Abstract
Circulating cell-free DNA (cfDNA) is extracellular DNA released into the bloodstream by apoptotic or necrotic tumor cells, with cfDNA determination proposed as a noninvasive, sensitive marker for the diagnosis of human cancer. We evaluated cfDNA quantification as a diagnostic and prognostic tool in dogs with various tumors. We quantified plasma cfDNA concentration by absolute real-time PCR of long interspersed nuclear elements in 50 dogs with malignant tumors, 13 dogs with benign tumors or nodules, and 11 healthy controls. Six patients with malignant tumors were followed-up, and plasma cfDNA was quantified throughout disease progression. We found that plasma cfDNA concentrations were significantly elevated in dogs with malignant tumors compared with dogs with benign nodules or healthy controls. The DNA integrity index (the ratio between long and short cfDNA fragments) was significantly lower in dogs with malignant tumors compared to healthy controls. Significantly higher cfDNA levels and a lower DNA integrity index were observed in dogs with lymphoma or leukemia, hemangiosarcoma, and distant metastasis; cfDNA levels correlated well with clinical stage and tended to increase during or before periods of disease progression, suggesting potential efficacy of cfDNA for the detection of distant metastasis and to monitor the clinical stage of neoplasia.
Keywords: cell-free DNA, dogs, liquid biopsy, tumor biomarker
Introduction
Various tumors occur naturally in dogs and cats, with cancer being among the most common causes of death in these animals. Veterinary oncology is required to provide early and effective cancer detection.14 Tumor biomarkers have been used in human medicine to predict the outcome of therapy response and prognosis,2,16,17 and such markers would be useful in the detection and monitoring of neoplasia in animals. Biomarkers, including their differential expression or mutation in microRNAs, circulating tumor DNAs, proteins, exosomes, and circulating tumor cells, are noninvasive means to identify the status of tumor development.16,28 However, available tumor biomarkers are limited in veterinary medicine.
Circulating cell-free DNA (cfDNA) is extracellular DNA released into the bloodstream as a result of apoptosis, necrosis, and secretion.10 Another important source of circulating tumor DNA is exosomes, which are cell-released membranous structures that contain proteins, lipids, and nucleic acids.5 Low levels of cfDNA can be detected in healthy individuals; however, higher levels have been detected in human patients with a number of diseases, including cancer.3,25 Changes in plasma cfDNA levels reflect tumor burden, with cfDNA methylation, cancer-derived mutation, and loss of heterozygosity representing potential biomarkers for cancer detection.34 Compared with a traditional tissue biopsy, analysis of plasma cfDNA has obvious advantages and provides a rapid, cost-effective, and noninvasive “liquid biopsy” method capable of potentially providing important complementary diagnostic information concerning therapeutic targets and drug-resistance mechanisms in cancer patients.33
cfDNA concentration has been quantified using various techniques, including UV spectrophotometry, fluorescence-based nucleic acid quantification, and quantitative PCR (qPCR).22 A study using dogs with sepsis, trauma, and neoplasia demonstrated excellent linearity between UV spectroscopy and fluorescence assays for cfDNA measurement.15 Regarding cfDNA, the 180-bp fragment reflects apoptosis, which is the prevalent mechanism associated with normal cell death, whereas necrosis produces longer DNA fragments and occurs more frequently in tumor cells.10 The degree of cfDNA integrity (i.e., the DNA integrity index) is the ratio between long and short cfDNA fragments.3 Since the 2000s, the DNA integrity index was proposed as a promising specific oncologic biomarker given its high degree of sensitivity.3,4,9
Increased blood cfDNA concentrations have been documented in dogs with immune-mediated hemolytic anemia, cancer, sepsis, gastric dilation-volvulus syndrome, and trauma1,11,15,29; however, the veterinary cancer literature in this area is limited. Therefore, we assessed the value of plasma cfDNA samples using long interspersed nuclear element-1 (LINE-1) quantification as a diagnostic marker and predictor of metastasis in dogs with various tumors.
Material and methods
Sample collection and processing
Blood samples from 63 dogs with various tumors and 11 healthy controls were collected at the Veterinary Medical Center at the Obihiro University of Agriculture and Veterinary Medicine (VMC-OUAVM) between January 2018 and January 2019. Our study was approved by the Institutional Animal Care and Use Committees at OUAVM (permission 18-2).
The neoplasia group comprised 50 dogs with malignancies and 13 dogs with benign tumors or nodules, with all of the diagnoses confirmed histologically or cytologically at the VMC-OUAVM. Staging of the malignancies was performed according to the World Health Organization staging system,21 including the use of computed tomography, 3-view thoracic radiography, abdominal ultrasound, and hematologic examinations. Six patients with malignant tumors underwent multiple blood collections over time in order to determine whether cfDNA levels were correlated with disease progression (Table 1). Definitions for the response to treatment and relapse criteria used at each visit were those described for lymphomas30 and other solid tumors20 based on physical examination and additional tests at the discretion of the clinician. Dogs in the control group were healthy patients arriving at the hospital for medical examination. Two mL of peripheral blood was collected in EDTA from each dog prior to biopsy or surgical excision; plasma was separated by centrifugation at 2,000 × g for 10 min at 4°C, transferred to new tubes, and centrifuged at 16,000 × g for 10 min at 4°C to remove cell debris. Plasma was stored at −30°C prior to DNA extraction, and all samples were processed within 4 h of blood collection.
Table 1.
Clinical characteristics of 6 dogs assessed during the follow-up study.
| Patient | Age (y) | Sex | Breed | Tumor site | Tumor type | Stage* | Treatment | Metastasis or recurrence, timing |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | CM | Mixed | Urinary bladder | TCC | T2N0M0 | Surgery and COX2i | Lung, day 102 |
| 2 | 10 | CM | FB | Subcutaneous | STS | T4N0M0 | Surgery and LDCPA | Local site, day 48 |
| 3 | 13 | SF | Mixed | Spleen | HS | T2N0M0, stage II | Surgery and DOX | Peritoneum, day 60 |
| 4 | 8 | M | Mixed | MC | LSA | Stage Vb | Chemotherapy | No recurrence |
| 5 | 4 | CM | FB | MC | LSA | Stage Va | Chemotherapy | Mandibular LN, day 220 |
| 6 | 8 | CM | CHH | Spleen, liver, PB, LN | Leukemia | † | Chemotherapy | Spleen, liver, PB, day 205 |
CHH = Chihuahua; CM = castrated male; COX2i = cyclooxygenase-2 inhibitor; DOX = doxorubicin; FB = French Bulldog; HS = hemangiosarcoma; LDCPA = low-dose cyclophosphamide; LN = lymph node; LSA = lymphoma; M = male; MC = multicentric; PB = peripheral blood; SF = spayed female; STS = soft tissue sarcoma; TCC = transitional cell carcinoma.
According to the WHO staging system.23
There is no staging system.
cfDNA extraction and quantification
cfDNA was isolated from 500 µL of plasma (MagMAX cell-free DNA isolation kit; Qiagen, Valencia, CA) according to the manufacturer’s instructions. cfDNA preparations were eluted in 50 µL of elution buffer and stored at −30°C until further analysis.
Plasma cfDNA concentrations were measured by qPCR in order to quantify short and long cfDNA fragments. To maximize quantification sensitivity, the LINE-1 retroposon (GenBank accession AY266086.1), which is the most abundant repeat sequence present in the canine genome,31 was used as a qPCR target. Two primer pairs were used as follows: LINE-99 primers amplified both the short (99 bp) and long (218 bp) fragments, and LINE-218 primers amplified only the long DNA fragments. The results obtained using the LINE-99 primers represent the total free plasma DNA, whereas results obtained using the LINE-218 primers reflect the amount of DNA released from non-apoptotic cells. The quantitative values from the 99-bp amplicon represent total cfDNA concentration (ng/mL), whereas the ratio of long to short fragments represented the DNA integrity index in each plasma sample.1 Primer sequences were as follows: LINE-99 forward (5′-AAATGCAATGAAACGCCGGG-3′), and LINE-218 forward (5′-TGGGAATGTGAACTGGTGCA-3′) and reverse (5′-TCTTTCGTTGGACACCGAGG-3′).15 The qPCR reaction was performed in a 20-µL reaction volume containing 500 nM of each primer, 1 µL of cfDNA template, and 10 µL of PowerUp SYBR Green master mix (ABI; Thermo Fisher Scientific, Waltham, MA), using a StepOne real-time PCR system (ABI; Thermo Fisher Scientific). Cycling was as follows: initial incubation at 50°C for 2 min and 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 3 s, and annealing/extension at 60°C for 30 s. A melting curve (60–95°C) was generated at the end of each run to verify specificity. A standard curve generated by 10-fold serial dilution (from 1–10,000 ng/mL) of genomic DNA obtained from the peripheral blood leukocytes of a healthy dog was used to determine the absolute equivalent amount of cfDNA in each sample. All samples were evaluated in triplicate, and a negative control (without template) was included in each plate.
Statistical analysis
The Mann–Whitney U test and Kruskal–Wallis test were applied to compare plasma cfDNA concentrations between groups. The Steel–Dwass test was performed on each pair of groups following the Kruskal–Wallis test. The diagnostic values of cfDNA for the prediction of distant metastasis were assessed by using receiver operation characteristic (ROC) curves, with a minimum area under the curve (AUC) of 0.7 required for consideration of the ROC model. All analyses were performed using JMP 13 software (SAS Institute, Cary, NC), and differences were considered statistically significant at p ≤ 0.05.
Results
Patient characteristics
The 50 patients with malignant tumors included 27 males (7 intact) and 23 females (4 intact), with a median age of 11 y (range: 1–16 y). The 13 patients with benign tumors or nodules included 7 males (3 intact) and 6 females (2 intact), with a median age of 10 y (range: 4–15 y). The 11 control dogs included 1 intact male and 10 females (6 intact), with a median age of 6 y (range: 1–12 y). The most common malignant tumor types were lymphoma or leukemia (n = 11), hemangiosarcoma (n = 8), urothelial carcinoma (n = 5; 4 dogs with transitional cell carcinoma of the bladder, 1 dog with prostate carcinoma), mammary carcinoma (n = 4), oral squamous cell carcinoma (n = 3), apocrine gland anal sac carcinoma (n = 3), hepatic tumor (n = 3; 1 dog with hepatic cell carcinoma, 1 dog with cholangiocarcinoma, and 1 dog with carcinoid), soft tissue sarcoma (n = 2; 1 dog with undifferentiated sarcoma, 1 dog with nerve sheath tumor), malignant melanoma (n = 2), gastrointestinal stromal tumor (n = 2), and 1 each of the following: mast cell tumor, osteosarcoma, ceruminous adenocarcinoma, salivary adenocarcinoma, apocrine adenocarcinoma, pheochromocytoma, and seminoma. Except for lymphoma or leukemia cases, distant metastasis at sampling was observed in 19 dogs (lung, n = 12; liver, n = 4; spleen, n = 2; and peritoneum, n = 1). The benign tumors or nodules included adenoma (n = 6; mammary gland, sebaceous gland, and rectal), leiomyoma (n = 2), and one each of the following: melanocytoma, histiocytoma, hematoma, bladder polyp, and epidermal cyst.
cfDNA concentrations and DNA integrity index
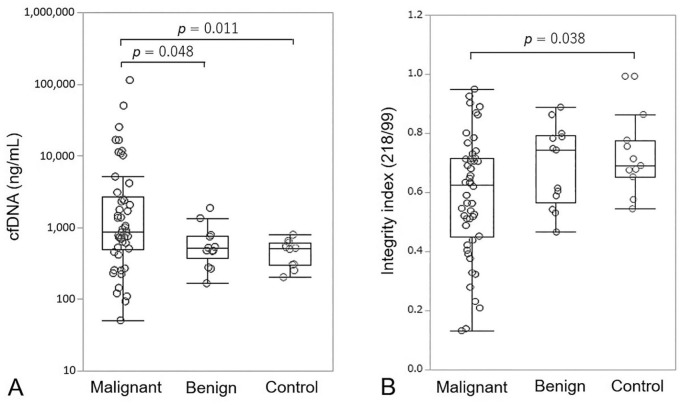
The median cfDNA concentration of LINE-99 elements in malignant, benign, and control dogs was 885 ng/mL (mean: 6,290 ng/mL; range: 51–115,000 ng/mL), 521 ng/mL (mean: 652 ng/mL; range: 166–1,870 ng/mL), and 524 ng/mL (mean: 481 ng/mL; range: 205–802 ng/mL), respectively (Fig. 1A). Plasma cfDNA concentrations were significantly elevated in dogs with malignant tumors compared with those with benign nodules (p = 0.048) and healthy controls (p = 0.011). The median DNA integrity index in malignant, benign, and control dogs was 0.62 (mean: 0.58; range: 0.14–0.95), 0.75 (mean: 0.69; range: 0.47–0.86), and 0.69 (mean: 0.72; range: 0.55–0.99), respectively (Fig. 1B). The integrity index was significantly lower in dogs with malignant tumors compared with healthy controls (p = 0.038).
Figure 1.
A. Box-plot of plasma cell-free DNA (cfDNA) concentrations in dogs with malignant tumors, benign diseases, and healthy controls. B. cfDNA integrity index. Each box indicates the 25th and 75th percentiles. The horizontal line inside the box indicates the median, and the whiskers indicate the extreme measured values.
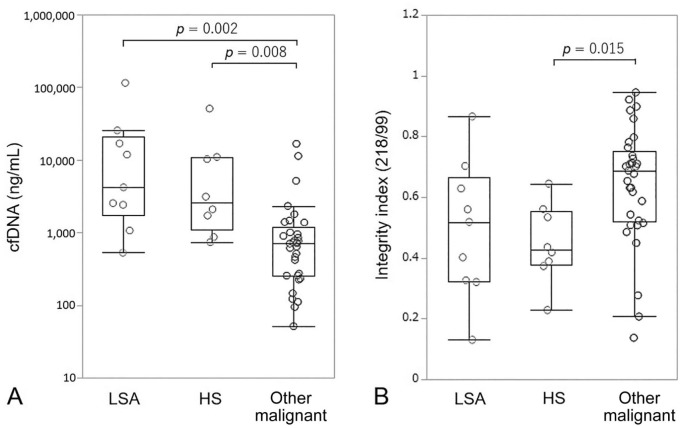
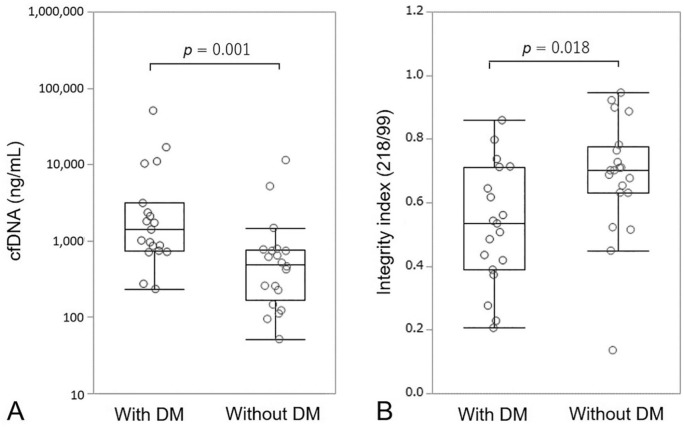
Among the 50 dogs with malignant tumors, lymphoma or leukemia, and hemangiosarcoma resulted in significantly higher cfDNA levels compared with other malignant tumors (Fig. 2A). The integrity index for dogs with hemangiosarcoma was significantly lower compared with dogs with other malignant tumors (Fig. 2B). Among 39 dogs with malignant tumors, except for those with lymphoma or leukemia, dogs with distant metastases had significantly higher cfDNA levels and a lower integrity index compared with dogs without distant metastases (Fig. 3).
Figure 2.
A. Box-plot of plasma cell-free DNA (cfDNA) concentrations in dogs with lymphoma or leukemia (LSA), hemangiosarcoma (HS), and other malignant tumors. B. cfDNA integrity index. Each box indicates the 25th and 75th percentiles. The horizontal line inside the box indicates the median, and the whiskers indicate the extreme measured values.
Figure 3.
A. Box-plot of plasma cell-free DNA (cfDNA) concentrations in malignant-tumor dogs with distant metastasis (DM) or without DM. B. cfDNA integrity index. Each box indicates the 25th and 75th percentiles. The horizontal line inside the box indicates the median, and the whiskers indicate the extreme measured values.
Diagnostic value of cfDNA for the prediction of distant metastasis
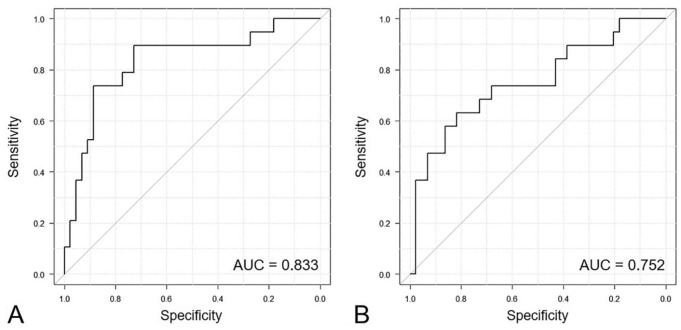
Except for lymphoma or leukemia cases, ROC curves were generated to distinguish distant metastasis patients from other patients and control dogs based on plasma cfDNA levels and the integrity index. The cutoff value for cfDNA concentration was 852 ng/mL, with an AUC of 0.833 (95% confidence interval [CI]: 0.714–0.952; p = 0.002), 73.7% sensitivity, and 88.6% specificity (Fig. 4A). The cutoff value of the integrity index was 0.62, with an AUC of 0.752 (95% CI: 0.611–0.893; p < 0.001), 63.2% sensitivity, and 81.8% specificity (Fig. 4B, Table 2).
Figure 4.
Receiver operating characteristic and area under the curve (AUC) of plasma cell-free DNA (cfDNA) concentrations (A) and cfDNA-integrity index (B) to distinguish patients with distant metastasis from other patients and controls. The AUC for cfDNA concentrations and the cfDNA integrity index was 0.833 (95% CI: 0.714–0.952; p = 0.002) and 0.752 (95% CI: 0.611–0.893; p < 0.001), respectively.
Table 2.
AUC of cfDNA and the DNA integrity index for screening distant metastasis.
| cfDNA | DNA integrity index | |
|---|---|---|
| AUC (95% CI) | 0.833 (0.714–0.952) | 0.752 (0.611–0.893) |
| Cutoff value | 852 ng/mL | 0.62 |
| Accuracy (%) | 84.1 | 71.4 |
| Sensitivity (%) | 73.7 | 63.2 |
| Specificity (%) | 88.6 | 81.8 |
| PPV (%) | 73.7 | 52.0 |
| NPV (%) | 88.6 | 84.2 |
AUC = area under the receiver operating characteristics curve; cfDNA = cell-free DNA; CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
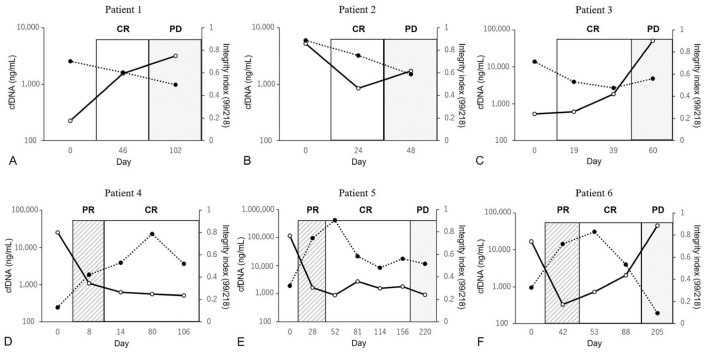
Plasma cfDNA dynamics and tumor progression
In several patients, cfDNA concentration increased along with the progression of tumor metastasis or recurrence, whereas the integrity index tended to decrease according to tumor progression, with this change inversely proportional to changes in cfDNA concentration (Fig. 5). Fluctuations in cfDNA obtained from several patients were observed before a period of disease progression, and the lead-time ranged from a few weeks to several months compared with clinical staging.
Figure 5.
Comparison of cell-free DNA (cfDNA) and cfDNA integrity index according to treatment response: cfDNA levels (solid line) and the cfDNA integrity index (dotted line) at various time points in 6 patients. The vertical axis on the left represents cfDNA concentration, and the vertical axis on the right represents the cfDNA integrity index. The horizontal axis labels represent the times of plasma-sampling day and tumor status. A. Patient 1, transitional carcinoma of the bladder treated with surgery and cyclooxygenase-2 inhibitor; B. Patient 2, undifferentiated sarcoma treated with surgery; C. Patient 3, splenic hemangiosarcoma treated with surgery and chemotherapy; D. Patient 4, lymphoma treated with chemotherapy; E. Patient 5, lymphoma treated with chemotherapy; F. Patient 6, leukemia treated with chemotherapy. CR = complete response; PD = progressive disease; PR = partial response.
Discussion
We found that patients with malignant tumors had significantly higher median plasma cfDNA concentration than dogs with benign tumors or nodules or healthy controls. Several studies reported higher cfDNA concentrations in multiple human cancer patients,26,32 and previous studies also revealed that circulating cfDNA in plasma from dogs with mammary neoplastic lesions and various neoplasias was significantly higher than in healthy dogs.1,15 Our data showed significantly elevated levels of plasma cfDNA in dogs with lymphoma or leukemia and hemangiosarcoma compared with those harboring other malignant tumors, which agreed with a previous result concerning lymphoid neoplasia.24 Possible explanations include the higher tumor burden associated with lymphoma or leukemia, with necrotic, apoptotic, and fragile cells frequently encountered in specimens collected from lymphoid neoplasms.24 Additionally, canine hemangiosarcoma is a neoplasm of vascular endothelial origin that has an aggressive biological behavior and a high rate of rapid and widespread metastasis.23 Histologically, the tumors are cellular with moderate-to-extensive areas of hemorrhage and necrosis.13 In fact, most of the hemangiosarcoma dogs included in our study had distant metastases. Hemangiosarcoma is a systemic disease; therefore, elevated plasma cfDNA concentration in dogs with hemangiosarcoma might be a consequence of tumor volume and tumor-cell necrosis.
We observed a significantly lower DNA integrity index in dogs with malignant tumors compared with that in healthy controls. Moreover, dogs with hemangiosarcoma had a lower DNA integrity index than dogs with other malignant tumors. In veterinary medicine, there are minimal data concerning the efficacy of the DNA integrity index, although a study of canine mammary tumors reported results similar to ours.1 Previous studies suggest that the extent of DNA fragmentation during apoptosis might vary between cancer and normal cells, and that increased DNA fragmentation in cancer cells undergoing apoptosis relative to that in non-cancerous cells might provide an explanation for the decreased DNA integrity index in cancer patients.7,18 In fact, some studies report either equivalent or lower DNA integrity indexes in human patients with cancer.3,18,19 However, it remains unknown whether this theory applies to canine tumors, with a previous review suggesting that the DNA integrity index is controversial as a blood-based biomarker of human cancers.3 Therefore, further studies are needed to evaluate the clinical utility of the DNA integrity index in veterinary medicine.
Our dogs with distant metastases had significantly higher plasma cfDNA concentrations and a lower DNA integrity index relative to patients with malignant disease but no distant metastases. Both markers showed good predictive capability, with the AUC for the cfDNA concentration generated by the LINE-99 primers higher than that for the DNA integrity index. Based on the cutoff value of 852 ng/mL for plasma cfDNA, we suggest that patients with distant metastasis might be discriminated from other dogs with 73.7% sensitivity and 88.6% specificity when using LINE-99 primers for qPCR analysis. Previous studies suggest that plasma cfDNA levels are correlated with larger tumor size, metastasis, and later cancer stage.6,17 Additionally, studies reported that elevated cfDNA levels were significantly correlated with poor outcomes, and that cfDNA quantification could be used as an effective biomarker to differentiate patients with several malignancies from healthy individuals.18,27,32 Our results from canine patients indicated cfDNA as a good screening tool for the detection of distant metastasis and possible utility when used in combination with existing diagnostic tools, including thoracic radiography and abdominal ultrasound, to improve diagnostic efficacy.
By tracking the plasma cfDNA concentration and DNA integrity index at several times in 6 patients, we found that cfDNA parameters correlated well with the clinical stage and tended to increase during or before periods of disease progression, suggesting comparability in monitoring the clinical stage. In contrast, we found that cfDNA parameters did not reflect the disease progression observed in patient 5. Patient 4 did not appear to develop disease progression during the study period. A previous study reported that cfDNA could be used to monitor disease progression in non–small-cell lung cancer patients32; however, several other studies demonstrated that circulating tumor DNA using mutated genes or the Epstein-Barr virus gene was superior to nonspecific cfDNA as an early marker of relapsed and refractory disease.8,12 To our knowledge, evaluation of the efficacy of cfDNA monitoring as a disease-response biomarker associated with several canine malignancies has not been reported previously. However, although the integrity index tended to decrease according to tumor progression in a few of our patients, mismatched changes with tumor progression were observed in other patients.
Our study had several limitations. First, the small sample size used for each analysis might have limited the extent to which differences between groups could be detected. Additionally, our study included various tumor types; therefore, our findings should be verified in a larger population and unified according to tumor type. In addition, our study was a retrospective analysis, and imaging modalities for each cancer stage were not standardized. This might have resulted in an underestimation of distant metastasis in some patients; therefore, a future prospective study involving standardized imaging modalities is necessary.
Acknowledgments
We thank the veterinary medical staff of the Veterinary Medical Center, Obihiro University of Agriculture and Veterinary Medicine for kindly and diligently collecting the patient information for use in this study.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michihito Tagawa  https://orcid.org/0000-0003-1645-4413
https://orcid.org/0000-0003-1645-4413
Hisashi Inokuma  https://orcid.org/0000-0003-1124-3094
https://orcid.org/0000-0003-1124-3094
References
- 1. Beffagna G, et al. Circulating cell-free DNA in dogs with mammary tumors: short and long fragments and integrity index. PLoS One 2017;12:e0169454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlsson J, et al. The potential role of miR-126, miR-21 and miR-10b as prognostic biomarkers in renal cell carcinoma. Oncol Lett 2019;17:4566–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng J, et al. Cell-free circulating DNA integrity based on peripheral blood as a biomarker for diagnosis of cancer: a systematic review. Cancer Epidemiol Biomarkers Prev 2017;26:1595–1602. [DOI] [PubMed] [Google Scholar]
- 4. Chudasama DY, et al. Prognostic value of the DNA integrity index in patients with malignant lung tumors. Oncotarget 2018;9:21281–21288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Rubis G, et al. , Circulating tumor DNA—current state of play and future perspectives. Pharmacol Res 2018;136:35–44. [DOI] [PubMed] [Google Scholar]
- 6. Desai A, et al. Quantification of circulating plasma cell free DNA fragments in patients with oral cancer and precancer. Gulf J Oncology 2018;1:11–17. [PubMed] [Google Scholar]
- 7. Giacona MB, et al. Cell-free DNA in human blood plasma: length measurements in patients with pancreatic cancer and healthy controls. Pancreas 1998;17:89–97. [DOI] [PubMed] [Google Scholar]
- 8. Hamakawa T, et al. Monitoring gastric cancer progression with circulating tumour DNA. Br J Cancer 2015;112:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hao TB, et al. Circulating cell-free DNA in serum as a biomarker for diagnosis and prognostic prediction of colorectal cancer. Br J Cancer 2014;111:1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jahr S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001;61:1659–1665. [PubMed] [Google Scholar]
- 11. Jeffery U, et al. Cell-free DNA and DNase activity in dogs with immune-mediated hemolytic anemia. J Vet Intern Med 2017;31:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones K, et al. Tumor-specific but not nonspecific cell-free circulating DNA can be used to monitor disease response in lymphoma. Am J Hematol 2012;87:258–265. [DOI] [PubMed] [Google Scholar]
- 13. Kim JH, et al. Pathobiology of hemangiosarcoma in dogs: research advances and future perspectives. Vet Sci 2015;2:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kycko A, Reichert M. Proteomics in the search for biomarkers of animal cancer. Curr Protein Pept Sci 2014;15:36–44. [DOI] [PubMed] [Google Scholar]
- 15. Letendre JA, Goggs R. Measurement of plasma cell-free DNA concentrations in dogs with sepsis, trauma, and neoplasia. J Vet Emerg Crit Care (San Antonio) 2017;27:307–314. [DOI] [PubMed] [Google Scholar]
- 16. Lin J, et al. Tumour biomarkers—tracing the molecular function and clinical implication. Cell Prolif 2019;52:e12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin LH, et al. Increased plasma circulating cell-free DNA could be a potential marker for oral cancer. Int J Mol Sci 2018;19:pii:E3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madhavan D, et al. Plasma DNA integrity as a biomarker for primary and metastatic breast cancer and potential marker for early diagnosis. Breast Cancer Res Treat 2014;146:163–174. [DOI] [PubMed] [Google Scholar]
- 19. Mead R, et al. Circulating tumour markers can define patients with normal colons, benign polyps, and cancers. Br J Cancer 2011;105:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen SM, et al. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2015;13;176–183. [DOI] [PubMed] [Google Scholar]
- 21. Owen LN. TNM Classification of Tumours in Domestic Animals. Geneva, Switzerland: World Health Organization, 1980. [Google Scholar]
- 22. Ponti G, et al. The value of fluorimetry (Qubit) and spectrophotometry (NanoDrop) in the quantification of cell-free DNA (cfDNA) in malignant melanoma and prostate cancer patients. Clin Chim Acta 2018; 479:14–19. [DOI] [PubMed] [Google Scholar]
- 23. Regan DP, et al. Role of monocyte recruitment in hemangiosarcoma metastasis in dogs. Vet Comp Oncol 2017;15:1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schaefer DM, et al. Quantification of plasma DNA as a prognostic indicator in canine lymphoid neoplasia. Vet Comp Oncol 2007;5:145–155. [DOI] [PubMed] [Google Scholar]
- 25. Spindler KL, et al. Cell-free DNA in healthy individuals, noncancerous disease and strong prognostic value in colorectal cancer. Int J Cancer 2014;135:2984–2991. [DOI] [PubMed] [Google Scholar]
- 26. Sunami E, et al. Quantification of LINE1 in circulating DNA as a molecular biomarker of breast cancer. Ann N Y Acad Sci 2008;1137:171–174. [DOI] [PubMed] [Google Scholar]
- 27. Szpechcinski A, et al. Cell-free DNA levels in plasma of patients with non-small-cell lung cancer and inflammatory lung disease. Br J Cancer 2015;113:476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tainsky MA. Genomic and proteomic biomarkers for cancer: a multitude of opportunities. Biochim Biophys Acta 2009;1796:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Troia R, et al. Cell-free DNA, high-mobility group box-1, and procalcitonin concentrations in dogs with gastric dilatation-volvulus syndrome. Front Vet Sci 2018; 5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vail DM, et al. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 2010;8;28–37. [DOI] [PubMed] [Google Scholar]
- 31. Wang W, Kirkness EF. Short interspersed elements (SINEs) are a major source of canine genomic diversity. Genome Res 2005;15:1798–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei L, et al. A quantitative analysis of the potential biomarkers of non-small cell lung cancer by circulating cell-free DNA. Oncol Lett 2018;16:4353–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeng H, et al. Liquid biopsies: DNA methylation analyses in circulating cell-free DNA. J Genet Genomics 2018; 45:185–192. [DOI] [PubMed] [Google Scholar]
- 34. Zhao Y, et al. Genome-wide methylation profiling of the different stages of hepatitis B virus-related hepatocellular carcinoma development in plasma cell-free DNA reveals potential biomarkers for early detection and high-risk monitoring of hepatocellular carcinoma. Clin Epigenetics 2014;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]