Short abstract
BTLA is a useful biomarker to characterize the immune states of sepsis patients. We investigated the association between genetic variations of BTLA and morbidity of sepsis and MODS in severe traumatic patient. Three tag single nucleotide polymorphisms of BTLA were genotyped in 562 severe trauma patients. To further elucidate the mechanism, mRNA stability, BTLA 3ʹ-UTR activity, and its expression on T lymphocytes were measured. Only rs1982809 which located in 3ʹ-UTR of BTLA showed a significant clinical relevance with the incidence rate of sepsis and MOD scores. The sepsis incidence and MOD score of rs1982809 CC genotype carriers were higher than TT carriers. The percentage of circulating BTLA + CD4 + CD3 + T lymphocytes was markedly lower in CC genotype carriers. Luciferase activity in plasmids containing C allele was lower than that of T allele. Thus, the differential expression of BTLA on T lymphocytes might be caused by the different 3ʹ-UTR activity induced by rs1982809 T/C. Therefore, rs1982809 is a useful clinical biomarker in the prognosis evaluating of sepsis and subsequent MODS. Moreover, it is also a functional single nucleotide polymorphism affecting the activity of BTLA 3ʹ-UTR and the expression of BTLA in peripheral blood T lymphocytes.
Impact statement
This work is useful in the field of genetic mechanism of severe post-traumatic complications, as it provides important evidence for the influence of BTLA gene polymorphism on sepsis and MODS susceptibility. The results are useful and of importance because rs1982809 is a useful clinical biomarker in the prognosis evaluating of sepsis and subsequent MODS. It is also a functional single nucleotide polymorphism affecting the activity of BTLA 3ʹ-UTR and the expression of BTLA in peripheral blood T lymphocytes.
Keywords: B and T lymphocyte attenuator, gene polymorphisms, trauma, sepsis, multiple organ dysfunction
Introduction
Traffic accidents, disasters, and terrorist incidents are the main causes of severe trauma, leading to disability and death among young people. Each year, traumatic injuries lead to more than five million deaths. Although the majority of patients with major trauma survive their injuries due to the great improvements in emergency care systems, they still encounter life-threatening complications including sepsis and multiple organ dysfunction syndrome (MODS).1,2 Sepsis is recently recognized as a global health priority by a WHO resolution.3 Based on a systematic review extrapolated results from global population, more than 19 million individuals suffer from sepsis and 6 million died annually.4 Prevention and treatment of sepsis and subsequent MODS are therefore very important. Single nucleotide polymorphism (SNP) is a key determinant of susceptibility and prognosis of infectious and non-infectious diseases.5 Increasing evidence shows that genetic factors play a key role in post-traumatic complications.6
B and T lymphocyte attenuator (BTLA) is a costimulatory molecule of CD28 superfamily. It has the similar structure and function to programmed death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4). It has been proved to inhibit the activation and proliferation of T lymphocyte.7–9 BTLA regulates T cell response negatively by recruiting SHP-1 and SHP-2 phosphatase.9 BTLA also participates in the reduction of pro-survival signaling of CD4 + T cells and plays an important role in innate immune immunity.10–12 Compared with wild-type, BTLA knockout mice showed strongly attenuated malaria infection and early clearance of infection, suggesting that co-inhibitory receptor BTLA played a key role in experimental malaria and weakened congenital and subsequent adaptive immune responses.13 Because of its ability to inhibit CD8 and CD4 T cell proliferation, differentiation, and/or activation, BTLA has also been implicated in contributing to many diseases, including certain kinds of cancer and some autoimmune diseases14–16 In addition to inhibiting the adaptive immune response, BTLA has also been indicated to affect the innate immune response and immune inflammatory response.17
T-lymphocyte dysfunction may lead to sepsis-related mortality and morbidity. In intensive care unit, the BTLA + CD4 + lymphocytes percentage in peripheral blood in patients with sepsis was significantly higher than that in patients without sepsis, which was related to subsequent secondary infection.18 It is reported that the BTLA + CD4 + CD3 + T lymphocytes’ percentage in healthy individuals is higher than that in severe sepsis patients.19 In addition, the BTLA + CD4 + CD3 + T lymphocytes percentage in the initial stage of sepsis is low, which is correlated to the severity and mortality of septic patients.19 BTLA can be used as a useful biomarker to predict the progress and prognosis of sepsis.20
In order to comprehensively evaluate the relationship between common gene mutations in BTLA gene and predisposition to sepsis and MODS, a group of tag single nucleotide polymorphisms (tagSNPs) were selected in BTLA gene. We studied the tagSNPs’ clinical correlation with sepsis and the MODS score incidence in severe traumatic patients. Our results showed that rs1982809 is clinically relevant to sepsis and MODS. We further demonstrated that the T to C mutation in rs1982809 influenced the activity of the BTLA 3ʹ-untranslated region (3ʹ-UTR), thus altering the transcription and translation of BTLA in peripheral blood T lymphocyte.
Materials and methods
Study population
Five hundred sixty-two uncorrelated severe traumatic patients were included in this research, they were all Han population from Guizhou province, southwestern China, and they were hospitalized in the Trauma Centre, the Affiliated Hospital of Guizhou Medical University during 1 January 2011 and 1 January 2017. The patients met the following three inclusion rules were included in the study: (1) 18–60 years old, (2) the injury severity score (ISS) ≥16 with at least one ISS ≥4 trauma and more than one additional ISS ≥3 injury to other parts of the body, (3) post-traumatic survival time ≥48 h. According to the established injury severity score and the simplified injury scale (AIS, latest edition 2005), the overall injury severity was classified to determine the severity of individual injuries.21 The exclusion criterion were: (1) rejected by the patients or their family members, (2) patients who had preexisting cardiovascular, respiratory, hepatic, renal, immunologic, or hematologic diseases, (3) a doctor’s advice to ambulatorily treat the patient or transfer within 24 hours after being hospitalized, and (4) unable to follow up. All protocols of this research got approval from the Ethical and Protocol Review Committee of Guizhou Medical University, Guiyang, China. Informed consent of patients and their near kin was obtained. Patients are kept confidential in accordance with the Guidelines for Human Subjects. Blood samples were obtained less than 24 h of admission.
The diagnostic criteria for sepsis complied with the criteria recommended by the third international consensus definitions for sepsis.22 MOD scores mean the total of the individual organ scores got at the same time in each hospitalization day.23 Briefly, six organ dysfunctions (pulmonary, renal, hepatic, neurologic, cardiac and hematologic) were scored from zero (no dysfunction) to four points (severe dysfunction) every day. The Marshall score ranged from 0 to 4 and the total score from 0 to 24. Failure of organ function was considered as three or more points. Daily laboratory and physiological values were used to calculate both scores. The definition of MODS is a Marshall – score of four or longer than two consecutive days. The calculating method of MODS scores is the total of individual organ scores obtained simultaneously per hospitalization day.
TagSNPs selection and genotyping
The BTLA gene of human is situated on chromosome 3, position 112,463,966–112,499,702. We included the BTLA gene exons and introns as well as 3000 bp (base pair) upstream of the transcription initiation site and 3000 bp downstream of the termination codon. Genetic variation data were obtained from a healthy Chinese Han Beijing (CHB) population from HapMap (www.hapmap.org). In total, we identified 21 SNPs with minor allele frequency (MAF) ≥0.05 in the CHB population from this database (Table 1). Haplotype blocks were constructed by Haploview software sets (Broad Institute of MIT and Harvard, Cambridge, MA). Based on genotyping data, the software package can calculate linkage disequilibrium (LD) and population haplotype.24 Tag SNPs were selected by Tagster (Version 2.0.2).25 Genotyping was achieved by an improved multiplex ligation detection reaction (iMLDR) technique.26 Without knowing the clinical data of the patients, genotyping was carried out by blind method; 20% of the samples were genotyped by direct sequencing to ensure the accuracy of iMLDR technique.
Table 1.
SNPs identified within the entire BTLA gene in Chinese Han population.
| rs number | Variation | MAF | Region |
|---|---|---|---|
| rs1982809 (Tag SNP) | T/C | 0.244 | 3ʹUTR |
| rs2171513 | C/T | 0.198 | 3ʹUTR |
| rs9288952 (Tag SNP) | A/G | 0.256 | Exon |
| rs2705544 | G/T | 0.198 | Intron |
| rs6438079 | A/G | 0.244 | Intron |
| rs2633562 | A/G | 0.179 | Intron |
| rs1386895 | A/G | 0.233 | Intron |
| rs4682410 | A/G | 0.244 | Intron |
| rs12632867 | C/G | 0.244 | Intron |
| rs9288953 (Tag SNP) | T/C | 0.456 | Intron |
| rs2705534 | G/T | 0.233 | Intron |
| rs2705535 | C/T | 0.202 | Intron |
| rs2952323 | C/G | 0.25 | Intron |
| rs2971204 | G/T | 0.211 | Intron |
| rs1844089 | C/T | 0.244 | Intron |
| rs2705565 | C/T | 0.198 | 5ʹUTR |
| rs2633582 | G/T | 0.244 | 5ʹUTR |
| rs2633580 | C/G | 0.233 | 5ʹUTR |
| rs2633579 | A/G | 0.211 | 5ʹUTR |
| rs2705566 | A/G | 0.264 | 5ʹUTR |
| rs2633578 | T/C | 0.232 | 5ʹUTR |
SNP: single nucleotide polymorphism; BTLA: B and T lymphocyte attenuator.
BTLA mRNA stability assay
To determine if the location of the SNPs in the 3ʹ-UTR has effect on the mRNA stability,27,28 we investigated the stability of BTLA mRNA in 10 rs1982809 TT-carrier patients and 10 rs1982809 CC-carrier patients. Actinomycin D (Sigma), a transcriptional inhibitor, was employed. Two milliliters of peripheral blood from each patient was mixed with 2 µg/mL actinomycin D uniformity to restrain trans splicing and RNA synthesis.29 Using QIAGEN RNeasy® Plus Kit (ID/Cat No.: 74136) and referencing the manufacturer’s instructions to extracting total RNA and its decay were analyzed by RT-qPCR. Primer pairs for BTLA (forward (F): TGGGTCATACCGCTGTTCTGCA, reverse (R): CTGCTTGCCATTTCGTCCTTGG), GAPDH (F: AGAAGGCTGGGGCTCATTTG, R: AGGGGCCATCCACAGTCTTC) were designed for amplifying different sequences at the 3ʹ-UTRs of each transcript. Using the iScript™ cDNA Synthesis kit (Cat: 1708890, Bio-Rad), first-strand cDNA was retrieved from 1 µg of RNA; 20 µl (microliters) reactions were provided by mixing together reverse transcriptase, gene-specific assay pool (20×, 2 µM), gene-specific enhancer solution, iScript Select reaction mix and RNA that diluted in RNase-free water in 1:1:2:4:12 ratio. Quantitative real-time PCR (qPCR) was accomplished by primers, synthesized cDNA and SsoFast™ EvaGreen® Supermix (#172–5204, Bio-Rad). Half-life (t1/2) of mRNA was obtained based on the equation (t1/2)= ln(2)/kdecay, in which kdecay is the mRNA decay constant.30 The rate constant of the decay of RNA equals the slope of the semi-logarithmic map of the change of the concentration of RNA with time. It is determined by the non-linear regression (least squares) analysis of three bioreplicated specimens, which adopt the first-order decay model (GraphPad Prism 6.0).
Flow cytometry analysis
Peripheral blood samples were transported to the lab within 1 h at 4 °C in an EDTA anticoagulant tube. Before using the peripheral blood for flow cytometric analysis or for in vitro assays, red blood cells were removed by lysis buffer (Thermo Fisher Scientific, cat. no. 00–4333). Then the cells were washed and the cell suspension was diluted to a density of 1–5 × 106 cells/mL in 4 °C PBS, 10% of FCS and 1% of sodium azide. After adding the conjugated primary antibody (1 µg/mL) and incubating it for 30 min in dark at 4 °C, the cells were washed again three times and centrifuged at 400 × g for 5 min and then resuspended in 500–1000 µL 4 °C PBS with FCS and sodium azide. Antibodies and their isotype controls: APC Mouse Anti-Human BTLA (BD Bioscience, San Jose, CA, USA), Isotype: APC Mouse IgG2a, κ Isotype Control (BD Bioscience); PE-labeled anti-CD4 (BD Bioscience), Isotype: PE Mouse IgG1, κ Isotype Control (BD Bioscience); FITC-labeled anti-CD3 (BD Bioscience), and Isotype: FITC Mouse IgG2a, κ Isotype Control (BD Bioscience). Samples were carried on a BD Accuri™ Flow Cytometer C6 (BD Biosciences, Inc.). More than 5 × 103 CD4 + T lymphocytes were analyzed in each sample.
Plasmid construction and detection of 3ʹ-UTR activity
The full-length 1022-base pair BTLA 3ʹ-untranslated region (3ʹ-UTR) was synthesized from genomic DNA PCR amplification, which was collected from a subject homozygous with the rs1982809 C allele. The primer sequences were 5ʹ-GCTCTCGAGTCCCGTCCCAGTAGTTGAAGGGTCCT GCTA-3′ (F) and 5′-TGCCAGCTGCTTCGAGCGAAGCC ATTGCTGC-3′ (R). XhoI as well as SalI restriction sites were introduced by PCR. After digestion with the XhoI and SalI restriction enzymes, the PCR products were directly inserted into a 3ʹ-UTR sequence with the luciferase gene in the pmirGLO vector. The pmirGLO BTLA/3’-UTR rs1982809C plasmid was constructed by the site-directed mutagenesis kit (Invitrogen, Carlsbad, CA). The mutation primers are 5ʹ-CTGACACAcAAAAGCGACCCGAATAACTTGCT -3ʹ (F) and 5ʹ-TCGCTTTTgTGTGTCAGGGGGTTTAAGAATCAG -3ʹ (R).
Human leukemia monocytic cell line, and THP-1 cells (ATCC, Cat#TIB-202) were incubated for 48 h and then transfected with the pmirGLO BTLA/3ʹ-UTR rs1982809T or pmirGLO BTLA/3ʹ-UTR rs1982809C plasmids using the Lipofectamine® 3000 (Invitrogen, California). Briefly, the Lipofectamine® 3000 Reagent was diluted and mixed with Opti-MEM Medium; 5 µg DNA was diluted in 250 µL Opti-MEM Medium and 10 µL P3000 Reagent was added for preparing the master mix of DNA. Then the added master mix of DNA was transferred to each tube with diluted Lipofectamine® 3000 Reagent (mix in a ratio of 1:1) and incubated the mixture for 15 min at ordinary temperature and then the DNA-lipid complex was added to the cells. Finally, the cells were incubated for 48–96 h at 37 °C. According to a standard protocol on a Varioskan Lux (Thermo Scientific, Helsinki, Finland), luciferase activity of transfected cells was analyzed by the Dual-Luciferase® Reporter Assay System (E1910, Promega) at the end of each experiment. Luminescence experiments were carried out more than three times, with three copies of each transfection, using six DNA preparations separately. The transfection efficiency was standardized based on testing the luciferase activity of the control pmirGLO plasmid.
Data analysis
All statistical data were analyzed by SPSS software version 19.0 (SPSS Company, Chicago, IL, USA). Continuous data are expressed in mean ± standard deviation (SD) or interquartile ranges and median. Classified data are expressed in counts and percentages. Comparing variables by Chi-squared test/Fisher's exact test, while analyzing continuous variables by the Mann–Whitney U test/Student’s t test. Hardy–Weinberg equilibrium of each SNP was analyzed by Chi-square tests. Calculating the odds ratio (OR) of 95% confidence interval by multivariate logistic regression model. It was tested that recessive, dominant, as well as allele dose models were adjusted according to the mixed variables of site of injury, ISS, sex ratio and age. Bilateral P below 0.05 was considered significant.
Results
Overall clinical characteristic of major trauma patients
Patients’ basic data are shown in Table 2. The patients’ average age was 42.9 ± 12.5. The sepsis morbidity rate was 36.8%. In the study cohort, the median time for the occurrence of sepsis was 6.0 days, the quartile range was 5.0 to 8.0 days; 65.9% of the patients had organ dysfunction, of which 129 (23.0%) and 86 (15.3%) had two or more organ dysfunction. Among MODS patients, sepsis occurrence accounted for 43.8%.The median onset time of MODS was 9.0 days in septic patients, and the interquartile range was 7.5 to 11.0 days. The median onset time of MODS was 6.0 days in non-septic patients, and the interquartile range was 4.0 to 7.0 days. The mainly injured sites were the head (40.9%), thorax (35.8%), abdomen (29.7%), and extremities (40.7%). Most patients had multiple injuries, such as two injury sites (44.8%), three injury sites (24.2%), and four injury sites (6.0%).
Table 2.
Overall clinical characteristics of patients with major trauma.
| Guizhou (n = 562) | |
|---|---|
| Age (yrs) | 42.9 ± 12.5 (19–61) |
| Male/female, n | 456/106 |
| Injured body regions, n (%) | |
| Head | 230 (40.9) |
| Thorax | 201 (35.8) |
| Abdomen | 167 (29.7) |
| Extremities | 229 (40.7) |
| Number of regions injured, n (%) | |
| Two | 252 (44.8) |
| Three | 136 (24.2) |
| Four | 34 (6.0) |
| ISS, n | 26.1±7.3 |
| ≥16, <25, n (%) | 351 (62.5) |
| ≥25, n (%) | 211 (37.5) |
| Organ dysfunction, n (%) | |
| One | 155 (27.6) |
| Two | 129 (23.0) |
| Three or above | 86 (15.3) |
| Sepsis, n (%) | 207 (36.8) |
ISS: injury severity score; n: number; yrs: years.
Tag SNP selection and genotype distribution
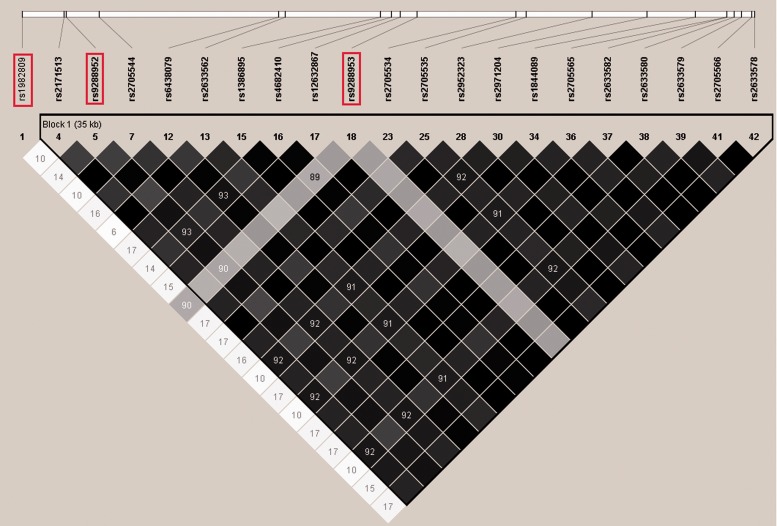
In CHB population, there were 21 SNPs in the BTLA gene, with MAF greater than or equal to 5% (www.hapmap.org) (Table 1), which constructed three haplotype blocks (Figure 1). Using Tagster, three tag SNPs were selected for genotyping (Figure 1). The MAF of rs1982809, rs9288952, and rs9288953 in traumatic patients was consistent with those of CHB population (data from HapMap data base). As they were in accordance with Hardy–Weinberg equilibrium (P > 0.05), the allele frequencies and genotype frequencies of the three variants remained constant in CHB population.
Figure 1.
Overview of selected htSNPs. The location of the 21 SNPs within the BTLA gene and 3 kb up- and downstream regions with a minor allele frequency ≥ 5%. The selected three htSNPs are indicated by boxes. A linkage disequilibrium (LD) plot of the 21 SNPs is displayed in an r2-black and white color scheme. Black represents very high LD (r2 = 1) and white indicates the absence of correlation (r2 =0) between SNPs.
Clinical significance of rs1982809 in traumatic patients
Among patients with different rs1982809, rs9288952, and rs9288953 genotypes, there were no significant differences of their ISS, sex ratio, and age (Table 3). In the three tagSNPs, the sepsis morbidity of rs1982809 C allele carriers was significantly higher than T allele carriers (P = 0.005). Meanwhile, rs1982809 C allele carriers had higher MOD scores (dominant effect: P = 0.011, recessive effect: P = 0.003). Regression analysis showed that there was no significant relation between other two tagSNPs and sepsis or MODS.
Table 3.
Clinical relevance of the 3 htSNPs among trauma patients in Guizhou.
| SNPs | Genotypes | N | Age(yr) | Gender (M/F) | ISS | Sepsis, n/% | MOD score |
|---|---|---|---|---|---|---|---|
| rs1982809 | TT | 307 | 41.9±16.7 | 231/76 | 25.2±7.3 | 97 (31.6) | 5.7±2.2 |
| TC | 206 | 41.8±15.7 | 185/21 | 26.2±8.5 | 82 (39.8) | 6.8±2.6 | |
| CC | 49 | 42.1±16.2 | 40/9 | 28.2±8.3 | 28 (57.1)a1 | 7.5±2.6a2,b1,c1 | |
| AA | 294 | 42.8±11.8 | 244/50 | 27.8±7.9 | 102 (34.7) | 5.4±2.5 | |
| rs9288952 | AG | 236 | 43.5±12.3 | 190/46 | 26.1±8.2 | 93 (39.4) | 6.8±2.8 |
| GG | 32 | 43.2±16.7 | 22/10 | 25.7±9.0 | 12 (37.5) | 6.4±2.8 | |
| TT | 164 | 43.5±11.3 | 137/27 | 25.7±7.8 | 62 (37.8) | 6.5±3.2 | |
| rs9288953 | TC | 263 | 42.6±12.4 | 205/58 | 25.9±8.3 | 103 (39.2) | 6.8±2.7 |
| CC | 135 | 43.7±15.4 | 114/21 | 26.5±7.7 | 42 (31.1) | 6.0±2.3 |
a1P = 0.005, a2P = 0.011 for dominant association (CC + TC vs. TT).
b1P = 0.003 for recessive association (CC vs. TT + TC).
c1P = 0.005 for allele dose association.
ISS: injury severity score; MOD: multiple organ dysfunction; N: number; M/F: male/female; SNP: single nucleotide polyorphism.
Effect of T to C mutation for rs1982809 on BTLA mRNA stability and the post-transcriptional regulation of the 3ʹ-untranslated regions
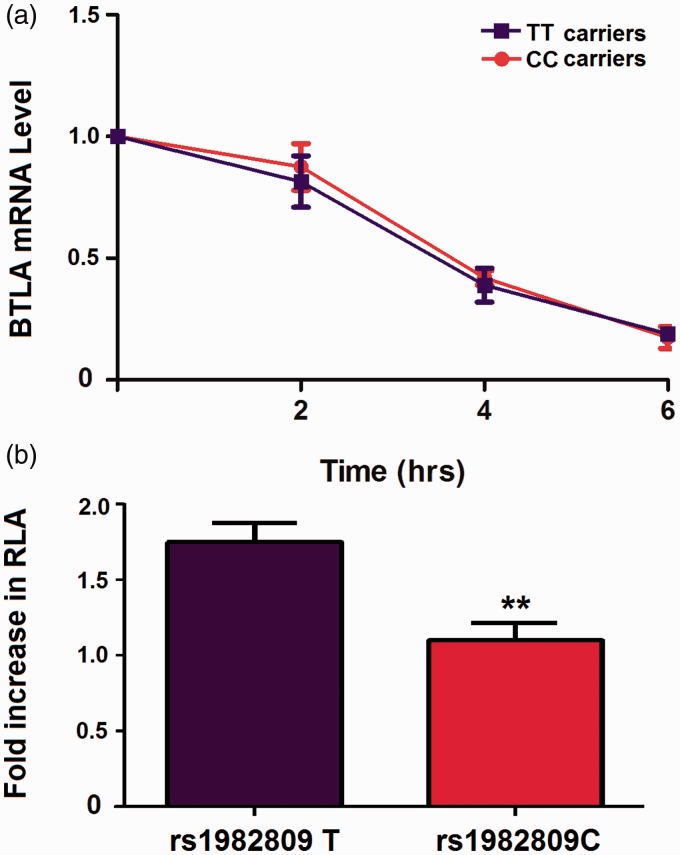
To determine if location of rs1982809 in BTLA 3ʹ-UTR can affect the stability of mRNA or mRNA translation efficiency, we first investigated the stability of BTLA mRNA in ten rs1982809 TT-carrier patients and 10 rs1982809 CC-carrier patients. Transcriptional level of BTLA was detected by RT-PCR in each blood sample. We used the peripheral blood volume corresponding to the same transcriptional level of BTLA to investigate the mRNA stability. Blood samples were treated with actinomycin D, an inhibitor of mRNA synthesis. BTLA transcript levels were quantified by RT-qPCR at 0, 2, 4, and 6 h. The percentage of mRNA that remained was plotted (Figure 2). There were no differences in the level of BTLA mRNA between the TT-carriers and the CC-carriers at 2, 4, and 6 h. Therefore, the mutation of the T to C in rs1982809 does not affect BTLA mRNA stability.
Figure 2.
Effect of T to C mutation for rs1982809 on BTLA mRNA stability and the activity of 3ʹ-UTR. (a) Transcriptional level of BTLA in peripheral blood of 10 rs1982809 CC-carrier patients and 10 rs1982809 TT-carrier patients was detected by RT-PCR. The peripheral blood volume was used corresponding to the same transcriptional level of BTLA to investigate the mRNA stability. Peripheral blood was treated with actinomycin D. Samples were removed at 2, 4, and 6 h. The values correspond to the mean ± SD. There were no differences in the BTLA mRNA levels between the CC-carrier patients and the TT-carrier patients (P = 0.86). (b) The luciferase activity of the transfected THP-1 was measured using a luciferase assay system. The relative luciferase activity (RLA) was significantly lower in the cells transfected with the construct containing the C allele than the cells transfected with the construct containing the T allele (**P < 0.01).
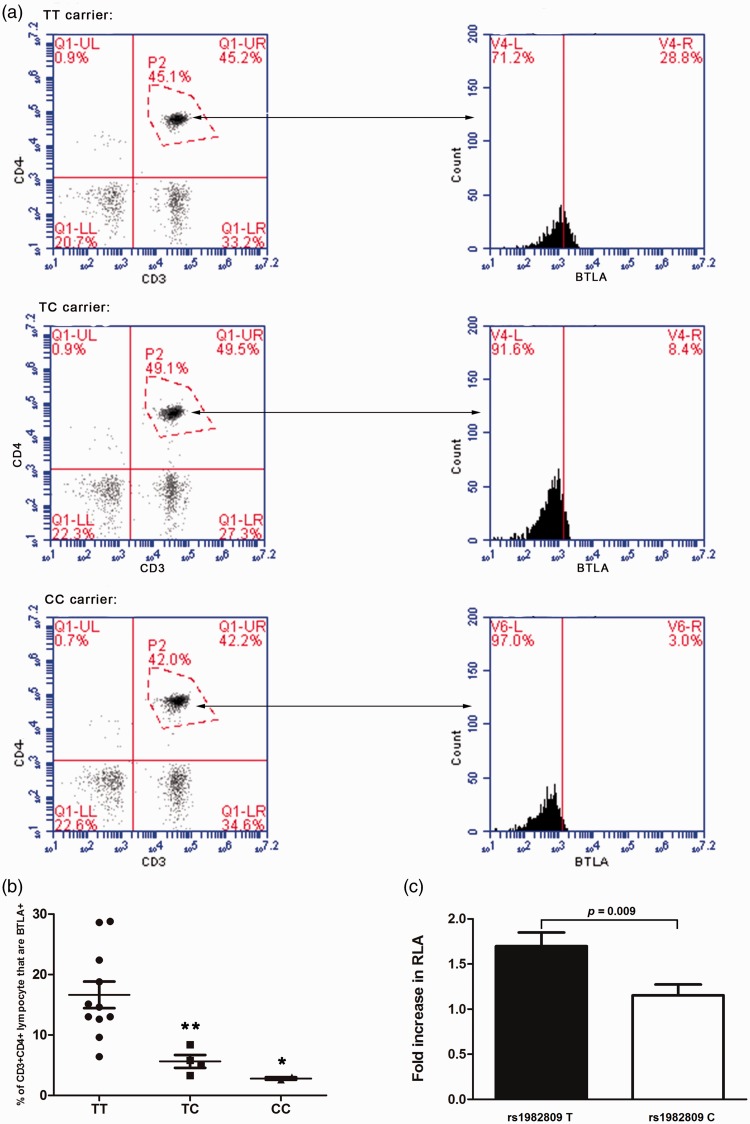
By the insertion of the full length sequence of BTLA rs1982809 T-3ʹ-UTR or rs1982809 C-3ʹ-UTR into a pmirGLO-vector, we elucidated the direct effect of rs1982809 T to C mutation on post-transcriptional regulation of BTLA 3ʹ-UTR. The activity of luciferases in the transfected THP-1 was detected by reporter gene assay. The results indicated that the luciferase activity in cells transfected with C alleles was significantly lower than that in cells transfected with T allele (P = 0.009, Figure 3(c)), which means that the mutation of the T to C in rs1982809 attenuated the activity of 3ʹ-UTR and the TT carriers may have higher expression of BTLA.
Figure 3.
rs1982809 affects BTLA 3ʹ-UTR activity and the expression of BTLA on T lymphocytes. (a) Representative flow dot plots of lymphocyte gating strategy and the percentage of BTLA on CD3+CD4+ T lymphocytes in the rs1982809 TT, TC, and CC carriers. (b) The percentage of BTLA on CD3+CD4+ T lymphocytes in the rs1982809 TT, TC and CC carriers. There was a stepwise reduction in the percentage of BTLA on CD3+CD4+ T lymphocytes from the TT, TC, and CC carriers (*P < 0.05, **P < 0.01). (c) The fluorescence intensity of BTLA+ on CD4+CD3+ T lymphocytes in peripheral blood from TT carriers, TC carriers, and CC carriers (*P < 0.05, **P < 0.01).
T to C mutation in rs1982809 affects the expression of BTLA on T lymphocytes and its molecular mechanism
As T lymphocyte is the main immunoregulatory cell in sepsis, we detected the expression of BTLA in peripheral blood T lymphocyte of traumatic patients by flow cytometry assay. The results showed that the circulating BTLA + CD4 + CD3 + T lymphocytes’ percentage was significantly lower in rs1982809 CC carriers than TT carriers (Figure 3(a)). The BTLA + CD4 + CD3 + T lymphocytes’ percentage in peripheral blood from TT carriers, TC carriers, and CC carriers was 34.53% ± 7.14%, 8.98% ± 3.32% and 3.65% ± 1.43% (Figure 3(b)). By flow cytometry assay, the fluorescence intensity of BTLA + on CD4 + CD3 + T lymphocytes was also detected. The results indicated that the fluorescence intensity was also higher in TT carriers than CC carriers. The mean fluorescence intensity in peripheral blood from TT carriers, TC carriers, and CC carriers was 1973.8 ± 115.4, 1680.7 ± 207.8 and 934.3 ± 182.0 (Figure 3(c)).
Discussion
BTLA, CTLA-4, and PD-1 are all coinhibitory receptors which can regulate T cell activation. Moreover, soluble BTLA (sBTLA) can be used as a dynamic prognostic indicator of sepsis and sepsis-induced immunosuppression.18,31 BTLA gene polymorphisms are reported to be associated with many diseases, especially tumors and autoimmune disease, such as colorectal cancer, lymphoblastic leukemia, rheumatoid arthritis, and breast cancer. rs1982809 has also been found to be associated with chronic lymphocytic leukemia (CLL) and renal cell carcinoma (RCC).32,33 Karabon et al.32 reported that rs1982809 is associated with the level of mRNA expression in CLL patients and confers susceptibility to CLL. Another study investigated the correlation between BTLA SNPs and predisposition to RCC. The study showed that rs1982809 of BTLA gene might be a biomaker for RCC.33
So far, our research is the first study to investigate the clinical correlation of BTLA gene variants and severe complications after major trauma. Rs1982809, rs9288952, and rs9288953 were identified as markers of the whole BTLA gene in Chinese Han population. The results indicated that among these tag SNPs, only the 3ʹ-UTR SNP rs1982809 indicated a strong clinical correlation, showing a high incidence of sepsis, MOD score, and strong immune inflammation in patients with C allele mutation. The 3ʹ-UTR is in charge of post-transcriptional regulation, such as the stability of mRNA, the translation of multiple transcripts, location of gene expression, translation efficiency, etc.34,35 Many researchers found that SNPs in the 3ʹ-UTR can affect the expression of target genes and is related to the development of disease.36,37 According to our research, the result of mRNA stability detection showed that the mutation of T to C in rs1982809 cannot affect BTLA mRNA stability. As T lymphocytes were the main effect cells in the immunological regulation of sepsis and the expression of BTLA on CD4 + T cells was associated with sepsis and secondary infection of patients in intensive care unit,18 we proposed that the mutation of rs1980809 can affect the expression of BTLA on lymphocytes. Thus, we detected BTLA expression on peripheral blood T lymphocytes from major trauma patients by flow cytometry assay. Our results showed that circulating BTLA + CD4 + CD3 + T lymphocytes percentage was significantly lower in rs1982809 CC carriers than TT carriers. To elucidate the mechanism, we constructed two plasmids containing either the rs1982809 T or C allele. By reporter gene analysis, we elucidated that the luciferase activity of cells transfected with C allele was significantly lower than that of cells transfected with T allele. Karabon et al.32 indicated that rs1982809C allele was associated with decreased expression of BTLA mRNA in T cells. According to our results, C carriers have higher BTLA promoter activity. This explains why G allele carriers (C allele carriers) have higher levels of RNA expression in T cells. C allele carriers in traumatic patients have a higher incidence of sepsis and a higher MOD score, which may be related to the differential expression of BTLA in T lymphocyte. Therefore, rs1982809 polymorphism is not only a useful clinical biomarker for evaluating the prognosis of sepsis and MODS, but also a functional SNP affecting the activity of BTLA 3'-UTR and the expression of BTLA in peripheral blood T lymphocytes.
Our research have some limitations. First, though the sample size is large enough, the association of rs1982809 with sepsis and MODS was detected only in the Guizhou population. Second, the treatment and care of traumatic patients can also affect the risk of sepsis and MODS. Despite the limited capacity associated with clinical association studies, our results demonstrate the functional significance of BTLA rs1982809 and reveal that it may be used to assess risk factors associated with sepsis and MODS in severe trauma patients.
ACKNOWLEDGMENTS
We thank Dalin Wen and Zhang Anqiang for their important contributions. D-LW and A-QZ were involved in the collection of clinical data and blood samples.
Authors’ contributions
Project administration, JXJ, LZ and JD; Writing-review & editing, LZ and JD; Investigation, LBG, CH and JLS; Writing-original draft, LBG; Methodology, CH and GXP; Resources, ZHD; Data curation, HXL.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval and consent to participate
The project was approved by the Ethics and Program Review Committee of Guizhou Medical University (GMU 2018064), and the informed consent of the patient or his close relatives was obtained.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Funds of China (81660317, 81571892), Project of Trauma, Burns and Combined Injury State Key Laboratory (SKLKF201802, no.SKLKF201502), the funding by the Military Medical University (2018XLC3057) and the “Miaopu” Talent Program.
References
- 1.Coimbra R, Fraga GP, Starling SV. World trauma congress: when dreams come true. World J Emerg Surg 2012; 7: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng L, Gu W, Zhang AQ, Zhang M, Zhang LY, Du DY, Huang SN, Jiang JX. A functional variant of lipopolysaccharide binding protein predisposes to sepsis and organ dysfunction in patients with major trauma. Ann Surg 2012; 255:147–57 [DOI] [PubMed] [Google Scholar]
- 3.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority – a WHO resolution. N Engl J Med 2017; 377:414–7 [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–72 [DOI] [PubMed] [Google Scholar]
- 5.Oliveira MB, de Vasconcellos JPC, Ananina G, Costa VP, de Melo MB. Association between IL1A and IL1B polymorphisms and primary open angle glaucoma in a Brazilian population. Exp Biol Med 2018; 243:1083–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu W, Jiang JX. Genetic polymorphisms and posttraumatic complications. Comp Funct Genomics 2010; 2010:814086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol 2003; 4:670–9 [DOI] [PubMed] [Google Scholar]
- 8.Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009; 229:114–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy KM, Nelson CA, Sedý JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol 2006; 6:671–81 [DOI] [PubMed] [Google Scholar]
- 10.Shui JW, Steinberg MW, Kronenberg M. Regulation of inflammation, autoimmunity, and infection immunity by HVEM-BTLA signaling. J Leukoc Biol 2011; 89:517–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4 + T cells. J Immunol 2005; 174:3377–85 [DOI] [PubMed] [Google Scholar]
- 12.Deppong C, Degnan JM, Murphy TL, Murphy KM, Green JM. B and T lymphocyte attenuator regulates T cell survival in the lung. J Immunol 2008; 181:2973–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler G, Steeg C, Pfeffer K, Murphy TL, Murphy KM, Langhorne J, Jacobs T. B and T lymphocyte attenuator restricts the protective immune response against experimental malaria. J Immunol 2011; 187:5310–9 [DOI] [PubMed] [Google Scholar]
- 14.Del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol 2010; 87:223–35 [DOI] [PubMed] [Google Scholar]
- 15.Paulos CM, June CH. Putting the brakes on BTLA in T cellmediated cancer immunotherapy. J Clin Invest 2010; 120:76–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Li J, He M, Zhang GL, Zhao Q. Distinct changes of BTLA and HVEM expressions in circulating CD4 + and CD8 + T cells in hepatocellular carcinoma patients. J Immunol Res 2018; 18:4561571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Brown NK, Ruddy MJ, Miller ML, Lee Y, Wang Y, Murphy KM, Pfeffer K, Chen L, Kaye J, Fu YX. B and T lymphocyte attenuator tempers early infection immunity. J Immunol 2009; 183:1946–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shubin NJ, Monaghan SF, Heffernan DS, Chung CS, Ayala A. B and T lymphocyte attenuator expression on CD4 + T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care 2013; 17:R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao R, Li CS, Fang Y, Zhao L, Hang C. Low B and T lymphocyte attenuator expression on CD4 + T cells in the early stage of sepsis is associated with the severity and mortality of septic patients: a prospective cohort study. Crit Care 2015; 19:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherwood ER, Hotchkiss RS. BTLA as a biomarker and mediator of sepsis induced immunosuppression. Crit Care 2013; 17:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 1974; 14:187–96 [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003; 31:1250–6 [DOI] [PubMed] [Google Scholar]
- 23.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med 1995; 23:1638–52 [DOI] [PubMed] [Google Scholar]
- 24.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet 2004; 74:106–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Kaplan NL, Taylor JA. TAGster: efficient selection of LD tag SNPs in single or multiple populations. Bioinformatics 2007; 23:3254–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai Q, Luo H, Li XP, Huang J, Zhou TJ, Yang ZH. XRCC1 and ERCC1 polymorphisms are related to susceptibility and survival of colorectal cancer in the Chinese population. Mutagenesis 2015; 30:441–9 [DOI] [PubMed] [Google Scholar]
- 27.Chen JM, Ferec C, Cooper DN. A systematic analysis of disease-associated variants in the 3’ regulatory regions of human protein-coding genes I: general principles and overview. Hum Genet 2006; 120:1–21 [DOI] [PubMed] [Google Scholar]
- 28.Pullmann R, Abdelmohsen K, Lal A, Martindale JL, Ladber RD, Gorospe M. Differential stability of thymidylate synthase 3’-untranslated region polymorphic variants regulated by AUF1. J Biol Chem 2006; 281:23456–63 [DOI] [PubMed] [Google Scholar]
- 29.Azevedo A, Toledo JS, Defina T, Pedrosa AL, Cruz AK. Leishmania major phosphoglycerate kinase transcript and protein stability contributes to differences in isoform expression levels. Exp Parasitol 2015; 159:222–6 [DOI] [PubMed] [Google Scholar]
- 30.Chen CY, Ezzeddine N, Shyu AB. Messenger RNA half-life measurements in mammalian cells. Meth Enzymol 2008; 448:335–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange A, Sunden-Cullberg J, Magnuson A, Hultgren O. Soluble B and T lymphocyte attenuator correlates to disease severity in sepsis and high levels are associated with an increased risk of mortality. PLoS One 2017; 12:e0169176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karabon L, Partyka A, Jasek M, Lech-Maranda E, Grzybowska-Izydorczyk O, Bojarska-Junak A, Pawlak-Adamska E, Tomkiewicz A, Robak T, Rolinski J, Frydecka I. Intragenic variations in BTLA gene influence mRNA expression of BTLA gene in chronic lymphocytic leukemia patients and confer susceptibility to chronic lymphocytic leukemia. Arch Immunol Ther Exp 2016; 64:137–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partyka A, Tupikowski K, Kolodziej A, Zdrojowy R, Halon A, Malkiewicz B, Dembowski J, Frydecka I, Karabon L. Association of 3’ nearby gene BTLA polymorphisms with the risk of renal cell carcinoma in the polish population. Urol Oncol 2016; 34:419.e13–9 [DOI] [PubMed] [Google Scholar]
- 34.Subramaniam K, Chen K, Joseph K, Raymond JR, Tholanikunnel BG. The 3ʹ-untranslated region of the beta2-adrenergic receptor mRNA regulates receptorsynthesis. J Biol Chem 2004; 279:27108–15 [DOI] [PubMed] [Google Scholar]
- 35.Izquierdo JM, Cuezva JM. Epigenetic regulation of the binding activity oftranslation inhibitory proteins that bind the 3ʹ untranslated region of beta-F1-ATPase mRNA by adenine nucleotides and the redox state. Arch Biochem Biophys 2005; 433:481–6 [DOI] [PubMed] [Google Scholar]
- 36.Doi K, Noiri E, Nakao A, Fujita T, Kobayashi S, Tokunaga K. Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J Am Soc Nephrol 2006; 17:823–30 [DOI] [PubMed] [Google Scholar]
- 37.Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Dujin CM, Witteman JC. ACE polymorphisms. Circ Res 2006; 98:1123–33 [DOI] [PubMed] [Google Scholar]