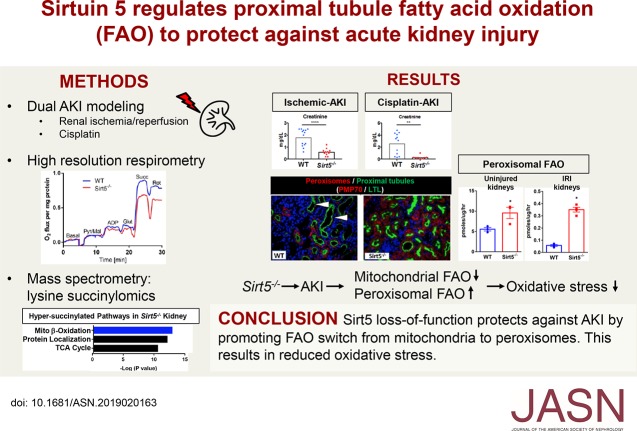
Significance Statement
Proximal tubular epithelial cells, a primary site of injury in AKI, are rich in mitochondria and peroxisomes, the two organelles that mediate fatty acid oxidation. Deletion of Sirtuin 5 (Sirt5) reverses posttranslational lysine acylation of several enzymes involved in fatty acid oxidation. The authors demonstrate that mice lacking Sirt5 had significantly improved kidney function and less tissue damage following either ischemia-induced or cisplatin-induced AKI compared with wild-type mice. These differences coincided with higher peroxisomal fatty acid oxidation compared with mitochondria fatty acid oxidation in the Sirt5-deficient proximal tubular epithelial cells. Their findings indicate that Sirt5 regulates the balance of mitochondrial versus peroxisomal fatty acid oxidation in proximal tubular epithelial cells to protect against injury in AKI. This novel mechanism has potential therapeutic implications for treating AKI.
Keywords: acute kidney injury, Sirtuins, Peroxisome, mitochondria, Fatty acid oxidation, Post translational modifications
Visual Abstract
Abstract
Background
The primary site of damage during AKI, proximal tubular epithelial cells, are highly metabolically active, relying on fatty acids to meet their energy demands. These cells are rich in mitochondria and peroxisomes, the two organelles that mediate fatty acid oxidation. Emerging evidence shows that both fatty acid pathways are regulated by reversible posttranslational modifications, particularly by lysine acylation. Sirtuin 5 (Sirt5), which localizes to both mitochondria and peroxisomes, reverses post-translational lysine acylation on several enzymes involved in fatty acid oxidation. However, the role of the Sirt5 in regulating kidney energy metabolism has yet to be determined.
Methods
We subjected male Sirt5-deficient mice (either +/− or −/−) and wild-type controls, as well as isolated proximal tubule cells, to two different AKI models (ischemia-induced or cisplatin-induced AKI). We assessed kidney function and injury with standard techniques and measured fatty acid oxidation by the catabolism of 14C-labeled palmitate to 14CO2.
Results
Sirt5 was highly expressed in proximal tubular epithelial cells. At baseline, Sirt5 knockout (Sirt5−/−) mice had modestly decreased mitochondrial function but significantly increased fatty acid oxidation, which was localized to the peroxisome. Although no overt kidney phenotype was observed in Sirt5−/− mice, Sirt5−/− mice had significantly improved kidney function and less tissue damage compared with controls after either ischemia-induced or cisplatin-induced AKI. This coincided with higher peroxisomal fatty acid oxidation compared with mitochondria fatty acid oxidation in the Sirt5−/− proximal tubular epithelial cells.
Conclusions
Our findings indicate that Sirt5 regulates the balance of mitochondrial versus peroxisomal fatty acid oxidation in proximal tubular epithelial cells to protect against injury in AKI. This novel mechanism might be leveraged for developing AKI therapies.
Proximal tubular epithelial cells (PTECs) are highly sensitive to damage after AKI largely because of its high metabolic rate.1,2,3 To meet energy demands, PTECs rely on fatty acid β-oxidation (FAO). Parallel FAO pathways exist in mitochondria and in peroxisomes, both of which are notably abundant in PTECs. Mitochondrial FAO directly links to the mitochondrial electron transport chain and is an oxygen-intensive source of energy. Concomitant with oxygen utilization is the formation of reactive oxygen species (ROS), which are thought to be particularly damaging during ischemic- and cisplatin-induced AKI.4,5 Limiting mitochondrial FAO limits ischemic injury in the heart6,7 and kidney.8
In contrast to mitochondria, little is known about the role of peroxisomes in AKI. Although peroxisomes do not produce energy, they metabolize long-chain fatty acids to acetate and other short-chain products. Because of their hydrophilicity, the metabolites can cross plasma membranes to either leave the cell or enter mitochondria to be oxidized to completion. Peroxisomes have been observed to physically interact with mitochondria and ablation of peroxisomal function has secondary effects on mitochondrial function.9 Peroxisomes may serve as a sink for mitochondrially produced ROS because of the abundance of catalase and other ROS-neutralizing enzymes in the peroxisome.10,11 Peroxisomes may also serve to protect PTECs from the accumulation of toxic long-chain fatty acids.12
Emerging evidence shows both FAO pathways are regulated by reversible post-translational modifications (PTMs), in particular by lysine acylation and the sirtuin deacylase enzymes that remove these PTMs.13,14 Mammalian sirtuins Sirt1–Sirt7 differ in their subcellular localization and substrate specificity. The nuclear/cytosolic sirtuin Sirt1 has been shown to protect against AKI by maintaining peroxisomes, upregulating catalase, and eliminating renal ROS.15 The mitochondrial sirtuin Sirt3 is also renoprotective through its role in improving mitochondrial dynamics.16 Although its role in regulating kidney FAO is not clear, Sirt3 promotes mitochondrial FAO in liver and heart.14,17 Sirt5, which is unique among the sirtuins in its substrate preference for succinyllysine, malonyllysine, and glutaryllysine,18,19 also promotes mitochondrial FAO in liver and heart.13,20 Intriguingly, Sirt5 was recently shown to localize to peroxisomes. In direct contrast to its effect on mitochondrial FAO, Sirt5 was observed to suppress peroxisomal FAO in vitro and in rodent liver.21 However, the role of Sirt5 in the kidney has not yet been studied. Here, we report that Sirt5−/− mice are protected against ischemic and cisplatin AKI. Although mitochondrial function is moderately suppressed in Sirt5−/− kidney, peroxisomal function is enhanced both before and after AKI. These results indicate that Sirt5 regulates the balance of mitochondrial versus peroxisomal FAO in PTECs to protect against PTEC injury in AKI. This novel mechanism may be leveraged for developing future AKI therapies.
Methods
Animals
Homozygous global Sirtuin 5 knockout mice (Sirt5−/−)22 were obtained from the Jackson Laboratory (B6;129-Sirt5tm1Fwa/J, stock no. 012757). B6/129S F2 strain wild-type (WT) mice were used as controls. To produce appropriate littermate controls Sirtuin 5 heterozygous mutant mice (Sirt5+/−) were bred together to produce WT (Sirt5+/+), Sirt5+/−, and Sirt5−/− mutants. Age-matched 10- to 14-week-old male mice were used throughout the study. The University of Pittsburgh Institutional Animal Care and Use Committee approved all experiments (approval no. 16088935).
Ischemic and Cisplatin AKI Models in Mice
Ischemic AKI was induced by a renal ischemia-reperfusion injury (IRI) model as previously described,23 with modifications. Briefly, mice were anesthetized with inhalant 2% isoflurane. Core body temperature of the mice was monitored with a rectal thermometer probe and was maintained at 36.8–37.2°C throughout the procedures with a water-heating circulation pump system (EZ-7150; BrainTree Scientific) and an infrared heat lamp (Shat-R-Shield). Buprenorphine (Par Pharmaceutical) was administered for pain control (0.1 mg/kg body wt, administered subcutaneously). With aseptic techniques, a dorsal incision was made to expose the kidney, and renal ischemia for 22 minutes was induced by unilateral clamping of the left kidney pedicle with a nontraumatic microvascular clamp (18055–04; Fine Science Tools). Renal reperfusion was visually verified. Delayed contralateral nephrectomy of the right kidney was performed at day 6.23 Mice were euthanized at day 7 to harvest blood and the injured kidney. Serum was separated from the blood and analyzed by the Kansas State Veterinary Diagnostic Laboratory for levels of creatinine and BUN.
To induce cisplatin AKI, mice were given a single dose of 20 mg/kg intraperitoneal cisplatin (Fresenius Kabi) or vehicle control of normal saline as described previously.24 Mice were euthanized on day 3 to harvest blood and the kidneys.
Cultured PTECs
Primary mouse PTECs were isolated from single-cell kidney suspension of 10- to 14-week-old male Sirt5−/−, Sirt5+/−, B6/129 strain WT, or 129 strain WT mice by Dynabeads FlowComp Flexi Kit (Thermo Fisher Scientific) conjugated to lotus tetragonolobus lectin (LTL) (L-1320; Vector Laboratories). Passage 3–6 PTECs were used for experiments. Human kidney proximal tubular epithelial cells (hPTECs) were obtained from American Type Culture Collection (Manassas, VA). Both types of cells were cultured as previously described,25 with modifications.
Mouse primary PTECs were plated at 106 cells per well and, under serum-restricted conditions, exposed to 24 hours of normoxia or hypoxia (FiO2 1%) in the hypoxia chamber (Coy Laboratory) or treated with 24 hours of 20 µM cisplatin or vehicle control of normal saline.
hPTECs were maintained in a 1:1 mix of DMEM (Thermo Fisher Scientific) and Ham F12 (Thermo Scientific) containing 5 mM glucose and supplemented with 2 mM GlutaMAX (Thermo Scientific), 1× insulin-transferrin-selenium (Gibco), 36 ng/ml hydrocortisone, and 0.1% (v/v) penicillin/streptomycin at 37°C in 5% CO2.
To knockdown Sirt5, the plasmid containing Sirt5 small interfering RNA (siRNA; 16708; Ambion) and the control plasmid (scrambled, AM4611; Thermo Fisher Scientific) were reverse-transfected into hPTECs using INTERFERin transfection reagent (409–10; Polyplus) according to the manufacturer’s protocol. hPTECs were used for experiments 48 hours after siRNA treatment. To inhibit acyl-CoA oxidase-1 (ACOX1) activity, we used 500 nM 10,12-tricosadiynoic acid (Sigma).
Combined Glucose–Oxygen Deprivation–Mediated In Vitro Ischemic AKI
hPTECs were subjected to an in vitro ischemic AKI model by combined glucose–oxygen deprivation (CGOD) insult. For the CGOD procedure, hPTECs in a 24-well or 96-well plates were exposed to 4–24 hours of normoxia or hypoxia (FiO2 1%) with defined glucose-free buffer (115 mM NaCl, 1.0 mM NaH2PO4, 26.2 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, and 0.8 mM MgSO4). After CGOD procedure, culture medium was replaced with glucose-containing buffer (+30 mM glucose) and hPTECs moved to a regular incubator for normoxic treatment in the next 24 hours to simulate “reperfusion.” The defined glucose-free buffer was used instead of glucose-free cell culture media because other components in medium, such as amino acids, can alter the response of cells to hypoxia.26,27
LDH Release Assay
Cell death was assessed by lactate dehydrogenase (LDH) efflux using the LDH release assay kit (Promega) in accordance with the manufacturer’s protocol. Cytotoxicity was expressed as the ratio of the LDH release in the treated cell medium to that of the maximal LDH release.
Western Blots
Kidney lysates were lysed in radioimmunoprecipitation assay buffer (Thermo Fisher Scientific) and the homogenates plated in triplicate to measure protein concentration using a Bradford assay kit (Bio-Rad). The following antibodies were used in this study: anti-Acox1 antibodies (1:1000, rabbit, ab59964; Abcam), pan-succinyllysine antibodies (1:1000, rabbit, PTM-401; PTM Biolabs), and anti-Sirt5 antibodies (rabbit, 1:50, 8782; Cell Signaling Technology). Anti-GAPDH or anti–α/β-tubulin were used as loading controls at 1:100.
Real-Time PCR
Real-time PCR analysis was performed as previously described,28 to determine mRNA level in whole kidneys. Complementary DNA was reverse-transcribed from 500 ng of total RNA with SuperScript First-Strand Synthesis System (Thermo Fisher Scientific). Real-time PCR analysis was performed with gene specific primer oligos, SsoAdvanced SYBR Green Supermix (Bio-Rad), and CFX96 Touch Real-Time PCR Detection System with C1000 Thermal Cycler (Bio-Rad). Cycling conditions were 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Rn18S was used for endogenous control. Expression was compared with Rn18S endogenous control and analyzed using the 2−∆∆Ct method. Primer sequences used are shown in Supplemental Table 1.
Tissue Section Analysis
Kidneys were fixed in 4% paraformaldehyde and embed in paraffin. These tissues were sectioned at 4 μm. The kidney sections were stained with hematoxylin and eosin (H&E) or Masson trichrome. H&E-stained slides were subject to histologic evaluation, and ×40 magnification images of renal cortex and medulla were obtained with a Leica DM2500 optical microscope. Semiquantitative scoring (0–4) for tubular injury was performed in terms of tubular dilation, proteinaceous cast formation, and loss of brush border. To quantify the Masson trichrome–stained, collagen-rich area, a tiling imaging system via TissueFAXS PLUS (TissueGnostics) were used to capture the entire kidney section with ×20 magnification objectives. Masson trichrome–positive area was quantified with NIS Elements software (Nikon). All tissue evaluation was performed in a blinded fashion.
Immunostaining were performed as previously described29 with paraffin-embedded tissues and with following primary antibodies or lectins: β-galactosidase (chicken, 1:200, ab9361; Abcam), endomucin (rat, 1:200, sc-65495; Santa Cruz Biotechnology), kidney injury molecule 1 (Kim-1; rat, 1:100, MAB1817; R&D Systems), thiazide-sensitive NaCl cotransporter (rabbit, 1:250, AB3553; Millipore), neutrophil gelatinase–associated lipocalin (NGAL; rat, 1:50, ab70287; Abcam), Peroxisomal Membrane Protein 70 (rabbit, 1:200, ab85550; Abcam), dolichos biflorus agglutinin (1:200, FL-1031; Vector Laboratories), or LTL (1:200, FL-1321; Vector Laboratories).
Palmitate Oxidation Assay
14C-palmitate (PerkinElmer) was conjugated to BSA and used at 125 μM in 200 µl reactions as described.14 14CO2 was captured on filter papers soaked in 1 M KOH and water-soluble 14C-labeled FAO products were separated by the method of Bligh and Dyer30 and subjected to scintillation counting. Peroxisomal FAO was defined as the rate of palmitate oxidation in the presence of the irreversible mitochondrial FAO inhibitor etomoxir (100 µM). Data were normalized to protein concentration.
Oroboros High-Resolution Respirometry
Freshly prepared kidney homogenates were analyzed with an Oroboros Oxygraph-2K using our previously published method.31 Complex I respiration was defined as malate/pyruvate/glutamate–driven oxygen consumption in the presence of ADP, whereas Complex II respiration was defined as succinate-driven oxygen consumption.
Quantitative Mass Spectrometry
Both 7-day renal IRI and contralateral control kidney tissues (n=3) were homogenized, trypsinized, and then succinylated peptides were enriched using the PTMScan Succinyl-Lysine Motif Kit (Cell Signaling Technologies). After reverse-phase high-performance liquid chromatography/electrospray ionization-tandem mass spectrometry (HPLC/ESI-MS/MS), succinyl enriched samples were analyzed by data-independent acquisition (e.g., SWATH) on a TripleTOF 6600, and site-specific changes in succinylation were quantified using Skyline as described. See experimental details in Supplemental Appendix 1.32–34
Statistical Analyses
Data are presented as mean±SEM. Prism 7.0C software (GraphPad) was used for statistical analysis. To determine whether sample data has been drawn from a normally distributed population, D’Agostino–Pearson omnibus test or Shapiro–Wilk test was performed. For parametric data, ANOVA with post hoc Turkey comparison was used for multiple group comparison and t test was used to compare two different groups. For nonparametric data, Mann–Whitney U test was used. The threshold of P<0.05 was set to consider data statistically significant.
Results
Sirt5−/− Kidneys Exhibit Protein Lysine Hypersuccinylation
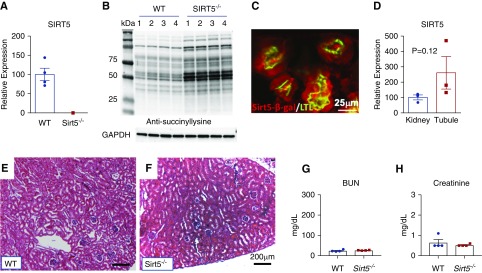
Sirt5 is highly expressed in kidney35 but its role in kidney function has not been examined. We confirmed that global Sirt5 knockout mice (Sirt5−/−) have no detectable Sirt5 mRNA in the kidney (Figure 1A). Similar to liver and heart,13,36 Sirt5 deletion in the kidney leads to accumulation of PTMs known as lysine succinylation, shown by pan anti-succinyllysine Western blotting for whole kidney lysates (Figure 1B). The mutant allele in Sirt5−/− mice bears a lacZ reporter cassette.22 β-galactosidase immunostaining revealed that Sirt5–β-galactosidase is colocalized in LTL-positive PTECs (Figure 1C), thiazide-sensitive NaCl cotransporter–positive distal tubular epithelial cells, and dolichos biflorus agglutinin–positive collecting ducts, but was absent from endomucin-positive microvasculature (Supplemental Figure 1). In WT mice, Sirt5 mRNA was expressed in the whole kidneys and was enriched in isolated primary PTECs (Figure 1D). At baseline, Sirt5−/− kidneys appear histologically (Figure 1, E and F) and functionally normal with unaltered serum creatinine and BUN compared with WT (Figure 1F).
Figure 1.
Sirt5−/− kidneys exhibit protein lysine hypersuccinylation but normal morphology and function. (A) Real-time PCR of Sirt5−/− kidney confirms no detectable Sirt5 mRNA in mouse kidney (n=4/group). (B) Western blotting of kidney lysates (50 µg) with anti-succinyllysine antibody was used to assess succinylation levels in mouse kidneys derived from WT or Sirt5−/− mice (n=4/group). (C) The Sirt5−/− mutant allele contains a lacZ cassette that allows for the interrogation of expression using an anti–β-galactosidase (β-gal) antibody (Sirt5–β-gal, red). Abundant expression of Sirt5 is colocalized in the proximal tubular epithelial cells (LTL, green). Scale bar, 25 µm. (D) Real-time PCR of whole kidney compared with isolated LTL-positive PTECs suggest that Sirt5 expression is enriched in PTECs (n=3/group). (E and F) H&E-stained kidneys of WT and Sirt5−/− kidneys at baseline. No discernable differences were noted between WT and Sirt5−/− kidneys. Scale bar, =200 µm. (G and H) Serum levels of (G) BUN and (H) creatinine were not significantly different at baseline between WT and Sirt5−/− mice (n=4/group). All data are presented as dot plots plus mean±SEM, t test.
Sirt5−/− Kidneys Are Protected against Ischemic AKI in Mice
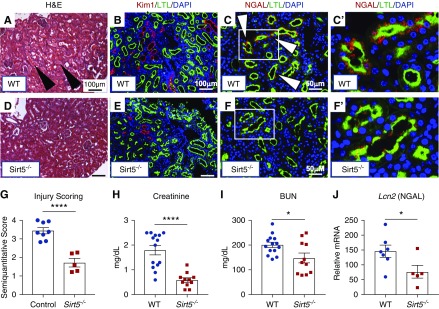
To test the role of Sirt5 in ischemic AKI, Sirt5−/− mice were subjected to a renal IRI-mediated ischemic AKI model and were euthanized 7 days later. H&E-stained histopathology revealed decreased proteinaceous casts, less severe tubular dilation, and reduced loss of brush border in Sirt5−/− kidneys (Figure 2, A, D, and G). Kim-1, which localizes to injured PTECs,37 was much more prominent in WT kidneys (Figure 2B versus Figure 2E). Further, NGAL, another marker for kidney tubular injury,38 was observed in many WT but was virtually absent in Sirt5−/− PTECs (Figure 2, C and C’ versus Figure 2, F and F’). Real-time PCR for Lcn2 (NGAL mRNA) confirmed its decreased mRNA level in Sirt5−/− kidneys (Figure 2J). Finally, kidney function was protected in Sirt5−/− mice, as evidenced by reduced serum creatinine and BUN (Figure 2, H and I). Interestingly, similar results were observed by using the heterozygous mutant (Sirt5+/−) and their littermate WT controls, suggesting that half Sirt5 gene dosage is sufficient to protect against ischemic AKI (Supplemental Figure 2). We also evaluated post-AKI fibrosis with the samples of day 7 after renal IRI. Although mRNA level of fibrosis markers (Col1a1 and Tgf-β1) were reduced in Sirt5−/− kidneys, little collagen-rich area was observed both in WT and Sirt5−/− kidneys (1.5%–2.3%, Supplemental Figure 3).
Figure 2.
Sirt5−/− kidneys are protected against ischemic AKI at 7 days after injury. (A, D, and G) H&E-stained kidneys of (A) WT and (D) Sirt5−/− kidneys that were subjected to the ischemic AKI model. The Sirt5−/− kidneys had decreased injury score as compared with WT kidneys, evidenced by proteinaceous casts in WT, dilated tubular structures, and vacuolization (arrowheads) that were decreased in Sirt5−/− kidneys (n=5–8/group). Scale bar, 100 µm. (B and E) Immunostaining for Kim-1 (red) in the PTECs (LTL, green) showed a large increase in the amount of Kim-1 expression in the (B) WT compared with very low levels in the (E) Sirt5−/− PTECs. Scale bar, 100 µm. (C and C’, inset) and (F and F’, inset) Immunostaining for NGAL (red) in PTECs (LTL) (arrowheads) was observed in many WT but were virtually absent from Sirt5−/− PTECs. Scale bar, 50 µm. (J) Real-time PCR for Lcn2 (NGAL mRNA) confirms the decrease of Lcn2 in Sirt5−/− kidneys compared with WT (n=5–7). (H and I) Serum levels of (I) creatinine and (J) BUN are decreased in the Sirt5−/− mice compared with WT, suggesting protected kidney function in the Sirt5−/− mice (n=11–14/group). All data are presented as dot plots plus mean±SEM. *P<0.05; ****P<0.001, t test.
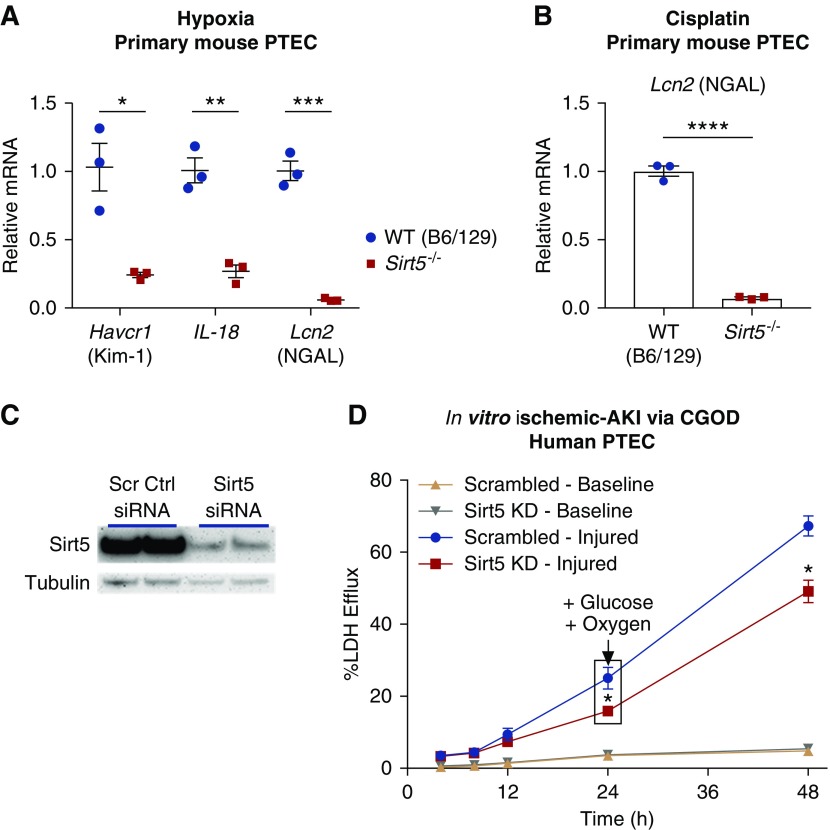
PTECs are sensitive to ischemic and cisplatin AKI, and are reliant upon mitochondria for energy metabolism; Sirt5 is known to regulate mitochondrial function and is enriched in PTECs (Figure 1). Therefore, we hypothesized that the protection against ischemic AKI in Sirt5−/− was occurring within PTECs. To test this, primary PTECs were isolated from Sirt5−/− and B6/129-strain WT mice and exposed to 24 hours of 1% hypoxic insult in vitro. After the hypoxia exposure, expression of the tubular injury markers Havcr1 (Kim-1 mRNA), Lcn2, and IL-18 was significantly reduced in Sirt5−/− PTECs (Figure 3A). Sirt5−/− mice are a mixed background of the C57Bl/6 and 129Sv strains, and 129Sv-strain mice have been shown to exhibit resistance to ischemic AKI.39 To determine if this is the case in cultured mouse PTECs, we subjected PTECs from WT B6/129, WT 129, and Sirt5−/− mice to 24 hours of 1% hypoxia. The results confirm that WT 129 is more protective than WT B6/129, evidenced by reduction of Havcr1 and Lcn2 mRNA; and that Sirt5−/− is more protective than WT 129, shown by reduction of Havcr-1, IL-18, and Lcn2 mRNA (Supplemental Figure 4). We further modeled ischemic AKI in vitro with primary hPTECs using a CGOD protocol.40 In this protocol, glucose and oxygen are deprived for the first 24 hours and then added back to “reperfuse” for an additional 24 hours. siRNA knocks down Sirt5 levels with an efficiency of approximately 80%, when compared with a scrambled siRNA control (Figure 3C). At the end of the 24 hours of CGOD and the subsequent 24-hour “reperfusion” period, hPTECs with Sirt5 knockdown demonstrated protection as evidenced by reduced LDH efflux (Figure 3D).
Figure 3.
Loss-of-function of Sirt5 in proximal tubular epithelial cells is protective against injury in vitro. (A) After 24 hours of 1% hypoxia, primary PTECs derived from Sirt5−/− kidneys exhibited reduced mRNA levels of injury markers Havcr1 (Kim-1 mRNA), Lcn2 (NGAL mRNA), and IL-18, compared with B6/129-strain WT PTECs (n=3/group). (B) After 24 hours, cisplatin-treated primary mouse PTECs from Sirt5−/− kidneys exhibited reduced mRNA levels of Lcn2, compared with WT PTECs (n=3/group). (C) Western blotting confirms siRNA knockdown (KD) of Sirt5 in primary hPTECs. (D) hPTECs are subjected to CGOD-mediated in vitro ischemic AKI model; Sirt5 KD hPTECs showed a marked decrease in LDH release (n=3/group). All data are presented as dot plots plus mean±SEM. *P<0.05; **P<0.01; ***P<0.001; ****P<0.001, t test.
Sirt5−/− Mice Are Protected against Cisplatin-Induced AKI
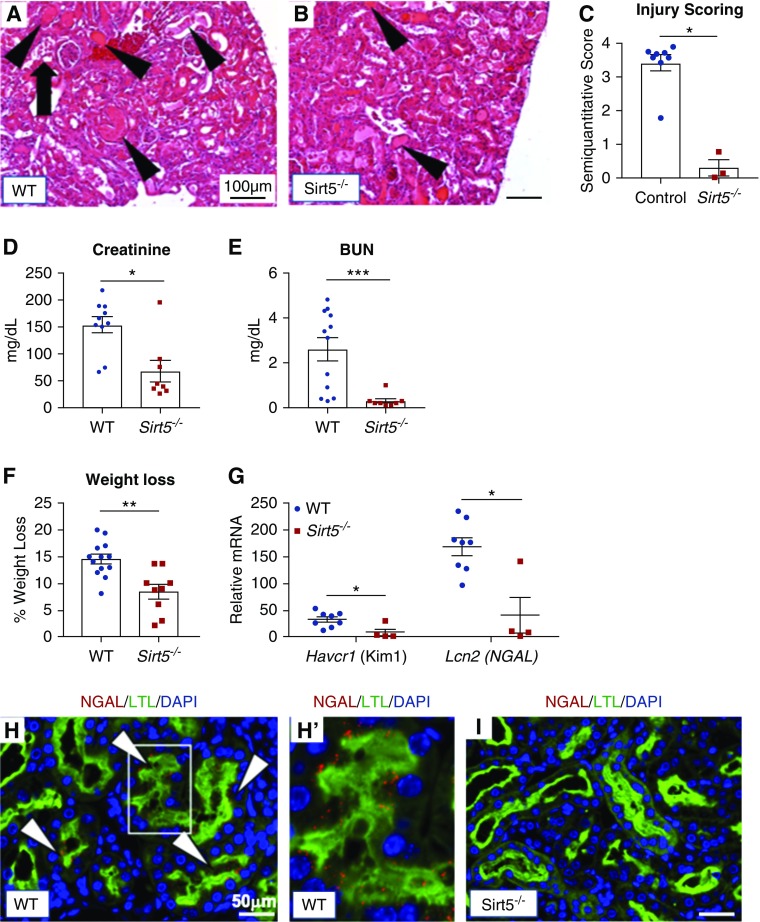
To determine if the protection afforded by Sirt5 loss-of-function extends to other forms of AKI, Sirt5−/− mice were challenged with a high dose (20 mg/kg body wt, administered intraperitoneally) of the nephrotoxin cisplatin and were euthanized 3 days later. Cisplatin specifically targets PTECs. H&E-stained kidney tissues demonstrated less severe tubular injury in cisplatin-treated Sirt5−/− mice compared with WT mice (Figure 4, A–C). Kidney function was also protected in Sirt5−/− mice as evidenced by reduced serum creatinine and BUN (Figure 4, D and E). During the 72 hours after cisplatin treatment, WT mice lost 14.5% body wt compared with just 8.4% among Sirt5−/− mice (Figure 4F), suggestive of a blunted response to cisplatin in the Sirt5−/− mice. Real-time PCR analysis for Havcr1 and Lcn2 confirmed decreased mRNA level of the tubular injury markers in Sirt5−/− kidney (Figure 4G). Consistent with this, many WT PTECs were colocalized with NGAL, but this was not observed in Sirt5−/− PTECs (Figure 4, H and I). Renoprotection was also confirmed in the PTECs as evidenced by the strong expression of Lcn2 in WT primary PTECs treated with 20 µM cisplatin in vitro as compared with Sirt5−/− PTECs (Figure 3B). Finally, cultured primary PTECs derived from heterozygous Sirt5+/− mice reduced mRNA level of Lcn2 after 24 hours of cisplatin treatment when compared with the PTECs derived from their littermate WT controls (Supplemental Figure 5).
Figure 4.
Sirt5−/− kidneys are protected against cisplatin-induced AKI at 3 days after injury. (A–C) H&E-stained kidneys of (A) WT and (B) Sirt5−/− kidneys that were subjected to the cisplatin AKI model. The Sirt5−/− kidneys had decreased injury score as compared with WT kidneys, evidenced by proteinaceous casts in WT (arrowheads), dilated tubular structures, and vacuolization (arrows) that were decreased in Sirt5−/− kidneys (n=3–8/group, Mann–Whitney U test). Scale bar, 100 µm. (D and E) Serum levels of (D) creatinine and (E) BUN are decreased in the Sirt5−/− mice compared with WT, suggesting protected kidney function in the Sirt5−/− mice (n=8–12/group, Mann–Whitney U test). (F) Sirt5−/− mice showed reduced weight loss than WT controls after the cisplatin injury (n=9–13/group, t test). (G) Real-time PCR for Havcr1 (Kim-1 mRNA) and Lcn2 (NGAL mRNA) confirm the reduction of Havcr1 and Lcn2 mRNA in Sirt5−/− kidneys compared with WT (n=4–8/group, Mann–Whitney U test). (H–I) WT and Sirt5−/− kidneys were immunostained for NGAL (red) and PTECs (LTL, green). WT kidneys (H and H’) had increased NGAL positive (arrows, red) in LTL-positive PTECs compared with Sirt5−/− kidneys (I). Scale bar, 50 µm. All data are presented as dot plots plus mean±SEM. *P<0.05; **P<0.01; ****P<0.001.
Mitochondrial FAO Enzymes Are Hypersuccinylated in Sirt5−/− Kidney
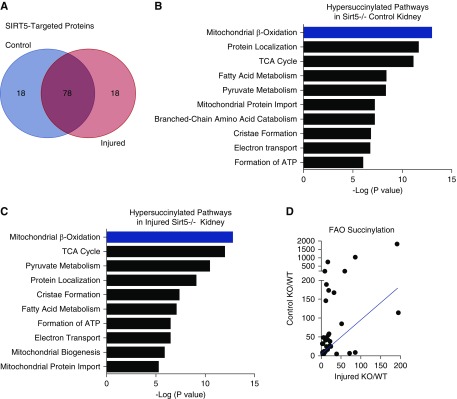
Sirt5 is a protein deacylase with a high affinity for succinyllysine, followed by glutaryllysine and malonyllysine.18,19 The lysine succinylome has not been characterized in kidney. Therefore, we used quantitative mass spectrometry to measure site-level lysine succinylation in Sirt5−/− and WT kidneys after 22 minutes of renal IRI and compared uninjured contralateral kidneys (Supplemental Figure 6). Altogether, a total of 1299 unique succinylation sites were identified in kidney (Supplemental Table 2A). Not all sites were present in all groups. In WT mice, there were 489 unique succinylated peptides in uninjured contralateral kidneys and 731 in the kidneys that had been subjected to 22 minutes of renal IRI 7 days previously (Supplemental Table 2, B and C). In Sirt5−/− mice the number of unique succinylated peptides in contralateral and injured kidneys was 1009 and 905, respectively (Supplemental Table 2, D and E). Thus, as expected, Sirt5−/− kidneys had more overall succinylated peptides than WT. However, although renal IRI induced an increase in lysine succinylation in WT, the opposite was seen in Sirt5−/− kidneys.
The small sample size (n=3/group) limited statistical power. Therefore, statistical comparisons of site-level succinylation were limited to uninjured WT versus uninjured Sirt5−/−, and injured WT versus injured Sirt5−/− kidneys. Not all succinylated lysine residues are targets for Sirt5 deacylation13; we defined Sirt5 target sites as those with statistically greater succinylation levels in Sirt5−/− kidneys compared with WT. In uninjured kidneys, 191 Sirt5 target sites were identified, spread across 96 different proteins, whereas in injured kidneys, 201 sites were found across 96 proteins (Supplemental Table 3, A and B). Overall, >95% of the succinylated kidney proteins targeted by Sirt5 were mitochondrial proteins.
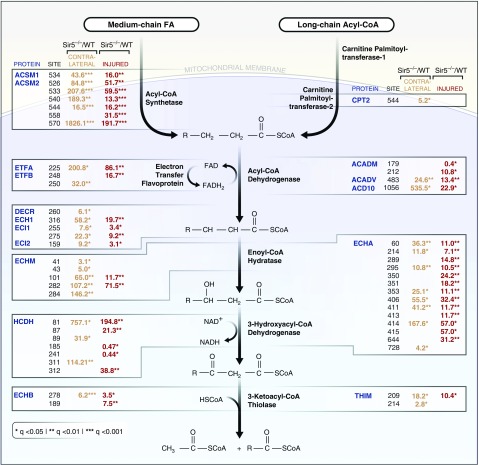
Reactome pathway analysis was performed on the lists of Sirt5 target proteins in injured and uninjured kidney. The top pathway hit was mitochondrial FAO (Figure 5, B and C). A total of 17 FAO-related proteins were found to be Sirt5 targets in uninjured kidney and 16 in injured kidney, with 15 of these proteins shared across injured and uninjured tissues. The specific lysine residues targeted by Sirt5 and the fold changes (Sirt5−/−:WT) are detailed in Figure 6. There are five key enzymatic steps to mitochondrial FAO, including activation to the metabolically active acyl-CoA derivative and four subsequent enzymatic steps for acyl-CoA chain-shortening. There are multiple enzymes at each of the five steps with different acyl-CoA chain-length specificities, i.e., long chain, medium chain, and short chain. Sirt5-targeted FAO proteins are spread across all five steps of the pathway. Interestingly, among the succinylated FAO peptides that were quantified in both the uninjured and injured kidneys, the degree of hypersuccinylation (Sirt5−/−:WT ratios) was always higher in the uninjured kidneys (Figures 5D and 6).
Figure 5.
Sirt5−/− kidneys have increased succinylation of mitochondrial proteins. (A) Venn diagram: 96 hypersuccinylated proteins were quantified in contralateral uninjured kidneys and 96 in 22-minute renal IRI kidneys; 78 out of 96 were common between groups. (B and C) Reactome pathway analysis was performed using the lists of hypersuccinylated proteins; shown are the top ten hits for contralateral uninjured (A) and 22-minute renal IRI kidneys (B). (D) The degree of hypersuccinylation at specific lysine residues on FAO proteins tended to be higher in contralateral control (uninjured) kidneys than in 22-minute IRI kidneys; the graph depicts the fold change in succinylation (Sirt5−/−:WT) in control (uninjured) kidneys (y axis) plotted against the ratios for the same lysine residues in injured kidneys (x axis). The blue line represents a 1:1 relationship, i.e., if succinylation were equal on FAO proteins across control and post-IRI. It can be seen that most points lie above this line, indicating higher relative succinylation in the contralateral controls. n=3/group.
Figure 6.
Mitochondrial FAO enzymes are hypersuccinylated in Sirt5−/− kidney. Schematic showing the steps of mitochondrial FAO and the relative enrichment in lysine succinylation on FAO enzymes as determined by mass spectrometry. For each protein, the lysine residues with statistically significant differences in succinylation are listed and the relevant fold-increases are provided (ratio of Sirt5−/−:WT signal) provided. Two statistical comparisons were made: uninjured contralateral control (WT versus Sirt5−/−, n=3) and 22-minute IRI (WT versus Sirt5−/−, n=3/group).
Total FAO Is Increased in Sirt5−/− Kidneys Both at Baseline and after Ischemic AKI
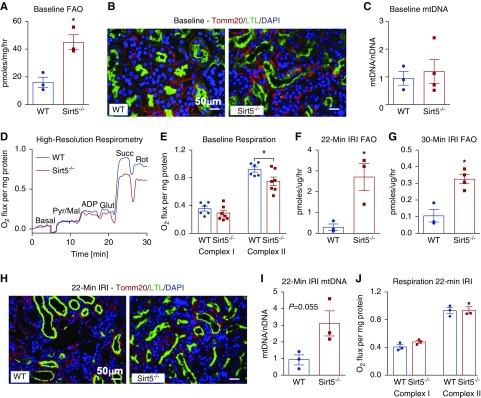
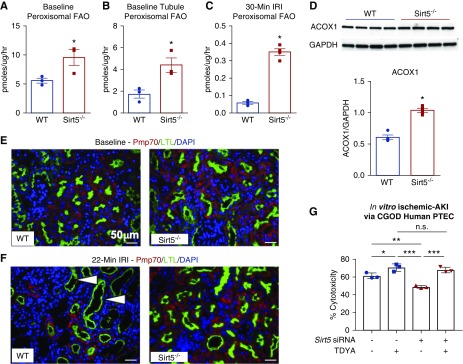
Our lysine succinylome analysis implicated FAO as a key pathway to confer renoprotection in Sirt5−/− kidneys. This pathway is known to be suppressed in Sirt5−/− liver and heart.13,20 To explore the effects of Sirt5 loss-of-function on kidney FAO, we followed the catabolism of 14C-labeled palmitate to 14CO2 in fresh kidney lysates prepared from uninjured kidneys. Contrary to expectation, baseline FAO rates were significantly elevated in Sirt5−/− kidneys (Figure 7A). This was not because of a greater number of mitochondria, as quantification of the Tomm20-positive mitochondria (Figure 7B) and the mitochondrial-to-nuclear DNA ratio (Figure 7C) indicated similar mitochondrial abundance across genotypes. Consistently, baseline FAO rates were trending to be increased (P=0.10) in cultured Sirt5−/− PTECs (Supplemental Figure 7). We also analyzed respiratory chain function, previously shown to be impaired in Sirt5−/− liver mitochondria,31 in kidney lysates using an Oroboros high-resolution respirometer. As in liver, uninjured Sirt5−/− kidney mitochondria demonstrated a significant decrease in respiration on the Complex II substrate succinate (Figure 7, D and E).
Figure 7.
FAO is enhanced in Sirt5−/− kidneys. (A) Total FAO is increased at baseline in whole kidney homogenates of Sirt5−/− mice compared with WT, as measured by 14C-palmitate oxidation. (n=3/group). (B) Immunostaining reveals equivalent numbers of Tomm20-positive mitochondria (red) in WT and Sirt5−/− PTECs (LTL, green) at baseline. Scale bar, 50 µm (C). Mitochondrial DNA (mtDNA) measured by real-time PCR was found to be equivalent between control and Sirt5−/− kidneys (n=3–4/group). (D and E) Baseline respirometry revealed that Complex II specifically and not Complex I of the electron transport chain was reduced in Sirt5−/− kidneys compared with WT (n=6–7/group). (F and G) After a moderate (22 minutes) (F) or severe (30 minutes) (G) renal IRI, Sirt5−/− kidneys displayed an increased rate of FAO (n=3–4/group). (H) After renal IRI, mitochondria (Tomm20, red) appear to be present in both WT and Sirt5−/− proximal tubules (LTL, green). Scale bar, 50 µm. (I) Mitochondrial DNA trended to be increased in Sirt5−/− kidneys compared with WT. (n=3/group). (J) No difference was observed in Complex I or Complex II utilization of the electron transport chain after renal IRI (n=3/group). All data are presented as dot plots plus mean±SEM. *P<0.05, t test.
We next repeated the same set of experiments on Sirt5−/− and WT kidney tissue isolated day 7 after renal IRI. FAO rates, overall, were an order of magnitude lower in kidneys subjected to 22 minutes of renal IRI compared with baseline (compare Figure 7F to Figure 7A). A more severe 30 minutes of renal IRI reduced FAO rates even further (Figure 7G), suggesting the pathway is highly sensitive to renal IRI. After either 22 or 30 minutes of renal IRI, Sirt5−/− kidneys still displayed significantly higher FAO than WT (Figure 7, F and G). Kidneys subjected to 22 minutes of renal IRI were also analyzed for mitochondrial content and mitochondrial respiration. There was a nonsignificant trend toward increased mitochondrial abundance in Sirt5−/− kidneys after renal IRI (P=0.06; Figure 6I), supported visually by immunostaining for Tomm20 (Figure 7H). Oroboros mitochondrial respiration measures in kidney lysates, normalized to total protein, showed equal mitochondrial respiratory capacity across genotypes.
Increased FAO in Sirt5−/− Kidneys Is Localized to Peroxisomes
There are two parallel pathways of FAO, one in mitochondria that directly transmits reducing equivalents into the electron transport chain and acetyl-CoA into the TCA cycle, and one in peroxisomes that partially chain-shortens long-chain fatty acids and releases short-chain products into the cytosol. These short-chain products can either be taken up by mitochondria and metabolized to completion or released from the cell. PTECs contain an abundance of peroxisomes and increased peroxisomal function has been previously linked to renoprotection.41,42 Recently, peroxisomal gain-of-function was reported in Sirt5−/− cell lines and mouse liver.13,21,31,43 We therefore interrogated Sirt5−/− kidneys for peroxisomal FAO at baseline and after renal IRI. The catabolism of 14C-palmitate was followed to 14CO2 and water-soluble (short-chain) products in kidney homogenates in the presence of etomoxir, an irreversible inhibitor of mitochondrial FAO. Etomoxir-resistant FAO is ascribed to peroxisomes.8 Sirt5−/− kidney homogenates at baseline displayed a significantly higher rate of peroxisomal FAO than WT kidney homogenates (Figure 8A). A similar result was obtained using freshly isolated primary mouse PTECs (Figure 8B) and a similar trend was observed using cultured hPTECs with Sirt5 siRNA knockdown (Supplemental Figure 8). After a severe 30 minutes of renal IRI, peroxisomal FAO was seven-fold higher in Sirt5−/− kidney homogenates (Figure 8C). The 30-minute renal IRI homogenates were Western-blotted for ACOX1, the rate-limiting enzyme of peroxisomal FAO. The abundance of ACOX1 was significantly higher in Sirt5−/− kidney (Figure 8D). Sirt5−/− kidneys were confirmed to have greater abundance of Peroxisomal Membrane Protein 70–positive peroxisomes in baseline and postrenal IRI tissues (Figure 8, E and F). To test whether inhibition of peroxisomal FAO abrogated the beneficial effect of Sirt5 inhibition, we used Sirt5 siRNA in cultured hPTECs ±10,12-tricosadiynoic acid, an irreversible peroxisomal FAO inhibitor, during in vitro ischemic AKI (Figure 8G). The combined Sirt5/peroxisomal FAO inhibition removed the protective role of Sirt5 knockdown confirming the role of peroxisomal FAO switching in driving the PTECs protection. These data confirm that Sirt5−/− kidneys undergo an FAO switch from mitochondria to peroxisomes.
Figure 8.
Increased FAO by Sirt5 loss-of-function is localized to peroxisomes. (A–C) Etomoxir-insensitive 14C-palmitate oxidation, which represents peroxisomal FAO, was measured in kidney homogenates at baseline (n=3/group) (A), in freshly isolated PTECs (n=3/group) (B), or after 30-minute renal IRI (n=3/group) (C), using t test. (D) Western blot analysis showed an upregulation of the rate-limiting peroxisomal FAO enzyme ACOX1 in Sirt5−/− kidney homogenates, using Mann–Whitney U test. (E and F) PMP70 (red) is a peroxisome marker, LTL (green) is a marker for PTECs and DAPI (blue). (E) shows baseline and (F) shows postrenal IRI; (n=4/group). At baseline (E) there appear to be more peroxisomes present in the proximal tubules (arrowheads) and this persists after injury. (F) Scale bar, 50 µm. (G) hPTECs was treated ±Sirt5 siRNA and ±10,12-tricosadiynoic acid (TDYA; ACOX1 inhibitor) during CGOD-mediated in vitro ischemic AKI and was evaluated its effect by LDH release (cell death marker). TDYA treatment abrogates the protective effect by Sirt5 siRNA (n=3/group, Tukey comparison). All data are presented as dot plots plus mean±SEM. *P<0.05; **P<0.01; ***P<0.001.
Discussion
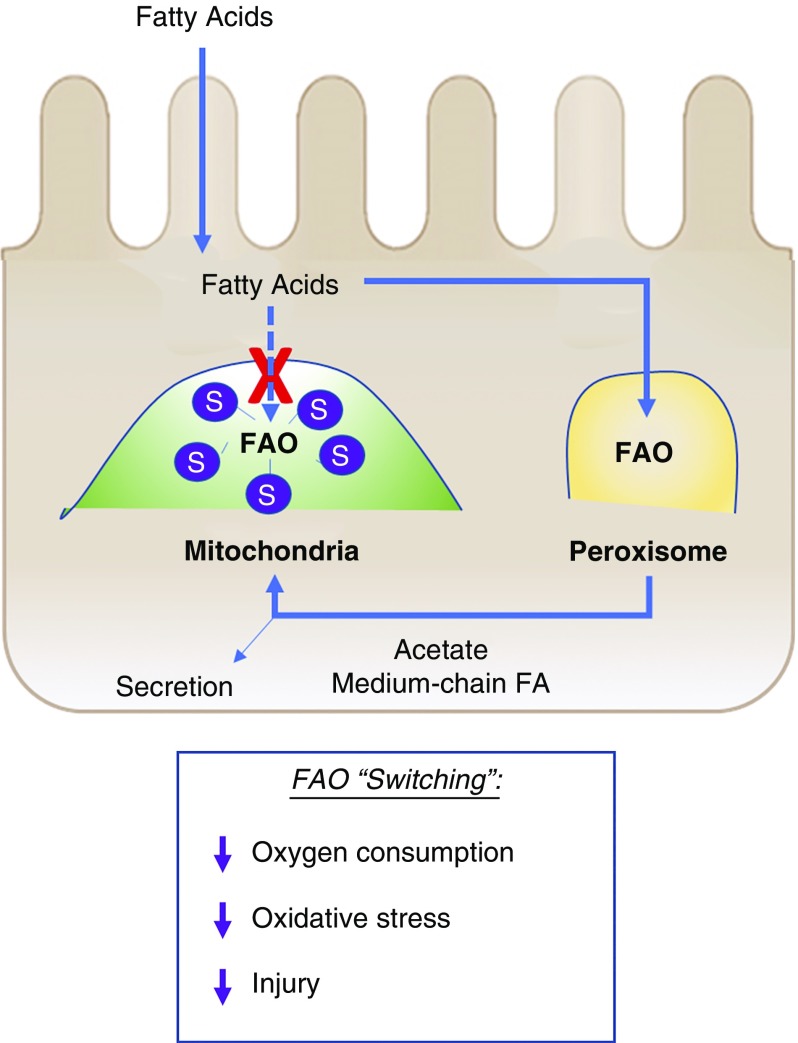
Current study interrogated the role of Sirt5 during AKI and found that loss-of-function of Sirt5 was renoprotective (Figure 9). This finding is opposite to those observed for Sirt1 and Sirt3, for which deletion exacerbates AKI.16,44 We propose that the beneficial nature of Sirt5 loss-of-function stems from the unique combination of only a minor loss of mitochondrial function combined with a significant peroxisomal gain-of-function. Deletion of Sirt5 in kidney caused only a modest reduction in mitochondrial function at baseline, i.e., approximately 10% loss of succinate-induced respiration and a suppression of mitochondrial FAO, and after injury, mitochondrial respiratory chain function was the same across genotypes. This could be because of the clear accumulation of protein succinylation in WT mitochondria after injury (731 succinylated peptides versus 489 at baseline), such that more than 50 peptides exhibited significantly higher succinylation in WT kidney compared with Sirt5−/− kidney after renal IRI (Supplemental Table 3C). Protein succinylation in mitochondria is determined by the concentration of succinyl-CoA, which chemically reacts with lysine residues on the surface of proteins, and the countering activity of Sirt5.45 We speculate that WT kidneys rely more upon mitochondria during AKI than Sirt5−/− kidneys, causing accumulation of succinyl-CoA and therefore greater protein succinylation, effectively closing the gap in mitochondrial function between WT and Sirt5−/− kidneys. Peroxisomal function, in contrast, remains enhanced in Sirt5−/− kidney after injury. Increased peroxisomal function has previously been linked to renoprotection in mice treated with etomoxir, in transgenic mice overexpressing Sirt1 in the kidney, and in animals treated with fibrates, a class of drugs that promotes peroxisomal proliferation.46
Figure 9.
Proposed model by which FAO switching in Sirt5−/− kidney mediates protection against AKI. Sirt5 loss-of-function blocks mitochondrial FAO via hypersuccinylation and thus reduced function of FAO key enzymes in the PTECs (see Figure 6). Metabolic adaptation to blocked mitochondrial FAO in PTECs involves compensatory FAO in the peroxisome, thereby reducing oxygen requirements, reducing oxidative stress, and leading to protection against injury. S, hypersuccinylation.
PTECs are the major site of injury during several forms of AKI, and are rivaled only by the liver in terms of peroxisome abundance.12 As previously reported for liver,21 we observed a peroxisomal gain-of-function, at least for the FAO pathway, in Sirt5−/− kidneys. Peroxisomes are also known to play an important role in the brain.47 Interestingly, Sirt5−/− mice are protected from ischemic stroke,48 whereas in the heart, Sirt5 deletion exacerbates ischemic injury.49 The heart contains only “microperoxisomes,” which are not thought to contribute significantly to FAO.50 We speculate that the capacity for peroxisomal FAO might explain why the kidney and brain, but not the heart, are privy to the protective effects of Sirt5 deficiency. Also, defective FAO in PTECs is responsible for interstitial fibrosis.51 We showed that although mRNA for two fibrosis markers are decreased in Sirt5−/− kidneys, no discernable difference was observed in tissue across genotypes. Thus, future studies will evaluate whether Sirt5 loss-of-function leads to fibrosis at later time points (28–42 days post AKI) with more severe ischemic insult, known to drive fibrosis.52
Therapeutically, because of the deleterious effect on the heart, inhibition of Sirt5 would need to be localized to the kidney. Recent advances have been made in targeting drug delivery to the kidney.53,54 Although our data in the Sirt5 heterozygous mice is therapeutically promising as a half gene dosage is sufficient to elicit protection and would likely not lead to off-target effects. Further, because of the unique substrate specificity of Sirt5, several Sirt5-specific inhibitors have been developed that demonstrate in vitro efficacy.55,56
In conclusion, our results demonstrated that Sirt5 loss-of-function ameliorated ischemic and cisplatin AKI in mice and humans because of a switch away from mitochondrial FAO toward peroxisomal FAO. Our findings uncover an attractive and novel candidate pathway modulated by Sirt5 for the treatment of AKI.
Disclosures
Dr. Sims-Lucas reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases, during the conduct of the study.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases R01 DK090242 (Dr. Sims-Lucas) and R56 DK121758-01 (Dr. Goetzman). The authors acknowledge support from the National Institutes of Health shared instrumentation grant for the TripleTOF system at the Buck Institute (1S10 OD016281 to Dr. Bradford Gibson). Dr. Basisty was supported by a postdoctoral fellowship from the Glenn Foundation for Medical Research. Dr. Chiba was supported by the American Heart Association postdoctoral fellowship (17POST33670685).
Supplementary Material
Acknowledgments
Chiba, Peasley, and Cargill designed and performed experiments, provided intellectual input, and wrote the manuscript. Maringer, Bharathi, Mukherjee, Zhang, and Yagobian performed experiments and provided intellectual input. Holtz and Schilling coordinated sample preparation for mass spectrometric analysis followed by data acquisition. Holtz, Basisty, and Schilling processed and interpreted mass spectrometry data and generated data results and graphical displays. Goetzman and Sims-Lucas designed and performed experiments, edited the manuscript, provided intellectual input, and oversaw the project. All authors approved the final version of this manuscript.
We would like to give special thanks to Dr. David R. Emlet for PTEC cultures, to the Center for Biologic Imaging for training and instrumentation of the tiling imaging, and to Michele Mulkeen at the Histology Core Laboratory for histology training, all at the University of Pittsburgh.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020163/-/DCSupplemental.
Supplemental Appendix 1. Supplemental materials and methods.
Supplemental Figure 1. Localization of Sirt5 in the kidney.
Supplemental Figure 2. Heterozygous deletion of Sirt5 (Sirt5+/−) kidneys are protective against ischemic AKI at 7 days after injury.
Supplemental Figure 3. Sirt5−/− kidneys may reduce interstitial fibrosis 7 days after ischemic AKI.
Supplemental Figure 4. Sirt5−/− proximal tubules is more protective than B6/129- or 129-strain WT cells against hypoxic insult.
Supplemental Figure 5. PTECs derived from heterozygous Sirt5 knockout kidneys (Sirt5+/−) are protective against cisplatin injury.
Supplemental Figure 6. Quantitative mass spectrometry workflow summary.
Supplemental Figure 7. FAO is enhanced in primary mouse PTECs derived from Sirt5−/− mouse.
Supplemental Figure 8. Trend in increased FAO by Sirt5 knockdown is localized to peroxisomes.
Supplemental Table 1. Primer sequences for real-time PCR analysis.
Supplemental Table 2. Relative quantification for succinylation sites identified in mouse kidneys.
Supplemental Table 3. Changes in succinylation sites identified in mouse kidneys.
References
- 1.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al.: Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chevalier RL: The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am J Physiol Renal Physiol 311: F145–F161, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malek M, Nematbakhsh M: Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev 4: 20–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2: 2490–2518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC: Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, et al.: A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48: 992–998, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Portilla D, Dai G, Peters JM, Gonzalez FJ, Crew MD, Proia AD: Etomoxir-induced PPARalpha-modulated enzymes protect during acute renal failure. Am J Physiol Renal Physiol 278: F667–F675, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Peeters A, Shinde AB, Dirkx R, Smet J, De Bock K, Espeel M,et al.: Mitochondria in peroxisome-deficient hepatocytes exhibit impaired respiration, depleted DNA, and PGC-1α independent proliferation. Biochim Biophys Acta 1853: 285–298, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Fransen M, Lismont C: Peroxisomes and cellular oxidant/antioxidant balance: Protein redox modifications and impact on inter-organelle communication. Subcell Biochem 89: 435–461, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Fransen M, Lismont C, Walton P: The peroxisome-mitochondria connection: How and why? Int J Mol Sci 18: E1126, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasko R: Peroxisomes and kidney injury. Antioxid Redox Signal 25: 217–231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, et al.: SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18: 920–933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al.: SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, et al.: Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem 285: 13045–13056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, et al.: Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest 125: 715–726, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sack MN: Emerging characterization of the role of SIRT3-mediated mitochondrial protein deacetylation in the heart. Am J Physiol Heart Circ Physiol 301: H2191–H2197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, et al.: Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 19: 605–617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, et al.: Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334: 806–809, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, et al.: SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 50: 919–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XF, Tian MX, Sun RQ, Zhang ML, Zhou LS, Jin L, et al.: SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep 19: e45124, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al.: Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skrypnyk NI, Harris RC, de Caestecker MP: Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp 78: 50495, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al.: A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH 4th, et al.: Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: Differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol 312: F284–F296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie LP, Zheng XY, Qin J, Tong YY: Amino acids protects against renal ischemia-reperfusion injury and attenuates renal endothelin-1 disorder in rats. Chin J Traumato 7: 87–90, 2004 [PubMed] [Google Scholar]
- 27.Weinberg JM: The effect of amino acids on ischemic and toxic injury to the kidney. Semin Nephrol 10: 491–500, 1990 [PubMed] [Google Scholar]
- 28.Di Giovanni V, Walker KA, Bushnell D, Schaefer C, Sims-Lucas S, Puri P, et al.: Fibroblast growth factor receptor-Frs2α signaling is critical for nephron progenitors. Dev Biol 400: 82–93, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukherjee E, Maringer K, Papke E, Bushnell D, Schaefer C, Kramann R, et al. : Endothelial marker-expressing stromal cells are critical for kidney formation. Am J Physiol Renal Physiol 313: F611–F620, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Bharathi SS, Rardin MJ, Lu J, Maringer KV, Sims-Lucas S, et al.: Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain. J Biol Chem 292: 10239–10249, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, et al. : Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: A new concept for consistent and accurate proteome analysis. Mol Cell Proteomics 11: O111.016717, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer JG, D'Souza AK, Sorensen DJ, Rardin MJ, Wolfe AJ, Gibson BW, et al.: Quantification of lysine acetylation and succinylation stoichiometry in proteins using mass spectrometric data-independent acquisitions (SWATH). J Am Soc Mass Spectrom 27: 1758–1771, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling B, Rardin MJ, MacLean BX, Zawadzka AM, Frewen BE, Cusack MP, et al.: Platform-independent and label-free quantitation of proteomic data using MS1 extracted ion chromatograms in skyline: Application to protein acetylation and phosphorylation. Mol Cell Proteomics 11: 202–214, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T, Lomb DJ, Haigis MC, Guarente L: SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137: 560–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadhukhan S, Liu X, Ryu D, Nelson OD, Stupinski JA, Li Z, et al.: Metabolomics-assisted proteomics identifies succinylation and SIRT5 as important regulators of cardiac function. Proc Natl Acad Sci U S A 113: 4320–4325, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV: Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol 286: F552–F563, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Bolignano D, Donato V, Coppolino G, Campo S, Buemi A, Lacquaniti A, et al.: Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis 52: 595–605, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lu X, Li N, Shushakova N, Schmitt R, Menne J, Susnik N, et al.: C57BL/6 and 129/Sv mice: Genetic difference to renal ischemia-reperfusion. J Nephrol 25: 738–743, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Haun SE, Murphy EJ, Bates CM, Horrocks LA: Extracellular calcium is a mediator of astroglial injury during combined glucose-oxygen deprivation. Brain Res 593: 45–50, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Gulati S, Singh AK, Irazu C, Orak J, Rajagopalan PR, Fitts CT, et al.: Ischemia-reperfusion injury: Biochemical alterations in peroxisomes of rat kidney. Arch Biochem Biophys 295: 90–100, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Negishi K, Noiri E, Sugaya T, Li S, Megyesi J, Nagothu K, et al.: A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int 72: 348–358, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Guedouari H, Daigle T, Scorrano L, Hebert-Chatelain E: Sirtuin 5 protects mitochondria from fragmentation and degradation during starvation. Biochim Biophys Acta Mol Cell Res 1864: 169–176, 2017 [DOI] [PubMed] [Google Scholar]
- 44.Gao R, Chen J, Hu Y, Li Z, Wang S, Shetty S, et al.: Sirt1 deletion leads to enhanced inflammation and aggravates endotoxin-induced acute kidney injury. PLoS One 9: e98909, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner GR, Bhatt DP, O'Connell TM, Thompson JW, Dubois LG, Backos DS, et al. : A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab 25: 823–837.e8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoonjans K, Staels B, Auwerx J: Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37: 907–925, 1996 [PubMed] [Google Scholar]
- 47.Berger J, Dorninger F, Forss-Petter S, Kunze M: Peroxisomes in brain development and function. Biochim Biophys Acta 1863: 934–955, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz-Canestro C, Merlini M, Bonetti NR, Liberale L, Wüst P, Briand-Schumacher S, et al.: Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int J Cardiol 260: 148–155, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Hershberger KA, Abraham DM, Martin AS, Mao L, Liu J, Gu H, et al.: Sirtuin 5 is required for mouse survival in response to cardiac pressure overload. J Biol Chem 292: 19767–19781, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrison EH, Walusimbi-Kisitu M: Properties and subcellular localization of myocardial fatty acyl-coenzyme A oxidase. Am J Physiol 255: H441–H445, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al.: Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21: 37–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kramann R, Tanaka M, Humphreys BD: Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol 25: 1924–1931, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams RM, Shah J, Tian HS, Chen X, Geissmann F, Jaimes EA, et al. : Selective nanoparticle targeting of the renal tubules. Hypertension 71: 87–94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hull TD, Agarwal A, Hoyt K: New ultrasound techniques promise further advances in AKI and CKD. J Am Soc Nephrol 28: 3452–3460, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalbas D, Liebscher S, Nowak T, Meleshin M, Pannek M, Popp C, et al.: Potent and selective inhibitors of human sirtuin 5. J Med Chem 61: 2460–2471, 2018 [DOI] [PubMed] [Google Scholar]
- 56.Rajabi N, Auth M, Troelsen KR, Pannek M, Bhatt DP, Fontenas M, et al.: Mechanism-based inhibitors of the human sirtuin 5 deacylase: Structure-activity relationship, biostructural, and kinetic insight. Angew Chem Int Ed Eng 56: 14836–14841, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.