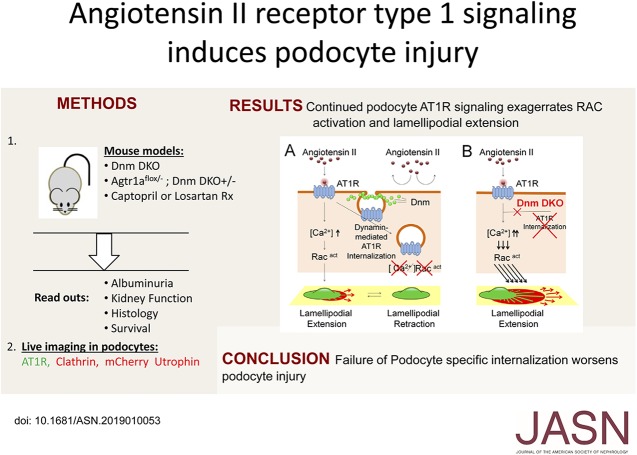
Significance Statement
The amelioration of proteinuria resulting from inhibition of the renin-angiotensin pathway is thought to be predominantly caused by reduction in intraglomerular pressure. However, because studies have produced conflicting findings, whether podocyte-associated angiotensin II receptor signaling directly contributes to podocyte injury remains unclear. Angiotensin II receptor type 1 (AT1R) is internalized by clathrin- and dynamin-mediated endocytosis, and in this study the authors used podocyte-specific Dynamin 1 and 2 double-knockout mice to examine the effect of angiotensin II stimulation on AT1R in these double-knockout mice. Loss of AT1R internalization accentuated Rac1 activation and membrane ruffling in Dnm double-knockout podocytes. Podocyte-specific deletion of the receptor in Dnm double-knockout mice demonstrated improved albuminuria and kidney function and attenuation of membrane abnormalities—findings suggesting that podocyte-associated AT1R signaling augments podocyte injury.
Keywords: podocyte, endocytosis, proteinuria
Visual Abstract
Abstract
Background
Inhibition of the renin-angiotensin system remains a cornerstone in reducing proteinuria and progression of kidney failure, effects believed to be the result of reduction in BP and glomerular hyperfiltration. However, studies have yielded conflicting results on whether podocyte-specific angiotensin II (AngII) signaling directly induces podocyte injury. Previous research has found that after AngII stimulation, β-arrestin–bound angiotensin II receptor type 1 (AT1R) is internalized in a clathrin- and dynamin-dependent manner, and that Dynamin1 and Dynamin2 double-knockout mice exhibit impaired clathrin-mediated endocytosis.
Methods
We used podocyte-specific Dyn double-knockout mice to examine AngII-stimulated AT1R internalization and signaling in primary podocytes and controls. We also examined the in vivo effect of AngII in these double-knockout mice through renin-angiotensin system blockers and through deletion of Agtr1a (which encodes the predominant AT1R isoform expressed in kidney, AT1aR). We tested calcium influx, Rac1 activation, and lamellipodial extension in control and primary podocytes of Dnm double-knockout mice treated with AngII.
Results
We confirmed augmented AngII-stimulated AT1R signaling in primary Dnm double-knockout podocytes resulting from arrest of clathrin-coated pit turnover. Genetic ablation of podocyte Agtr1a in Dnm double-knockout mice demonstrated improved albuminuria and kidney function compared with the double-knockout mice. Isolation of podocytes from Dnm double-knockout mice revealed abnormal membrane dynamics, with increased Rac1 activation and lamellipodial extension, which was attenuated in Dnm double-knockout podocytes lacking AT1aR.
Conclusions
Our results indicate that inhibiting aberrant podocyte-associated AT1aR signaling pathways has a protective effect in maintaining the integrity of the glomerular filtration barrier.
Blocking the renin-angiotensin system (RAS) has remained the mainstay for mitigating the progression of proteinuric kidney diseases.1,2 RAS blockers, such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), have been shown to reduce proteinuria beyond just improving BP control in patients with CKD both with and without diabetes.3,4 The protective effect of these drugs is ascribed to inhibiting vascular smooth muscle cell constriction of the glomerular efferent arteriole, thus reducing intraglomerular pressure and proteinuria.5,6 However, investigators have also shown the direct effects of RAS specifically on podocytes. In vitro, angiotensin II (AngII) has been shown to induce elevated calcium concentrations in podocytes through intracellular calcium release,7,8 along with evoking nonselective cationic conductance by Transient Receptor Potential Canonical (TRPC) 5 and 6 channels.9,10 In vivo, overexpression of angiotensin II receptor type 1 (AT1R) in rat podocytes induces proteinuria and glomerular disease,9,11 due to the activation of the calcineurin-NFAT pathway.12 However, conflicting results exist where podocyte-associated AT1R deletion has limited protective effects in mouse models of glomerular injury.13,14
AT1R belongs to the G protein–coupled receptor (GPCR) family.15 A ligand-bound AT1R results in dissociation of the G protein complex, which transduces G protein–mediated signaling. The ligand-bound AT1R is also phosphorylated at the carboxyl terminal cytoplasmic domain of the receptor by G protein–coupled receptor kinase.16 Phosphorylated AT1R can bind to β-arrestin, which induces clathrin-mediated AT1R internalization. β-arrestin–bound AT1R induces a conformational change, which inhibits G protein activation, resulting in receptor desensitization. It has been shown that β-arrestin plays a role not only in GPCR internalization but in signal transduction.17
Normally, after AngII stimulation, β-arrestin–bound AT1R is internalized in a clathrin- and dynamin-dependent manner.18,19 Thus, we hypothesized that AT1R would remain at the plasma membrane when Dnm1 and 2 were genetically deleted and result in continuous AT1R-mediated signal transduction inducing progression of podocyte injury. Compared with control podocytes, we also observed that the loss of podocyte-associated Dnm1 and 2 resulted in prolonged AngII stimulation resulting in increased Rac1 activation and lamellipodial extension. Therefore, in this study, we leveraged our podocyte-specific Dnm1 and Dnm2 double-knockout (Dnm DKO) mice, which exhibited impaired clathrin-mediated endocytosis due to the inability of clathrin-coated pits to undergo membrane fission.20,21 AT1R consists of two isoforms in mice (Angiotensin II receptor type 1a [Atr1a] and Agtr1b) and Agtr1a is mainly expressed in kidney. In order to study the role of podocyte-associated Agtr1a in vivo, we generated podocyte-specific Agtr1a KO mice on the background of Dnm DKO mice to elucidate whether loss of podocyte-associated angiotensin signaling would improve the progressive proteinuric phenotype observed in the Dnm DKO mice. Podocyte-specific loss of Agtr1a in the Dnm DKO mice demonstrated reduced proteinuria, and improvement in both glomerulosclerosis and kidney failure. These results suggest that podocyte-associated AT1aR signaling augments podocyte injury.
Methods
Antibodies and Plasmids
Rabbit anti-clathrin light chain (catalog: AB9884), Mouse anti-cortactin (catalog: 05–180), and Mouse anti-Rac1 (catalog: 05–389; EMD Millipore Corporation); Rabbit anti-WT1 (catalog: sc-192; Santa Cruz Biotechnology); Rabbit anti-phosphorylated ERK (catalog: 4376) and Mouse anti-ERK (catalog: 4696; Cell Signaling technology); Mouse anti-GFP (catalog: 11814460001; Roche); and Alexa Fluor 488 goat anti-mouse IgG antibody (catalog: A11029) and Alexa Fluor 594 goat anti-rabbit IgG antibody (catalog: A11012; Invitrogen) were purchased commercially. GFP human AT1R plasmid was a kind gift from Koichi Yamamoto (Osaka University, Osaka, Japan).22 RFP-clathrin, GFP-Dynamin 2, and mCherry-utrophin plasmid were kind gifts from Pietro De Camilli (Yale University, CT).
Generation of Mice
Agtr1afl/fl or Agtr1a−/− mice (purchased from the Jackson Laboratory) were mated with our Pod Cre Dnm1fl/fl Dnm2fl/+ mice21 to generate podocyte-specific Agtr1a, Dnm1, and Dnm2 knockout mice (Pod-Cre; Agtr1afl/−; Dnm1fl/fl Dnm2fl/fl mice); global Agtr1a knockout podocyte-specific Dnm1 and Dnm2 knockout mice (Agtr1a−/−; Pod-Cre Dnm1fl/fl Dnm2fl/fl mice); and global Agtr1a heterozygote, podocyte-specific Dnm1 and Dnm2 knockout mice (Agtr1a+/−; Pod-Cre; Dnm1fl/fl Dnm2fl/fl mice). Captopril (25 mg/kg body wt per day by oral gavage) or losartan (25 mg/kg body wt per day by oral gavage) treatment was started in Dnm DKO mice at 2 weeks of age.
Biochemical Measurements, Urine Albumin, Urine Creatinine, and Plasma Creatinine
Urine samples were collected from control, Dnm DKO, Agtr1afl/− Dnm DKO, Agtr1a+/− Dnm DKO, and Agtr1a−/− Dnm DKO mice. Urine albumin was quantified in duplicates using Albumin ELISA Quantitation kit, according to the manufacturer’s protocol (Bethyl Laboratories Inc.). Plasma and urine creatinine were measured in duplicate for each sample at the time points indicated in the figures using an ELISA quantification kit (Bioassay Systems, Hayward, CA) at an absorbance of 490 nm (Microplate Reader; Bio-Rad, Japan).
Cell Culture
Isolation of podocytes from day 1 to 3 control, Dnm DKO, and Agtr1afl/− Dnm DKO pups was performed as described previously in our laboratory.23 Mouse glomeruli were collected using Dynabeads and the glomeruli were passed through 100-μm cell strainer (BD Biosciences) and plated on type 1 collagen–coated plates at 37°C. Subculture of primary podocytes was performed by detaching the glomerular cells with 0.25% trypsin-EDTA (Invitrogen), followed by sieving through a 40-μm cell strainer (Falcon; BD Biosciences) and culturing on type 1 collagen–coated dishes as previously described.23 Primary podocyte enrichment was confirmed by WT1 staining, and passage 1 or 2 was used in all experiments. Primary podocytes in culture were treated with 0.1 μM AngII (Sigma Aldrich), 10 μM losartan (ARB; Santacruz), 50 μM NSC23766 (Rac1 inhibitor; Millipore), 20 nM Gö 6976 (conventional PKC inhibitor; Millipore), or 30 μM CN585 (calcineurin inhibitor; Millipore) for the designated time points.
Quantitative PCR Analysis
Total RNA was extracted from the primary podocytes using Trizol (Thermo Fisher Scientific). The RNA concentration was measured by spectrophotometry (Nanodrop Technologies). Total RNA (1 μg) was used for reverse-transcription by a high-capacity cDNA Synthesis Kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). The quantitative PCR amplifications were performed using Power SYBR green PCR Master Mix (Applied Biosystems) with a 7300 AB real-time PCR machine (Applied Biosystems).
Immunoblotting
Primary podocytes were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.6), 500 mM NaCl, 0.1% SDS, 0.5% deoxycholate, 1% Triton X-100, 0.5 mM MgCl2, phosphatase inhibitor cocktail (Sigma), 1 mM PMSF, and protease inhibitor cocktail (Roche Diagnostics). Protein concentrations were quantified using the Bradford Assay. Equal amounts of podocyte lysates were separated by SDS–PAGE and transferred to PVDF membranes (EMD Millipore). The membrane was blocked with 5% nonfat milk or 3% BSA (Sigma Aldrich) in Tris-buffered saline and Tween 20 (TBS-T) and incubated with the appropriate primary antibody at 4°C overnight. After three washes with TBS-T, the appropriate peroxidase-labeled anti-IgG secondary antibody (Bio-Rad) was added and signals were detected using enhanced chemiluminescence reagents (Bio-Rad) and exposed with Odyssey (LI-COR Bioscience). For quantification, densitometry was performed using ImageJ software (National Institutes of Health [NIH]).
BP and Heart Rate Measurement
For tail cuff measurements, BP was measured using a noninvasive BP system (Kent Scientific Inc.). After a 5-day training period (15 minutes each day), mice were placed in a restraining holder maintained at 32°C. The tail cuff was inflated and deflated on a fixed cycle and three consecutive measurements were performed per mouse and the values were averaged.
Rac1 Activation Assay
Rac1 activation assay was performed in control, Dnm DKO, and Agtr1a fl/− Dnm DKO primary podocytes as per the manufacturer’s protocol (Millipore) after stimulation with AngII, losartan, NSC23766, Gö 6983, or CN 585 for 3, 4, 7, 7, or 7 hours, respectively. The lysate was incubated with PAK-agarose followed by SDS–PAGE and immunoblotting with the Rac1 antibody (Millipore).
Live Cell Imaging
Live cell imaging was performed as described previously.21 Briefly, GFP-hAT1R and mRFP-clathrin, or mCherry-utrophin with or without GFP-Dynamin 2 or GFP-hAT1R, was expressed in control, Dnm DKO, or Agtr1afl/− Dnm DKO primary podocytes by electroporation (Nepa21, NEPAGENE). Transfected cells were seeded in glass-bottomed 35-mm dishes (no. 1.5 thickness; MatTek) and we obtained confocal images focusing directly at the base/bottom of the primary podocytes 24 hours after transfection using the same method as has been previously reported by De Camilli’s laboratory on clathrin-coated pits, which showed that both AT1R (green) and clathrin (red) puncta were located on the plasma membrane.21,24 AngII, losartan, NSC23766, and CN585 treatments started 2, 3, 6 and 6 hours before starting live imaging, respectively. Before live imaging, medium was replaced with an imaging buffer (containing 136 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, and 10 mM HEPES [pH 7.4]). Cells were imaged using an Andor spinning confocal microscope with ×60 oil immersion objectives for 1 hour. For quantification, maximum ruffling area in the primary podocyte during each video divided by initial podocyte cell area was measured by ImageJ software.
Calcium Imaging
Calcium imaging was performed as per the manufacturer’s protocol (Invitrogen). Briefly, primary culture podocytes were seeded in glass-bottomed 35-mm dishes (no. 1.5 thickness; MatTek) and incubated at 37°C for 15 minutes with 2 μM Fluo4 and 0.04% pluronic F127 (Invitrogen). The cells were washed three times with Hank’s balanced salt solution (Gibco) and incubated for 10 minutes at 37°C. Cells were treated with AngII 30 seconds after starting the video, which was taken by an Andor spinning confocal microscope for 10 minutes. For quantification, maximum fluorescence intensity in each primary podocyte was divided by initial fluorescence intensity (before AngII treatment) (F0) using ImageJ software.
Kidney Histology
Mice were anesthetized by intraperitoneal injection of ketamine and xylazine followed by perfusion fixation with 4% paraformaldehyde with or without glutaldehyde through the left ventricle. Sections were sent to the Yale Pathology Core Tissue Services for hematoxylin and eosin, periodic acid–Schiff, and trichrome staining. To evaluate glomerular sclerosis and interstitial fibrosis, kidney sections with trichrome staining were assessed as previously described.25 Briefly, the severity of glomerulosclerosis in each glomerulus was scored in a blinded manner as follows: 0=no sclerosis; 1=sclerosis of <10% of the glomeruli; 2=sclerosis of 10%–25% of the glomeruli; 3=sclerosis of 25%–50% of the glomeruli; 4=sclerosis of >50% of glomeruli. To evaluate interstitial fibrosis, 20 fields for each section were examined on Masson trichrome–stained sections. Semiquantitative analysis in each field was assessed as follows: 0=no fibrosis; 1=fibrosis of <10% of areas; 2=fibrosis of 10%–25% of areas; 3=fibrosis of 25%–50% of areas; 4=fibrosis of >50% of areas.
Immunofluorescence Staining
Primary podocytes on type 1 collagen–coated coverslips were washed with PBS and fixed with 4% PFA for 15 minutes. The cells were permeabilized with 0.1% Triton X in PBS for 5 minutes, followed by blocking with 3% BSA in PBS for 1 hour. Immunostaining was performed with primary antibodies overnight at 4°C, followed by Alexa Fluor 488– and/or Alexa Fluor 594–conjugated secondary antibodies, followed by washing and mounting with Slowfade (Invitrogen). For quantification, cortactin immunofluorescence–positive length in each podocyte was divided by the podocyte perimeter using ImageJ software as previously described.26
Statistical Analysis
All data are represented as mean±SEM. Statistical significance was determined with P value <0.05 by one-way ANOVA with Dunnett’s correction.
Study Approval
The Yale University IACUC approved all animal experiments (2017–11196). All work was carried out in accordance with the principles and procedures outlined in the NIH guidelines for the care and use of experimental animals.
Results
Clathrin-Mediated AT1R Internalization Is Arrested in Dnm DKO Primary Podocytes after AngII Stimulation
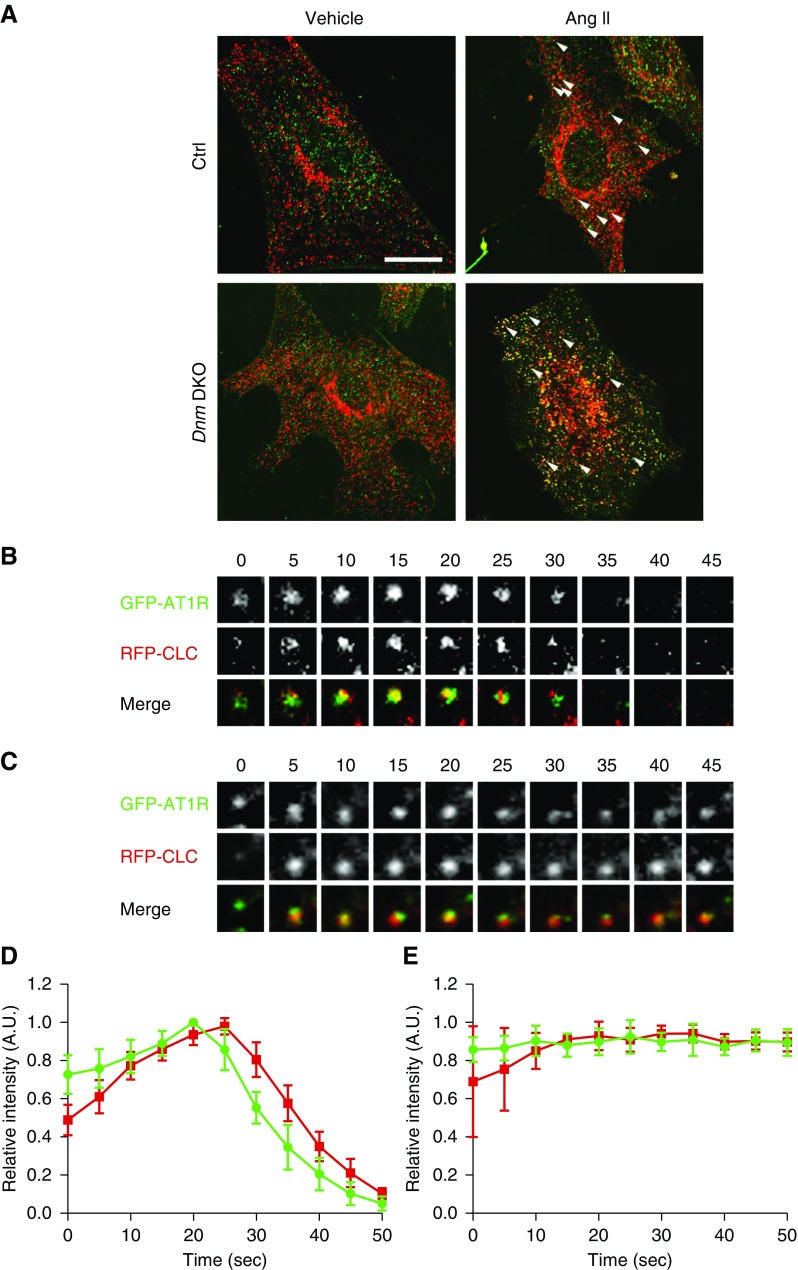
We initially examined the roles of dynamin 1 and dynamin 2 in clathrin-mediated AT1R internalization in primary podocytes. Because of the lack of reliable AT1R antibodies,27 we utilized GFP-tagged human AT1R expressed in control or podocyte-specific Dnm DKO primary podocytes. AT1R (green) was colocalized with clathrin (red) after AngII stimulation in both control and Dnm DKO primary podocytes (Figure 1A). After AngII treatment, in control primary podocytes AT1R was subsequently internalized after clathrin’s arrival (Figure 1B, quantified in Figure 1D, and Supplemental Video 1). However, in the Dnm DKO primary podocytes, AT1R remained at the plasma membrane due to failure of internalization as demonstrated by live imaging, montages, and intensity plots (Figure 1C, quantified in Figure 1E, and Supplemental Video 2).
Figure 1.
Clathrin-dependent AT1R internalization after AngII stimulation is inhibited in primary podocytes lacking Dnm1 and Dnm2. (A) Representative immunofluorescence images of GFP-AT1R (green) and clathrin (red) in control and Dnm DKO primary podocytes overexpressing GFP-hAT1R after stimulation with vehicle or AngII for 5 minutes. Arrowheads show clathrin-merged AT1Rs. Scale bar, 10 μm. (B) Selected frames from a time series of GFP-AT1R (green) and RFP-clathrin (red) acquired from 0 to 45 seconds in control primary podocytes. (C) Selected frames from a time series of GFP-AT1R (green) and RFP-clathrin (red) acquired from 0 to 45 seconds in Dnm DKO primary podocytes. (D) Fluorescence intensity as a function of time for GFP-AT1R and RFP-clathrin in control primary podocytes stimulated with AngII. (E) Fluorescence intensity as a function of time for GFP-AT1R (green) and RFP-clathrin (red) in Dnm DKO primary podocytes stimulated with AngII. A.U., arbitrary units; Ctrl, control.
AngII-Induced ERK Phosphorylation Is Sustained in Dnm DKO Primary Podocytes
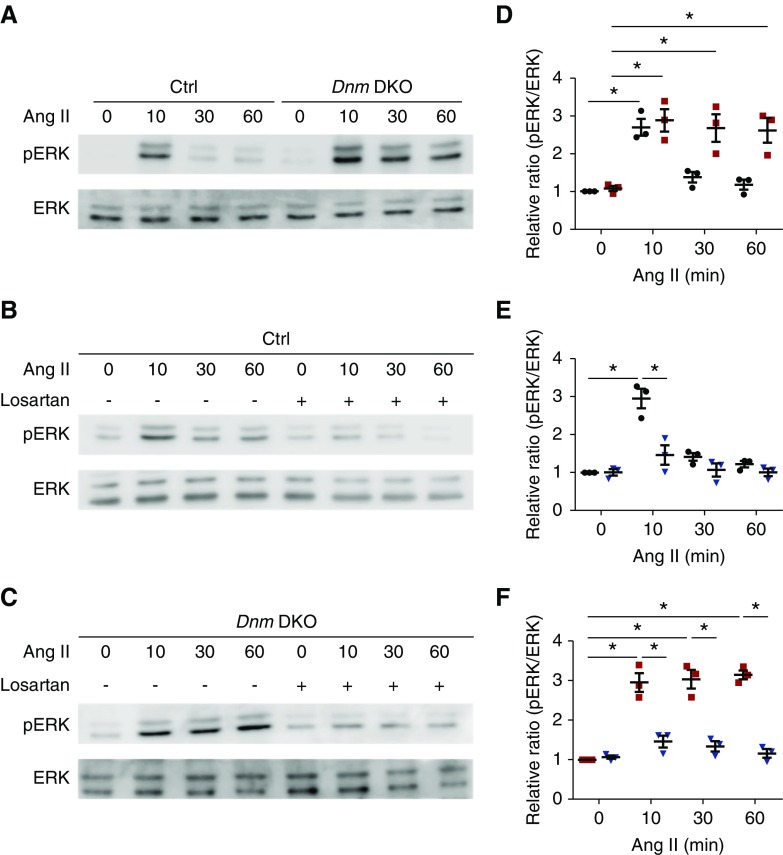
To examine whether failure of ligand-bound AT1R internalization affects its downstream signaling in Dnm DKO primary podocytes, we examined activation of ERK, a known AT1R downstream signaling target,17,28 in both control and Dnm DKO primary podocytes. In control primary podocytes, AngII stimulation resulted in peak ERK phosphorylation at 10 minutes, which resolved by 30 and 60 minutes. Conversely, in Dnm DKO primary podocytes, ERK phosphorylation was sustained even at 30 and 60 minutes after AngII stimulation (Figure 2A, quantified in Figure 2D). We next tested whether the angiotensin II receptor blocker (ARB) losartan would mitigate AngII-induced ERK activation. In both control and Dnm DKO primary podocytes, losartan blocked ERK activation (Figure 2, B and C, quantified in Figure 2, E and F). These results suggest that in Dnm DKO primary podocytes, the ligand-bound AT1R remains at the membrane, resulting in sustained ERK phosphorylation that can be reversed by ARB treatment. We next examined whether AngII can induce phosphorylation of EGF receptor (EGFR), where increases have been associated with worsened podocyte injury.29,30 In Dnm DKO podocytes, there was prolonged EGFR phosphorylation of Y1068 after AngII stimulation (Supplemental Figure 1A, and quantified in Supplemental Figure 1B).
Figure 2.
Lack of dynamin1 and 2 in podocytes reveals sustained AngII-induced extracellular regulated kinase (ERK) phosphorylation. (A) Representative immunoblots with pERK and ERK in control and Dnm DKO primary podocytes stimulated with AngII. (B) Representative immunoblots with pERK and ERK in control primary podocytes stimulated with AngII ± losartan. (C) Representative immunoblots with pERK and ERK in Dnm DKO primary podocytes stimulated with AngII ± losartan. (D) Quantification of the immunoblots in (A). Black and red dots represent control and Dnm DKO primary podocytes, respectively. *P<0.05. n=3. (E) Quantification of the immunoblots in (B). Blue and black dots represent control primary podocytes stimulated with AngII ± losartan, respectively. *P<0.05. n=3. (F) Quantification of the immunoblots in (C). Blue and red dots represent Dnm DKO primary podocytes stimulated with AngII ± losartan, respectively. *P<0.05. n=3. Ctrl, control.
Podocyte-Specific Deletion of Agtr1a Displays Improvement in Proteinuria and Kidney Function in Dnm DKO Mice
Because our in vitro studies demonstrated that Dnm DKO primary podocytes had prolonged AT1R activation, we next sought to determine whether loss of AT1R in the Dnm DKO mice would play a protective role. As a proof of principal, we first examined whether systemic AT1R signaling affected the progression of proteinuria and kidney failure in Dnm DKO mice. In order to accomplish this goal, we generated an Agtr1a global KO (Agtr1a−/−) (purchased from the Jackson Laboratory), which results in reduced BP but no overt kidney phenotype, on the background of Dnm DKO mice, or treated the Dnm DKO mice with the ACEI captopril. Genetic deletion of Agtr1a or chemical inhibition with ACEI reduced proteinuria, progression of glomerulosclerosis, and kidney failure compared with Dnm DKO mice (Supplemental Figure 2, A–D). Global deletion of Agtr1a or treatment with captopril reduced mean arterial BP without overt effects on heart rate in Dnm DKO mice (Supplemental Figure 2, E and F). Next, to examine the importance of sustained podocyte-associated AT1R signaling in Dnm DKO mice, we generated podocyte-specific Agtr1a, Dnm1, and Dnm2 knockout mice (Pod-Cre Agtr1afl/− Dnm1fl/fl Dnm2 fl/fl [Agtr1afl/− Dnm DKO]) (Figure 3A) by crossing Agtr1afl/fl and Agtr1a−/− mice (purchased from the Jackson Laboratory) with the previously described Dnm DKO mice.21 Agtr1afl/− Dnm DKO mice were used to eliminate concerns as to whether Cre recombinase would completely delete all three genes simultaneously. To first examine whether the global Agtr1a heterozygote mice affect the renal phenotype in Dnm DKO mice, Agtr1a+/− Dnm DKO mice were generated and demonstrated no improvement in survival, urine albumin, plasma creatinine, glomerular sclerosis, and interstitial fibrosis compared with Dnm DKO mice (Supplemental Figure 3, A–F), suggesting that loss of only one allele in Agtr1a had no protective effect on the Dnm DKO mice. To initially confirm Agtr1a deletion in podocytes, RT–PCR was performed, which demonstrated reduced podocyte Agtr1a mRNA expression in the Agtr1afl/− Dnm DKO primary podocytes (Figure 3B). AngII-induced ERK phosphorylation was not observed in the Agtr1afl/− Dnm DKO primary podocytes (Supplemental Figure 4A and quantified in Supplemental Figure 4B). In comparison with Dnm DKO mice after 4 weeks of age, there was a trend toward Agtr1afl/− Dnm DKO mice maintaining body weight (Figure 3C). Agtr1afl/− Dnm DKO mice also showed longer survival than Dnm DKO mice, which all died by 50 days of age (Figure 3D). Furthermore, Dnm DKO mice had robust proteinuria and kidney failure, which were improved in the Agtr1afl/− Dnm DKO mice (Figure 3, E and F). To examine whether BP changes could account for these beneficial effects after podocyte-specific Agtr1a deletion, we measured BP and heart rate at 4 weeks of age by noninvasive tail cuff measurements. No differences in mean arterial BP or heart rate were observed among these mice (Figure 3, G and H). Therefore, the observed beneficial effects of survival, reduced proteinuria, and kidney failure due to the deletion of podocyte-associated Agtr1a are likely independent of BP control. Next, to determine whether systemic blockade of AT1R signaling in the Agtr1afl/− Dnm DKO mice would improve albuminuria and kidney failure, captopril treatment was initiated. Treatment with captopril did not significantly improve body weight (Supplemental Figure 5A), albuminuria (Supplemental Figure 5B), or kidney failure (Supplemental Figure 5C) in the Agtr1afl/− Dnm DKO mice.
Figure 3.
Loss of podocyte specific Angiotensin II type 1a receptor (Agtr1a) on the Dnm DKO mouse background improves albuminuria and renal function. (A) Agtr1a Neo, Agtr1a flox, Dnm1, Dnm2, and Podocin-Cre genotypes confirmed by tail genotyping. (B) Agtr1a mRNA expression in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) mouse primary podocytes by RT–PCR. *P<0.05. n=3. (C) Body weight at 2, 4, and 6 weeks of age in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) mice. *P<0.05. n=5. (D) Survival curves of control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO mice (blue). n=5. (E) Urine albumin normalized to urine creatinine at 2, 4, and 6 weeks of age in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) mice. *P<0.05. n=5. (F) Plasma creatinine levels at 4 weeks of age in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) mice. *P<0.05. n=5. (G and H) Mean arterial BP (G) and heart rate (H) measured by tail cuff at 4 weeks of age in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) mice. n=10. BW, body weight; Ctrl, control.
Deletion of Agtr1a in Podocytes Partially Improved Glomerulosclerosis, Interstitial Fibrosis, and Foot Process Effacement in Dnm DKO Mice
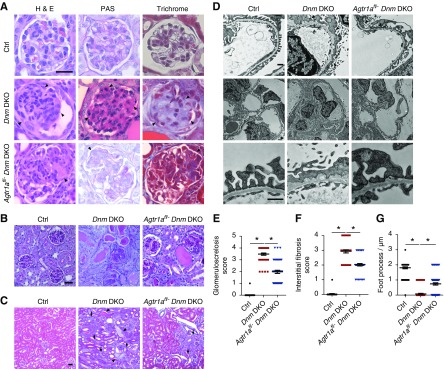
Compared with the Dnm DKO mice, which developed global glomerulosclerosis and severe interstitial fibrosis at 4 weeks of age, there was improvement in the Agtr1afl/− Dnm DKO mice (Figure 4, A–C, quantified in Figure 4, E and F). To examine the ultrastructure of the podocyte foot processes, we next performed electron microscopy which also demonstrated improved foot process effacement in the Agtr1afl/− Dnm DKO mice when compared with Dnm DKO mice (Figure 4E, quantified in Figure 4G).
Figure 4.
Genetic deletion of Agtr1a in podocytes improves glomerulosclerosis and interstitial fibrosis in the Dnm DKO mice. (A) Representative light microscopy images (hematoxylin and eosin [H&E], periodic acid–Schiff [PAS], and trichrome) of a single glomerulus from control, Dnm DKO, and Agtr1afl/− Dnm DKO mice at 4 weeks of age. Arrowheads show mesangial matrix deposition and mesangial cell proliferation. Scale bar, 50 μm. (B) Representative PAS images of several glomeruli from control, Dnm DKO, and Agtr1afl/− Dnm DKO mice at 4 weeks of age. Scale bar, 50 μm. (C) Representative trichrome staining of kidney interstitium in control, Dnm DKO, and Agtr1afl/− Dnm DKO mice at 4 weeks of age. Arrowheads show dilated tubules and proteinaceous casts, and arrows display interstitial fibrosis. Scale bar, 25 μm. (D) Representative transmission electron micrograph images in control, Dnm DKO, and Agtr1afl/− Dnm DKO mice at 4 weeks of age. Arrowheads demonstrate foot process effacement. Scale bar, 1 μm. (E) Quantification of glomerulosclerosis in (A and B). Black, red, and blue dots represent control, Dnm DKO, and Agtr1afl/− Dnm DKO mice, respectively. *P<0.05. n=3 different mice were used in independent experiments. (F) Quantification of interstitial fibrosis in (B). Black, red, and blue dots represent control, Dnm DKO, and Agtr1afl/− Dnm DKO mice, respectively. *P<0.05. n=3 different mice were used in independent experiments. (G) Quantification of the number of foot processes in (D). Black, red, and blue dots represent control, Dnm DKO, and Agtr1afl/− Dnm DKO mice, respectively. *P<0.05. n=3 different mice were used in independent experiments. Ctrl, control.
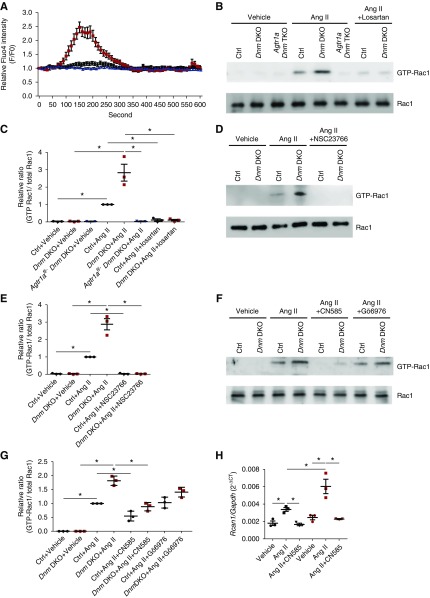
AngII Induces Calcium Influx and Calcineurin-Mediated Rac1 Activation in Dnm DKO Podocytes
Previous studies have demonstrated that AngII induces calcium influx in podocytes31,32 and Rac1 activation in smooth muscle cells.10,33–35 Hence, we examined AngII-mediated calcium influx by using the calcium indicator Fluo-4 in control and Dnm DKO primary podocytes. After AngII treatment, Dnm DKO primary podocytes showed increased calcium influx when compared with control primary podocytes. This effect was reduced in the Agtr1afl/− Dnm DKO primary podocytes (Figure 5A). We first examined the time course of Rac1 activation in enriched podocytes after AngII stimulation (Supplemental Figure 6A, quantified in Supplemental Figure 6B). We next determined that Rac1 activation was also increased in AngII-stimulated Dnm DKO primary podocytes when compared with control primary podocytes (Figure 5B). This effect was abrogated by losartan treatment, deletion of Agtr1a, or NSC23766 (Rac1 inhibitor) treatment (Figure 5, B and D, quantified in Figure 5, C and E). As previously reported, we next examined whether the Rac1 activation was induced by calcium-induced activation of conventional protein kinase C (cPKC) or calcineurin.36,37 After AngII stimulation in control and Dnm DKO primary podocytes, Rac1 activation could be mitigated by pretreatment with CN585 (calcineurin inhibitor), but not with Gö 6983 (cPKC inhibitor) (Figure 5F, quantified in Figure 5G). Further supporting our findings, AngII stimulation increased Rcan1 mRNA expression, which is a known marker of calcineurin-NFAT activation (Figure 5H).38 This suggests that AngII-induced Rac1-activation occurs in a calcineurin-dependent manner, which is accentuated in Dnm DKO primary podocytes when compared with control primary podocytes.
Figure 5.
AngII induces calcium influx and calcineurin-mediated Rac1 activation in Dnm DKO primary podocytes. (A) The intracellular calcium dynamics shown by calcium imaging tracings in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) primary podocytes stimulated with AngII using Fluo-4 AM. n=3. (B) Representative immunoblot images of GTP-Rac1 and total Rac1 in control, Dnm DKO primary podocytes stimulated with AngII ± losartan, and Agtr1afl/− Dnm DKO primary podocytes stimulated with AngII. (C) Quantification of the immunoblots in (B). Black, red, and blue dots represent control, Dnm DKO, and Agtr1afl/− Dnm DKO primary podocytes, respectively. *P<0.05. n=3. (D) Representative immunoblot images of GTP-Rac1 and total Rac1 in control and Dnm DKO primary podocytes stimulated with AngII ± NSC23766. (E) Quantification of immunoblots in (D). Black and red dots represent control and Dnm DKO primary podocytes, respectively. *P<0.05. n=3. (F) Representative immunoblot images of GTP-Rac1 and total Rac1 in control and Dnm DKO primary podocytes stimulated with AngII ± CN585 or Gö 6976. (G) Quantification of immunoblots in (F). Black and red dots represent control and Dnm DKO primary podocytes, respectively. *P<0.05. n=3. (H) Rcan1 mRNA expression in control and Dnm DKO primary podocytes stimulated with AngII and CN585. Black and red dots represent control and Dnm DKO primary podocytes, respectively. *P<0.05. n=3. Ctrl, control.
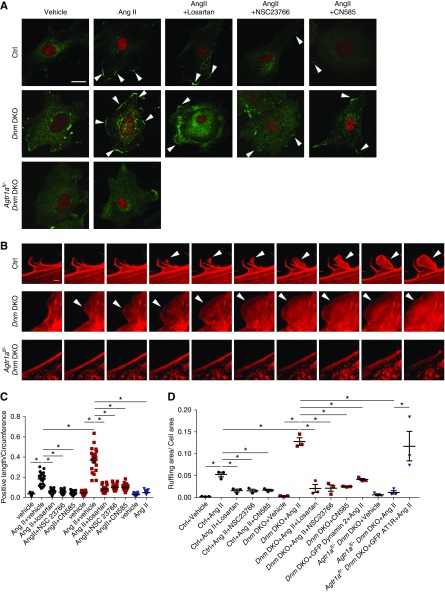
AngII Induces Lamellipodia and Membrane Ruffling in Dnm DKO Podocytes
Because robust AngII-mediated Rac1 activation in Dnm DKO primary podocytes was observed, we examined whether Rac1-induced lamellipodial extension occurred in AngII-stimulated control, Dnm DKO, and Agtr1afl/− Dnm DKO primary podocytes. Rac1 activation has been suggested to play an important role in the pathogenesis of podocyte injury,39–42 whereas lamellipodial protrusion may mimic what may occur during foot process effacement.43 There was a striking increase in lamellipodial formation demonstrated by cortactin staining in AngII-stimulated Dnm DKO primary podocytes when compared with control podocytes. This observation was attenuated in Agtr1afl/− Dnm DKO primary podocytes or Dnm DKO primary podocytes treated with losartan, NSC23766, or CN585 (Figure 6A, quantified in Figure 6C). Live imaging of AngII-stimulated Dnm DKO primary podocytes overexpressing mCherry-utrophin (F-actin marker) revealed striking membrane ruffling when compared with control primary podocytes. However, the deletion of podocyte-associated Agtr1a or treatment with chemical inhibitors losartan, NSC23766, and CN585 reduced membrane ruffling (Figure 6B [montages], quantified in Figure 6D, Supplemental Videos 3–6). Furthermore, in Dnm DKO primary podocytes, the re-expression of dynamin 2 reduced AngII-induced membrane ruffling, whereas re-expression of AT1R exacerbated AngII-induced membrane ruffling in Agtr1a Dnm DKO primary podocytes (Figure 6D). These results suggest that podocyte membrane ruffling is dependent on the AngII-induced AT1R-mediated calcineurin signaling pathway which was augmented after the deletion of podocyte-associated Dynamin1 and 2.
Figure 6.
Rac1-dependent lamellipodia is observed in Dnm DKO primary podocytes with AngII. (A) Representative immunofluorescence images of cortactin (green) and WT1 (red) in control, Dnm DKO, and Agtr1afl/− Dnm DKO primary podocytes stimulated with vehicle or AngII ± losartan, NSC23766, or CN585. Arrowheads display cortactin-positive cell edge. Scale bar, 10 μm. (B) Representative montage images of utrophin (red) in control, Dnm DKO, and Agtr1afl/− Dnm DKO primary podocytes stimulated with AngII. Arrowheads show membrane ruffling. Scale bar, 1 μm. (C) Quantification of cortactin-positive length divided by cell circumference in (A). Black, red, and blue dots represent control, Dnm DKO, and Agtr1afl/− Dnm DKO primary podocytes, respectively. *P<0.05. n=3. (D) Quantification of maximum ruffling area divided by total cell area during live imaging in control (black), Dnm DKO (red), and Agtr1afl/− Dnm DKO (blue) primary podocytes. *P<0.05. n=3. Ctrl, control.
Discussion
ACEIs and ARBs are the standard of care for therapy of glomerular diseases.44,45 The main mechanism attributed to mitigating albuminuria is through the reduction of intraglomerular pressure due to vasodilation of the efferent arteriole.5,6 There is a large body of evidence to suggest that AngII stimulation within cultured human and mouse podocytes results in profound effects on the actin cytoskeleton and induces apoptosis.46–49 These studies suggest that inhibition of AngII may have direct effects on the podocytes to maintain the integrity of the glomerular filtration barrier. However, the biologic significance of podocyte-associated AT1R in vivo remains unclear. Rats overexpressing AT1R in podocytes developed albuminuria and ultrastructural evidence of foot process effacement,11 but mice with podocyte-specific genetic ablation of Agtr1a did not show reduced albuminuria or podocyte injury after immunotoxin-induced nephropathy.14 Furthermore, deletion of podocyte Agtr1a did not result in improvement of proteinuria in AngII-infused mice although changes in BP may have confounded the results.14 In mouse models of HIV-1 nephropathy, losartan (ARB) reduced proteinuria and glomerulosclerosis; however, a lack of these effects was observed by deletion of podocyte Agtr1a.13 In another study, using a murine lupus model, the loss of AT1aR resulted in worsened albuminuria and increased death, possibly due to increased AT1bR expression.50 However, in these kidney injury models, whether exaggerated podocyte-specific AT1R stimulation actually occurred was not examined, making the interpretation of these results difficult. Hence, in this study we were able to capitalize on our Dnm DKO podocytes, where clathrin-mediated endocytosis was arrested,51,52 to show sustained membrane localization and continued stimulation of AT1R. We were also able to demonstrate that the loss of podocyte-associated AT1aR in the Dnm DKO mice reduced proteinuria and podocyte injury. We further demonstrated that in enriched Dnm DKO primary podocytes there was an increase in calcium signaling, which validates previous findings where AngII stimulation in podocytes resulted in increased intracellular calcium concentrations likely due to increased expression of TRPC59 or TRPC612,53 and release from the cytoplasmic stores.7,8 We also observed that increased calcium in primary podocytes elicited NFAT and Rac1 activation, and these pathways have been reported to induce glomerulosclerosis.54,55 Furthermore, AngII-stimulated podocytes have previously been shown to induce podocyte contractility.56,57 In validation of these findings, an increase in lamellipodial extension was observed in control primary podocytes, but more strikingly in Dnm DKO primary podocytes after AngII stimulation. AngII stimulation has been reported to induce podocyte apoptosis and autophagy.58,59 However, our control and Dnm DKO mice did not demonstrate increased autophagosomes by ultrastructural examination or evidence of apoptosis by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining, respectively (data not shown).
Clathrin-mediated endocytosis plays an essential role in internalization of AT1R, along with adrenergic receptors, endothelin receptors, calcium sensing receptors, and EGFRs.60–64 Several investigators have demonstrated that endothelin receptor signaling plays a critical role in progression of podocyte injury,65,66 whereas others have shown that AngII stimulation can transactivate EGFR.67 Because deletion of podocyte-associated Agtr1a results in only partial improvement of proteinuria and kidney failure in Dnm DKO mice, other receptors and pathways are likely also contributing to podocyte injury in these transgenic mice. Limitations do exist in this study. It remains unclear whether sustained podocyte-associated AT1R signaling in the Dnm DKO mice correlates with progression of human glomerular disease. Yet, the specific loss of AT1R in our model suggests that inhibiting podocyte-associated AT1R signaling may have implications in reducing albuminuria and kidney failure.
Hence, our study supports the important role of podocyte AT1aR signaling in the progression of podocyte injury through the induction of Rac1 activation in a calcineurin-dependent manner. Understanding podocyte-specific signaling pathways may further open new avenues for future therapeutic regimens for primary podocytopathies.
Disclosures
None.
Funding
The work was supported by grants from Osaka Medical Research Foundation for Intractable Diseases and from Sumitomo Life Welfare and Culture Foundation to Dr. Inoue. The work was also supported by the National Institutes of Health (DK083294 and DK093269), the Department of Defense (W81XWH-17-1-0662 to Dr. Ishibe), and the George O’Brien Kidney Center at Yale (P30DK079310).
Supplementary Material
Acknowledgments
Dr. Inoue, Dr. Tian, and Dr. Velazquez performed the research. Dr. Cross, Mr. Groener, Dr. Hassan, Dr. Inoue, Dr. Ishibe, Mr. Li, Dr. Mundel, Dr. Pedigo, Ms. Shin, Dr. Soda, Dr. Tian, Dr. Yamamoto, Dr. Y. Wang, and Dr. Z. Wang designed the research and analyzed the data. Dr. Ishibe wrote the manuscript. All authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019010053/-/DCSupplemental.
Supplemental Figure 1. EGF receptor (EGFR) activation was prolonged in Dnm DKO primary podocytes compared with control after angiotensin II treatment.
Supplemental Figure 2. Deletion of Agtr1a or treatment with angiotensin II–converting enzyme inhibitor captopril improves albuminuria and glomerulosclerosis in Dnm DKO mice.
Supplemental Figure 3. Heterozygous Agtr1a does not affect albuminuria and kidney failure in Dnm DKO mice.
Supplemental Figure 4. Deletion of Agtr1a blocks angiotensin II–induced ERK phosphorylation in Dnm DKO primary podocytes.
Supplemental Figure 5. Captopril improved albuminuria and kidney failure in Agtr1afl/− Dnm DKO mice.
Supplemental Figure 6. Rac1 activation after angiotensin II stimulation.
Supplemental Video 1. AT1R and clathrin dynamics in control primary podocytes stimulated with AngII.
Supplemental Video 2. AT1R and clathrin dynamics in Dnm DKO primary podocytes stimulated with AngII.
Supplemental Video 3. AngII-induced ruffling in control primary podocytes.
Supplemental Video 4. AngII-induced ruffling in Dnm DKO primary podocytes.
Supplemental Video 5. AngII-induced ruffling in Agtr1afl/− Dnm DKO primary podocytes.
Supplemental Video 6. AngII-induced ruffling in Dnm DKO primary podocytes treated with losartan.
References
- 1.Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al.: Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 334: 939–945, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 5.Imanishi M, Yoshioka K, Konishi Y, Okumura M, Okada N, Sato T, et al.: Glomerular hypertension as one cause of albuminuria in type II diabetic patients. Diabetologia 42: 999–1005, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Myers BD, Deen WM, Brenner BM: Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ Res 37: 101–110, 1975 [DOI] [PubMed] [Google Scholar]
- 7.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, et al.: Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J Clin Invest 99: 2772–2781, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitschke R, Henger A, Ricken S, Gloy J, Müller V, Greger R, et al.: Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int 57: 41–49, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, et al.: A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358: 1332–1336, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, et al.: Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, et al.: Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu A, Zhong J, Miyazaki Y, Hosoya T, Ichikawa I, Matsusaka T: ARB protects podocytes from HIV-1 nephropathy independently of podocyte AT1. Nephrol Dial Transplant 27: 3169–3175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaka T, Asano T, Niimura F, Kinomura M, Shimizu A, Shintani A, et al.: Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor-independent. Hypertension 55: 967–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pierce KL, Premont RT, Lefkowitz RJ: Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Oppermann M, Freedman NJ, Alexander RW, Lefkowitz RJ: Phosphorylation of the type 1A angiotensin II receptor by G protein-coupled receptor kinases and protein kinase C. J Biol Chem 271: 13266–13272, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Lefkowitz RJ, Shenoy SK: Transduction of receptor signals by beta-arrestins. Science 308: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Morinelli TA, Walker LP, Velez JC, Ullian ME: Clathrin-dependent internalization of the angiotensin II AT1A receptor links receptor internalization to COX-2 protein expression in rat aortic vascular smooth muscle cells. Eur J Pharmacol 748: 143–148, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Cunha FM, Berti DA, Ferreira ZS, Klitzke CF, Markus RP, Ferro ES: Intracellular peptides as natural regulators of cell signaling. J Biol Chem 283: 24448–24459, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takei K, McPherson PS, Schmid SL, De Camilli P: Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature 374: 186–190, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, et al.: Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Kakino A, Takeshita H, Hayashi N, Li L, Nakano A, et al.: Oxidized LDL (oxLDL) activates the angiotensin II type 1 receptor by binding to the lectin-like oxLDL receptor. FASEB J 29: 3342–3356, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, et al.: Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol 21: 1145–1156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nández R, Balkin DM, Messa M, Liang L, Paradise S, Czapla H, et al.: A role of OCRL in clathrin-coated pit dynamics and uncoating revealed by studies of Lowe syndrome cells. ELife 3: e02975, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan H, Tian X, Inoue K, Chai N, Liu C, Soda K, et al.: Essential role of X-box binding protein-1 during endoplasmic reticulum stress in podocytes. J Am Soc Nephrol 27: 1055–1065, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Schlondorff J, Higgs HN, Pollak MR: Inverted formin 2 regulates actin dynamics by antagonizing Rho/diaphanous-related formin signaling. J Am Soc Nephrol 24: 917–929, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benicky J, Hafko R, Sanchez-Lemus E, Aguilera G, Saavedra JM: Six commercially available angiotensin II AT1 receptor antibodies are non-specific. Cell Mol Neurobiol 32: 1353–1365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, et al.: Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A 102: 1442–1447, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bollée G, Flamant M, Schordan S, Fligny C, Rumpel E, Milon M, et al.: Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat Med 17: 1242–1250, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Chen JK, Harris RC: EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol 26: 1115–1125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A: Podocyte injury in diabetic nephropathy: Implications of angiotensin II-dependent activation of TRPC channels. Sci Rep 5: 17637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, et al.: Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest 124: 2050–2058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz U, Thömmes K, Beier I, Wagner W, Sachinidis A, Düsing R, et al.: Angiotensin II-induced stimulation of p21-activated kinase and c-Jun NH2-terminal kinase is mediated by Rac1 and Nck. J Biol Chem 276: 22003–22010, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Jiang M, Bujo H, Ohwaki K, Unoki H, Yamazaki H, Kanaki T, et al.: Ang II-stimulated migration of vascular smooth muscle cells is dependent on LR11 in mice. J Clin Invest 118: 2733–2746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greka A, Mundel P: Calcium regulates podocyte actin dynamics. Semin Nephrol 32: 319–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, Collard JG: Calcium signaling regulates translocation and activation of Rac. J Biol Chem 278: 39413–39421, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pandey D, Goyal P, Dwivedi S, Siess W: Unraveling a novel Rac1-mediated signaling pathway that regulates cofilin dephosphorylation and secretion in thrombin-stimulated platelets. Blood 114: 415–424, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lee MY, Garvey SM, Baras AS, Lemmon JA, Gomez MF, Schoppee Bortz PD, et al.: Integrative genomics identifies DSCR1 (RCAN1) as a novel NFAT-dependent mediator of phenotypic modulation in vascular smooth muscle cells. Hum Mol Genet 19: 468–479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robins R, Baldwin C, Aoudjit L, Côté JF, Gupta IR, Takano T: Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int 92: 349–364, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Brähler S, Yu H, Suleiman H, Krishnan GM, Saunders BT, Kopp JB, et al.: Intravital and kidney slice imaging of podocyte membrane dynamics. J Am Soc Nephrol 27: 3285–3290, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, et al.: ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagase M, Fujita T: Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013 [DOI] [PubMed] [Google Scholar]
- 43.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, et al.: Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest 122: 674–692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wheeler DC, Becker GJ: Summary of KDIGO guideline. What do we really know about management of blood pressure in patients with chronic kidney disease? Kidney Int 83: 377–383, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Xu R, Sun S, Huo Y, Yun L, Huang S, Li G, et al.: Effects of ACEIs versus ARBs on proteinuria or albuminuria in primary hypertension: A meta-analysis of randomized trials. Medicine (Baltimore) 94: e1560, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenk LK, Möller-Kerutt A, Klosowski R, Wolters D, Schaffner-Reckinger E, Weide T, et al.: Angiotensin II regulates phosphorylation of actin-associated proteins in human podocytes. FASEB J 31: 5019–5035, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, et al.: Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Ren Z, Yang Q, Ding G: Csk regulates angiotensin II-induced podocyte apoptosis. Apoptosis 21: 846–855, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, et al.: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65: 30–39, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, et al.: Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 119: 943–953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue K, Ishibe S: Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am J Physiol Renal Physiol 309: F398–F405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferguson SM, Raimondi A, Paradise S, Shen H, Mesaki K, Ferguson A, et al.: Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell 17: 811–822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckel J, Lavin PJ, Finch EA, Mukerji N, Burch J, Gbadegesin R, et al.: TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol 22: 526–535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Jarad G, Tripathi P, Pan M, Cunningham J, Martin DR, et al.: Activation of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc Nephrol 21: 1657–1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, et al.: Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saleem MA, Zavadil J, Bailly M, McGee K, Witherden IR, Pavenstadt H, et al.: The molecular and functional phenotype of glomerular podocytes reveals key features of contractile smooth muscle cells. Am J Physiol Renal Physiol 295: F959–F970, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, et al.: Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med (Berl) 86: 1379–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Jia J, Ding G, Zhu J, Chen C, Liang W, Franki N, et al.: Angiotensin II infusion induces nephrin expression changes and podocyte apoptosis. Am J Nephrol 28: 500–507, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, et al.: ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol 299: C488–C496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodman OB Jr., Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, et al.: β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383: 447–450, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Chun M, Lin HY, Henis YI, Lodish HF: Endothelin-induced endocytosis of cell surface ETA receptors. Endothelin remains intact and bound to the ETA receptor. J Biol Chem 270: 10855–10860, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H: Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem 275: 17596–17604, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Grant MP, Stepanchick A, Cavanaugh A, Breitwieser GE: Agonist-driven maturation and plasma membrane insertion of calcium-sensing receptors dynamically control signal amplitude. Sci Signal 4: ra78, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Vieira AV, Lamaze C, Schmid SL: Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274: 2086–2089, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Lenoir O, Milon M, Virsolvy A, Hénique C, Schmitt A, Massé JM, et al.: Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol 25: 1050–1062, 2014. 24722437 [Google Scholar]
- 66.Garsen M, Lenoir O, Rops AL, Dijkman HB, Willemsen B, van Kuppevelt TH, et al.: Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol 27: 3545–3551, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flannery PJ, Spurney RF: Transactivation of the epidermal growth factor receptor by angiotensin II in glomerular podocytes. Nephron, Exp Nephrol 103: e109–e118, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.