Significance Statement
Although complement blockade is highly effective for preventing recurrence of atypical hemolytic uremic syndrome (HUS) after kidney transplant, debates regarding the use of eculizumab prophylaxis continue because of its very high cost. An individualized strategy—using eculizumab prophylaxis specifically in patients with moderate- to high-risk kidney transplants, determined by complement analysis and a medical history of a previous recurrence—was implemented in France in 2011 and subsequently adopted more widely. In the authors’ retrospective study of patients with atypical HUS in France, they found that prophylactic use of eculizumab almost abolished the risk of recurrence and significantly increased graft survival, especially in high-risk transplants. It also led to a substantial expansion after 2012 of the transplanted population among patients with atypical HUS and ESKD. These findings support use of eculizumab prophylaxis based on pretransplant risk stratification.
Keywords: complement, hemolytic uremic syndrome, kidney transplantation
Visual Abstract
Abstract
Background
Atypical hemolytic uremic syndrome (HUS) is associated with high recurrence rates after kidney transplant, with devastating outcomes. In late 2011, experts in France recommended the use of highly individualized complement blockade–based prophylaxis with eculizumab to prevent post-transplant atypical HUS recurrence throughout the country.
Methods
To evaluate this strategy’s effect on kidney transplant prognosis, we conducted a retrospective multicenter study from a large French nationwide registry, enrolling all adult patients with atypical HUS who had undergone complement analysis and a kidney transplant since January 1, 2007. To assess how atypical HUS epidemiology in France in the eculizumab era evolved, we undertook a population-based cohort study that included all adult patients with atypical HUS (n=397) between 2007 and 2016.
Results
The first study included 126 kidney transplants performed in 116 patients, 58.7% and 34.1% of which were considered to be at a high and moderate risk of atypical HUS recurrence, respectively. Eculizumab prophylaxis was used in 52 kidney transplants, including 39 at high risk of recurrence. Atypical HUS recurred after 43 (34.1%) of the transplants; in four cases, patients had received eculizumab prophylaxis and in 39 cases they did not. Use of prophylactic eculizumab was independently associated with a significantly reduced risk of recurrence and with significantly longer graft survival. In the second, population-based cohort study, the proportion of transplant recipients among patients with ESKD and atypical HUS sharply increased between 2012 and 2016, from 46.2% to 72.3%, and showed a close correlation with increasing eculizumab use among the transplant recipients.
Conclusions
Results from this observational study are consistent with benefit from eculizumab prophylaxis based on pretransplant risk stratification and support the need for a rigorous randomized trial.
Hemolytic uremic syndrome (HUS) is a rare yet potentially life-threatening disease that results from thrombotic microangiopathy in target organs, including the kidneys.1 HUS was referred to as atypical when etiologic investigations fail to identify coexisting disease, medical conditions, or treatments associated with secondary HUS.1,2 Genetically determined or acquired dysregulation of the complement alternative pathway (CAP) has been found in up to 70% of patients with atypical HUS (aHUS). Before the era of complement blockade, aHUS was associated with high rates of progression toward ESKD and post-transplant recurrence.3 In this context, patients with aHUS who have experienced early and devastating recurrences with previous allografts were contraindicated for subsequent transplantation and were maintained for years on dialysis.
However, two recent breakthroughs have made kidney transplantation a much safer and suitable option.4–6 First, advances in deciphering the clinical significance of variants in CAP-related genes have permitted the individualization of risk assessment for post-transplant aHUS recurrence.3,7,8 Second, eculizumab, a C5-targeted complement blocker, has revolutionized the treatment of aHUS episodes, including those occurring after kidney transplantation.9–11 Together, these major strides forward set the stage for treatment tailoring and prompted the French aHUS Study Group to issue recommendations in 2011 about how to individualize aHUS management after transplantation.10 A highly personalized prophylactic strategy was advised throughout the country based on an individual risk assessment, depending primarily on complement testing and the history of recurrence with previous allografts.10 Similarly, the 2016 Kidney Disease Improving Global Outcomes (KDIGO) guidelines advocated the use of prophylactic complement blockade based on the recurrence risk, according to approximately the same criteria.12 Recently, a large analysis of the Global aHUS Registry suggested that the pretransplant initiation of eculizumab resulted in a better allograft function than that of the post-transplant initiation of eculizumab, but these conclusions were limited by the caveats inherent to any poorly phenotyped registry study.13 In particular, this study did not investigate post-transplant events, in addition to dialysis requirements, nor did it assess the outcomes according to the risk stratification.13
The present study aimed to retrospectively investigate the effect of the individualized prophylaxis of aHUS recurrence on post-transplant outcomes in an extensively characterized population and to evaluate the resulting epidemiologic changes of the disease at a nationwide level.
Methods
Patients and Studies
Two studies were undertaken, wherein the patients were identified and enrolled from the nationwide aHUS registry maintained by the French Reference Laboratory for CAP and Diacylglycerol kinase epsilon (DGKE) investigations. HUS was characterized either by the classic triad combining thrombocytopenia, hemolytic mechanical anemia, and AKI, or by a kidney biopsy disclosing thrombotic microangiopathy (TMA) whenever HUS presented with incomplete clinical manifestations. Once routine laboratory investigations and/or renal histology had demonstrated a process of TMA, the diagnosis of aHUS was made based on a stepwise diagnostic approach, which aimed to rule out thrombotic thrombopenic purpura (TTP) and other HUS forms.1 The diagnostic procedure was subsequently reviewed and approved at the centralized Reference Laboratory before proceeding with complement investigations. The diagnosis of aHUS was made by default when etiologic investigations failed to identify coexisting diseases, medical conditions, or treatments associated with secondary HUS.1 The diagnostic algorithm depends on the age of HUS onset because the incidences of TTP, related to ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats-13) deficiency, and of noninfectious causes of HUS are much lower in children than in adults. In child-onset HUS, a specific emphasis is placed on the exclusion of Shiga toxin–producing Escherichia coli (STEC)–associated HUS (STEC-HUS), based on negative stool culture for STEC and negative testing for Shiga toxin genes. Streptococcus pneumoniae–related HUS is only suspected in the setting of sepsis, whereas normal plasma homocysteine and methylmalonic acid levels are used to eliminate cobalamin C deficiency in non–STEC-HUS. In adult-onset HUS, the diagnostic approach is less straightforward and should exclude not only STEC-HUS but also acquired TTP, based on an ADAMTS13 activity level of >5%, as well as other secondary forms associated with coexisting diseases. To this end, further etiologic investigations may be required, guided by anamnesis (medical history, medications) and clinical examination, including anti-phospholipid antibodies, anti-nuclear antibodies, HIV test, screening for monoclonal gammopathy (in individuals older than 50 years old), and for disseminated solid cancers (especially mucin-producing adenocarcinoma). This stringent diagnostic process has been resulting in high rates of positive genetic testing in complement genes among the French aHUS cohort (65%–70%).
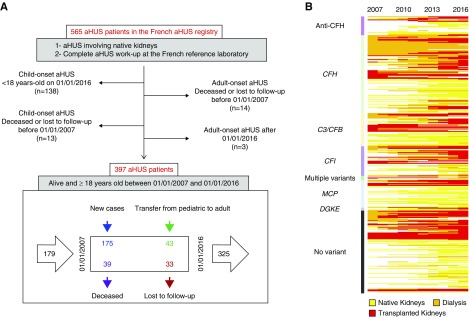
Study 1 included patients with aHUS who underwent transplantation since January 1, 2007 to ensure there was homogeneity in the immunosuppressive regimens, donor-specific antibody screening (using a solid-phase assay), and rejection classification. In study 1, all patients fulfilling the following criteria were included: (1) a diagnosis of aHUS involving native kidneys; (2) exhaustive aHUS workup at the French Reference Laboratory, including complement investigations and DGKE sequencing (only for those with very early-onset disease, i.e., <2 years old); and (3) at least one adult-onset kidney transplantation at a French transplant center since January 1, 2007 (Supplemental Figure 1). Additional data about baseline characteristics and the post-transplant course were collected through a questionnaire that was sent out to all transplant centers. The risk stratification (high/moderate/low) for aHUS recurrence was based on a classification described in previous reports.10,12 Briefly, “high risk” referred to the transplantations performed in patients who had experienced a recurrence with a previous allograft and/or were harboring a pathogenic variant of the complement factor H (CFH)/C3/complement factor B (CFB) genes. Transplantations in patients who had either a negative complement screening result or a pathogenic variant in the CFI gene or detectable circulating anti-CFH antibody were considered at “moderate risk.” Finally, “low-risk” transplantations encompassed the following situations: isolated mutations in the MCP14 and DGKE15 genes, or anti-CFH antibodies no longer detected at the time of transplantation in patients in whom positive anti-CFH antibody testing had been previously identified as the sole complement abnormality associated with aHUS. Supplemental Table 1 shows the history of post-transplant aHUS recurrence, as well as immunologic and genetic investigations, that were used to assign each transplantation to a given risk category. Clinical recurrence was defined as any kidney graft dysfunction with at least two hematologic features that were suggestive of an active TMA process (low platelet count, increased LDH, schizocytes, anemia or low haptoglobin levels) or with histologic evidence of TMA. The presence of the histologic features of TMA on protocol biopsies in the absence of the above-listed criteria was considered subclinical recurrence.
Study 2 was meant to assess, over a decade, the proportion of patients with aHUS who were on dialysis and who underwent transplantation, according to eculizumab exposure. A population-based study was conducted among the whole French adult aHUS cohort during a 10-year period. Patients meeting the following inclusion criteria were enrolled: (1) aHUS involving native kidneys; (2) exhaustive aHUS workup at the French Reference Laboratory, including complement investigations and DGKE sequencing (only for those with very early-onset disease, i.e., <2 years old); and (3) alive and aged 18 or older from January 1, 2007 to January 1, 2016. Whenever necessary, the investigators reached out to nephrology, transplant, and dialysis centers to track supposedly living patients for whom updated follow-up information was not available until January 1, 2016. This proactive inquiry decreased the number of patients who were lost to follow-up from 77 to 33.
Complement Investigations
The complement evaluation and genetic analysis were performed as part of the usual workup for patients diagnosed with HUS. The plasma concentrations of C3, C4, factor B, factor H, and factor I, as well as MCP expression on granulocytes were quantified as previously described.16 All coding sequences of the CFH, complement factor I (CFI), MCP, C3, CFB, and thrombomodulin (THBD) genes were analyzed by direct sequencing analysis or by next generation sequencing. Screening for the CFH/CFHR1 hybrid gene and assessment of CFH/CFHR copy numbers were performed using multiplex ligation–dependent probe amplification.17 Rare variants were defined as having a gene frequency of 0.1% or lower in the general population. Among rare variants, pathogenic variants were those associated with well established protein dysfunction (based on in vitro assay), and/or reduced plasma levels, and/or those located in a disease-related functional domain.12 The other rare variants were referred to as variants of uncertain significance.
Statistical Analyses
Mean±SD and frequencies are provided for the description of the continuous and categoric variables, respectively, unless otherwise stated. The characteristics were compared between two groups (eculizumab prophylaxis or none) using the Mann–Whitney U test for quantitative variables and the Fisher exact test for the qualitative variables.
Kaplan–Meier analysis, with the log-rank test, was used to examine the association between recurrence-free survival or death-censored graft survival and several variables. Recurrence-free survival was censored at the time of graft loss, death, last follow-up, or at the latest 3000 days post-transplant. More specifically, for the comparison between eculizumab prophylaxis and no prophylaxis (Figure 1A), recurrence-free survival was censored at the time of prophylaxis withdrawal. Graft survival was censored at the time of death, last follow-up, or at the latest 3000 days post-transplant.
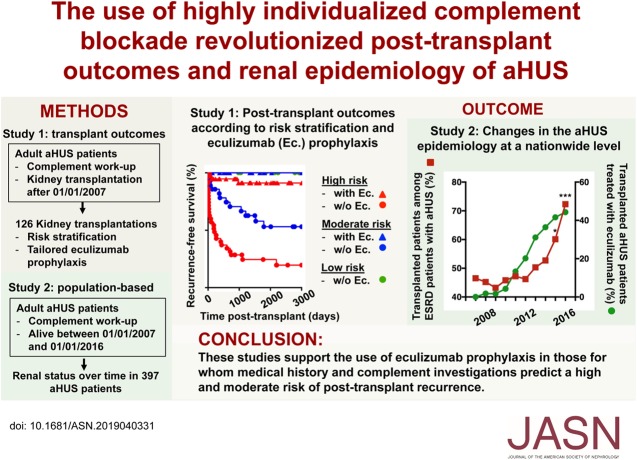
Figure 1.
Risk factors for aHUS recurrence. (A) Recurrence-free survival curves according to risk stratification (top panel) and use of eculizumab prophylaxis (bottom panel). Log-rank test, ****P<0.001. (B) Results of multivariate Cox regression models for the HRs and 95% CIs. Ec., eculizumab; proph./prophyl., prophylaxis.
Cox proportional hazard models were used to quantify the hazard ratio (HR) and 95% confidence intervals (CIs) for the factors associated with recurrence or graft loss. To assess the association between risk stratification and recurrence in the Cox model, we could not compare high- versus moderate- versus low-risk groups because this model failed to converge due to the limited number of cases (n=9) and events (0 recurrences) for the low-risk group. Hence, we pooled low and moderate risk to account for all cases. The associations of some selected variables with the event of interest were first assessed in univariate regression analyses. All variables with a P value of 0.1 or less were then included in a multivariate Cox model.
The value of P<0.05 was considered statistically significant and all of the tests were two sided. All analyses were performed using R software (version 3.6.1) with the survival package or GraphPad Prism software (GraphPad Software, version 7.0d, La Jolla CA).
Results
Characteristics of the Study Population
A total of 126 transplantations performed in 116 adult patients with aHUS fulfilled the inclusion criteria (Table 1), 15 of which were already included in a previous report.3 aHUS recurrence was prevented with prophylactic eculizumab in 52 of the transplantations, whereas prophylactic plasmapheresis alone was used in 22 of the remaining 74 transplantations (Table 1). We first sought to compare the epidemiologic and clinical characteristics between two groups of transplantations depending on whether eculizumab prophylaxis was used. The group with eculizumab prophylaxis, compared with the other group, was associated with greater frequencies of complement/DGKE abnormalities (86.5 versus 51.3, P<0.001) and high-risk transplantations (75.0 versus 47.3, P=0.003). With the exception of a significantly lower frequency of extended-criteria donors (ECDs) in those with eculizumab prophylaxis, the two groups were otherwise very similar, especially in terms of donor and recipient ages, cold-ischemia time, presensitization, and immunosuppressive regimens (Table 1).
Table 1.
Characteristics at the time of transplantation and transplant outcomes
| Kidney Transplantations | Eculizumab Prophylaxis (n=52) | No Eculizumab Prophylaxis (n=74) | P value |
|---|---|---|---|
| At the time of transplantation | |||
| Rank 1/2/3/>3 | 33/10/5/4 | 53/18/2/1 | — |
| Complement and DGKE abnormalities (%) | 45 (86.5) | 38 (51.3) | <0.001 |
| CFH-H3 (%)a | 8 (17.0) | 12 (16.7) | NS |
| High recurrence risk (%) | 39 (75.0) | 35 (47.3) | 0.003 |
| CFH variant | 23 (44.2) | 18 (24.3) | 0.02 |
| C3/CFB variant | 6 (11.5) | 5 (6.7) | NS |
| Multiple variant | 3 (5.7) | 2 (2.7) | NS |
| Previous recurrence (% of two or more KTx) | 18/19 (94.7) | 16/21 (76.2) | NS |
| Moderate recurrence risk (%) | 13 (25) | 30 (40.5) | NS |
| CFI variant | 7 (13.4) | 6 (8.1) | NS |
| Multiple variant | 1 (1.9) | 0 (0) | NS |
| No variant | 3 (5.7) | 24 (32.4) | 0.0003 |
| Anti-CFH | 2 (3.8) | 0 (0) | NS |
| Low recurrence risk (%) | 0 (0) | 9 (12.1) | 0.02 |
| MCP variant | 0 (0) | 5 (6.7) | 0.08 |
| DGKE variant | 0 (0) | 3 (4.0) | NS |
| Anti-CFH (no longer detected) | 0 (0) | 1 (1.3) | NS |
| Mean (±SD) recipient age in years | 39.5 (±12.5) | 42.5 (±12.5) | NS |
| Mean (±SD) donor age in years | 42.1 (±13.4) | 47.3 (±16.3) | NS |
| Living donor (%) | 11 (21.1) | 6 (8.1) | NS |
| ECD donor (%) | 8 (15.4) | 24 (32.4) | 0.04 |
| Mean (±SD) cold-ischemia time in hours | 16.2 (±9.9) | 17.7 (±7.6) | NS |
| Preformed DSA (%) | 9 (17.3) | 13 (17.5) | NS |
| rATG induction (%) | 37 (71.1) | 46 (62.2) | NS |
| CNI-based maintenance immunosuppression regimen (%) | 50 (96.1) | 72 (97.3) | NS |
| Prophylactic plasmapheresis alone (%) | — | 22 (29.7) | — |
| Post-transplant outcomes | |||
| Biopsy-proven rejection (%) | 11 (21.1) | 16 (21.6) | NS |
| Clinical aHUS recurrence (%) | 1b (1.9) | 30 (40.5) | <0.001 |
| Subclinical TMA lesions (%) | 3 (5.7) | 9 (12.1) | NS |
| Death with functioning allograft (%) | 2 (3.8) | 9 (12.1) | NS |
| Death-censored graft loss (%) | 2 (3.8) | 28 (37.8) | <0.001 |
| Median (range) follow-up in months | 56.6 (0.03–108) | 70.1 (0–150) | 0.06 |
KTx, kidney transplants; DSA, donor-specific antibody; rATG, rabbit anti-thymoglobulin; CNI, calcineurin inhibitor.
CFH haplotype H3 in the homozygous state.
Occurred after eculizumab prophylaxis discontinuation.
Recurrence Rates and Risk Factors
Clinical and subclinical aHUS recurrence occurred after 30 (40.5%) and 9 (12.1%) of the transplantations without eculizumab prophylaxis, respectively. The hallmark hematologic features of aHUS never occurred under ongoing eculizumab prophylaxis, except in one patient after eculizumab withdrawal. However, graft biopsies disclosed TMA lesions in three other patients, despite uninterrupted eculizumab prophylaxis. Overall, the recurrence rate, including clinical and subclinical forms, was significantly lower in the prophylaxis group than in the other group (Figure 1A, Table 1). To identify the risk factors associated with aHUS recurrence, univariate (Supplemental Figure 2) and multivariate (Figure 1B) analyses were conducted, including the following variables: the type of prophylaxis (plasma alone/eculizumab), type of donors (ECD), risk stratification (high versus moderate/low), history of relapse in previous transplantation, complement abnormalities, cold-ischemia time, and biopsy-proven acute rejection (BPAR). Notably, no difference was observed in terms of the recurrence rate between moderate- and high-risk transplantations (Figure 1A), but the latter were treated more often with eculizumab prophylaxis (Table 1). To eliminate confounding effects, ECD, BPAR, risk stratification, and plasma or eculizumab prophylaxis were entered into the Cox model (Figure 1B). A high-risk assessment (HR=3.94, P<0.001) and prophylactic eculizumab (HR=0.05, P<0.001) were independently associated with an increased and reduced risk of recurrence, respectively.
Treatment of Overt aHUS Recurrence with Eculizumab
The three patients who developed mild TMA lesions upon eculizumab prophylaxis were maintained with the same regimen. In addition, 29 patients started eculizumab after a median time of 32 (range: 0–690) days after the occurrence of post-transplant aHUS recurrence, with the clinical features of TMA occurring in 24 of the patients. Eculizumab was given either as a first-line therapy (n=12) or because plasma therapy had failed to obtain the full and/or sustained remission of aHUS (n=17). Recurrence that was treated with eculizumab occurred in 19 and 10 high- and moderate-risk transplantations, respectively. Eculizumab therapy significantly improved death-censored graft survival when compared with that in patients with aHUS recurrence who were treated with plasmapheresis alone (Supplemental Figure 3), even when eculizumab treatment was delayed by >1 week after aHUS recurrence (Supplemental Figure 3). However, early-onset eculizumab treatment tended to be associated with better graft survival than delayed treatment (Supplemental Figure 3). Despite the improved graft outcomes in the eculizumab era, aHUS recurrence nonetheless remained associated with an accelerated graft-loss rate (Figure 2A).
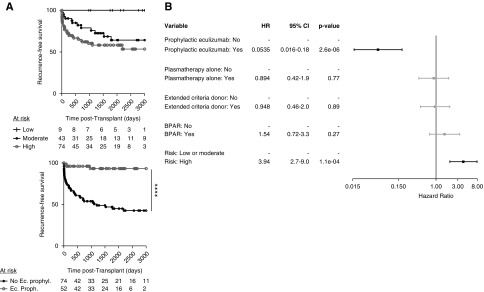
Figure 2.
Risk factors for death-censored graft loss. (A) Death-censored graft according to the use of eculizumab, either as a prophylaxis or as a treatment after aHUS recurrence (top panel). Death-censored graft survival according to the occurrence of aHUS recurrence (bottom panel). Log-rank test, *P<0.05; **P<01; ***P<0.001. (B) Results of multivariate Cox regression models for HRs and 95% CIs. AMR, antibody-mediated rejection; D0, day zero; DSA, donor-specific antibody; No Ec., no eculizumab treatment at any time in the post-transplant course; No recur., no recurrence; Post-Tx. Ec., use of eculizumab after post-transplant aHUS recurrence; Prophyl. Ec., eculizumab prophylaxis; Recur., recurrence.
Risk Factors for Graft Loss
Importantly, graft survival was significantly better in patients with eculizumab prophylaxis than in those who started eculizumab after aHUS recurrence (P<0.02) (Figure 2A). We next explored the risk factors associated with kidney graft loss in patients with aHUS. In univariate analysis (Supplemental Figure 4), aHUS recurrence (HR=3.26, P=0.002), ECD kidney allografts (HR=2.17, P=0.04), donor age (HR=1.04, P=0.005), BPAR (HR=2.63, P=0.009), preformed donor-specific antibodies (HR=2.64, P=0.02), and biopsy-proven, antibody-mediated rejection (HR=2.63, P=0.009) were significantly associated with an increased risk of graft failure; whereas eculizumab prophylaxis was protective against graft failure (HR=0.13, P=0.006). In multivariate analysis, aHUS recurrence (HR=4.08, P<0.02) and eculizumab prophylaxis (HR=0.19, P=0.04) remained independently associated with the increased and decreased risk of graft loss, respectively; whereas preformed donor-specific antibodies (HR=3.03, P=0.06) and postrecurrence eculizumab treatment (HR=0.31, P=0.05) fell short of statistical significance (Figure 2B).
Treatment Tailoring Based on the Risk Stratification
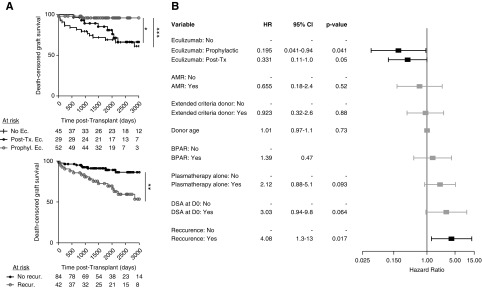
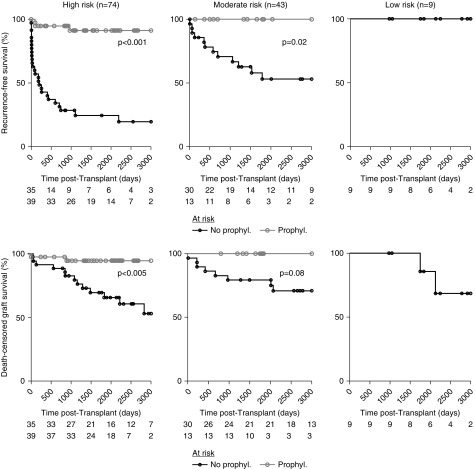
We further investigated the effect of individualized management on transplant outcomes. Eculizumab was approved by the European and French regulatory authorities in 2011, after which a national meeting was held to provide recommendations that subsequently spread throughout the country and were published.10 Since then, eculizumab prophylaxis has been used in the high- and moderate-risk groups accordingly, but it has not yet been used in the low-risk groups (Figure 3, A and B). Eculizumab was associated with both a reduced recurrence rate and better graft survival compared with those of patients who did not receive eculizumab prophylaxis (Figures 1 and 2). In fact, the rate of post-transplant recurrence decreased after 2011 in parallel with the use of eculizumab prophylaxis (Figure 3A). However, subgroup analysis disclosed heterogeneity within the high- and moderate-risk groups (Figure 3B). After the recommendations were issued, eculizumab prophylaxis was used in 37/49 (75.5%), 8/9 (89%), and only 3/11 (27%) of high-risk transplantations, moderate-risk transplantations with complement abnormalities, and moderate-risk transplantations without complement abnormalities, respectively (Figure 3B). However, it is worth noting that those without pathogenic variants in genes encoding circulating complement regulators (i.e., CFH, CFI, C3, and CFB) but who were harboring two copies of the at-risk CFH haplotype displayed the same recurrence rate as the CFI-variant carriers (Supplemental Figure 5). Although eculizumab prophylaxis significantly decreased the aHUS recurrence rate within the high- and moderate-risk groups (Figure 4), it was more compellingly and significantly associated with improved graft survival in the high-risk subgroup than in the moderate-risk group (Figure 4).
Figure 3.
Compliance with recommendations for tailored therapy based on individualized risk stratification. (A) Frequency of kidney transplantations performed every year, for which the post-transplant course was complicated by aHUS recurrence (black circles) and for which eculizumab prophylaxis was administered (gray circles). (B) Number of kidney transplantations with or without eculizumab prophylaxis over time, according to the risk stratification. KTx, kidney transplantations.
Figure 4.
Recurrence and graft loss rates, according to risk stratification, genetic background, and the use of eculizumab prophylaxis. Recurrence-free and death-censored graft survivals, according to the pretransplant risk stratification and the use of eculizumab prophylaxis. Prophyl., use of eculizumab prophylaxis; No prophyl., no use of eculizumab prophylaxis.
Eculizumab Discontinuation
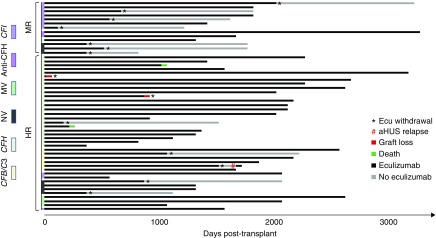
Treatment duration remains controversial, but preliminary data suggest that genetic background may critically determine the risk of relapse after treatment cessation. However, information about eculizumab withdrawal after transplantation is very scarce. We aimed to investigate whether the risk stratification also determined the rate and timing of eculizumab discontinuation. In the prophylactic eculizumab group, eculizumab was discontinued in 12 patients with a functioning allograft and in two others at the time of graft loss (Figure 5). Among those with a living allograft, the discontinuation rate was significantly lower in the high-risk than in the moderate-risk groups (5/37 [13.5%] versus 7/13 [53.9%], P=0.007). One relapse of aHUS occurred after prophylaxis interruption in the high-risk transplantations, but none occurred in their moderate-risk counterparts (Figure 5).
Figure 5.
Discontinuation of prophylactic eculizumab therapy. Courses of prophylactic eculizumab therapies, according to the risk stratification and genetic background. Ecu, eculizumab; HR, high-risk; MR, moderate risk; MV, multiple complement variants; NV, no genetic variants.
In the postrecurrence eculizumab group, eculizumab was discontinued in four patients with a functioning allograft and in five others at the time of graft loss (Supplemental Figure 6). Among the four patients with a living allograft, two experienced aHUS relapse after 36 and 329 days, respectively, leading to graft failure soon after, despite eculizumab reintroduction (Supplemental Figure 6).
Dramatic Changes in the aHUS Epidemiology at a Nationwide Level
A total of 397 adult individuals with aHUS, alive between January 1, 2007 and January 1, 2016 were included in the cohort (Figure 6A, Table 2). The renal status of each patient was monitored over time (Figure 6B). New adult-onset patients (n=175) and those who turned 18 years old (n=43) were included in the cohort, whereas the patients who were lost to follow-up (n=33) and who died (n=39) exited the cohort (Figure 6A). The positive balance of patients who entered and exited the cohort resulted in a gain of 146 adult patients with aHUS who were identified over a decade (Figure 6A). Most of the patients who were lost to follow-up were those with functioning native kidneys (Supplemental Figure 7A), with either no mutations or with MCP mutations (Supplemental Figure 7B). In contrast, those who died during the study period were primarily receiving dialysis (Supplemental Figure 7A) and most frequently had CAP dysregulation (Supplemental Figure 7B).
Figure 6.
Population-based aHUS cohort study. (A) Flowchart depicting the selection process of the cohort from the French aHUS registry. The patients who died or were lost to follow-up before the beginning of the study period (January 1, 2007) and those who developed the disease after the end of the study period (January 1, 2016) were excluded from the study. Similarly, the patients with child-onset disease who did not turn 18 years old before the end of the study were not included. The bottom panel shows the 397 patients enrolled in the French adult aHUS cohort over 10 years, including 218 and 72 patients entering and exiting the cohort, respectively, during the study. (B) Renal status, according to genetic background, among the whole cohort of 397 adult patients with aHUS.
Table 2.
Characteristics of the study 2 population
| Characteristics of the Adult aHUS Cohort (January 1, 2007 to January 1, 2016) | Data |
|---|---|
| Total number of patients in the cohort | 397 |
| Female/male sex ratio | 254/143 |
| Age at time of aHUS onset (median yr [range]) | 29.3 (0.1–79.7) |
| Child-onset aHUS (%) | 82 (20.6) |
| Age when entering the 2007–2016 cohort (median yr [range]) | 34.5 (18–79.7) |
| Complement/DGKE abnormality (%) | 260 (65.5) |
| CFH | 99 (24.9) |
| CFI | 38 (9.6) |
| CFB | 2 (0.5) |
| C3 | 40 (10.1) |
| MCP | 45 (11.3) |
| DGKE | 4 (1.0) |
| Combined CAP abnormalities | 8 (2.0) |
| Anti-CFH antibody | 24 (6.0) |
| Patients who reached ESKD before the end of the study period (%) | 224 (56.4) |
| Death before the end of the study period (%) | 39 (9.8) |
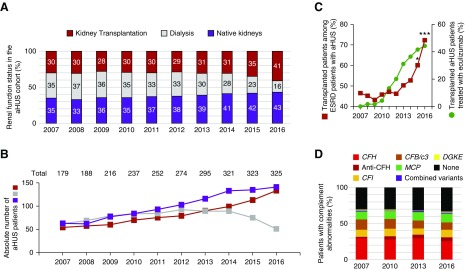
We aimed to explore the effect of eculizumab use on aHUS epidemiology. Since 2012, the proportion and absolute numbers of patients who had aHUS with functional kidney allografts and who were receiving long-term dialysis have been steadily increasing and decreasing, respectively (Figure 7, A and B). Between 2012 and 2016, the proportion of patients who received a transplant among the patients with aHUS and ESKD increased from 46.2% to 72.3% (P<0.001) (Figure 7C). In addition, the proportion of the patients who received a transplant who were actively treated with eculizumab increased from 19.0% in 2012 to 44.4% in 2016 (Figure 7C). Over the same period, the frequency of complement abnormalities remained remarkably constant among the French aHUS cohort (Figure 7D).
Figure 7.
Growing frequency of recipients of kidney transplants among patients with aHUS and with ESKD paralleled the use of eculizumab. (A) Frequencies and (B) absolute numbers of patients with aHUS with functioning kidney allografts (red), who were under dialysis (light gray), or with functioning native kidneys (purple) over time. The numbers of living patients on January 1 of each year are reported. The total number of patients with aHUS for each year is indicated above the bars in (B). Notably, any transient change in the renal status (such as an immediate transplant failure) or short-term eculizumab treatment that did not continue over the following year was not captured in the census conducted on January 1 of each year. (C) Frequency of patients receiving a transplant among the patients with aHUS and ESKD (left y axis) and the frequency of recipients of kidney transplants who were treated with eculizumab on January 1 of each year (right y axis). The Fischer exact test was used to compare the frequencies between 2012 and the subsequent years; *P<0.05, ***P<0.001. (D) Abnormalities in the CAP over time in the French aHUS cohort.
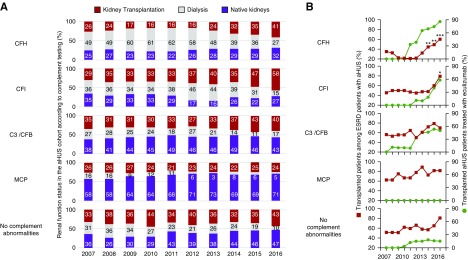
Importantly, the renal epidemiology varied greatly across the different genes that were involved (Figure 8). Whereas the proportion of CFH-variant carriers who were on dialysis was approximately 60% before the eculizumab era, it decreased to 27% in January 2016 (Figure 8A). Meanwhile, the proportion of patients who had the CFH variant and a functioning allograft, 85.7% of whom were given eculizumab in early 2016, increased from 21.7% to 60.3% of the patients with ESKD (Figure 8B). Similarly, patients with variants in CFI, C3/CFB, or with no mutations represented a growing fraction of patients who received a transplant (Figure 8A). In contrast, patients who received a renal transplant and who had the MCP variant were not treated with eculizumab (Figure 8B), and their proportion barely changed over this period (Figure 8, A and B).
Figure 8.
Renal status over time according to complement genetics and eculizumab therapy. (A) Frequencies of patients with aHUS with functioning kidney allografts (red), who were under dialysis (light gray), or with functioning native kidneys (purple), according to the genetic background, over time. (B) Frequency of patients who received transplants among the patients with aHUS and ESKD (left y axis), and the frequency of recipients of kidney transplants who were treated with eculizumab on January 1 of each year (right y axis), according to the genetic background. The Fischer exact test was used to compare the frequencies between 2012 and the subsequent years; *P<0.05, **P<0.01, ***P<0.001.
Discussion
The nationwide studies described here demonstrate that the outcome of kidney transplantation in patients with aHUS has dramatically improved since the approval of eculizumab. Moreover, we show there have been unprecedented and compelling changes in the epidemiology of aHUS. With the exception of the carriers of the MCP variant, for whom the post-transplant outcome was not affected by eculizumab use,18 the frequency of patients who received a transplant among the patients with aHUS sharply increased between 2012 and 2016, with a concurrent decrease in the population who were receiving dialysis.
Nearly 60% of the deaths among patients with aHUS during the study period occurred in patients undergoing chronic dialysis. In this respect, it is worth noting that the proportion of patients with aHUS with a pathogenic variant of CFH who received chronic dialysis was as high as 60% until 2012. A voluntarist policy of kidney transplantation in the aHUS population should eventually save life years, especially among patients with CFH variants, and should therefore be strongly advocated.
This paradigm shift resulted from the implementation of an individualized risk stratification and a highly tailored therapeutic strategy based on complement blockade. The recurrence and graft loss rates in patients without eculizumab prophylaxis confirmed the risk stratification that was implemented in France in late 2011 and was adopted by the 2016 KDIGO accurately identified three groups of patients with significantly different post-transplant outcomes. Multivariate analysis confirmed that patients who were high risk, based on a pretransplant risk assessment, were independently associated with a greater risk of aHUS recurrence in comparison with that of the patients with a moderate/low risk. The aHUS recurrence rates were approximately 50% and 80% in the moderate- and high-risk groups, respectively, supporting the use of prophylactic treatment, at least formally, in the latter. In contrast, the lack of recurrence among the carriers of the isolated variants of the MCP14 or DGKE15 genes, encoding for membrane-anchored proteins, definitely emphasized the uselessness of prophylaxis in this low-risk subpopulation. Prophylactic eculizumab significantly improved graft survival, especially for high-risk transplantations.
The rationale for prophylaxis, instead of therapy for overt recurrence, stemmed from three observations. First, aHUS recurrence usually occurs very early in the post-transplant course3 and is likely precipitated by an ischemia-reperfusion–induced endothelial insult.6,18 Second, the renal function recovery upon eculizumab therapy for an overt aHUS episode is less striking in the recipients of kidney transplants than in patients who have not received a transplant.19,20 Indeed, our study showed a better graft survival in patients treated with prophylactic therapy compared with those who received eculizumab after a recurrence. Similarly, the recent Global Registry analysis found significantly better early graft function in patients with prophylactic eculizumab treatment compared with that in patients who began eculizumab treatment post-transplant.13 However, in this respect, it was previously shown that the recovery of renal graft function critically depended on the timing of eculizumab administration after aHUS recurrence.11 Our current study also showed that the administration of eculizumab within a week after aHUS recurrence tended to be associated with better outcomes than delayed treatment. One may speculate that prompt initiation of eculizumab after aHUS recurrence would decrease the differences in terms of graft survival between prophylaxis and postrecurrence treatment. Third, “cryptic aHUS,”21 which is defined by the progression of a TMA process in the absence of the hallmark hematologic features, is increasingly recognized.5,11 Subintrant TMA may lead to irreversible renal injury that is discovered late during the process, including at the time of an impending graft failure.9,21,22
However, the use of prophylactic complement blockade therapy was challenged by others23 who were legitimately concerned about the very high cost of the treatment. A Dutch study explored alternative strategies, including living donation, low-dose calcineurin inhibitors, and strict BP control, with the goal of minimizing the burden of the endothelial-damaging factors that trigger the onset of the disease. A total of 17 living-donor kidney transplantations, 15 of which met the qualifying high-risk criteria, were performed in patients with aHUS, and only one of these patients experienced a recurrence.23 Although these results are very intriguing, they still call for caution. In our cohort, among the 17 patients who received a living-donor kidney after 2007, ten fulfilled the high-risk criteria, and seven of these patients were accordingly given eculizumab prophylaxis. The three prophylaxis-free patients harbored a pathogenic variant in the CFH gene. Two of the patients experienced a recurrence, eventually leading to graft loss at 559 and 1289 days post-transplant.
We must acknowledge that the conclusions drawn from a retrospective nationwide study may be limited by heterogeneous management and confounding factors across centers. However, this study also demonstrates that the recommendations issued in 2011 to promote treatment tailoring based on complement analysis have been broadly incorporated into clinical practice throughout the country. The use of prophylactic eculizumab was more frequent in the high-risk transplantations than in the moderate-risk transplantations and, among the latter, prophylaxis was primarily used in those with genetic abnormalities. In addition, eculizumab prophylaxis was withdrawn more frequently and earlier in the moderate-risk transplantations than in the high-risk transplantations. As a consequence, the proportion of patients who received a transplant and who were treated with eculizumab greatly varied across the patients with different genetic backgrounds. Our data also suggest that risk stratification could be further refined based on the presence or absence of a homozygous at-risk CFH haplotype (CFH-H3; tgtgt). The patients with neither pathogenic variants in circulating complement regulators (CFH, CFI, C3/CFB) nor two copies of the at-risk CFH haplotype had a reduced recurrence rate, consistent with a previous report.22 This suggests that eculizumab prophylaxis may be dispensable in this subset of patients. A growing amount of data indicates that the treatment duration might also be tailored based on genetic background.24–26 Our data suggest eculizumab prophylaxis could be safely interrupted, at least in moderate-risk transplantations. This study lays important groundwork for a future prospective randomized trial that aims to address treatment duration based on risk stratification. In this respect, significant clues could also be obtained by the propensity score–matching comparison of patients with long-term versus short-term treatment, irrespective of risk stratification, across countries using different treatment policies.
In this study, we estimate the use of eculizumab in 81 patients, 52 of whom were given prophylaxis, corresponded to 336 patient years of treatment, for a total cost of >150 million euro. We acknowledge that the high cost of eculizumab may limit its broad use. We previously established that its costs far exceeded those of plasma therapy, except for the youngest children,11 but a comprehensive cost-utility analysis including the assessment of quality-adjusted life years and preserved ability to work has yet to be performed. Proper evaluation of the optimal duration of treatment according to the genetic background may also provide clues to limit the expenses. Furthermore, one may expect upcoming changes in pricing when promising new C5 blockers enter the market.
In summary, this study strongly supports the use of eculizumab prophylaxis in those for whom complement genetics and a past medical history predict a high and moderate risk of post-transplant recurrence. Similarly, our study suggests eculizumab prophylaxis could be interrupted based on an individual risk assessment.
Disclosures
Prof. Zuber reports personal fees from Alexion Pharmaceuticals France, outside of the submitted work. Dr. M. Frimat reports grants from AIRG (Association pour l’Information et la Recherche sur les Maladies Rénales Génétiques) France, outside of the submitted work. Prof. Kamar reports personal fees from Abbvie, Amgen, Chiesi, Fresenius Medical Care, Gilead, Merck Sharp and Dohme (MSD), Neovii, Novartis, Roche, Sanofi, and Shire, oustide of the submitted work. Prof. Thierry reports personal fees from Chiesi, personal fees from Astellas, personal fees from Sanofi, outside of the submitted work. Dr. Delmas reports personal fees from Alexion and personal fees from Sanofi Ablynx, outside of the submitted work. Prof. Massy reports grants and other from Amgen, other from Sanofi-Genzyme, grants from the French Government, grants from Amgen, grants from MSD, grants from Sanofi-Genzyme, grants from GlaxoSmithKline, grants from Lilly, grants from Fresenius Medical Care, grants and other from Baxter, grants from Outsuka, other from Daiichi, and other from Astellas, outside of the submitted work. Prof. Loirat reports personal fees from Alexion and personal fees from Roche, outside of the submitted work. Prof. Fakhouri reports personal fees from Alexion Pharmaceutic. Prof. Fremeaux-Bacchi reports grants, personal fees, and other from Alexion Pharmaceuticals, outside of the submitted work.
Funding
Prof. Zuber’s research is supported by the Emmanuel Boussard Foundation. Prof. Zuber, Prof. Fakhouri, Prof. Loirat, Prof. Rondeau, Prof. Le Quintrec, and Prof. Frémeaux Bacchi received fees from Alexion Pharmaceuticals for invited lectures and/or board membership. Prof. Frémeaux Bacchi is the recipient of a research grant from Alexion Pharmaceuticals.
Supplementary Material
Acknowledgments
Prof. Zuber, Dr. M. Frimat, Prof. Caillard, Prof. Kamar, Dr. Gatault, Prof. Couzi, Prof. Jourde-Chiche, Dr. Chatelet, Dr. Bertrand, Dr. Sberro Soussan, Prof. L. Frimat, Dr. Colosio, Prof. Thierry, Dr. Rivalan, Dr. Albano, Dr. Arzouk, Dr. Cornec-Le Gall, Dr. Elias, Dr. El Karoui, Dr. Chauvet, Dr. Rerolle, Dr. Delmas, Prof. Hourmant, Prof. Loirat, Prof. Fakhouri, Prof. Pouteil Noble, Prof. Peraldi, Prof. Legendre, Prof. Rondeau, Prof. Le Quintrec, and Prof. Frémeaux Bacchi designed the study. Prof. Zuber, Dr. M. Frimat, Prof. Caillard, Prof. Kamar, Dr. Gatault, Dr. Petitprez, Prof. Couzi, Prof. Jourde-Chiche, Dr. Chatelet, Dr. Gaisne, Dr. Bertrand, Prof. Bamoulid, Dr. Louis, Dr. Sberro Soussan, Dr. Westeel, Prof. L. Frimat, Dr. Colosio, Prof. Thierry, Dr. Rivalan, Dr. Albano, Dr. Arzouk, Dr. Cornec-Le Gall, Dr. Claisse, Dr. Elias, Dr. El Karoui, Dr. Chauvet, Dr. Coindre, Dr. Rerolle, Dr. Tricot, Dr. Sayegh, Dr. Garrouste, Dr. Charasse, Dr. Delmas, Prof. Massy, Prof. Hourmant, Dr. Servais, Prof. Loirat, Prof. Fakhouri, Prof. Pouteil Noble, Prof. Peraldi, Prof. Legendre, Prof. Rondeau, Prof. Le Quintrec, and Prof. Frémeaux Bacchi collected and analyzed the data. Prof. Zuber and Dr. Petitprez performed statistical analyses and drew figures. Prof. Zuber drafted the manuscript which was revised and approved by all coauthors.
The investigators are grateful to all of our colleagues who agreed to share updated information about their patients, namely Prof. Jean-François Augusto, Dr. Véronique Baudouin, Dr. Guillaume Bobrie, Prof. Bernard Boudailliez, Dr. Catherine Boudray, Prof. Olivia Boyer, Dr. Laura Braun-Parvez, Dr Françoise Broux, Prof. Matthias Buchler, Ms. Marion Causeret, Prof. Gabriel Choukroun, Dr. Anne-Laure Faller, Dr. Thomas Guincestre, Dr. Maxime Hoffmann, Dr. Aurélie Hummel, Dr. Josiane Joule, Dr. Aurélie Le Guillou, Prof. Christophe Mariat, Dr. Marie Matignon, Dr. Vincent Meunier, Dr. Clement Mottola, Prof. Christiane Mousson, Dr. Alain Nony, Dr. Marion Sallee, Dr. Johan Schikowski, Dr. Séverine Poulain, Dr. François Provost, Dr. Pascale Siohan, Dr. Elisabeth Tomkiewicz, Prof. Michel Tsimaratos, Prof. Tim Ulinski, Dr. Marc Ulrich, Dr. David Verhelst, Dr. Laurence Vrigneaud, and Dr. Alain Wynckel.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019040331/-/DCSupplemental.
Supplemental Figure 1. Flow-chart of Study 1.
Supplemental Figure 2. Risk factors for aHUS post-transplant recurrence, univariate analysis.
Supplemental Figure 3. Death-censored graft survival after aHUS recurrence, according to eculizumab treatment and its timing.
Supplemental Figure 4. Risk factors for graft loss, univariate analysis.
Supplemental Figure 5. Recurrence-free survival in moderate-risk transplantations, according to genetic background, in the absence of eculizumab prophylaxis.
Supplemental Figure 6. Discontinuation of post-recurrence eculizumab therapy.
Supplemental Figure 7. Renal status and complement genetic background for the patients who left the cohort during the study period.
Supplemental Table 1. Detailed clinical, immunological and genetic information used to assign each transplantation to a given risk category.
References
- 1.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C: Haemolytic uraemic syndrome [published correction appears in Lancet 390: 648, 2017]. Lancet 390: 681–696, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Le Quintrec M, Zuber J, Moulin B, Kamar N, Jablonski M, Lionet A, et al.: Complement genes strongly predict recurrence and graft outcome in adult renal transplant recipients with atypical hemolytic and uremic syndrome. Am J Transplant 13: 663–675, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Matar D, Naqvi F, Racusen LC, Carter-Monroe N, Montgomery RA, Alachkar N: Atypical hemolytic uremic syndrome recurrence after kidney transplantation. Transplantation 98: 1205–1212, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Sheerin NS, Kavanagh D, Goodship TH, Johnson S: A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM 109: 27–33, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Zuber J, Le Quintrec M, Morris H, Frémeaux-Bacchi V, Loirat C, Legendre C: Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando) 27: 117–125, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bresin E, Daina E, Noris M, Castelletti F, Stefanov R, Hill P, et al.: International Registry of Recurrent and Familial HUS/TTP : Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: Prognostic significance of genetic background. Clin J Am Soc Nephrol 1: 88–99, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al.; Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al.: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G : Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 8: 643–657, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Zuber J, Le Quintrec M, Krid S, Bertoye C, Gueutin V, Lahoche A, et al.: French Study Group for Atypical HUS : Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant 12: 3337–3354, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, et al.: Conference Participants : Atypical hemolytic uremic syndrome and C3 glomerulopathy: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 91: 539–551, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Siedlecki AM, Isbel N, Vande Walle J, James Eggleston J, Cohen DJ; Global aHUS Registry : Eculizumab use for kidney transplantation in patients with a diagnosis of atypical hemolytic uremic syndrome. Kidney Int Rep 4: 434–446, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresin E, Rurali E, Caprioli J, Sanchez-Corral P, Fremeaux-Bacchi V, Rodriguez de Cordoba S, et al.: European Working Party on Complement Genetics in Renal Diseases : Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol 24: 475–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, et al.: Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, et al.: Genetics and outcome of atypical hemolytic uremic syndrome: A nationwide French series comparing children and adults. Clin J Am Soc Nephrol 8: 554–562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira-Martins P, El Sissy C, Bordereau P, Gruber A, Rosain J, Fremeaux-Bacchi V: Defining the genetics of thrombotic microangiopathies. Transfus Apheresis Sci 54: 212–219, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Frémeaux-Bacchi V, Legendre C: New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol 7: 23–35, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Fakhouri F, Hourmant M, Campistol JM, Cataland SR, Espinosa M, Gaber AO, et al.: Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: A single-arm, open-label trial. Am J Kidney Dis 68: 84–93, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, et al.: Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int 87: 1061–1073, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belingheri M, Possenti I, Tel F, Paglialonga F, Testa S, Salardi S, et al.: Cryptic activity of atypical hemolytic uremic syndrome and eculizumab treatment. Pediatrics 133: e1769–e1771, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Le Quintrec M, Zuber J, Noel LH, Thervet E, Frémeaux-Bacchi V, Niaudet P, et al.: Anti-Factor H autoantibodies in a fifth renal transplant recipient with atypical hemolytic and uremic syndrome [published correction appears in Am J Transplant 9: 2205 and 2647, 2009. Am J Transplant 9: 1223–1229, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Duineveld C, Verhave JC, Berger SP, van de Kar NCAJ, Wetzels JFM: Living donor kidney transplantation in atypical hemolytic uremic syndrome: A case series. Am J Kidney Dis 70: 770–777, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Ardissino G, Testa S, Possenti I, Tel F, Paglialonga F, Salardi S, et al.: Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: A report of 10 cases. Am J Kidney Dis 64: 633–637, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Fakhouri F, Fila M, Provôt F, Delmas Y, Barbet C, Châtelet V, et al.: Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol 12: 50–59, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA: Eculizumab cessation in atypical hemolytic uremic syndrome. Blood 130: 368–372, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.