Abstract
Feline progressive histiocytosis (FPH) is an uncommon and infrequently reported cutaneous histiocytic proliferative disorder, whose clinical presentation is solitary or multiple cutaneous nodules and papules, with late-course internal metastasis. We describe herein the clinical, epidemiologic, histologic, and immunohistochemical features of this entity, and document the outcome of FPH based on a retrospective study of 26 cases. Female and male cats were affected equally. Lesions were evident either as solitary (16 of 26 cases) or multiple (10 of 26 cases) nonpruritic and alopecic nodules or plaques, preferentially located on the legs and extremities (73%). Follow-up was complete for 19 cats, and ranged from 41 to 1,449 d. Nine died of FPH with a median overall survival of 96 d (range: 41–238 d). The disease recurred in 14 cats after surgical excision of the nodules, and the median disease-free survival was 175 d (range: 21–1,449 d). Five of the 26 cats were alive at the end of the study, and 4 had no progression of the disease. Histologically, lesions were characterized by poorly circumscribed, unencapsulated histiocytic infiltration of dermis and subcutis. Epitheliotropism was observed in 11 (42%) cats. Atypical histiocytes diffusely and consistently expressed MHC II, CD18, and Iba1. Statistically significant higher E-cadherin expression was observed in epitheliotropic cases compared to non-epitheliotropic cases. A negative correlation between overall survival and proliferation index was evident, thus suggesting Ki67 as a promising prognostic marker.
Keywords: cats, feline progressive histiocytosis, histiocytosis, immunohistochemistry, Ki67, skin
Introduction
Feline histiocytic disorders are uncommon and infrequently reported. Such disorders include feline progressive histiocytosis (FPH),2,11,23,28,31 feline pulmonary Langerhans cell histiocytosis,6 and feline localized15,22,25,27,29,30,33 or disseminated4,7-10,14,16 histiocytic sarcoma. These diseases derive either from dendritic cells (DCs) or macrophages, which differentiate from a common CD34+ stem cell precursor.19 Various DC sublineages home to specific tissues.2 In the skin, they include dermal DCs and epidermal Langerhans cells (LCs).19 Dermal DCs are part of interstitial DCs, which can be encountered in perivascular locations in many organs.2
The differentiation between these different histiocytic sublineages is not possible with routine histopathology and requires immunohistochemistry (IHC). Macrophages typically express CD18, CD11b, or CD11d and low CD1a. Dermal interstitial DCs express CD1a, CD18, CD11c/b, and CD90. LCs express CD1a, CD18, CD11c, and E-cadherin.19 Assessment of CD1 and CD11 requires either frozen sections or air-dried smears.
FPH is a rare cutaneous histiocytic disease, typically affecting middle-aged to older cats. Initially, it occurs with either solitary or multiple firm, nonpruritic, and nonpainful skin nodules, mostly located on the head, lower extremities, or trunk. The lesions may wax and wane but spontaneous regression does not occur, and the condition can progress to a malignant histiocytic sarcoma–like form with metastases to lymph nodes and various internal organs including liver, spleen, kidneys, lungs, and bone marrow.2,31 No breed predilection has been described, whereas data regarding sex predilection are controversial, with a slight predilection for females noted.2 Immunophenotyping done by others revealed expression of CD1a, CD1c, CD18, and major histocompatibility complex (MHC) class II, supporting a DC origin. Data regarding E-cadherin expression, a feature of LCs, by atypical histiocytes are controversial, with most studies describing a lack of expression of this marker,2,19 whereas one study23 found 4 of 5 cases immunoreactive for E-cadherin, making FPH cell origin uncertain. CD90 expression has not been examined given that feline-specific anti-CD90 antibodies are not available.3 In order to give insights into a possible interstitial DC origin versus LC origin of FPH, another parameter that could be assessed is the presence of Birbeck granules, intracytoplasmic structures that can be seen by electron microscopy and that would support a LC origin.24 According to current literature,19,23 FPH lacks the presence of these intracytoplasmic organelles.
We describe herein the clinical, epidemiologic, histologic, and immunohistochemical features of a series of FPH cases, and compare our findings with existing data. Furthermore, the proliferation index of examined lesions was assessed and correlated with overall survival, in order to investigate it as a prognostic marker. Increased cellular proliferation rate has been recognized as one of the hallmarks of cancer.12 Accordingly, the evaluation of proliferative activity, assessed either by mitotic index or by different immunohistochemical markers, is universally regarded as a valuable prognostic tool in several tumor entities in domestic animals, such as canine mast cell tumors,1,17,32 melanomas,5,26 and canine and feline mammary carcinomas,13,20 to name just a few.
Materials and methods
Animals and tissue samples
The databanks of the IDEXX Saint-Denis laboratory (France) were searched for presumptive cases of FPH submitted by veterinary practitioners from 2012 to 2016. Submitted samples encompassed punch biopsies and excisional biopsies of cutaneous nodules. Tissue samples from 26 cats were included (Table 1) based on histologic features consistent with a histiocytic disorder and IHC confirmation of a FPH diagnosis. In one cat, multiple samples were collected at different times during the evolution of the disease. In one cat, a lymph node sample was also submitted.
Table 1.
Clinical data at the time of diagnosis of 26 cats with feline progressive histiocytosis.
| Cat | Breed | Sex | Age (y) | No. of skin lesions | Location(s) of skin lesions |
|---|---|---|---|---|---|
| 1 | DSH | SF | 9 | 1 | Distal leg |
| 2 | DSH | SF | 13 | 4 | Back, leg, tail |
| 3 | DSH | SF | 4 | 1 | Tail |
| 4 | Mix Chartreux | CM | 2 | 1 | Flank |
| 5 | DSH | CM | 11 | 8 | Distal leg |
| 6 | DSH | SF | 10 | 1 | Digit |
| 7 | DSH | CM | 14 | 2 | Flank, distal leg |
| 8 | DSH | SF | 13 | 1 | Digit |
| 9 | DSH | SF | 4 | 1 | Digit |
| 10 | DSH | CM | 1 | 1 | Head |
| 11 | DSH | SF | 10 | 1 | Distal leg |
| 12 | BSH | CM | 9 | 4 | Proximal leg |
| 13 | DSH | SF | 8 | 1 | Distal leg |
| 14 | DSH | CM | 5 | 1 | Distal leg |
| 15 | DSH | F | 10 | 2 | Thorax |
| 16 | DSH | CM | 6 | 2 | Flank, scapula |
| 17 | DSH | CM | 12 | 1 | Back |
| 18 | DSH | SF | 12 | 8 | Head, neck |
| 19 | DSH | CM | 6 | 2 | Leg |
| 20 | Unknown | F | 15 | 1 | Scapula |
| 21 | DSH | CM | 10 | 1 | Abdomen |
| 22 | DSH | SF | 13 | 2 | Tail |
| 23 | DSH | CM | 8 | 1 | Distal leg |
| 24 | Ragdoll | SF | 8 | 1 | Abdomen |
| 25 | DSH | CM | 14 | 1 | Head (maxilla) |
| 26 | Mix Chartreux | CM | 14 | 2 | Leg |
BSH = British Shorthair; CM = castrated male; DSH = Domestic Shorthair; F = female; SF = spayed female.
Clinical history and follow-up
Case data were collected during tissue sample submission. More precise clinical history and follow-up data (up to 1,449 d) were collected through a questionnaire sent to veterinary practitioners.
Histologic examination
Tissue samples were fixed in 10% neutral-buffered formalin, processed routinely, and sections stained with hematoxylin and eosin (H&E) or used for IHC. Histochemical special stains, including Giemsa, Ziehl–Neelsen, toluidine blue, periodic acid–Schiff (PAS), and Schmorl, were performed to rule out possible differential diagnoses based on morphologic cellular features at the time of diagnosis.
The markers used for IHC were CD18, MHC II, E-cadherin, Ki67, Iba1, and c-kit (Table 2). Enzyme-induced (CD18) or heat-induced (other antibodies) epitope retrieval was performed before incubating the slides with the primary antibody for 1 h at room temperature. After incubation with the appropriate secondary biotinylated antibody, slides were labeled by the avidin–biotin–peroxidase procedure with a commercial immunoperoxidase kit (Vectastain Elite standard kit; Vector Laboratories, Burlingame, CA). The immunoreaction was developed with 3,3’-diaminobenzidine substrate (Vector Laboratories) for 5 min, and sections were counterstained with Mayer hematoxylin. Negative controls were prepared by replacing the primary antibody with an irrelevant one, and known positive control sections were included in each immunolabeling assay.
Table 2.
Immunohistochemical markers used to characterize feline progressive histiocytosis lesions.
| Antibody | Manufacturer | Clone/code no. | Dilution |
|---|---|---|---|
| CD18 | LABL | Fe3.9F2 | 1:10 |
| MHC II | Dako | ab25333 | 1:150 |
| Iba1 | Wako | 019-19741 | 1:2,000 |
| E-cadherin | BDTL | Clone 36 | 1:250 |
| Ki67 | Dako | Clone MIB-1 | 1:600 |
| CD117/c-kit | Dako | A4502 | 1:200 |
BDTL = Transduction Laboratories, Lexington, KY; Dako = Dako, Agilent Technologies, Glostrup, Denmark; LABL = Leukocytes Antigen Biology Laboratory, Davis, CA; Wako = Wako Chemicals, Richmond, VA.
Mitotic counts (MCs) were performed on H&E-stained sections in 10 randomly selected 40× microscopic fields, corresponding to a total area of 2.37 mm2, as recommended.18 The proliferation index was calculated as the percentage of Ki67-positive histiocytic cells out of the total number of histiocytic cells. For each sample, a minimum of 1,000 cells were counted, evaluated in at least 8 high-power fields randomly selected within the bulk of the lesion. The percentage of E-cadherin immunoreactive atypical cells was evaluated independently by 2 pathologists (L Minoli and V Castiglioni). Cases in disagreement were reviewed together to reach a consensus.
Statistics
Statistical analysis was performed (Prism v.6.0; GraphPad Software, La Jolla, CA). The normality of data was assessed by the Shapiro–Wilk test, and nonparametric tests were applied when indicated. Differences in E-cadherin expression between lesions that did or did not display epitheliotropism were analyzed by the Mann–Whitney test. Disease-free survival (DFS) was calculated as the time between surgery and recurrence of the disease. This was calculated only for cats that received surgery and had a follow-up of > 60 d.
Only cases with an observation period > 2 y, or which had died earlier of the disease, were included in the survival analysis, and the overall survival (OS) was defined as the time (in days) between diagnosis and death or last follow-up. Correlation analyses (MC vs. OS; Ki67 index vs. OS; E-cadherin expression vs. OS) were performed by computing a Spearman rank correlation coefficient. Differences in survival were analyzed by a log-rank test.
Results
Clinical features
Cats were 1–15 y old, with a mean age of 9.3 y. Sixteen cats were 9–15 y old (61%), 8 were 4–9 (31%), and 2 were < 4 y old (8%). Thirteen cats were female (2 intact and 11 spayed), and 13 were castrated males. The breed was reported for 25 cats, and included 21 Domestic Shorthair (84%), 1 Ragdoll, 1 British Shorthair, and 2 mixed Chartreux cats (Table 1).
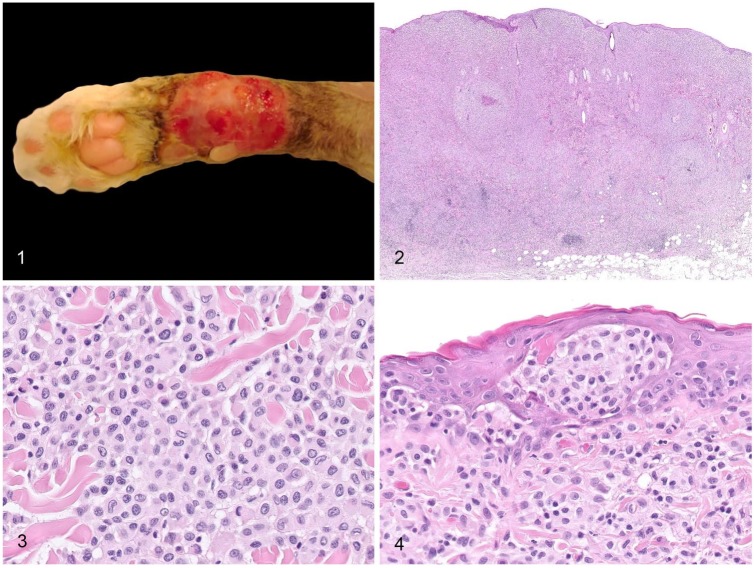
All 26 cats had cutaneous nodules that were either solitary (n = 16) or multiple (n = 10) at the time of diagnosis. Nodules were most frequently located on legs and extremities (digits, tail, and head; n = 19, 73%). Other locations such as flank, abdomen, back, and scapula were observed less frequently. Nodules were 0.4–6 cm diameter. Lesions were grossly described by veterinary practitioners as nodules or plaques, partially or completely alopecic, nonpruritic and nonpainful, and occasionally ulcerated (Fig. 1).
Figures 1–4.
Gross and histologic features of feline progressive histiocytosis skin nodules. Figure 1. Large alopecic and ulcerated plaque on the distal limb of a cat. Image provided by MicenVet. Figure 2. Cutaneous and subcutaneous densely cellular, unencapsulated, and poorly circumscribed histiocytic nodule, with clusters of reactive lymphocytes at the deep periphery of the lesion. H&E. Figure 3. The atypical cells are round-to-polygonal with distinct cell borders, abundant pale eosinophilic homogeneous to finely granular cytoplasm, and a central-to-paracentral, oval-or-indented nucleus with marginated chromatin. Anisocytosis and anisokaryosis are mild-to-moderate. H&E. Figure 4. Skin nodule with intraepidermal aggregates of atypical cells (epitheliotropism). H&E.
Outcome and clinical follow-up was complete for 19 cats, and ranged from 41 to 1,449 d (Table 3); of these, 9 cats died of FPH, after local recurrence or progression of the disease, defined as the development of additional skin nodules or confirmed metastasis in lymph nodes, assessed either by cytology (n = 1), autopsy (n = 1), and/or CT scan (n = 2). The median survival time of the cats that died as a result of FPH was 96 d (range: 41–238 d). Metastases were clinically suspected in the lymph node, pleura, and central nervous system in 3 other cats.
Table 3.
Outcome and follow-up of 26 cats with feline progressive histiocytosis.
| Cat | Local recurrence | Additional skin nodules | Metastasis | Outcome | Follow-up (d) | DFS (d) | OS at last follow-up (d) |
|---|---|---|---|---|---|---|---|
| 1 | + | + | + | Deceased (FPH) | 84 | 49 | 84 |
| 2 | – | – | ND | Unknown | 980 | ≥980 | ≥980 |
| 3 | – | – | ND | Alive | 1,449 | ≥1,449 | 1,449 |
| 4 | – | – | ND | Alive | 1,438 | ≥1,438 | 1,438 |
| 5 | + | – | – | Euthanized | 788 | ND | 788 |
| 6 | + | + | ND | Alive | 1,218 | ND | 1,218 |
| 7 | – | + | Suspected | Euthanized (FPH) | 127 | 21 | 127 |
| 8 | – | – | ND | Alive | 1,147 | ≥1,147 | 1,147 |
| 9 | – | + | Suspected | Euthanized | 76 | 60 | 76 |
| 10 | – | – | ND | Euthanized | 852 | 852 | 852 |
| 11 | – | + | + | Euthanized (FPH) | 96 | 0 | 96 |
| 12 | + | + | ND | Euthanized (FPH) | 175 | 53 | 175 |
| 13 | – | – | ND | Euthanized | 45 | ND | 45 |
| 14 | – | – | ND | Unknown | 12 | ≥12 | U |
| 15 | U | U | ND | Unknown | 0 | U | U |
| 16 | – | – | ND | Alive | 321 | ≥321 | 321 |
| 17 | U | U | ND | Unknown | 0 | U | U |
| 18 | – | + | ND | Euthanized (FPH) | 41 | ND | 41 |
| 19 | – | – | Suspected | Euthanized | 126 | ND | 126 |
| 20 | U | U | ND | Unknown | 0 | U | U |
| 21 | – | + | ND | Euthanized (FPH) | 66 | 0 | 66 |
| 22 | + | + | ND | Euthanized | 735 | 309 | 735 |
| 23 | – | – | ND | Unknown | 8 | ≥8 | ≥8 |
| 24 | – | – | ND | Unknown | 1,010 | U (≥1,010) | U (≥1,010) |
| 25 | – | – | + | Euthanized (FPH) | 113 | ND | 113 |
| 26 | + | + | ND | Euthanized (FPH) | 238 | 208 | 238 |
Deceased/euthanized (FPH) = dead of feline progressive histiocytosis; DFS = disease-free survival; ND = not determined; OS = overall survival; U = unknown.
Local recurrence at the site of surgery or progression of the disease occurred in 14 cats. All 14 cases were characterized by one or more of the following risk factors: presence of neoplastic emboli or lymph nodal metastasis at first presentation, infiltrated excisional margins at surgery, and incomplete or no surgery performed. None of the cats with clean margins at surgery (n = 7) experienced local recurrence of the disease, although a number of these had infiltrated subcutaneous tissue at first presentation.
The DFS was calculated only for cats that received surgery and had a follow-up > 60 d. For cats that experienced local recurrence of the disease, median DFS was 175 d (range: 21–1,449 d); for cats without recurrence, it was ≥ 995 d (range: 321–1,447 d).
Four cats did not undergo surgery and were treated with other therapies. Two of the 4 cats received masitinib and local radiotherapy, with an early size diminution of the primary skin nodule; both rapidly developed additional skin nodules and lymph nodal metastasis. Two cats were treated with corticosteroids and antibiotics, without excision of the nodule; 1 died after 113 d as a result of progression of the disease; the other is still alive (OS = 1,218 d) at time of manuscript submission. No spontaneous regressions were observed.
Histopathologic features
Lesions were characterized by a densely cellular, nonencapsulated, and poorly circumscribed atypical cellular proliferation (Fig. 2). Twenty-five cats had dermal involvement, 12 with extension in the subcutis; one lesion was only subcutaneous. The lesions were composed of solid areas of round-to-polygonal cells, ~15–20 µm diameter, with distinct cell borders, a low nuclear-to-cytoplasmic ratio, and abundant pale eosinophilic homogeneous to finely granular cytoplasm (Fig. 3). Nuclei were central-to-paracentral, oval or indented, 9–12 µm diameter, with marginated chromatin and 1 or 2 nucleoli. Anisocytosis and anisokaryosis were mild to moderate. Mitoses were 0–8 per high-power field, sometimes with bizarre morphology. Multinucleate atypical giant cells were present in a few cases.
Clusters of small reactive lymphocytes were scattered multifocally throughout the proliferations of atypical cells (Fig. 2), admixed with fewer neutrophils. Infiltration of eosinophils and/or mast cells was observed in a few cases. Erosions and ulcerations of the epidermis were observed in 10 cases.
In 11 cats (42%), intraepidermal aggregates of atypical cells (epitheliotropism) were observed (Fig. 4). Among the 14 cases that had dermal involvement but no epitheliotropism, 6 had a small zone of non-infiltrated superficial dermis (grenz zone).
Intralymphatic or intravascular aggregates of atypical cells (interpreted as emboli) were observed in 6 cats. Of these, 5 developed additional skin nodules. The outcome is unknown for the sixth cat with emboli.
Cellular pleomorphism was marked in 4 cases, and all developed progressive disease. In one case, additional skin nodules were evaluated histologically 3 mo after the first submission. Early lesions showed marked cellular pleomorphism and intralymphatic emboli. Later nodules also had cellular pleomorphism, multiple emboli, and a higher mitotic index. In one case (cat 1), the regional (popliteal) lymph node was submitted for histologic examination and confirmed to be infiltrated.
Immunohistochemistry
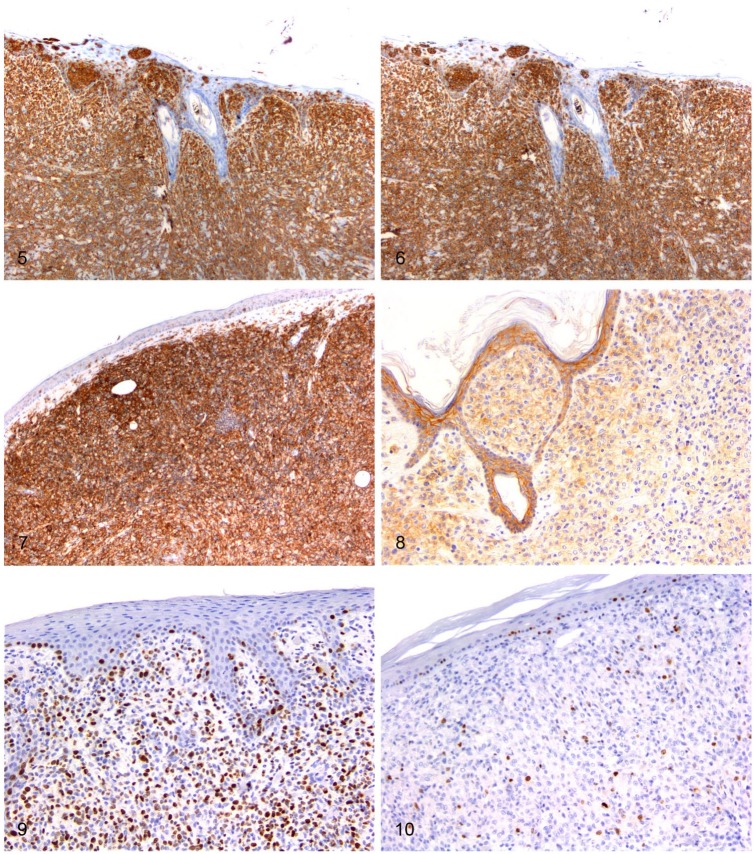
All samples were diffusely positive for MHC II and Iba1 (Table 4; Figs. 5, 6). CD18 immunostaining was positive in 25 cases (Fig. 7).
Table 4.
Immunophenotype and percentage of positive cells in 26 cats with feline progressive histiocytosis.
| Cat | MHC II | Iba1 | CD18 | c-kit | E-cadherin | Ki67 | |
|---|---|---|---|---|---|---|---|
| +/– | % of positive cells | % of positive cells | |||||
| 1 | |||||||
| Skin nodule | + | + | + | NT | + | 5–10 | 21 |
| Lymph node | + | NT | + | NT | + | <5 | 30 |
| 2 | + | + | – | NT | + | 40–70 | 5 |
| 3 | + | + | + | NT | + | 90 | 3 |
| 4 | + | + | + | – | + | <5 | 7 |
| 5 | + | + | + | – | + | 80 | 4 |
| 6 | + | + | + | NT | – | 0 | 4 |
| 7 | + | + | + | – | + | 5–10 | 22 |
| 8 | + | + | + | – | + | 95 | 3 |
| 9 | + | + | + | – | + | 60 | 7 |
| 10 | + | + | + | – | + | <5 | 16 |
| 11 | + | + | + | NT | + | <5 | 15 |
| 12 | + | + | + | – | +/– | 0–10 | 60 |
| 13 | + | + | + | – | + | 75 | 11 |
| 14 | + | + | + | – | + | 90 | 15 |
| 15 | + | + | + | – | +/– | 0–5 | 31 |
| 16 | + | + | + | – | + | 90 | 21 |
| 17 | + | + | + | – | – | 0 | 15 |
| 18 | + | + | + | – | + | <5 | 16 |
| 19 | + | + | + | – | + | <5 | 16 |
| 20 | + | + | + | – | + | 90 | 11 |
| 21 | + | + | + | – | + | 10 | 54 |
| 22 | + | + | + | – | + | 80 | 9 |
| 23 | + | + | + | – | + | 10 | 5 |
| 24 | + | + | + | – | + | >90 | 19 |
| 25 | + | + | + | – | + | <5 | 45 |
| 26 | + | + | + | – | + | 25 | 22 |
NT = not tested.
Figures 5–10.
Immunohistochemical features of feline progressive histiocytosis skin nodules. Immunohistochemistry; chromogen: 3,3’-diaminobenzidine, hematoxylin counterstain. Figure 5. Dermal atypical cells are diffusely positive for MHC II. Figure 6. Dermal atypical cells are diffusely positive for Iba1. Figure 7. Dermal atypical cells are diffusely positive for CD18. Figure 8. Dermal and epitheliotropic atypical cells are positive for E-cadherin. Figure 9. Dermal atypical cells have a Ki67 index > 15% (~60%). Figure 10. Dermal atypical cells have a Ki67 index ≤ 15% (~5%).
E-cadherin was expressed in 24 cases, with a highly variable positivity of <5 to >90% of the atypical cells; 14 expressed E-cadherin in >10% of the cells, 2 in 5–10% of the cells, and 6 cases had scattered immunoreactivity in <5% of the cells. In the remaining 2 cases, E-cadherin was negative except for scattered intralesional positive atypical cells accounting for 5–10%, depending on the skin nodule examined.
Among the 11 cases that were characterized by epitheliotropism, 8 had >60% of the cells immunoreactive for E-cadherin (Fig. 8). On the other hand, of the 15 cases without epitheliotropism, 12 were either negative or had <10% E-cadherin immunoreactivity. Accordingly, although the total percentage range of E-cadherin expressing cells was similar in lesions with or without epitheliotropism, the medians of the 2 groups differed significantly (p ≤ 0.05): the median value of E-cadherin expressing cells was 80% in epitheliotropic lesions and 5% in non-epitheliotropic ones.
Survival analysis
There was no correlation between overall survival and E-cadherin expression. Ki67 scored from 1 to 60% of tumor cells (Figs. 9, 10). A negative correlation between OS and proliferation index was observed (Spearman rank correlation coefficient (ρs) = −0.618; p < 0.01). Accordingly, cases were divided into 2 groups, based on the proliferation index: Ki67 ≤ 15% and Ki67 > 15%. Survival analysis demonstrated a statistically significant (p ≤ 0.05) difference in OS between the 2 groups (median survival time = 980 d for Ki67 ≤ 15% group, and median survival time = 127 d for Ki67 > 15% group). There was a positive correlation (ρs = 0.708; p < 0.01) between MC and proliferation index, but no statistically significant correlation was observed between MC and OS.
Discussion
Our epidemiologic and clinical results support some of the previous published data on FPH.2,11,19,23 One study2 observed a slight predominance of females over males; we found no such predominance in our study. Unlike the findings of that study,2 at the time of diagnosis, the majority of cats in our study had a single cutaneous nodule. The most frequent sites of involvement in our cases were the distal legs and extremities (digits, tail, head), consistent with previous data.2
Based on our case series, wide surgical excision seems to be an effective treatment, given that none of the 7 cats with clean surgical margins experienced local recurrence of the disease. Thus, the presence of neoplastic emboli or lymph nodal metastasis at first presentation, infiltrated excisional margins at surgery, incomplete or no surgery, could be considered as risk factors for progression of the disease. In addition, on histologic examination, marked pleomorphism, high mitotic index, and emboli were associated with progressive disease. These features were more severe in samples collected later in the disease in one cat, as was the Ki67 index. These findings support previous observations2 and suggest a potential link between duration of the disease, cellular atypia, and proliferative activity. We did not examine this link in our study, but this might be useful to investigate in future studies.
The clinical differential diagnoses of early stages of FPH are mycobacterial and fungal diseases, cutaneous xanthomas, cutaneous lymphocytosis, or other round cell tumors.23 A 2014 study assessed the cytologic features of FPH.23 Based on cytomorphologic features, the authors could rule out granulomatous inflammation, xanthoma, cutaneous lymphocytosis, and poorly differentiated mast cell tumor.23 They concluded that, in cases of clinical presentation supporting a FPH diagnosis, cytologic assessment could be highly suggestive of a FPH diagnosis. Immunophenotyping is a useful diagnostic tool for definitive diagnosis and helps rule out multiple differential diagnostic entities.
In advanced forms of FPH, the histologic lesions are not discernible from primary histiocytic sarcoma.2 Cells tend to have marked anisocytosis and anisokaryosis, more numerous multinucleate giant cells, and higher mitotic index. Diagnosis of late-course FPH rather than primary histiocytic sarcoma relies on recognition of the slowly progressive clinical course of FPH.2
All but one of our cases were positive for CD18 immunostaining, confirming a leukocytic origin for 25 of 26 cases. In addition, the histiocytic origin of the cells was confirmed in all lesions by strong and diffuse immunoreactivity for MHC II and Iba1, a pan-histiocyte marker, considered specific and reliable also in feline tissues.21 The CD18-negative case was included in the study because morphologic features and immunoreactivity for MHC II and Iba1 supported the diagnosis of FPH. The lack of immunoreactivity for CD18 was considered a fixation and/or processing artifact, given that the lack of immunoreactivity for this marker also affected infiltrating leukocytes.
In 24 of 26 cases, variable percentages of atypical cells were immunoreactive for E-cadherin. All 11 cases with epitheliotropism expressed E-cadherin with a statistically significant higher percentage of immunoreactive cells, compared to non-epitheliotropic cases. These results support the findings of another study.23 Based on our immunohistochemical results, in which most of the lesions expressed MHC II, Iba1, and E-cadherin, a LC origin is considered likely. Further studies are needed to definitively confirm the LC versus interstitial DC origin of FPH, given that existing studies are based on limited numbers of animals. Another aspect in this regard deserving further assessment is the presence of intracytoplasmic Birbeck granules. We did not look for these structures because we did not have access to electron microscopy. Current literature accounts for only 3 cases of FPH investigated for Birbeck granules, failing to identify their presence.19,23
Considering the statistically significant negative correlation observed between OS and proliferation index, Ki67 could be regarded as a promising prognostic marker; however, further studies are needed to define the most clinically useful cutoff value. Although not statistically significant, a similar trend was observed for OS versus MC, therefore suggesting a potential prognostic value also for this parameter.
Acknowledgments
We thank Prof. Eugenio Scanziani and the MAPLab team for their technical support and their immunohistochemistry expertise, as well as all veterinary practitioners who provided case materials for our study. We also thank Prof. Silvana Mattiello (University of Milan) for statistical support.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclose no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Margaux Coste  https://orcid.org/0000-0002-0277-3723
https://orcid.org/0000-0002-0277-3723
References
- 1. Abadie JJ, et al. Immunohistochemical detection of proliferating cell nuclear antigen and Ki-67 in mast cell tumors from dogs. J Am Vet Med Assoc 1999;215:1629–1634. [PubMed] [Google Scholar]
- 2. Affolter VK, Moore PF. Feline progressive histiocytosis. Vet Pathol 2006;43:646–655. [DOI] [PubMed] [Google Scholar]
- 3. Affolter VK, Moore PF. Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol 2000;22:40–48. [DOI] [PubMed] [Google Scholar]
- 4. Bell R, et al. Dynamic tracheal collapse associated with disseminated histiocytic sarcoma in a cat. J Small Anim Pract 2006;47:461–464. [DOI] [PubMed] [Google Scholar]
- 5. Bergin IL, et al. Prognostic evaluation of Ki67 threshold value in canine oral melanoma. Vet Pathol 2011;48:41–53. [DOI] [PubMed] [Google Scholar]
- 6. Busch MDM, et al. Feline pulmonary Langerhans cell histiocytosis with multiorgan involvement. Vet Pathol 2008;45:816–824. [DOI] [PubMed] [Google Scholar]
- 7. Cortese L, et al. Morphological characterisation of malignant histiocytosis in a cat. Folia Morphol (Warsz) 2008;67:299–303. [PubMed] [Google Scholar]
- 8. Court EA, et al. Malignant histiocytosis in a cat. J Am Vet Med Assoc 1993;203:1300–1302. [PubMed] [Google Scholar]
- 9. Freeman L, et al. Clinical vignette. Malignant histiocytosis in a cat. J Vet Intern Med 1995;9:171–173. [DOI] [PubMed] [Google Scholar]
- 10. Gafner F, Bestetti GE. Feline Maligne Histiozytose und Lysozymnachweis [Feline malignant histiocytosis and lysozyme detection]. Schweiz Arch Tierheilkd 1988;130:349–356. German. [PubMed] [Google Scholar]
- 11. Gelberg HB. Diagnostic exercise: multiple skin nodules in a cat. Vet Pathol 2013;50:569–571. [DOI] [PubMed] [Google Scholar]
- 12. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 13. Hughes K, Dobson JM. Prognostic histopathological and molecular markers in feline mammary neoplasia. Vet J 2012;194:19–26. [DOI] [PubMed] [Google Scholar]
- 14. Ide K, et al. Disseminated histiocytic sarcoma with excessive hemophagocytosis in a cat. J Vet Med Sci 2009;71:817–820. [DOI] [PubMed] [Google Scholar]
- 15. Ide T, et al. Histiocytic sarcoma in the brain of a cat. J Vet Med Sci 2010;72:99–102. [DOI] [PubMed] [Google Scholar]
- 16. Kraje AC, et al. Malignant histiocytosis in 3 cats. J Vet Intern Med 2001;15:252–256. [DOI] [PubMed] [Google Scholar]
- 17. Maglennon GA, et al. Association of Ki67 index with prognosis for intermediate-grade canine cutaneous mast cell tumours. Vet Comp Oncol 2008;6:268–274. [DOI] [PubMed] [Google Scholar]
- 18. Meuten DJ, et al. Mitotic count and the field of view area: time to standardize. Vet Pathol 2016;53:7–9. [DOI] [PubMed] [Google Scholar]
- 19. Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol 2014;51:167–184. [DOI] [PubMed] [Google Scholar]
- 20. Pena LL, et al. Immunohistochemical detection of Ki-67 and PCNA in canine mammary tumors: relationship to clinical and pathological variables. J Vet Diagn Invest 1998;10:237–246. [DOI] [PubMed] [Google Scholar]
- 21. Pierezan F, et al. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J Comp Pathol 2014;151:347–351. [DOI] [PubMed] [Google Scholar]
- 22. Pinard J, et al. Histiocytic sarcoma in the tarsus of a cat. Vet Pathol 2006;43:1014–1017. [DOI] [PubMed] [Google Scholar]
- 23. Pinto da Cunha N, et al. Cytologic and immunocytochemical characterization of feline progressive histiocytosis. Vet Clin Pathol 2014;43:428–436. [DOI] [PubMed] [Google Scholar]
- 24. Saint-André Marchal I, et al. Immunophenotypic characterization of feline Langerhans cells. Vet Immunol Immunopathol 1997;58:1–16. [DOI] [PubMed] [Google Scholar]
- 25. Scurrell E, et al. Ocular histiocytic sarcoma in a cat. Vet Ophthalmol 2013;16(Suppl 1):173–176. [DOI] [PubMed] [Google Scholar]
- 26. Smedley RC, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol 2011;48:54–72. [DOI] [PubMed] [Google Scholar]
- 27. Smoliga J, et al. Myelopathy caused by a histiocytic sarcoma in a cat. J Small Anim Pract 2005;46:34–38. [DOI] [PubMed] [Google Scholar]
- 28. Solc M, et al. Feline progressive dendritic cell histiocytosis in a domestic long hair feline. Vet Rec Case Rep 2017;5:e000428. [Google Scholar]
- 29. Tanimoto T, et al. Histiocytic sarcoma in a cat. Nihon Juigaku Zasshi 1988;50:291–293. [DOI] [PubMed] [Google Scholar]
- 30. Teshima T, et al. Amputation for histiocytic sarcoma in a cat. J Feline Med Surg 2012;14:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Treggiari E, et al. Clinical outcome, PDGFRβ and KIT expression in feline histiocytic disorders: a multicentre study. Vet Comp Oncol 2017;15:65–77. [DOI] [PubMed] [Google Scholar]
- 32. Webster JD, et al. Cellular proliferation in canine cutaneous mast cell tumors: associations with c-KIT and its role in prognostication. Vet Pathol 2007;44:298–308. [DOI] [PubMed] [Google Scholar]
- 33. Wong VM, et al. Primary nasal histiocytic sarcoma of macrophage-myeloid cell type in a cat. J Comp Pathol 2012;147:209–213. [DOI] [PubMed] [Google Scholar]