Short abstract
Stroke remains a devastating disease with limited treatment options, despite our growing understanding of its pathology. While ischemic stroke is traditionally characterized by a blockage of blood flow to the brain, this may coincide with reduced blood circulation to the eye, resulting in retinal ischemia, which may in turn lead to visual impairment. Although effective treatment options for retinal ischemia are similarly scarce, new evidence suggests that deleterious changes to mitochondrial structure and function play a major role in both cerebral and retinal ischemia pathologies. Prior studies establish that astrocytes transfer healthy mitochondria to ischemic neurons following stroke; however, this alone is not enough to significantly mitigate the damage caused by primary and secondary cell death. Thus, stem cell-based regenerative medicine targeting amelioration of ischemia-induced mitochondrial dysfunction via the transfer of functional mitochondria to injured neural cells represents a promising approach to improve stroke outcomes for both cerebral and retinal ischemia. In this review, we evaluate recent laboratory evidence supporting the remedial capabilities of mitochondrial transfer as an innovative stroke treatment. In particular, we examine exogenous stem cell transplants in their potential role as suppliers of healthy mitochondria to neurons, brain endothelial cells, and retinal cells.
Impact statement
Stroke constitutes a global health crisis, yet potent, applicable therapeutic options remain effectively inaccessible for many patients. To this end, stem cell transplants stand as a promising stroke treatment and as an emerging subject of research for cell-based regenerative medicine. This is the first review to synthesize the implications of stem cell-derived mitochondrial transfer in both the brain and the eye. As such, this report carries fresh insight into the commonalities between the two stroke-affected organs. We present the findings of this developing area of research inquiry with the hope that our evaluation may advance the use of stem cell transplants as viable therapeutic alternatives for ischemic stroke and related disorders characterized by mitochondrial dysfunction. Such lab-to-clinic translational advancement has the potential to save and improve the ever increasing millions of lives affected by stroke.
Keywords: Stroke, stem cell transplants, mitochondria, brain endothelial cells, neurons, retinal cells
Introduction
Stroke, a leading cause of mortality and disability throughout the modern world, imposes a major health and economic burden.1 However, despite the widespread prevalence of stroke, its immense costs, and our ever-broadening insight into stroke pathology, tissue plasminogen activator (tPA) remains the only drug-based stroke therapy approved by the Food and Drug Administration (FDA).2 The restrictive time frame for safe and effective tPA treatment—4.5 h following stroke onset—due to high risk for hemorrhagic transformation severely limits the number of patients eligible for its therapeutic benefits.2 While endovascular thrombectomy represents another available therapeutic option for stroke, strict criteria for its usage and considerable danger of hemorrhagic evolution similarly hinder its utility, further exacerbating this dearth of treatment options.3–5
While tPA and endovascular intervention are thus restricted to acute phase treatment of stroke, physical therapy and cognitive rehabilitation can be effective in the long term. However, various post-stroke consequences may impede recovery. Specifically, visual impairment is a prominent and common sequela of stroke which may complicate functional outcomes in both the short and long term.6,7 In fact, 92% of all stroke victims suffer some degree of visual impairment,6 while vision problems persisting up to 90 days after stroke onset plague 20.5% of patients.8 While it may be expected that ischemic stroke—usually affecting only one hemisphere of the brain—coincides with elevated risk of monocular vision loss, the reverse is also evident.8–13 Approximately 16% of post-stroke visual impairments are primarily attributable to retinal ischemia, which displays a similar pathology to other common vascular diseases of the eye (e.g. central retinal artery occlusion, retinal vein occlusion, diabetic retinopathy, and glaucoma) and indeed to ischemia of the brain.14–19
Accumulating evidence indicates that the dysfunction of mitochondria contributes to the pathological progression of stroke, retinal ischemia, and various neurological diseases, including Huntington disease, Parkinson disease, Alzheimer disease, and fragile X-associated tremor/ataxia syndrome.20–28 During cerebral and retinal ischemia, the lack of oxygen and nutrients prevents mitochondrial regeneration of adenosine triphosphate (ATP), depriving the cell of energy for metabolism and initiating a cascade of cell death processes.29–33 A novel observation of host repair mechanisms indicates that astrocytes initiate transfer of functional mitochondria to neurons in order to protect them and delay cell death within these ischemic conditions.34 On its own, this natural mechanism is largely unable to confer sufficient neuroprotection during stroke. However, the evident capacity of stem cell transplants to serve as surrogate sources of healthy mitochondria for ischemia-threatened cells lays the foundations for an innovative approach to stem cell-based repair of mitochondrial dysfunction in stroke.35 In this review, we analyze and progress the auspicious potential of stem cells as crucial reservoirs of healthy mitochondria not only for neurons, but also for brain endothelial cells and retinal cells of the eye.
The role of mitochondria in stroke pathology
During ischemic stroke, glucose and oxygen deprivation—the result of insufficient blood circulation in cerebral tissue—causes deleterious structural changes to mitochondria, compromising oxidative metabolism and exacerbating the loss of neural cells as well as the inflammatory response.2,35 Following stroke, ischemia-induced mitochondrial impairments inhibit regeneration of ATP. Because mitochondrial oxidative phosphorylation accounts for 92% of overall cellular ATP generation, stroke-damaged cells may therefore lack sufficient energy to properly maintain metabolic functions.35,36 Moreover, defective oxidative metabolism in dysfunctional mitochondria precipitates the overproduction of reactive oxygen species (ROS) and, in turn, reactive nitrogen species (RNS) following the return of normal oxygen concentrations during reperfusion.35 Oxidative stress resulting from the disproportionate increase in ROS and RNS destructively alters proteins, lipids, and deoxyribonucleic acid (DNA).35 In response to this critical threat posed to cellular ultrastructure, mitochondria possess mechanisms at various levels of their structural hierarchy to protect against oxidative stress.35 At the level of mitochondrial networks, for example, the dynamic processes of mitochondrial fusion and fission sequester functional mitochondria from oxidative damage, while systematically eliminating dysfunctional mitochondria by mitophagy.35 Further down this hierarchy, at the level of mitochondrial membrane structure, the excessively permeable membrane of severely damaged mitochondria permits the passage of pro-apoptotic molecules into the cytoplasm, resulting in apoptotic cell death.35
Mitochondrial and cytosolic creatine kinase (CK) isoenzymes—which comprise an important homeostatic mechanism in tissues with high ATP demand such as brain and muscle—are also particularly susceptible to damage by ROS and RNS.37–41 CK enzymes, primarily those sequestered within mitochondria, reversibly phosphorylate creatine to maintain high intracellular concentrations of phosphocreatine.41–44 Because phosphocreatine diffuses slightly faster than ATP, cytosolic CK enzymes form an intracellular energy buffer by rapidly converting phosphocreatine back to creatine and regenerating ATP.41–44 Impairments to cellular energy metabolism, often resulting from ischemia and oxidative stress, are characterized by upregulated expression of mitochondrial CK to delay ATP depletion, and the localization of mitochondrial CK complexes within mitochondria compounds their vulnerability to oxidative damage, potentially leading to a buildup of crystalline mitochondrial CK inclusion bodies and exacerbating mitochondrial dysfunction and energy deficits.37,38,41 Thus, interconversion of creatine to phosphocreatine plays a large role in cellular energetics, and rescue of this fragile process may be a prime target for mitochondria-mediated cell therapy.
Taken together, mitochondrial impairment due to oxidative stress likely plays a pivotal role in cell death processes during stroke. In the context of mitochondrial repair in stroke, stem-cell based regenerative medicine holds promise based on stem cell transplants’ extensive repertoire of therapeutic benefits, ranging from cell replacement and trophic support to anti-inflammation and stimulation of endogenous neurorestorative processes.45 With these aspects of stem cells’ therapeutic features, we now aim to evaluate the prospects of stem cell-based mitochondria transfer as a treatment for stroke.
Stem cells as a source of healthy mitochondria
The ability of stem cells to convey healthy mitochondria to damaged cells is a novel finding and could prove to be beneficial for stroke therapy.46,47 Mitochondria are transferred from stem cells to injured cells via tunneling nanotubes, microvesicles, gap junctions, cell fusion, or direct uptake.46,47 In pathological conditions, endogenous mitochondrial transfer has restored damaged cell function; however, further investigation is warranted to establish the necessary conditions and molecular signals for optimally inducing mitochondrial release from stem cells.46,47 A variety of cell types have demonstrated the ability to convey healthy mitochondria, such as pulmonary alveoli, astrocytes, neurons, and bone marrow-derived mesenchymal stem cells (BM-MSCs).34,48,49
Since stem cell-based mitochondrial transfer is a relatively new potential treatment for stroke, an ideal cell type has not yet been established. A potential candidate is the BM-MSC-derived endothelial progenitor cells (EPCs), which comprise populations of immature endothelial cells circulating the human bloodstream.50 Through migration to the brain and restoration of the blood–brain barrier (BBB), EPCs exhibit positive regenerative effects on brain vasculature.51 While recent evidence suggests that the transfer of EPC-derived mitochondria to ischemic brain endothelial cells contributes significantly to the promotion of angiogenesis and BBB repair, it cannot be ruled out that other effects of EPC transplants, such as the secretion of various angiogenic factors, also play a major role in these regards. For example, EPCs secrete the pro-angiogenic enzyme thymidine phosphorylase, an important regulator of the angiogenic potential of EPC cultures and colony-forming units.52 Moreover, the mechanism through which EPCs convey mitochondria is unknown. Therefore, three questions may be asked to determine the efficacy of EPC-based mitochondrial transfer as a potential treatment: one, can EPCs release mitochondria; two, can brain endothelial cells accept and avail themselves of these mitochondria; and three, can stem cell-derived mitochondria restore cell viability and function.
To address the first question, human EPCs were identified by representative markers including vWF, lectin-UEA, CD34, and Flk-1 after subjection to an in vitro stroke model.50 Centrifuge and Western blot analysis of the supernatant and particle fractions reveal that EPC-conditioned media enriches TOM40 mitochondrial membrane protein and increases ATP levels.50 Furthermore, flow cytometry with MitoTracker Red and electron microscopy displays mitochondria in EPC-derived extracellular vesicles.50 Measurement of oxygen consumption levels indicates that these extracellular mitochondria are viable.50 Likewise, media derived from other cell populations, including endothelial cells, human astrocytes, and pericytes, exhibits similar vascular function in the neurovascular unit.50 Flow cytometry of the conditioned media using markers MitoTracker Deep Red, CD63, and JC-1 respectively for each cell type reveals that EPC-derived particles contain levels of extracellular mitochondria similar to other cell types.50 In all, these findings indicate that EPCs support a mode of action for releasing active extracellular mitochondria, thus resolving the first question.
That EPCs may release viable extracellular mitochondria raises the questions of whether these mitochondria may be transferred into the desired cells and whether this would confer any benefits. In response to the first part, confocal microscopy reveals that EPC-derived extracellular mitochondria can indeed be relayed to brain endothelial cells.50 In response to the second part, several observations suggest an answer. For one, capillary-like structures spontaneously form on the brain endothelial cells upon exposure to EPC-conditioned media.50 Matrigel assay of these structures indicates that both particle fractions and supernatant can increase angiogenesis in the EPC-conditioned media groups, whereas the empty and ATP-loaded liposome controls produce no variation.50 Aside from angiogenesis, barrier function constitutes another important role in the brain endothelium. To this end, tight junctions and adherens may be assessed by using occludin and VE-cadherin as markers of each respectively. While Western blot analysis demonstrates similar levels of protein expression for both molecules, regardless of condition, immunocytochemistry reveals that EPC-conditioned media particle fraction elevates VE-cadherin membrane localization, suggesting greater levels of adherens.50 Furthermore, an endothelial permeability assay using a transwell system evinces that EPC-derived particles with mitochondria enclosed may decrease brain endothelial permeability, whereas liposome controls do not.50 To more closely approximate actual ischemic conditions, an in vitro stroke model may be used to conduct further tests of EPC-derived mitochondria’s neuroprotective effects. After subjecting brain endothelial cells to oxygen and glucose deprivation (OGD) and treating with EPC-derived particles, the affected endothelium exhibited upregulation of TOM40, a mitochondrial protein.50 Western blot analysis further indicates that the EPC-derived particle treatment restores intracellular mitochondrial DNA (mtDNA) and ATP levels, and also reduces endothelial permeability.50 Moreover, administering EPC-derived extracellular mitochondria, which were isolated through fluorescence-activated cell sorting (FACS), to OGD-subjected brain endothelial cells results in increased cell viability and endothelial tightness, in line with previous findings. Taken together, these results suggest that EPC mitochondria can be transferred to brain endothelial cells and consequently recover mitochondrial capability.
The remaining questions primarily relate to the lingering gaps in knowledge concerning the relationship between mitochondrial incorporation and endothelial functional improvement, as well as identifying the mechanism underlying the therapeutic influences of mitochondrial transfer. To investigate these gaps, endothelial cells in the brain were subjected to proteome analysis after exposure to OGD, with the goal of distinguishing the cells that integrated EPC-derived mitochondria from the cells that did not.50 FACS demonstrates a greater amount of angiogenesis and BBB proteins, such as Serpin E1, plasminogen, FGF-4, and bFGF, in cells with foreign mitochondria than in cells without.50 Thus, the transferred mitochondria’s mtDNA may increase expression of genes that protect the endothelium in response to OGD. In sum, once functioning mitochondria carried in EPC-derived particles are secreted, they can be incorporated into endothelial cells in the brain, where they then enhance angiogenesis and ameliorate BBB function post-OGD exposure in vitro. Overall, stem cells, such as EPCs, that are able to convey mitochondria may serve as therapeutic tools for improving mitochondrial function in stroke and other disorders.
The incorporation of mitochondria derived from stem cells into ischemic cells may serve as an effective new strategy for treating stroke. However, a black box remains as to the mechanism behind the observed neuroprotection conferred by the transfer of mitochondria from stem cells into ischemic neurons. Thus, it is necessary to establish a causal relationship between the transferred mitochondria and the ensuing neuroprotection. Moreover, it must be demonstrated whether stem cells’ capacity to release the functioning mitochondria that induce recovery of cellular bioenergetics can be translated to ischemic tissue. To this end, mitochondrial transfer into impaired neurons may be distinguished by immunofluorescent imaging in vitro and in vivo. Furthermore, Seahorse or the Clark electrode assays may indicate mitochondrial functionality from both non-transplanted and transplanted stroke tissue. These methods are employed to both visually inspect the transfer of healthy mitochondria as well as measure the recovery of cellular bioenergetics mediated by these mitochondria. Although long-term graft survival may be low, that mitochondrial transfer from transplanted stem cells into ischemic neurons may transpire in the short term while granting lasting ameliorative effects may explain the observed long-term neuroprotective effects.
Recognizing the therapeutic potential of mitochondrial transfer from stem cells, the functional benefit bestowed by grafted stem cells beyond that which is already granted by astrocytes requires greater elucidation. Astrocytes indeed transfer healthy mitochondria into impaired neurons,34 yet this astrocyte-mediated transfer is brief and does not engender potent, reliable neuroprotection. Thus, the secondary cell death that occurs in stroke cannot be effectively inhibited with the astrocyte-transfer alone and necessitates the complement of stem cell-derived transfers. Stem cell-induced mitochondrial transfer strategies may demonstrate that EPCs confer greater neuroprotection than astrocytes in this respect. To this end, direct comparison between the mitochondria from endogenous astrocytes and from exogenous stem cells should elucidate this efficacy difference.
After validation of stem cell-mediated transfer of mitochondria into ischemic neurons, the role of electron transport chain (ETC) complexes I–IV in conferring neuroprotection can be determined by applying corresponding inhibitors to examine mitochondrial activity in isolation. Additionally, generating cells containing malfunctioning mitochondria (e.g. Rho0 cells) may serve as another way to avoid confounds and confirm mitochondria as the principal mechanism behind the observed neuroprotection. Comparing the neuroprotection afforded in ischemic neurons after EPC co-culture or transplantation with the neuroprotection provided in Rho0 cells with ETC inhibitors may elucidate this mechanism. In both the EPC and Rho0 cultures, during the early period (1–14 days), graft survival in the brain was moderate (<1%), and, in the later phase (1–3 months), graft survival was too low to be discerned.50 Notwithstanding this low survival, EPC-mediated mitochondrial transfer should be considered more important for neuroprotection than graft survival. To this end, the Rho0 culture did not afford neuroprotection, supporting the hypothesis advanced here. Further extensive studies examining molecules correlated with both cell death and cell viability are warranted as a means of continuing investigation of the role of healthy mitochondria transfers in alleviating stroke-induced secondary cell death.
Although BM-MSCs generally display robust safety profiles compared to other stem cell types, the potential adverse effects of EPCs must be considered. While EPCs do not evidently elicit tumorigenesis directly, neovascularization of pre-existing tumors may facilitate tumor angiogenesis and metastasis.53,54 Thus, EPC transplantation is likely an unsuitable treatment option for stroke patients with tumors.54 Additionally, EPC-induced cerebral neovascularization via vascular endothelial growth factor signaling has been associated with elevated levels of brain edema.55 A few studies suggest that EPCs may also secrete pro-inflammatory molecules (e.g. interleukin-8 and monocyte chemotactic protein-1) and recruit monocytes and macrophages to the stroke brain, thereby exacerbating inflammatory neural cell loss.56–58 However, these findings are contradicted by many other studies that evince that MSCs and EPCs may instead reduce inflammation.59,60 This has been supported by multiple proposed mechanisms, including BBB regulation,51 splenic involvement,61,62 vasculome inflammation-related gene suppression,63 and endogenous T-regulatory cell populations.64,65 Altogether, these studies provide strong evidence of EPCs’ and MSCs’ net anti-inflammatory effects, and thus contend that EPCs may be safe for clinical trials. Even so, in light of this discrepancy and the other aforementioned potential caveats, employing animal models in further laboratory studies may clarify both the short and long-term pathophysiological effects of EPCs and provide a better assessment of their net safety and efficacy as a future stroke therapy.
Stem cell-mediated mitochondrial transfer to treat retinal ischemia
Thus far, we have primarily focused on present literature describing stem cell-based mitochondrial transfer to neurons and endothelial cells of the ischemic brain. However, comprehensive improvement of functional outcomes for stroke victims often does not concern the brain alone. Specifically, the eye is another organ particularly susceptible to stroke damage, and visual impairment in the aftermath of stroke is not only prevalent, but may also significantly impede recovery.6,7 Retinal ischemia contributes considerably to post-stroke visual impairments, and evidence implicates mitochondrial activity as a key determinant of retinal cell survival and death in this ocular disease,24,25 paralleling the case of ischemic stroke.
Acknowledging the overlapping pathological hallmarks between cerebral and retinal ischemia, the role of mitochondrial dysfunction in the progression of cerebral and retinal ischemia following stroke represents an appealing cell death pathway in further understating stroke pathology and its treatment.66 Furthermore, the potential of MSC treatment to restore mitochondrial function may prove beneficial in attenuating ischemic retinal cell loss.66 To this end, both a middle cerebral artery occlusion (MCAO) rat model and a retinal pigment epithelium (RPE) cell culture model of OGD were employed.66 The noteworthy findings of this study are the observations that MCAO in vivo and OGD in vitro recapitulate many of the pathological symptoms of retinal ischemia.66 In vivo, laser Doppler flow following MCAO demonstrates an 80% reduction in blood perfusion in the ipsilateral hemisphere of the brain and the ipsilateral eye, representative of cerebral and retinal ischemia, respectively, and in line with prior investigations.66–68 Following reperfusion, excessive, ischemia-induced retinal vascularization enables restoration of blood flow to the eye approximately 5 min faster than to the affected cerebral hemisphere.66,69,70 Despite this initial divergence, however, the lack of collateral circulation to the eye likely synchronizes retinal and hemispheric perfusion rates up to three days after MCAO.71–73 Immunohistochemical analysis at days 3 and 14 following MCAO reveals deficient blood flow to the retina correlating with diminished survival of retinal ganglion cells and exacerbated optic nerve degeneration, paralleling reduced, post-stroke, hemispheric blood flow.66 Additionally, immunocytochemistry indicates that in vitro OGD insult similarly induces RPE cell death.66 That ischemic insult and the ensuing aggravated retinal cell loss coincide with mitochondrial dysfunction both in vivo and in vitro therefore suggests that the accumulation of ultrastructural defects in these key cell organelles may approximate retinal ischemia pathology.66
Equally compelling as the observation of mitochondrial dysfunction after experimental stroke are the apparent therapeutic effects produced by stem cell treatment. The MSC co-culture regimen attenuates the extent of retinal ganglion cell loss and ameliorates deterioration of mitochondrial structure and function in both experimental models, possibly because MSCs can transfer their healthy mitochondria to the endangered retinal cells.66 Compared to the vehicle treatment, in vivo transplantation of intravenously delivered MSCs rescues mitochondrial respiration and significantly mitigates ganglion cell loss and optic nerve damage at the 14-day mark.66 Similarly, relative to non-treated retinal cells, RPE cells co-cultured with MSCs exhibit improved survivability following OGD, which likely arises from the restored network morphology, dynamics, and respiratory capacity of their mitochondria.66 The dynamic and interconnected morphology of mitochondrial networks, which serve to shelter mitochondrial DNA, enhance respiration, and promote mitochondrial signaling, may be regulated by the homeostatic balance of mitochondrial fusion and fission.66 When mitochondrial fission considerably exceeds fusion, the mitochondrial network fragments into isolated, rounded mitochondria.66 Closer in vitro examination of mitochondrial dynamics and network morphology reveals that RPE cells co-cultured with MSCs display more extensive mitochondrial networks, as well as fewer numbers of isolated, rounded mitochondria.66 Furthermore, co-culture with MSCs salvages expression levels of the fusion protein mitofusin-2, which is downregulated by OGD insult.66,74,75 However, OGD-induced upregulation of the fission protein dynamin-related protein-1 is not affected by MSC treatment.67,77 Additionally, this study provides the first evidence that exogenous MSCs moderate the mitochondrial membrane depolarization resulting from OGD.66
As mentioned previously, interconversion of creatine and phosphocreatine protects against lapses in the cellular energy supplies under normal conditions, but may be damaged under ischemic conditions and may exacerbate mitochondrial dysfunction. To answer for this deficit, creatine supplementation has demonstrated therapeutic properties in numerous neurodegenerative disorders.78 This indicates that rescue of endangered mitochondria via creatine supplementation renders system-wide benefits. This is further supported by stroke models that reveal that creatine treatment can improve histological outcomes.79,80 Aside from the direct treatment implications, these studies also reinforce mitochondrial functioning as a key mediator for cerebral and retinal ischemia pathology. To this end, stem cells’ transfer of healthy mitochondria facilitates restoration of mitochondrial function and may thus similarly abrogate stroke pathology.
In conclusion, the transfer of mitochondria by exogenous MSCs may restore respiratory output in ischemic retinal cells and thereby mitigate cell loss. Future studies should aim to determine the precise mechanism by which MSCs convey mitochondria to retinal ganglion cells, as well as to thoroughly characterize the metabolic and proteomic properties of MSC-derived mitochondria post-delivery into ischemic retinal cells. Furthermore, EPCs’ affinity for BBB repair positions them as an especially attractive MSC subtype. Their record as safe and effective cell-based therapeutics, as well as proficient donors of healthy mitochondria, warrants further investigation in the context of retinal ischemia treatments. A critical study limitation is the omission of detailed assessment of the specific phenotypic properties of MSCs, especially the EPC subpopulation, that may mediate mitochondrial transfer in retinal ischemia.66 Although the function and characterization of EPC-derived mitochondria are discussed in depth in the context of cerebral stroke, the role of EPCs as key drivers of mitochondrial transfer in retinal ischemia remains to be determined. Future endeavors should concentrate on this important functional characterization of MSCs and EPCs to bridge this gap in knowledge on mitochondria-mediated regenerative medicine for ischemic diseases.
Technical challenges facing clinical translation
Notwithstanding the therapeutic prospects of stem cell-mediated mitochondrial transfer as a treatment for both cerebral and retinal ischemia, translating this approach to clinical trials will require the resolution of a number of technical problems. To this end, we propose applying the STEPS recommendations as a framework for future research in this area.81–85 In short, it is of upmost importance for future laboratory research to establish the safest and most effective stem cell type for mitochondrial transfer in stroke and retinal ischemia.81–85 Secondly, future studies should also aim to clarify if mitochondrial transfer represents the principal mechanism by which stem cell transplants protect and restore ischemic neurons and retinal cells, and determine the extent to which direct cell replacement and bystander effects of stem cells—including anti-inflammation, trophic support, and endogenous stem cell recruitment60,86–88—are also necessary to significantly improve neurological and functional outcomes.81–85 Lastly, methodological specifications, such as the route and timing of stem cell delivery, will likely need to be optimized in animal models of cerebral and retinal ischemia before clinical testing can be initiated.81–85
Conclusion
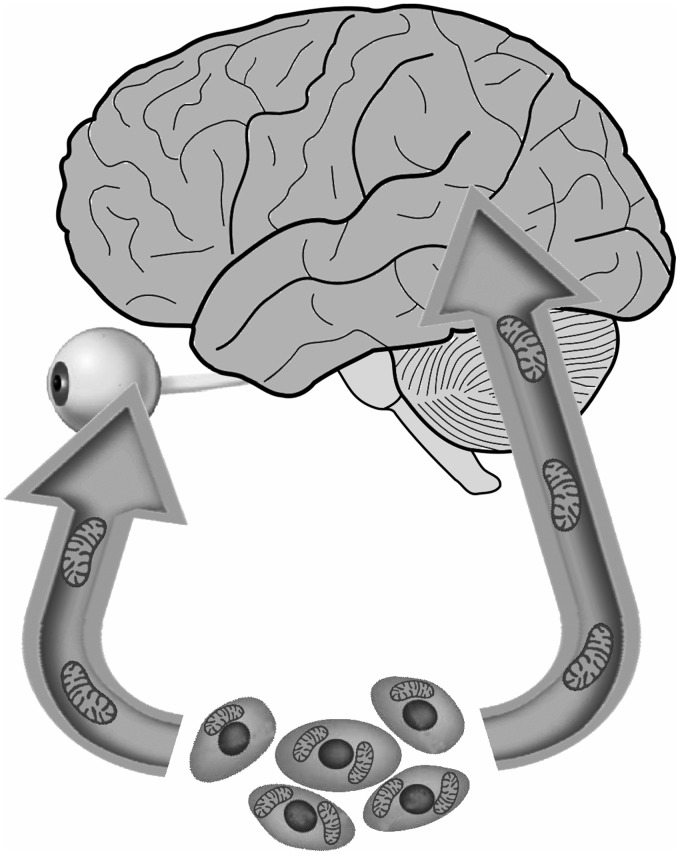
Although stroke continues to be a major cause of death and disability worldwide, treatment options remain severely limited. Among its numerous debilitating effects, stroke may restrict blood flow to the eye and cause retinal ischemia, a significant source of stroke-related visual impairments. Mitochondrial dysfunction characterizes the pathological progression of both stroke and retinal ischemia.20–25 Therefore, the transfer of functional mitochondria from exogenous stem cells to endangered neural and retinal cells represents an exciting therapeutic approach to mitigate cell loss in cerebral and retinal ischemia (Figure 1). Previously, we have reviewed the transfer of mitochondria, but this article was limited to the brain only.89 In this review, we examined recent laboratory findings demonstrating promising results toward these ends. Following OGD insult in vitro, EPCs successfully supply mitochondria to brain endothelium cells and restore ATP levels in the brain endothelium culture, indicating that transferred mitochondria remain functional.33 Moreover, in both a rat model of MCAO and an RPE cell culture model of OGD, MSC transplants transfer healthy mitochondria to retinal cells, ameliorating ischemia-induced impairment of mitochondrial structure and function within those cells and improving their survival.66
Figure 1.
Stem cells may transfer healthy mitochondria into endangered cells in the brain and eye, attenuating stroke progression.
Despite evident therapeutic potential to treat stroke and retinal ischemia, future research must clarify whether mitochondrial transfer, as opposed to other benefits mediated by stem cells, is the primary determinant of cell survival. Furthermore, optimizing treatment parameters such as timing of transplantation and route of delivery, as well as combining this novel approach of stem cell-based mitochondrial transfer with tPA, may further enhance post-ischemia outcomes in the brain and eye.
Authors’ contributions
All authors participated in drafting and editing this manuscript.
DECLARATION OF CONFLICTING INTERESTS
C Borlongan was funded and received royalties and stock options from Astellas, Asterias, Sanbio, Athersys, KMPHC, and International Stem Cell Corporation; and also received consultant compensation for Chiesi Farmaceutici. He also holds patents and patent applications related to stem cell biology and therapy. The other authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
FUNDING
C Borlongan is funded by National Institutes of Health (NIH) R01NS071956, NIH R01NS090962, NIH R21NS089851, NIH R21NS094087, and Veterans Affairs Merit Review I01 BX001407.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD. Heart disease and stroke statistics – 2018 update: a report from the American Heart Association. Circulation 2018; 137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 2.Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010; 17:197–218 [DOI] [PubMed] [Google Scholar]
- 3.Kaesmacher J, Kaesmacher M, Maegerlein C, Zimmer C, Gersing AS, Wunderlich S, Friedrich B, Boeckh-Behrens T, Kleine JF. Hemorrhagic transformations after thrombectomy: risk factors and clinical relevance. Cerebrovasc Dis 2017; 43:294–304 [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Gao X, Yao Z, Feng X, He H, Xue J, Gao P, Yang L, Cheng X, Chen W, Yang Y. Permeability surface of deep Middle cerebral artery territory on computed tomographic perfusion predicts hemorrhagic transformation after stroke. Stroke 2017; 48:2412–8 [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Van der Lugt AA, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, Cardona P. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a metaanalysis. JAMA 2016; 316:1279–89 [DOI] [PubMed] [Google Scholar]
- 6.Rowe FJ. Stroke survivors’ views and experiences on impact of visual impairment. Brain Behav 2017; 7:e00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sand KM, Midelfart A, Thomassen L, Melms A, Wilhelm H, Hoff JM. Visual impairment in stroke patients – a review. Acta Neurol Scand Suppl 2013; 196:52–6 [DOI] [PubMed] [Google Scholar]
- 8.Zhang LY, Zhang J, Kim RK, Matthews JL, Rudich DS, Greer DM, Lesser RL, Amin H. Risk of acute ischemic stroke in patients with monocular vision loss of vascular etiology. J Neuro Ophthalmol 2018; 38:328–33 [DOI] [PubMed] [Google Scholar]
- 9.Brown SM, Vasudevan A. Acute retinal arterial ischemia: an emergency often ignored. Am J Ophthalmol 2014; 158:1353. [DOI] [PubMed] [Google Scholar]
- 10.Dattilo M, Newman NJ, Biousse V. Acute retinal arterial ischemia. Ann Eye Sci 2018; 3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helenius J, Arsava EM, Goldstein JN, Cestari DM, Buonanno FS, Rosen BR, Ay H. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol 2012; 72:286–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauda F, Neugebauer H, Reiber L, Jüttler E. Acute silent brain infarction in monocular visual loss of ischemic origin. Cerebrovasc Dis 2015; 40:151–6 [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol 2014; 157:1231–8 [DOI] [PubMed] [Google Scholar]
- 14.Bradshaw SE, Gala S, Nanavaty M, Shah A, Mwamburi M, Kefalas P. Systematic literature review of treatments for management of complications of ischemic Central retinal vein occlusion. BMC Ophthalmol 2016; 16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelho K, Mogilevskaya M, Losada-Barragan M, Vargas-Sanchez JK. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmol 2019; 39:259–71 [DOI] [PubMed] [Google Scholar]
- 16.Nashine S, Liu Y, Kim BJ, Clark AF, Pang IH. Role of C/EBP homologous protein in retinal ganglion cell death after ischemia/reperfusion injury. Invest Ophthalmol Vis Sci 2014; 56:221–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res 2004; 23:91–147 [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Dia Care 2017; 40:412–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma DD, Cugati S, Lee AW, Chen CS. A review of Central retinal artery occlusion: clinical presentation and management. Eye 2013; 27:688–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 2012; 342:619–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentice H, Modi JP, Wu JY. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid Med Cell Longev 2015; 2015:964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010; 67:181–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen H, Aum D, Mashkouri S, Rao G, Vega Gonzales-Portillo JD, Reyes S, Borlongan CV. Growth factor therapy sequesters inflammation in affording neuroprotection in cerebrovascular diseases. Exp Rev Neurother 2016; 16:915–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborne NN. Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res 2010; 90:750–7 [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, Ellisman MH, Weinreb RN, Ju WK. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Invest Ophthalmol Vis Sci 2011; 52:2837–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Napoli E, Song G, Wong S, Hagerman R, Giulivi C. Altered bioenergetics in primary dermal fibroblasts from adult carriers of the FMR1 premutation before the onset of the neurodegenerative disease fragile X-associated tremor/ataxia syndrome. Cerebellum 2016; 15:552–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabezas-Opazo FA, Vergara-Pulgar K, Pérez MJ, Jara C, Osorio-Fuentealba C, Quintanilla RA. Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev 2015; 2015:509654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napoli E, Wong S, Hung C, Ross-Inta C, Bomdica P, Giulivi C. Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington’s disease. Hum Mol Genet 2013; 22:989–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vosler PS, Graham SH, Wechsler LR, Chen J. Mitochondrial targets for stroke: focusing basic science research toward development of clinically translatable therapeutics. Stroke 2009; 40:3149–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narendra DP, Youle RJ. Neurodegeneration: trouble in the cell's powerhouse. Nature 2012; 483:418–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youle RJ, Van Der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012; 337:1062–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014; 112:24–49 [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH. Extracellular mitochondria for therapy and diagnosis in acute Central nervous system injury. JAMA Neurol 2018; 75:119–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016; 535:551–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol 2018; 16:263–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarasov AI, Griffiths EJ, Rutter GA. Regulation of ATP production by mitochondrial Ca2+. Cell Cal 2012; 52:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta 2006; 1762:164–80 [DOI] [PubMed] [Google Scholar]
- 38.Stachowiak O, Dolder M, Wallimann T, Richter C. Mitochondrial creatine kinase is a prime target of peroxynitrite-induced modification and inactivation. J Biol Chem 1998; 273:16694–9 [DOI] [PubMed] [Google Scholar]
- 39.Koufen P, Ruck A, Brdiczka D, Wendt S, Wallimann T, Stark G. Free radical-induced inactivation of creatine kinase: influence on the octameric and dimeric states of the mitochondrial enzyme (Mib-CK). Biochem J 1999; 344:413–7 [PMC free article] [PubMed] [Google Scholar]
- 40.Koufen P, Stark G. Free radical induced inactivation of creatine kinase: sites of interaction, protection, and recovery. Biochim Biophys Acta 2000; 1501:44–50 [DOI] [PubMed] [Google Scholar]
- 41.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta 2010; 1802:80–91 [DOI] [PubMed] [Google Scholar]
- 42.Jacobus WE, Lehninger AL. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem 1973; 248:4803–10 [PubMed] [Google Scholar]
- 43.Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EI. Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharmacol 1978; 56:691–706 [DOI] [PubMed] [Google Scholar]
- 44.Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science 1981; 211:448–52 [DOI] [PubMed] [Google Scholar]
- 45.Tajiri N, Duncan K, Antoine A, Pabon M, Acosta SA, de la Pena I, HernadezOntiveros DG, Shinozuka K, Ishikawa H, Kaneko Y, Yankee E. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci 2014; 8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis 2018; 9:924–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paliwal S, Chaudhuri R, Agrawal A, Mohanty S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 2018; 25:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012; 18:759–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Babenko V, Silachev D, Popkov V, Zorova L, Pevzner I, Plotnikov E, Sukhikh G, Zorov D. Miro1 enhances mitochondria transfer from multipotent mesenchymal stem cells (MMSC) to neural cells and improves the efficacy of cell recovery. Molecules 2018; 23:e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, Arai K, Rosell A, Lo EH. Protective effects of endothelial progenitor cell‐derived extracellular mitochondria in brain endothelium. Stem Cells 2018; 36:1404–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaneko Y, Tajiri N, Shinozuka KE, Glover LL, Weinbren N, Cortes LV, Borlongan C. Cell therapy for stroke: emphasis on optimizing safety and efficacy profile of endothelial progenitor cells. Curr Pharm Des 2012; 18:3731–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, Astroulakis Z, Xiao Q, Hill J, Xu Q, Mayr M. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circ Res 2009; 104:32–40 [DOI] [PubMed] [Google Scholar]
- 53.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, Mittal V. Bone marrow‐derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev 2007; 21:1546–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao S, Luo C, Cao B, Hu H, Wang S, Yue H, Chen L, Zhou Z. Endothelial progenitor cells for ischemic stroke: update on basic research and application. Stem Cells Int 2017; 2017:2193432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slevin M, Kumar P, Gaffney J, Kumar S, Krupinski J. Can angiogenesis be exploited to improve stroke outcome? mechanisms and therapeutic potential. Clin Sci 2006; 111:171–83 [DOI] [PubMed] [Google Scholar]
- 56.Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L, Foucault-Bertaud A, Velly L, Dignat-George F, Pisano P. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev and Rep 2011; 7:208–20 [DOI] [PubMed] [Google Scholar]
- 57.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004; 24:288–93 [DOI] [PubMed] [Google Scholar]
- 58.Van der Strate BW, Popa ER, Schipper M, Brouwer LA, Hendriks M, Harmsen MC, van Luyn MJ. Circulating human CD34+ progenitor cells modulate neovascularization and inflammation in a nude mouse model. J Mol Cell Cardiol 2007; 42:1086–97 [DOI] [PubMed] [Google Scholar]
- 59.Napoli E, Borlongan CV. Stem cell recipes of bone marrow and fish: just what the stroke doctors ordered. Stem Cell Rev Rep 2017; 13:192–7 [DOI] [PubMed] [Google Scholar]
- 60.Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017; 158:94–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Acosta SA, Tajiri N, Hoover J, Kaneko Y, Borlongan CV. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 2015; 46:2616–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu K, Lee JY, Kaneko Y, Tuazon JP, Vale F, van Loveren H, Borlongan CV. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica 2019; 104:1062–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Acosta SA, Lee JY, Nguyen H, Kaneko Y, Borlongan CV. Endothelial progenitor cells modulate inflammation-associated stroke vasculome. Stem Cell Rev Rep 2019; 15:256–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neal EG, Acosta SA, Kaneko Y, Ji X, Borlongan CV. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J Cereb Blood Flow Metab 2019; 39:1750–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zarriello S, Neal EG, Kaneko Y, Borlongan CV. T-regulatory cells confer increased myelination and stem cell activity after stroke-induced white matter injury. J Clin Med 2019; 8:pii: E537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen H, Lee JY, Sanberg PR, Napoli E, Borlongan CV. Eye opener in stroke. Stroke 2019; 50:2197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res 2004; 1010:108–16 [DOI] [PubMed] [Google Scholar]
- 68.Taninishi H, Jung JY, Izutsu M, Wang Z, Sheng H, Warner DS. A blinded randomized assessment of laser Doppler flowmetry efficacy in standardizing outcome from intraluminal filament MCAO in the rat. J Neurosci Methods 2015; 241:111–20 [DOI] [PubMed] [Google Scholar]
- 69.Shih YY, De La Garza BH, Huang S, Li G, Wang L, Duong TQ. Comparison of retinal and cerebral blood flow between continuous arterial spin labeling MRI and fluorescent microsphere techniques. J Magn Reson Imaging 2014; 40:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hui F, Nguyen CT, He Z, Vingrys AJ, Gurrell R, Fish RL, Bui BV. Retinal and cortical blood flow dynamics following systemic blood-neural barrier disruption. Front Neurosci 2017; 11:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ritzel RM, Pan SJ, Verma R, Wizeman J, Crapser J, Patel AR, Lieberman R, Mohan R, McCullough LD. Early retinal inflammatory biomarkers in the Middle cerebral artery occlusion model of ischemic stroke. Mol Vis 2016; 22:575–88 [PMC free article] [PubMed] [Google Scholar]
- 72.Allen RS, Sayeed I, Oumarbaeva Y, Morrison KC, Choi PH, Pardue MT, Stein DG. Progesterone treatment shows greater protection in brain vs. retina in a rat model of Middle cerebral artery occlusion: progesterone receptor levels may play an important role. Restor Neurol Neurosci 2016; 34:947–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xiao J, Zhou X, Jiang T, Zhi ZN, Li Q, Qu J, Chen JG. Unilateral cerebral ischemia inhibits optomotor responses of the ipsilateral eye in mice. J Integr Neurosci 2012; 11:193–200 [DOI] [PubMed] [Google Scholar]
- 74.Chen XL, Zhang GP, Guo SL, Ding JQ, Lin JJ, Yang Q, Li ZY. Mfn2-mediated preservation of mitochondrial function contributes to the protective effects of BHAPI in response to ischemia. J Mol Neurosci 2017; 63:267–74 [DOI] [PubMed] [Google Scholar]
- 75.Shi Y, Yi C, Li X, Wang J, Zhou F, Chen X. Overexpression of Mitofusin2 decreased the reactive astrocytes proliferation in vitro induced by oxygen-glucose deprivation/reoxygenation. Neurosci Lett 2017; 639:68–73 [DOI] [PubMed] [Google Scholar]
- 76.Flippo KH, Gnanasekaran A, Perkins GA, Ajmal A, Merrill RA, Dickey AS, Taylor SS, McKnight GS, Chauhan AK, Usachev YM, Strack S. AKAP1 protects from cerebral ischemic stroke by inhibiting Drp1-dependent mitochondrial fission. J Neurosci 2018; 38:8233–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zuo W, Zhang S, Xia CY, Guo XF, He WB, Chen NH. Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology 2014; 86:103–15 [DOI] [PubMed] [Google Scholar]
- 78.Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci 2008; 1147:395–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stöckler-Ipsiroglu G, Wallimann T, Priller J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J Cereb Blood Flow Metab 2007; 27:452–9 [DOI] [PubMed] [Google Scholar]
- 80.Kitzenberg D, Colgan SP, Glover LE. Creatine kinase in ischemic and inflammatory disorders. Clin Transl Med 2016; 5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, Hess DC, Kondziolka D. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med 2008; 3:249–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke 2009; 40:S146–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Borlongan CV. Concise review: stem cell therapy for stroke patients: are we there yet? Stem Cells Transl Med 2019; 8:983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chopp M, Steinberg GK, Kondziolka D, Lu M, Bliss TM, Li Y, Hess DC, Borlongan CV. Who's in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant 2009; 18:691–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke 2015; 46:588–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chau M, Zhang J, Wei L, Yu SP. Regeneration after stroke: stem cell transplantation and trophic factors. Brain Circ 2016; 2:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nguyen H, Zarriello S, Coats A, Nelson C, Kingsbury C, Gorsky A, Rajani M, Neal EG, Borlongan CV. Stem cell therapy for neurological disorders: a focus on aging. Neurobiol Dis 2019; 126:85–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, Case C, Borlongan CV. Stem cell recruitment of newly formed host cells via a successful seduction? filling the gap between neurogenic niche and injured brain site. PLoS One 2013; 8:e74857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russo E, Napoli E, Borlongan CV. Healthy mitochondria for stroke cells. Brain Circ 2018; 4:95–8 [DOI] [PMC free article] [PubMed] [Google Scholar]