Significance
The primary source of vitamin D in man is production in the skin following exposure to sunlight or artificially produced ultraviolet (UV) light. Despite the importance of this source, the mechanisms surrounding the transport of cutaneous vitamin D into the circulation have not been clearly defined. We have examined the vitamin D response to UVB in mutant mice lacking vitamin D binding protein (DBP). We have shown that in the absence of DBP, UVB does not correct vitamin D deficiency. Further, injection of small amounts of recombinant DBP to DBP−/− mice restores the response to vitamin D produced in skin. These results demonstrate a requirement for DBP in the utilization of vitamin D produced in the epidermis by UV light.
Keywords: vitamin D, vitamin D binding protein, UV light
Abstract
Vitamin D is produced in the skin following exposure to sunlight. Ultraviolet (UV) B (UVB, 280–310 nm) results in isomerization of 7-dehydrocholesterol to previtamin D that spontaneously isomerizes to vitamin D. This pool of skin-derived vitamin D is the major source of vitamin D for animals. However, the mechanisms by which it becomes available remain undefined. It has been assumed that cutaneous vitamin D is transported into the circulation by vitamin D binding protein (DBP), but experimental evidence is lacking. To determine whether cutaneous vitamin D is transported by DBP, we utilized DBP−/− mice that were made vitamin D-deficient. These animals lack measurable 25(OH)D in blood and are hypocalcemic. As controls, DBP+/+ animals were vitamin D depleted and made equally hypocalcemic. UV irradiation of DBP+/+ animals restored serum calcium and serum 25(OH)D while the same treatment of DBP−/− animals failed to show either a serum calcium or 25(OH)D response despite having normal vitamin D production in skin. Intravenous injection of small amounts of recombinant DBP to the vitamin D-deficient DBP−/− mice restored the response to UV light. These results demonstrate a requirement for DBP to utilize cutaneously produced vitamin D.
The primary source of vitamin D in man is production in the skin following exposure to sunlight. Ultraviolet (UV) B (UVB, 280–310 nm) results in the conversion of 7-dehydrocholesterol to previtamin D, which isomerizes to vitamin D (1, 2). Biologically, vitamin D itself must undergo a 2-step activation pathway to produce the functional hormone, 1,25-dihydroxyvitamin D (1,25(OH)2D). The active hormone is a critical regulator of calcium and phosphorous homeostasis and may be involved in additional biological functions. Skin production of vitamin D is still considered the major source of vitamin D, even with the availability of a variety of synthetic vitamin D preparations and fortified foodstuffs (3, 4). Despite its established importance, little is known concerning the manner in which vitamin D becomes available from the epidermis.
Vitamin D binding protein (DBP), also known as GC-globulin, is the primary transport protein for vitamin D metabolites in the blood (5, 6). However, the important finding that DBP−/− mice are clearly responsive to orally administered vitamin D did not support an essential role of DBP in vitamin D metabolism and function (7, 8). Although the lack of DBP did not alter tissue distribution, uptake, metabolism, or biological potency of vitamin D, it did result in diminished levels of circulating 25-hydroxyvitamin D (25(OH)D) and 1,25(OH)2D (7, 8).
We have now discovered that DBP−/− mice are unable to respond to vitamin D produced in skin by UVB irradiation, while DBP+/+ mice clearly respond. Further, the intravenous (i.v.) injection of small amounts of recombinant DBP restores the systemic vitamin D response to UVB irradiation. These results clearly demonstrate the essential role of DBP in the utilization of vitamin D produced in skin by UVB irradiation and presumably sunlight.
Results
Generation of Vitamin D-Deficient Hypocalcemic Mice.
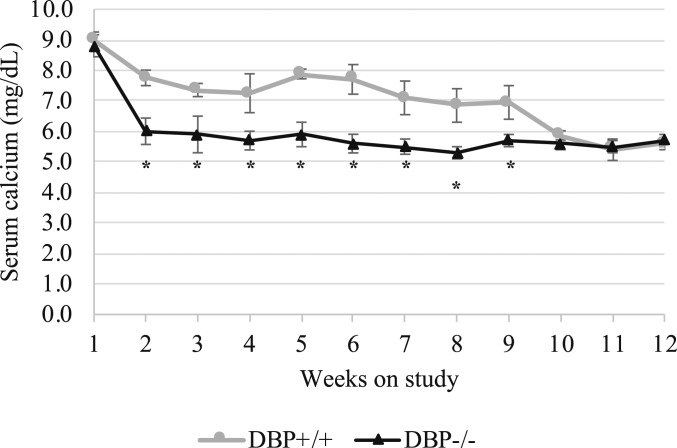
For all experiments, DBP+/+ and DBP−/− mice were depleted of vitamin D and made equally hypocalcemic after an 11-wk period of vitamin D depletion. They were fed an adequate (0.47% Ca) calcium, vitamin D-free diet for 1 wk followed by 3 wk on the same diet but containing no added calcium (0.02% Ca). DBP+/+ and DBP−/− mice were severely hypocalcemic when placed on a low calcium diet after repeating the above procedure once or twice, respectively (Fig. 1). That DBP−/− mice were more susceptible to vitamin D deficiency is consistent with a previous observation (8). As a result of their accelerated deficiency, they were maintained on the adequate calcium, vitamin D-free diet until DBP+/+ mice were equally hypocalcemic. Serum calcium at the end of this procedure was 5.6 ± 0.2 mg/dL for DBP+/+ mice and 5.5 ± 0.2 mg/dL for DBP−/− mice (n = 14, ± SEM). Serum 25(OH)D measurements taken at the same time were below detection (<4 ng/mL) for both groups, confirming vitamin D deficiency.
Fig. 1.
Serum calcium of mice during the depletion period. DBP+/+ and DBP−/− were equally hypocalcemic after a period of alternating dietary calcium with no dietary vitamin D. Mice were weaned at 3 wk of age and were placed on purified, vitamin D-free diets with alternating dietary calcium content. On study week 1, they were fed an adequate (0.47% Ca) calcium, vitamin D-free diet for 1 wk followed by 3 wk on the same diet but containing no added calcium (0.02% Ca). This procedure was repeated once for DBP−/− mice (weeks 1–8), after which they were maintained on the adequate calcium, vitamin D-free diet until week 12. The procedure was repeated twice for DBP+/+ mice in order to ensure mice reached the same level of hypocalcemia (weeks 1–12). Blood was collected for calcium analysis after 1 wk on each diet (n = 14, ± SEM, *P < 0.05 vs. DBP+/+).
UVB Exposure Failed to Rescue DBP−/− Mice from Vitamin D Deficiency.
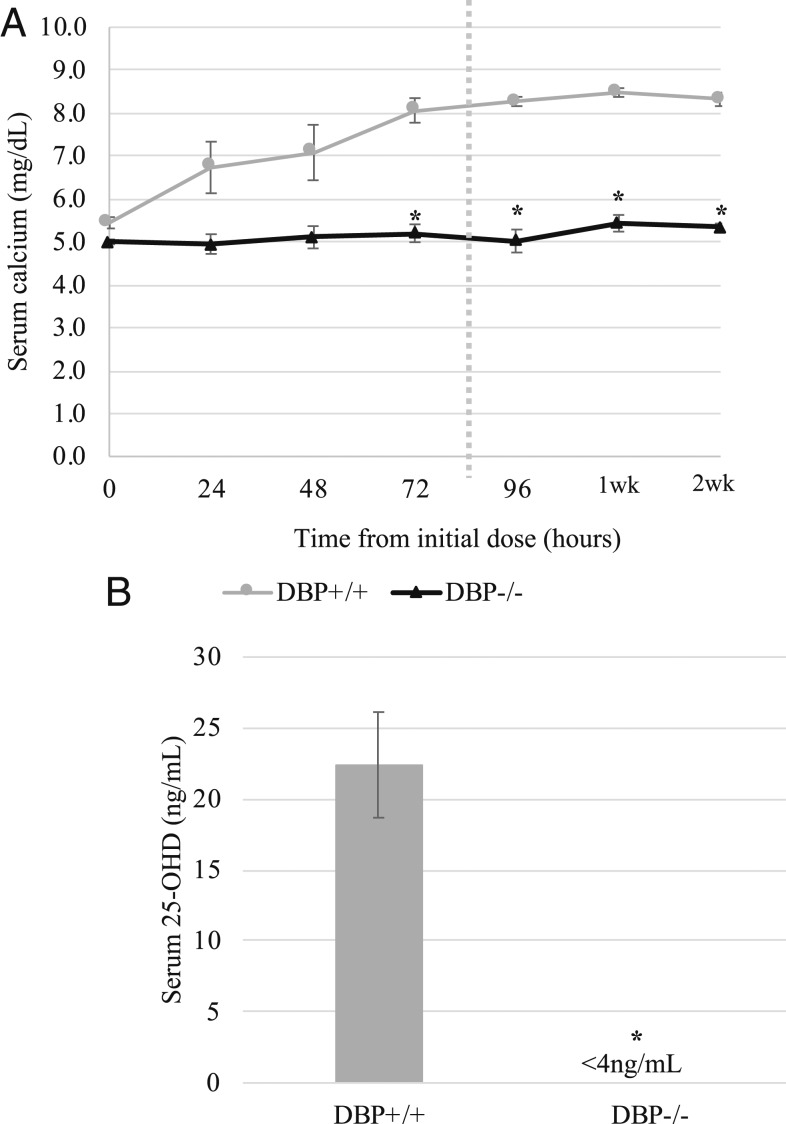
UVB treatment was successful at correcting hypocalcemia and restoring circulating 25(OH)D in vitamin D-deficient, hypocalcemic DBP+/+ mice, but not in DBP−/− mice. Repeated UVB exposure resulted in a steady increase in serum calcium levels in DBP+/+ mice, beginning within 24 h of the first UVB dose (Fig. 2A). After 4 daily treatments of 8 kJ UVB, a maximum tolerated dosing regimen, blood calcium levels had increased from 5.5 ± 0.2 mg/dL to near normal levels (8.4 ± 0.2 mg/dL). Serum calcium of the DBP−/− mice remained unchanged by UVB treatment throughout the duration of the experiment. To detect any potential delayed effect, serum calcium was monitored for a 2-wk period after cessation of UVB treatment but remained low in the DBP−/− mice while remaining high in the DBP+/+ mice (Fig. 2A). Circulating 25(OH)D measurements taken 24 h after the final UVB treatment were below detection (<4 ng/mL) in DBP−/− mice but were normal in the DBP+/+ mice (Fig. 2B).
Fig. 2.
UVB treatment failed to correct hypocalcemia and circulating 25(OH)D levels in vitamin D-deficient DBP−/− mice. Mice were made vitamin D-deficient and hypocalcemic as described under Experimental Mice. Animals were irradiated once daily for 4 d. Blood was collected for calcium analysis 24 h after each treatment. (A) Repeated UVB treatment normalized serum calcium in vitamin D-deficient DBP+/+ mice but had no effect on DBP−/− mice. Dotted line indicates end of UV treatment. One- and 2-wk timepoints represent serum calcium 1 wk and 2 wk following cessation of UVB treatment (n = 5 for DBP+/+ mice, n = 6 for DBP−/− mice, ± SEM, *P < 0.05 vs. DBP+/+). (B) At the end of the treatment period, serum 25(OH)D levels were undetectable in DBP−/− mice. Blood was collected for serum 25(OH)D measurement 24 h after the final treatment (n = 3 for DBP+/+ mice, n = 2 pools of 2 DBP −/− mice, ± SEM, *P < 0.05 vs. DBP+/+).
Vitamin D Is Not Mobilized from the Skin of DBP−/− Mice.
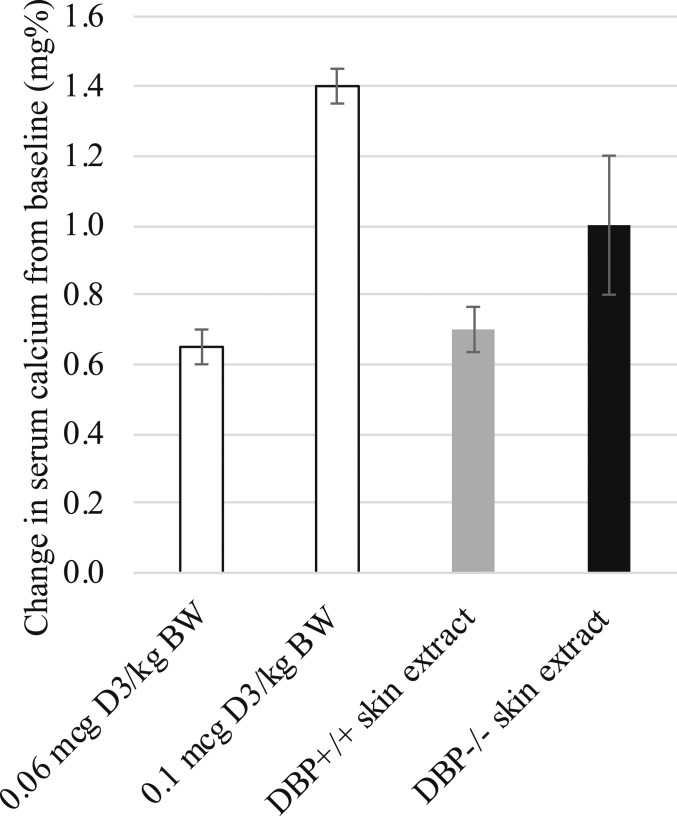
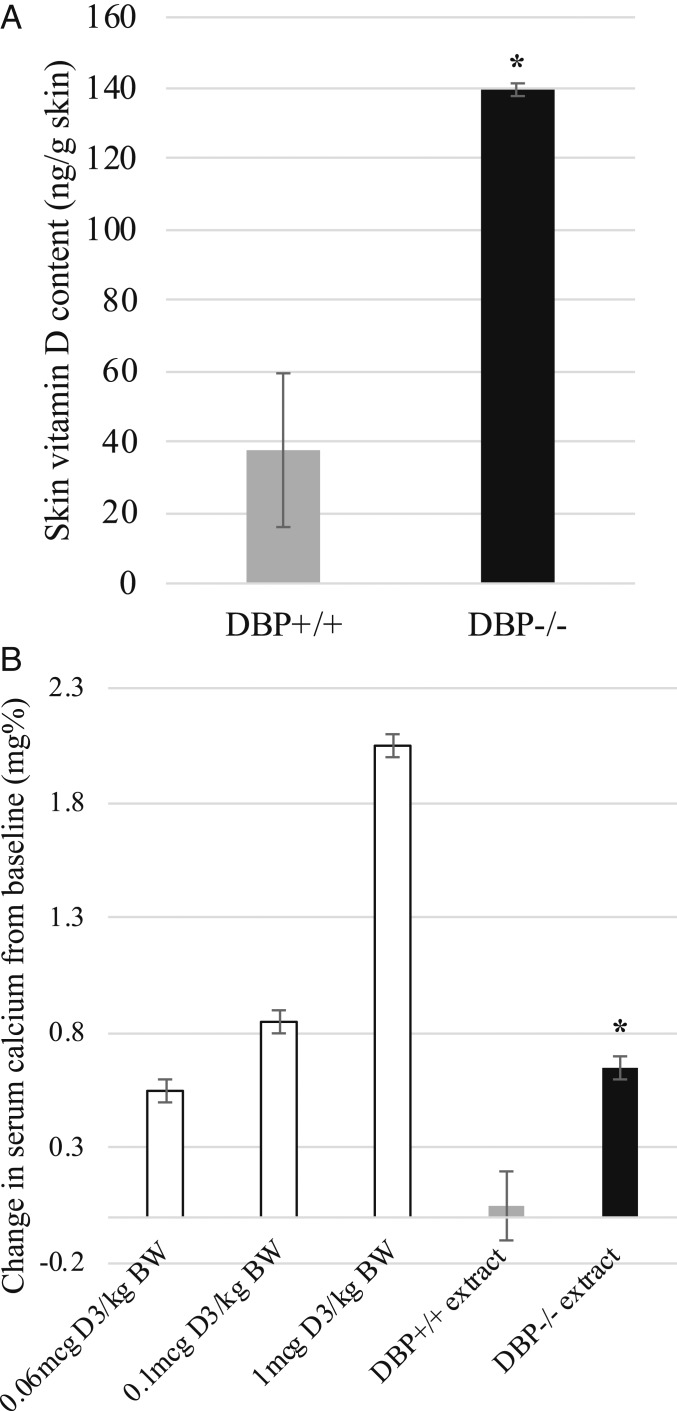
Vitamin D content of skin was analyzed at different timepoints following UVB exposure using a biological assay for vitamin D activity or measured by high-performance liquid chromatography (HPLC). Vitamin D-deficient, hypocalcemic test rats responded to intraperitoneal (i.p.)-administered skin lipid extracts obtained from DBP+/+ and DBP−/− mice immediately following UVB exposure with comparable increases in serum calcium. Based on this assessment, samples contained similar vitamin D activity, indicating that vitamin D production was normal in both DBP−/− and the DBP+/+ group (Fig. 3). HPLC analysis of skin lipid extracts obtained 48 h following the last of 4 daily treatments, a maximum tolerated dose regimen, revealed that skin vitamin D content decreased in the DBP+/+, but not in DBP−/− mouse skin (Fig. 4A). These samples were reanalyzed using a biological assay. Vitamin D activity detected in DBP−/− skin extract was similar to that detected immediately following the single UVB exposure, and no activity was detected in DBP+/+ skin extract (Fig. 4B).
Fig. 3.
Vitamin D-deficient rats responded to i.p. injection of skin lipid extracts from DBP−/− mice with comparable, if not greater, increases in serum calcium compared to those administered DBP+/+ mouse skin extracts. Skin was obtained from mice immediately following UVB irradiation. Isolated lipid extracts were administered daily for 3 d. Two control groups were included that were administered vitamin D in place of skin extracts. Blood was collected for calcium measurement 24 h prior to the first injection and 24 h after the final injection (n = 2 rats per experimental group, n = 2 mice per extract [0.6 g skin total], ± SEM).
Fig. 4.
Vitamin D content in skin was lower in DBP+/+ mice than in DBP−/− mice 48 h following 4 daily UVB treatments. (A) HPLC analysis of skin lipid extracts revealed vitamin D content was lower in DBP+/+ mice compared to DBP−/− mice (n = 3, ± SEM, *P < 0.05 vs. DBP+/+). (B) Serum calcium response of vitamin D-deficient rats administered either DBP−/− mouse skin extract or DBP+/+ skin extract. Vitamin D activity remained in the DBP−/− while in contrast, no vitamin D activity was detected in DBP+/+ mouse skin extracts. Three control groups were included that were administered vitamin D in place of skin extracts (n = 2 rats per experimental group, n = 2 mice per extract, ± SEM, *P < 0.05 vs. DBP+/+ extract).
Administration of DBP Protein to DBP−/− Mice Restores Responsiveness to Cutaneously Produced Vitamin D.
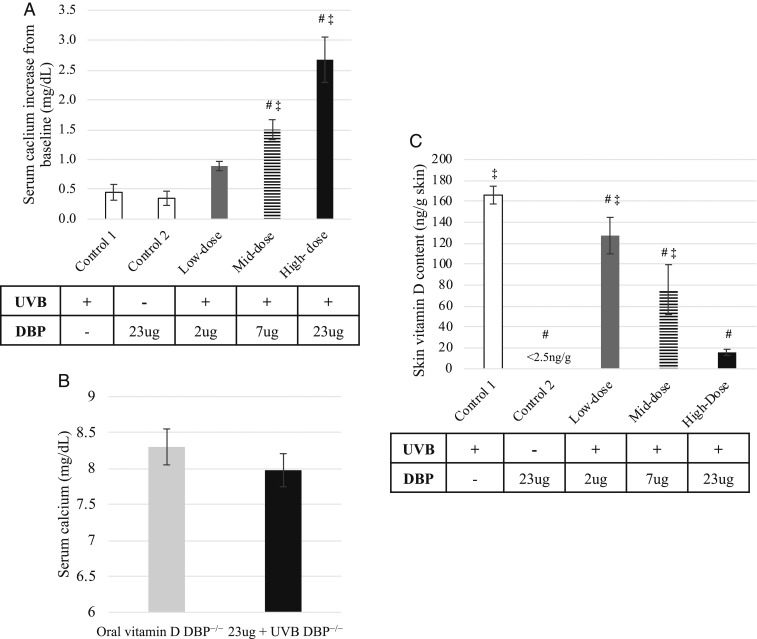
Intravenous injection of increasing amounts of recombinant mouse-DBP prior to UVB exposure of vitamin D-deficient, hypocalcemic DBP−/− mice resulted in corresponding increases in serum calcium. Two daily DBP injections and UVB treatments of DBP−/− mice resulted in an increase in serum calcium (Fig. 5A). No change in serum calcium was detected in the control group administered the highest amount of recombinant protein (23 µg) but not exposed to UVB or in the control group that received saline prior to UVB treatment. UVB combined with 7 µg of DBP prior to each treatment resulted in a 1.5 mg% increase in serum calcium after 2 UVB treatments, which is similar to that observed in DBP+/+ mice treated with UVB alone (Fig. 2A). Serum calcium was normalized in vitamin D-deficient DBP−/− mice injected with 23 µg of DBP after 2 UVB treatments compared to vitamin D (orally) sufficient DBP−/− mice that had been maintained on standard chow diet (Fig. 5B). HPLC analysis of skin lipid extracts obtained 48 h following the final UVB treatment revealed a corresponding decrease in skin vitamin D content with increasing amounts of administered recombinant DBP compared to the control mice administered saline, supporting restored mobilization of vitamin D from skin (Fig. 5C).
Fig. 5.
Repletion of DBP by i.v. injection of recombinant DBP prior to UVB treatments resulted in increased serum calcium and decreased skin vitamin D levels in DBP−/− mice. Serum calcium measurements were taken 24 h after the last of 2 daily treatments. (A) Injection of increasing amounts of recombinant mouse-DBP prior to UVB treatment resulted in a corresponding increase in serum calcium of DBP−/− mice (n = 4 for control dose groups, n = 5 recombinant protein dose groups, ± SEM, #P < 0.05 vs. control 1, ‡P < 0.05 vs. control 2). (B) Serum calcium was normalized in DBP−/− mice administered 2 daily injections of 23 µg of DBP together with UVB treatment compared to vitamin D (orally) sufficient DBP−/− mice maintained on standard chow diet (n = 5 for 23 µg dose group, n = 3 for control group, ± SEM). (C) HPLC analysis of skin lipid extracts obtained 48 h following UVB irradiation revealed a corresponding decrease in skin vitamin D content with increasing amounts of DBP injected compared to control 1 (n = 3, ± SEM, #P < 0.05 vs. control 1, ‡P < 0.05 vs. control 2).
Discussion
Our results demonstrate that DBP is absolutely required for the utilization of vitamin D produced in the epidermis by UV light. Most convincing is that in the absence of DBP, UVB exposure had no effect on vitamin D deficiency or the resultant hypocalcemia while UVB treatment of vitamin D-deficient DBP+/+ mice restored serum calcium and 25(OH)D to normal. Direct measurement of vitamin D in skin confirmed that UV successfully produces vitamin D in both DBP+/+ and DBP−/− mice. In agreement with a previous report, levels of UV-generated vitamin D decreased in the skin of DBP+/+ mice over a 2-d period following UV irradiation (9); however, the vitamin D levels were unchanged in the skin of DBP−/− mice. Of particular significance is the fact that injection of recombinant DBP restored serum calcium of the irradiated DBP−/− mice, thus recapitulating the DBP+/+ phenotype. Taken together, these results demonstrate that DBP is required for the utilization of cutaneous produced vitamin D.
The observation that these DBP knockout mice are phenotypically normal with detectable 25(OH)D and 1,25(OH)2D levels when maintained on vitamin D containing diets indicates that DBP is not required for the absorption of vitamin D from the gut (7, 8). Indeed, absorption and transport of vitamin D from the gut on chylomicrons has been repeatedly demonstrated (5, 10, 11). As animals in previous studies were maintained on diets containing vitamin D, this likely explains why this epidermal function of DBP has been overlooked. The presence of 25(OH)D and 1,25(OH)2D also suggests DBP is not required for their release from the liver or kidney.
In the circulation, virtually 100% of vitamin D metabolites are bound with ∼90% being associated with DBP and the remaining 10% associated with albumin (5, 10, 12). Although the ability to bind alternative carriers creates the possibility of a compensatory transport mechanism for cutaneous vitamin D in the absence of DBP, the data presented here show that the transport is DBP specific. It is possible that a saturating concentration of vitamin D could be reached within the keratinocytes that would enable free ligand to enter the circulation or enable binding of secondary transporters with lesser affinity, thus circumventing the requirement of DBP. However, this is unlikely as in vivo production of vitamin D is limited; in our experiments, skin vitamin D content was equivalent whether mice received a single or multiple UVB treatments.
DBP is highly polymorphic with 3 major variants representing the predominating polymorphisms in humans (Gc1S, Gc1F, and Gc2) (13–15). The biological consequences of the gene variations have not been delineated, but impact on serum DBP concentration has been reported (16). Gene frequencies of the major forms are associated with distinct distributions among ethnic groups and geographic location, prompting speculation of a relationship with sun exposure and vitamin D (17, 18). Additionally, as these DBP variants differ in affinity for 25(OH)D and 1,25(OH)2D, attempts have been made to relate specific alleles to serum 25(OH)D level, although to date no apparent relationship has been defined (19).
Prior to the advent of food fortification techniques and oral supplements, vitamin D was mostly obtained by UV light catalyzed synthesis in the skin. While it is now possible to replace cutaneous synthesis using oral supplements, cutaneous-derived vitamin D is more biologically efficient and serves as a more sustained source without the risk of toxicity (4, 20–22). Sun exposure remains a major determinant for vitamin D status (2, 23). As vitamin D deficiencies can compromise several biological activities, an understanding of the routes whereby we obtain vitamin D is crucial. This report provides strong experimental evidence of a DBP-specific mechanism for the utilization of cutaneous vitamin D. Exploration of the nature of the specific transfer of cutaneously generated vitamin D to the DBP transport protein is likely to be the subject of a future investigation.
Methods
Experimental Mice.
All experiments were conducted in accordance with the Research Animal Resources Committee of the College of Agricultural & Life Sciences, University of Wisconsin–Madison. Animals were maintained in the Department of Biochemistry vivarium with a 12 h:12 h light:dark cycle. Fluorescent bulbs in animal housing and procedure rooms were covered by filters, which eliminate the wavelengths that result in vitamin D production in skin. DBP+/− mouse embryos were generously provided by N.E.C. (Perelman School of Medicine, University of Pennsylvania) and rederived at the University of Wisconsin Genomic Editing and Animal Models Core (University of Wisconsin–Madison) (8). DBP+/− breeders were maintained on standard laboratory chow 5051 (Purina Mills). Genotyping of offspring was performed by Transnetyx (Cordova, TN). To generate vitamin D-deficient animals, DBP+/+ and DBP−/− mice were placed on purified diets devoid of vitamin D at the time of weaning and were fed purified diets containing either 0.47% Ca/0.3% P or 0.02% Ca/0.3% P during depletion. The mice were maintained for 1 wk on the 0.47% Ca/0.3% P diet followed by 3 wk on the 0.02% Ca/0.3% P diet. This was repeated until mice were determined to be deficient by serum calcium and serum 25(OH)D measurements.
UVB Radiation.
UVB radiation was carried out as previously described (24). The dorsal surface of each mouse was shaved using an electric razor ∼24 h prior to each experiment. Irradiation was performed using a bank of 4 UVB lamps that emit from 280 to 330 nm with a peak at 310 nm (Solarc Systems). The radiation output was measured by placing a UV radiometer equipped with a UVX-31 sensor with a calibration point of 310 nm and bandpass 280–340 nm (UVP LLC) at 3 locations within the cage to reproduce position of the animals. An average output was calculated and the time adjusted to ensure exposure to 8 kJ/m2 per treatment. Cages were rotated between available positions to ensure dose was equivalent between groups. Mice were irradiated once daily for up to 4 d, when signs of UV-induced erythema were observed. Blood was collected for a baseline serum calcium measurement ∼24 h prior to the start of each experiment. Additional measurements were collected at indicated times.
For the initial examination of UVB response in DBP−/− mice, 2 separate experiments were carried out using either (i) nonlitter-matched C57BL/6J (Jackson Laboratory) mice or (ii) litter-matched DBP+/+ mice as the control group. Because no statistical difference was observed between the 2 experiments, the data were combined. All other experiments were performed with litter-matched DBP+/+ mice. No statistical difference between males and females was observed; therefore, males and females were combined to yield 1 single group.
Serum Analysis.
Blood was collected from the retro-orbital sinus for all reported measurements. Serum calcium was determined by atomic absorption using a PerkinElmer 900H spectrophotometer following 1:40 dilution of the serum with 0.1% LaCl3. Serum 25(OH)D concentrations were measured by the DiaSorin Liaison 25 OH Vitamin D Total Assay.
HPLC Analysis of Skin Vitamin D Content.
DBP−/− and DBP+/+ mice were euthanized by CO2 asphyxiation, and the UV-exposed region of dorsal skin was isolated. Tissue was minced using razor blades and homogenized in PBS using a Beadmill24 homogenizer (Fisher Scientific). The entire volume was subjected to a Bligh-Dyer extraction using CH2Cl2 in place of CHCl3. The CH2Cl2 layer was dried under argon gas. The oil remaining was washed with 2:1 CH2Cl2:H2O. The CH2Cl2 layer was taken to dryness as before, and residual oil was dissolved in 99% hexane/1% isopropanol. 1,2-[3H]-D was added prior to each extraction to monitor extraction efficiency. Lipid extracts were applied to a straight-phase HPLC column (Zorbax SIL, 4.6 × 250 mm, Agilent) run at a flow rate of 0.75 mL/min of 99% hexane/1% isopropanol and monitored at 265 nm. The retention time was based on comigration with the tritiated vitamin D3. The peak corresponding to vitamin D3 was collected, dried under argon gas, resuspended in methanol, and applied to a reversed-phase HPLC column (YMC-Pack ODS-AM, 4.6 × 250 mm) run at a flow rate of 1 mL/min of 95% methanol/5% water and monitored at 265 nm. Retention time was based on comigration with the tritiated vitamin D3. Total vitamin D was determined based on the area under the curve. The limit of detection for this method was 1 ng and the limit of quantitation was 2.5 ng.
Biological Assay for Vitamin D Activity in Skin.
Vitamin D-deficient and hypocalcemic test rats were generated by feeding vitamin D-free diets. Animals were fed an adequate (0.47% Ca) calcium, vitamin D-free diet for 1 wk followed by 3 wk on the same diet but containing no added calcium (0.02% Ca). This procedure was repeated twice, and blood calcium was used as an indicator for functional vitamin D deficiency.
Skin lipid extracts were analyzed for vitamin D activity using a vitamin D-deficient rat model. Skin samples were homogenized and lipid extracts were obtained as described above. Extracts were pooled so that each sample represented the same total skin weight. Samples were administered in 5% ethanol in propylene glycol via i.p. injection. Rats received a single injection once daily for 3 d, and vitamin D activity was assessed by measuring the change in serum calcium in response to administered extracts. Limit of quantification for this method was 60 ng.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical analysis was performed using the mixed model procedure with Tukey’s adjustment using SAS Version 9.4 (SAS Institute). A value of P < 0.05 was considered statistically significant.
Data Availability.
All data are available on Figshare (DOI: 10.6084/m9.figshare.10269704).
Acknowledgments
We thank Mindy Kendrick for conducting serum analysis of calcium, Steven Marling for his assistance with UVB irradiation design, Kat Hodgeman for assistance on the animal work, Amy Irving and Debra Noltner for their assistance in preparation of the manuscript, and the University of Wisconsin College of Agriculture and Life Sciences statistical consulting group. This work was supported by a fund from the Wisconsin Alumni Research Foundation.
Footnotes
Competing interest statement: J.S. is an employee of DiaSorin Inc. that performed the measurement of 25(OH)D in a blinded manner.
References
- 1.Esvelt R. P., Schnoes H. K., DeLuca H. F., Vitamin D3 from rat skins irradiated in vitro with ultraviolet light. Arch. Biochem. Biophys. 188, 282–286 (1978). [DOI] [PubMed] [Google Scholar]
- 2.DeLuca H. F., Vitamin D., Historical overview. Vitam. Horm. 100, 1–20 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Haddad J. G. Jr, Hahn T. J., Natural and synthetic sources of circulating 25-hydroxyvitamin D in man. Nature 244, 515–517 (1973). [DOI] [PubMed] [Google Scholar]
- 4.Terushkin V., et al. , Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J. Am. Acad. Dermatol. 62, 929.e1–929.e9 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Rikkers H., DeLuca H. F., An in vivo study of the carrier proteins of 3H-vitamins D3 and D4 in rat serum. Am. J. Physiol. 213, 380–386 (1967). [DOI] [PubMed] [Google Scholar]
- 6.Rikkers H., Kletziens R., DeLuca H. F., Vitamin D binding globulin in the rat: Specificity for the vitamins D. Proc. Soc. Exp. Biol. Med. 130, 1321–1324 (1969). [DOI] [PubMed] [Google Scholar]
- 7.Zella L. A., Shevde N. K., Hollis B. W., Cooke N. E., Pike J. W., Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology 149, 3656–3667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safadi F. F., et al. , Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Invest. 103, 239–251 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takada K., Takashima A., Shimoi Y., A study of the photobiogenesis of cholecalciferol in vivo and the constraint on its 25-hydroxylation in rat. J. Steroid Biochem. 14, 1361–1367 (1981). [DOI] [PubMed] [Google Scholar]
- 10.Haddad J. G., Matsuoka L. Y., Hollis B. W., Hu Y. Z., Wortsman J., Human plasma transport of vitamin D after its endogenous synthesis. J. Clin. Invest. 91, 2552–2555 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachter D., Finkelstein J. D., Kowarski S., Metabolism of vitamin D. I. preparation of radioactive vitamin D and its intestinal absorption in the rat. J. Clin. Invest. 43, 787–796 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikle D. D., et al. , Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 63, 954–959 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Hirschfeld J., Jonsson B., Rasmuson M., Inheritance of a new group-specific system demonstrated in normal human sera by means of an immuno-electrophoretic technique. Nature 185, 931–932 (1960). [DOI] [PubMed] [Google Scholar]
- 14.Cleve H., Constans J., The mutants of the vitamin-D-binding protein: More than 120 variants of the GC/DBP system. Vox Sang. 54, 215–225 (1988). [DOI] [PubMed] [Google Scholar]
- 15.Constans J., Viau M., Group-specific component: Evidence for two subtypes of the Gc1 gene. Science 198, 1070–1071 (1977). [DOI] [PubMed] [Google Scholar]
- 16.Lauridsen A. L., Vestergaard P., Nexo E., Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clin. Chem. 47, 753–756 (2001). [PubMed] [Google Scholar]
- 17.Kamboh M. I., Ferrell R. E., Ethnic variation in vitamin D-binding protein (GC): A review of isoelectric focusing studies in human populations. Hum. Genet. 72, 281–293 (1986). [DOI] [PubMed] [Google Scholar]
- 18.Mourant A. E., Tills D., Domaniewska-Sobczak K., Sunshine and the geographical distribution of the alleles of the Gc system of plasma proteins. Hum. Genet. 33, 307–314 (1976). [DOI] [PubMed] [Google Scholar]
- 19.Bikle D. D., Schwartz J., Vitamin D binding protein, total and free vitamin D levels in different physiological and pathophysiological conditions. Front. Endocrinol. (Lausanne) 10, 317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser D. R., The physiological economy of vitamin D. Lancet 1, 969–972 (1983). [DOI] [PubMed] [Google Scholar]
- 21.Webb A. R., DeCosta B. R., Holick M. F., Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J. Clin. Endocrinol. Metab. 68, 882–887 (1989). [DOI] [PubMed] [Google Scholar]
- 22.Jones G., Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 88, 582S–586S (2008). [DOI] [PubMed] [Google Scholar]
- 23.Pilz S., et al. , Rationale and plan for vitamin D food fortification: A review and guidance paper. Front. Endocrinol. (Lausanne) 9, 373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irving A. A., Marling S. J., Plum L. A., DeLuca H. F., Suppression of experimental autoimmune encephalomyelitis by ultraviolet light is not mediated by isomerization of urocanic acid. BMC Neurosci. 18, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on Figshare (DOI: 10.6084/m9.figshare.10269704).