted from Houttuynia cordatas. Earlier evidence have shown that NB displays antibacterial, anti-inflammatory, antiviral and antiplatelet aggregation properties.23,24,32 Probstle et al. showed that NB had an inhibitory effect on cyclooxygenase, representing an underlying mechanism for inhibiting platelet aggregation. Recent reports also demonstrated that Houttuynia cordate extracts can improve memory function in dementia, Parkinson’s disease, and epilepsy. These data favored a protective property of NB against neuronal injury.25,27 Consistent with the aforementioned studies, we also confirm that NB preserves cell viability, reduces LDH release, maintains normal morphology, and inhibits apoptosis in H2O2-induced oxidative damages in HT22 cells.
Accumulating evidence has suggested that abundant ROS are associated with neuronal injury, and oxidative stress is an important pathological culprit in stroke, AD, and PD.35,36 In our study, H2O2 was used in HT22 cells to evoke oxidative stress-mediated neuronal injury. To better mimic the in vivo brain injury, neuronal death severity in murine models is considered. In the middle cerebral artery occlusion (MCAO) surgery that caused murine cerebral ischemia which was published previously, we noted a 50% decrease in Bcl-2 expression along with a 100% rise in caspase-12 in response to H2O2 challenge compared to the normal animals.37 Meanwhile, about 50% hippocampal neuronal death was reported after acute cerebral ischemia.38 Besides, similar cerebral injury severity has been reported elsewhere in other murine model of neurological illness, such as PD and AD.39,40 Our data showed that 69–79% of HT-22 cells survived after 100 µM H2O2 exposure, while 55%–67% of the cells survived at 300 µM H2O2. Therefore, we used 300 µM H2O2 in HT-22 cells, the level of which was also used to induce neuronal damage elsewhere.41 MDA is a poisonous species generated by lipid peroxidation, and SOD is a representative defensive enzyme which eliminates endogenous free radical. Scavenging free radicals by SOD and GSH protect the brain against neuronal injury.42
Erythroid 2-related factor 2 (Nrf2), as a critical transcription factor, is used for equalizing oxidation reaction. In addition, HO-1 plays as a consequential Nrf2-ARE pathway downstream gene. Heme oxygenase (HO) as a “speed-limiting” enzyme for heme catabolism, can catalyze the reaction of heme transferring to biliverdin, Fe2+, and CO. HO-1, as one of the subtypes of HO, is an antioxidant enzyme that antagonizes oxidative stress.43 It can be induced by various oxidative stress factors such as cytokines, ultraviolet rays, lipopolysaccharides, hydrogen peroxide. Multiple publications confirm that HO-1, which can directly manipulate the capability of protecting affliction from oxidation, is essential for the survival of organisms.44 Red blood cell destruction-derived heme is cytotoxic to organisms upon oxidative stress. HO-1 plays a cytoprotective role by catalyzing the degradation of heme. It can also reduce tissue lipid peroxidation and endogenous ROS production to alleviate oxidative stress. It is confirmed that HO-1 alleviates cerebral ischemia–reperfusion injury through inhibiting inflammation, oxidative stress, cell apoptosis and promoting angiogenesis.42 Therefore, we detected HO-1 to reveal the protective outcome of NB. In this paper, boosting SOD activity level while bringing down the formation of MDA in NB pre-treated cells was revealed. In addition, this study exhibited that H2O2 may upregulate HO-1, an effect that was overtly augmented by NB.
The size of cells, as a typical feature of cellular lineage, and their regulation serve as a conservative function to maintain integrity and functions. Condition-induced cell volume variation may disturb cellular homeostasis and trigger cell death.45 It is reported that the variation of cell size is related with plenty neuropathological situations like brain injury, ischemia, and trauma. Losing control of cell volume and the associated cell swelling may cause neuronal injury or apoptosis.46 In our study, stimulation of HT22 cells with H2O2 increased the cell volume during the initial 8 h in association with H2O2-induced apoptosis. Previous evidence showed that the dendritic and somatic cells can be enlarged to lead neuronal cell death by the stimulation of excitotoxicity, which promotes insistent Cl− influx independent of GABA.47 It was also shown that exogenously applied H2O2 induced cell swelling of HTC and Hela cells dependent of Cl− channel activation.13 The results in this paper suggested that the neuroprotective outcome of NB may present in two distinct stages. At early stage, NB resists oxidative stress and reduces the oxidative stress-evoked cell volume increase (such as cell swelling) to maintain the inner homeostasis. While, at the later stage of oxidative stress-induced cell swelling, NB inhibits volume-regulating Cl− channel to suppress apoptotic cell shrinkage to antagonize cell apoptosis.
Cell volume regulation is not only related with the net influx and efflux of water, but also has a relationship with solutes across plasma membranes. To equalize cell volume, a proper ion transporter concentration should be maintained, as illustrated by K+, Cl−, and some of organic osmolytes. By responding to an acute cell volume increasing over a threshold, VSOR Cl− channel is triggered to contribute a major pathway for anion transfer within the cell volume control process.48 In addition, chloride channels may function in the regulation of apoptosis.13 It is demonstrated that activated VSOR Cl− current is associated with cell apoptosis caused by H2O2 in cultured mesangial cells.49 Meanwhile, VSOR Cl− channel is tightly associated with the homeostasis of neuronal cell, and activation of VSOR Cl− channel may cause intracellular environmental disorder, which leads to both acute and chronic cerebral edema in cerebral ischemia/reperfusion, and neurodegeneration such as PD and AD.11 Patch clamp experiments were performed to record the immediate Cl− current after the challenge by H2O2, and it required high-dose agents to stimulate rapid and acute change of channel action. In the patch clamp experiments, we used higher dose of H2O2 (500 µmol/L) and NB (500 µmol/L). In other cellular or molecular experiments, chronic neuronal injuries were induced, while neurons were exposed to acute stimulations in patch clamp experiments. Therefore, we used H2O2 at 500 µmol/L in patch clamp experiments and 300 µmol/L H2O2 in other cell experiments. Our results demonstrated that NB may block the currents of VOSR Cl− channel triggered by H2O2-induced in HT22 cells, and NB had comparable efficiency with DCPIB in inhibiting VSOR Cl− current. DCPIB treatment increased the cell viability and reduced LDH release in H2O2-exposed cells, indicating that inhibiting VOSR channel promoted neuronal survival.
PI3K/Akt has a tight relationship with neuronal injury and the cell apoptosis caused by oxidative stress.50–52 Previous study demonstrated that function of chloride channel blockers DIDS and DCPIB was closely related to the activation PI3K/Akt in cardiomyocytes.17 In this study, NB was found to significantly upregulate the level of H2O2-induced Akt phosphorylation, and such effect was blocked by the PI3K/Akt inhibitor LY294002. Besides, PI3K/Akt inhibition abolished the neuroprotection offered by NB. Previous studies showed that H2O2 suppressed Akt phosphorylation in neurons,53 which was different from our current results. In this study, 300 µmol/L of H2O2 promoted the Akt phosphorylation and increased the amount of p-Akt (S473) in the total of p-Akt (S473)/Akt. We considered that the activated Akt signaling may function as a compensating mechanism for neurons to resist the H2O2-induced injury involving ROS system. However, this inner compensating mechanism is insufficient to eliminate H2O2-induced cell damages. In our study, NB pretreatment further potentiated the level of p-Akt and promoted neuronal survival. Besides, the inductive effect of H2O2 on p-Akt was confirmed in study by Wang et al.54 in SH-SY5Y cells. Therefore, we concluded that NB can protect cell from apoptosis, and the activation of p-AKT can mediate oxidative stress in part.
In summary, our results revealed that NB effectively alleviates neuronal injury through its inhibitory effects on oxidative stress and VSOR Cl− currents. Moreover, PI3K/Akt signaling mediated the neuroprotective outcome of NB. Therefore, NB may carry some promises as a harm-free candidate for treating stroke and additional neurodegeneration illness.
Authors’ contributions
XJ and XMW conceived and planned the whole project. XJ drafted of the manuscript. XJ, YL, XL, CH, DTL and LMH conducted experiments. XJ and RX analyzed and interpreted the data. XJ, XL, CH, XMW have put great efforts to revise the manuscript. All authors checked and approved the submission.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant number 81870184) and Major Basic Research Projects in Natural Science Foundation of Shaanxi Province (grant number 2016ZDJC-13).
References
- 1.Di Pietro V, Lazzarino G, Amorini AM, Tavazzi B, D'Urso S, Longo S, Vagnozzi R, Signoretti S, Clementi E, Giardina B, Lazzarino G, Belli A. Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic Biol Med 2014; 69:258–64 [DOI] [PubMed] [Google Scholar]
- 2.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology 2000; 7:153–63 [DOI] [PubMed] [Google Scholar]
- 3.Troyano A, Sancho P, Fernandez C, de Blas E, Bernardi P, Aller P. The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ 2003; 10:889–98 [DOI] [PubMed] [Google Scholar]
- 4.Gardner AM, Xu FH, Fady C, Jacoby FJ, Duffey DC, Tu Y, Lichtenstein A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic Biol Med 1997; 22:73–83 [DOI] [PubMed] [Google Scholar]
- 5.Model MA. Possible causes of apoptotic volume decrease: an attempt at quantitative review. Am J Physiol Cell Physiol 2014; 306:C417–24 [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SF, Okada Y, Nilius B. Biophysics and physiology of the volume-regulated anion channel (VRAC)/volume-sensitive outwardly rectifying anion channel (VSOR). Pflugers Arch 2016; 468:371–83 [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann EK, Holm NB, Lambert IH. Functions of volume-sensitive and calcium-activated chloride channels. IUBMB Life 2014; 66:257–67 [DOI] [PubMed] [Google Scholar]
- 8.Qiu Z, Dubin AE, Mathur J, Tu B, Reddy K, Miraglia LJ, Reinhardt J, Orth AP, Patapoutian A. SWELL1, a plasma membrane protein, is an essential component of volume-regulated anion channel. Cell 2014; 157:447–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science 2014; 344:634–8 [DOI] [PubMed] [Google Scholar]
- 10.Mongin AA. Volume-regulated anion channel-a frenemy within the brain. Pflugers Arch 2016; 468:421–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akita T, Okada Y. Characteristics and roles of the volume-sensitive outwardly rectifying (VSOR) anion channel in the Central nervous system. Neuroscience 2014; 275:211–31 [DOI] [PubMed] [Google Scholar]
- 12.Okada Y, Sato K, Numata T. Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 2009; 587:2141–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varela D, Simon F, Riveros A, Jorgensen F, Stutzin A. NAD(P)H oxidase-derived H2O2 signals chloride channel activation in cell volume regulation and cell proliferation. J Biol Chem 2004; 279:13301–4 [DOI] [PubMed] [Google Scholar]
- 14.Browe DM, Baumgarten CM. Angiotensin II (AT1) receptors and NADPH oxidase regulate Cl- current elicited by β1 integrin stretch in rabbit ventricular myocytes. J Gen Physiol 2004; 124:273–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ. Bromocriptine activates NQO1 via Nrf2-PI3K/akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res 2008; 57:325–31 [DOI] [PubMed] [Google Scholar]
- 16.Tiong CX, Lu M, Bian JS. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/akt pathway. Br J Pharmacol 2010; 161:467–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Wang B, Zhang WW, Liu JN, Shen MZ, Ding MG, Wang XM, Ren J. Modulation of staurosporine-activated volume-sensitive outwardly rectifying Cl- channel by PI3K/akt in cardiomyocytes. Curr Pharm Des 2013; 19:4859–64 [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Ohtaki H, Nakamachi T, Shioda S, Okada Y. Anion channel blockers attenuate delayed neuronal cell death induced by transient forebrain ischemia. J Neurosci Res 2007; 85:1427–35 [DOI] [PubMed] [Google Scholar]
- 19.Ricci L, Valoti M, Sgaragli G, Frosini M. Protection by taurine of rat brain cortical slices against oxygen glucose deprivation- and reoxygenation-induced damage. Eur J Pharmacol 2009; 621:26–32 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto S, Kita S, Iyoda T, Yamada T, Iwamoto T. New molecular mechanisms for cardiovascular disease: cardiac hypertrophy and cell-volume regulation. J Pharmacol Sci 2011; 116:343–9 [DOI] [PubMed] [Google Scholar]
- 21.d'Anglemont de Tassigny A, Berdeaux A, Souktani R, Henry P, Ghaleh B. The volume-sensitive chloride channel inhibitors prevent both contractile dysfunction and apoptosis induced by doxorubicin through PI3kinase, akt and erk 1/2. Eur J Heart Fail 2008; 10:39–46 [DOI] [PubMed] [Google Scholar]
- 22.Han Q, Liu S, Li Z, Hu F, Zhang Q, Zhou M, Chen J, Lei T, Zhang H. DCPIB, a potent volume-regulated anion channel antagonist, attenuates microglia-mediated inflammatory response and neuronal injury following focal cerebral ischemia. Brain Res 2014; 1542:176–85 [DOI] [PubMed] [Google Scholar]
- 23.Chou SC, Su CR, Ku YC, Wu TS. The constituents and their bioactivities of Houttuynia cordata. Chem Pharm Bull 2009; 57:1227–30 [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Ryu SY, No J, Choi SU, Kim YS. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharm Res 2001; 24:518–21 [DOI] [PubMed] [Google Scholar]
- 25.Huh E, Kim HG, Park H, Kang MS, Lee B, Oh MS. Houttuynia cordata improves cognitive deficits in cholinergic dysfunction Alzheimer's disease-like models. Biomol Ther 2014; 22:176–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HG, Jeong HU, Hong SI, Oh MS. Houttuyniae herba attenuates kainic acid-induced neurotoxicity via calcium response modulation in the mouse hippocampus. Planta Med 2015; 81:1697–704 [DOI] [PubMed] [Google Scholar]
- 27.Park H, Oh MS. Houttuyniae herba protects rat primary cortical cells from Aβ25-35-induced neurotoxicity via regulation of calcium influx and mitochondria-mediated apoptosis. Hum Exp Toxicol 2012; 31:698–709 [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Cao Y, Shen M, Wang B, Zhang W, Liu Y, He X, Wang L, Xia Y, Ding M, Xu X, Ren J. DIDS reduces ischemia/reperfusion-induced myocardial injury in rats. Cell Physiol Biochem 2015; 35:676–88 [DOI] [PubMed] [Google Scholar]
- 29.Shen M, Wang L, Wang B, Wang T, Yang G, Shen L, Wang T, Guo X, Liu Y, Xia Y, Jia L, Wang X. Activation of volume-sensitive outwardly rectifying chloride channel by ROS contributes to ER stress and cardiac contractile dysfunction: involvement of CHOP through Wnt. Cell Death Dis 2014; 5:e1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Shen M, Guo X, Wang B, Xia Y, Wang N, Zhang Q, Jia L, Wang X. Volume-sensitive outwardly rectifying chloride channel blockers protect against high glucose-induced apoptosis of cardiomyocytes via autophagy activation. Sci Rep 2017; 7:44265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R, Xiao Y, Liu Y, Wang B, Li X, Huo C, Jia X, Hou L, Wang X. Fluorescence-based high throughput screening technologies for natural chloride ion channel blockers. Chem Res Toxicol 2018; 31:1332–8 [DOI] [PubMed] [Google Scholar]
- 32.Dai SH, Chen T, Wang YH, Zhu J, Luo P, Rao W, Yang YF, Fei Z, Jiang XF. Sirt3 attenuates hydrogen peroxide-induced oxidative stress through the preservation of mitochondrial function in HT22 cells. Int J Mol Med 2014; 34:1159–68 [DOI] [PubMed] [Google Scholar]
- 33.Szczeklik K, Krzysciak W, Domagala-Rodacka R, Mach P, Darczuk D, Cibor D, Pytko-Polonczyk J, Rodacki T, Owczarek D. Alterations in glutathione peroxidase and superoxide dismutase activities in plasma and saliva in relation to disease activity in patients with Crohn's disease. J Physiol Pharmacol 2016; 67:709–15 [PubMed] [Google Scholar]
- 34.Jayakumar AR, Panickar KS, Murthy CR, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci 2006; 26:4774–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo A, Monteiro L, Lima RM, Oliveira DM, Cerqueira MD, El-Bacha RS. Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives. Oxid Med Cell Longev 2011; 2011:467180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan PH. Oxygen radicals in focal cerebral ischemia. Brain Pathol 1994; 4:59–65 [DOI] [PubMed] [Google Scholar]
- 37.Cao G, Zhou H, Jiang N, Han Y, Hu Y, Zhang Y, Qi J, Kou J, Yu B. YiQiFuMai powder injection ameliorates cerebral ischemia by inhibiting endoplasmic reticulum stress-mediated neuronal apoptosis. Oxid Med Cell Longev 2016; 2016:5493279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu JL, Li C, Che LH, Xu N. Lentivirus-mediated gene silencing of lymphocyte function-associated antigen 1 inhibits apoptosis of hippocampal neurons in rats with acute cerebral ischemia after cerebral lymphatic blockage. Cell Physiol Biochem 2018; 51:1069–86 [DOI] [PubMed] [Google Scholar]
- 39.Gao Q, Ou Z, Jiang T, Tian YY, Zhou JS, Wu L, Shi JQ, Zhang YD. Azilsartan ameliorates apoptosis of dopaminergic neurons and rescues characteristic Parkinsonian behaviors in a rat model of Parkinson's disease. Oncotarget 2017; 8:24099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Wu J, Yu C, Tang Y, Liu J, Chen H, Jin B, Mei Q, Cao S, Qin D. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of Alzheimer's disease. Nutrients 2017; 9:pii: E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Zhang J, Wang S, Qiu J, Yu C. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting α-synuclein expression via the p38 MAPK pathway. Int J Mol Med 2017; 40:1772–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol 2004; 207:3221–31 [DOI] [PubMed] [Google Scholar]
- 43.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999; 103:129–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nitti M, Piras S, Brondolo L, Marinari UM, Pronzato MA, Furfaro AL. Heme oxygenase 1 in the nervous system: does it favor neuronal cell survival or induce neurodegeneration? Int J Mol Sci 2018; 19:pii:E2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasantes-Morales H. Channels and volume changes in the life and death of the cell. Mol Pharmacol 2016; 90:358–70 [DOI] [PubMed] [Google Scholar]
- 46.Sardini A, Amey JS, Weylandt KH, Nobles M, Valverde MA, Higgins CF. Cell volume regulation and swelling-activated chloride channels. Biochim Biophys Acta 2003; 1618:153–62 [DOI] [PubMed] [Google Scholar]
- 47.Inoue H, Okada Y. Roles of volume-sensitive chloride channel in excitotoxic neuronal injury. J Neurosci 2007; 27:1445–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akita T, Fedorovich SV, Okada Y. Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 2011; 28:1181–90 [DOI] [PubMed] [Google Scholar]
- 49.Jiao JD, Xu CQ, Yue P, Dong DL, Li Z, Du ZM, Yang BF. Volume-sensitive outwardly rectifying chloride channels are involved in oxidative stress-induced apoptosis of mesangial cells. Biochem Biophys Res Commun 2006; 340:277–85 [DOI] [PubMed] [Google Scholar]
- 50.Cheng Y, Cawley NX, Loh YP. Carboxypeptidase E/NFα1: a new neurotrophic factor against oxidative stress-induced apoptotic cell death mediated by ERK and PI3-K/AKT pathways. PLoS One 2013; 8:e71578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duarte AI, Santos P, Oliveira CR, Santos MS, Rego AC. Insulin neuroprotection against oxidative stress is mediated by akt and GSK-3β signaling pathways and changes in protein expression. Biochim Biophys Acta 2008; 1783:994–1002 [DOI] [PubMed] [Google Scholar]
- 52.Uranga RM, Katz S, Salvador GA. Enhanced phosphatidylinositol 3-kinase (PI3K)/akt signaling has pleiotropic targets in hippocampal neurons exposed to iron-induced oxidative stress. J Biol Chem 2013; 288:19773–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Q, Gou WL, Zhang R. FAM3A protects HT22 cells against hydrogen peroxide-induced oxidative stress through activation of PI3K/akt but not MEK/ERK pathway. Cell Physiol Biochem 2015; 37:1431–41 [DOI] [PubMed] [Google Scholar]
- 54.Wang XJ, Wang LY, Fu Y, Wu J, Tang XC, Zhao WM, Zhang HY. Promising effects on ameliorating mitochondrial function and enhancing akt signaling in SH-SY5Y cells by (M)-bicelaphanol A, a novel dimeric podocarpane type trinorditerpene isolated from celastrus orbiculatus. Phytomedicine 2013; 20:1064–70 [DOI] [PubMed] [Google Scholar]