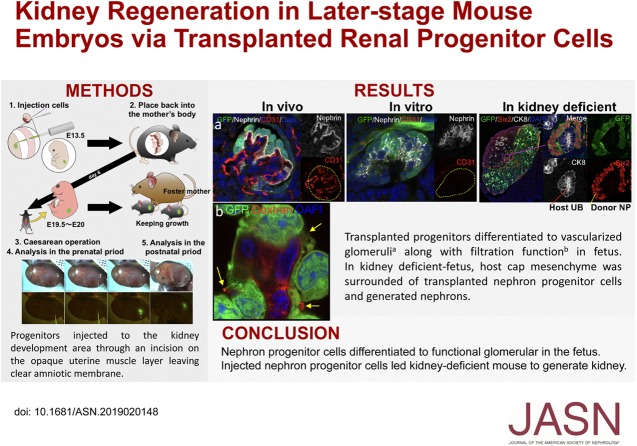
Significance Statement
Although researchers have attempted to regenerate organs by transplanting stem cells into embryos of another species, there is no established method for transplanting cells into the midstage embryo near the onset of organogenesis or information on kidney regeneration resulting from this approach. The authors describe a method to allow transplantation of renal progenitor cells (embryonic stage–matched cells) into the retroperitoneal cavity of mouse fetuses without lethality. The transplanted cells differentiated to glomeruli with capillary loops (comprising blood vessels from the host) and filtration function. In a mouse model of fetuses with atrophic kidneys, transplanting renal progenitor cells into such fetuses produced a transplanted-cell neonephron. These findings demonstrate potential kidney regeneration through transplanting renal progenitor cells to the site of natural kidney development.
Keywords: kidney regeneration, kidney development, pluripotent stem cell, progenitor, stem cell, nephron
Visual Abstract
Abstract
Background
The limited availability of donor kidneys for transplantation has spurred interest in investigating alternative strategies, such as regenerating organs from stem cells transplanted into animal embryos. However, there is no known method for transplanting cells into later-stage embryos, which may be the most suitable host stages for organogenesis, particularly into regions useful for kidney regeneration.
Methods
We demonstrated accurate transplantation of renal progenitor cells expressing green fluorescent protein to the fetal kidney development area by incising the opaque uterine muscle layer but not the transparent amniotic membrane. We allowed renal progenitor cell–transplanted fetuses to develop for 6 days postoperatively before removal for analysis. We also transplanted renal progenitor cells into conditional kidney-deficient mouse embryos. We determined growth and differentiation of transplanted cells in all cases.
Results
Renal progenitor cell transplantation into the retroperitoneal cavity of fetuses at E13–E14 produced transplant-derived, vascularized glomeruli with filtration function and did not affect fetal growth or survival. Cells transplanted to the nephrogenic zone produced a chimera in the cap mesenchyme of donor and host nephron progenitor cells. Renal progenitor cells transplanted to conditional kidney-deficient fetuses induced the formation of a new nephron in the fetus that is connected to the host ureteric bud.
Conclusions
We developed a cell transplantation method for midstage to late-stage fetuses. In vivo kidney regeneration from renal progenitor cells using the renal developmental environment of the fetus shows promise. Our findings suggest that fetal transplantation methods may contribute to organ regeneration and developmental research.
The rate of CKD is estimated to be about 10% worldwide. Currently, RRT is the only long-term solution, but over 2 million people die each year due to its limited availability.1 As the number of patients with CKD continues to climb each year, RRT has—by necessity—been limited to patients with ESKD. Thus, new therapeutic strategies are required to treat both CKD and ESKD.
One potential option is to regenerate a kidney from exogenous stem cells using an animal fetus as a scaffold for organ regeneration. Previously, successful regeneration of human kidney tissue was reported by transplanting human mesenchymal stem cells with overexpressed glial cell line–derived neurotrophic factor into the fetal rat kidney development area in vitro.2,3 The technique of organ regeneration using animal fetuses or embryos has therefore been attracting increasing attention.4,5 Another technique, that of transplanting induced pluripotent stem cells to blastocysts in kidney-deficient mice, has successfully regenerated kidneys from exogenous cells.6 These methods use chimerism to co-opt the complex system of native organ development. We assumed that progenitor cells are suitable as a cell source for organ regeneration. Kidney is considered to originate from ureteric bud (UB), stromal progenitor cells (SPCs), and the nephron progenitor cells (NPCs) of three renal progenitor cells (RPCs).7 An advantage of using NPCs as a cell source for regenerating kidneys is that they can be derived from pluripotent stem cells, propagated, and stored.8–10 In addition, isochronicity (donor/host cells matched by generational stage) is considered important for success with chimerism-based techniques.5,11,12 Thus, we believe that when transplanting RPCs, the most suitable host stages for organogenesis are later embryonic stages, particularly those around nephrogenesis (after midstage mouse embryo, i.e., after embryonic day 11.5 [E11.5]).13,14 We demonstrated the regeneration of nephrons was possible with exogenous rat NPCs using a transgenic mouse fetal kidney that conditionally eliminates NPCs.13,15 That is, the host embryonic kidney was genetically modified to inhibit nephrogenesis, thereby creating an empty organ niche. Implanted exogenous NPCs have been reported to show growth in organ culture13; however, it remains unknown whether the exogenous renal lineage progenitors would continue to develop inside a living embryo without taking out the metanephros (MN).
There is no current method for delivering the exogenous cells into the organ niche of a midstage developing embryo that will allow it to continuously develop to neonatality. We therefore focused on the potential use of the transuterine injection method known as the exo utero method.16 This is a method used in neuroscience to deliver a drug solution nonlethally to the nascent cerebral ventricle. However, no published studies have used this technique to deliver cells into the retroperitoneal cavity (within the renal development area). Because the retroperitoneal cavity is narrower than the cerebral ventricle and the cell suspension is more viscous than the drug solution, accurate cell transplantation into the renal development area was impossible with the conventional method without critically damaging the fetus. Here we developed a technique to deliver cells to the renal development area in the retroperitoneal cavity of a later-stage mouse embryo. We examined the subsequent differentiation of the transplanted RPCs and assessed any effects on host fetal growth. In addition, we sought to determine whether exogenous RPCs could commit coordinative nephrogenesis in an embryo genetically modified to prevent endogenous nephrogenesis.
Methods
Mouse Husbandry and Genetic Characterization
Animal experiments followed the Guidelines for the Proper Conduct of Animal Experiments of the Science Council of Japan (2006) and were approved by the Institutional Animal Care and Use Committee of the Jikei University School of Medicine (protocol numbers: 2015-078, 2016-027, 2017-051, 27-14, 27-69, 28-29, II-28-8). All efforts were made to minimize animal suffering. C57BL6/NCrSlc, C3H/HeSlc, and C57BL/6-Tg(CAG-EGFP) (green fluorescent protein [GFP] mice) mice were purchased from SLC Japan (Shizuoka, Japan). C57BL/6-Gt (ROSA) 26Sor (tm1[HBEGF]Awai)/J mice (iDTR) were purchased from Jackson Laboratory (Bar Harbor, ME).17 Six2-GFP-Cre transgenic mice (Six2Cre mice) were gifted by A.P. McMahon.18 Six2 mice were crossed with iDTR mice to obtain bigenic offspring (Six2Cre/iDTR mice). Mice were bred using timed matings; 12 pm on the day of vaginal plug detection was considered 0.5 days postcoitum. Pregnant mice were housed individually thereafter.
Genomic DNA was obtained from tail biopsies, and genotyping was performed using primers (Supplemental Methods).
Exo Utero Cell Transplantation
Mice at E13.5 were anesthetized with isoflurane and administered ritodrine hydrochloride (1.4 mg/kg body wt, intraperitoneally; Kissei Pharmaceutical) for uterine muscle relaxation.16 A median incision was then made and the intact uterus was gently pulled out from the peritoneal cavity, just far enough to allow visualization and access for transplantation. The uterine muscle layer opposite the placenta was incised with scissors along the major axis of the uterus (Supplemental Movie 1).16 The presence of a fetus was confirmed inside the transparent amniotic membrane and a puncture target was set based on the base of the hind limb. The fetus was punctured perpendicularly to the body surface using a three-axis manipulator (YOU-1; Narishige; Supplemental Figure 1B), and the depth of the puncture was approximately 1.5 mm. Because it was difficult to pass the glass needle for cell injection through the amniotic membrane, a guide hole was first made in the amniotic membrane with a thin steel needle with a diameter of 0.18 mm (J-type number 2, 0.18×30 mm; SEIRIN Corporation, Shizuoka, Japan) (Supplemental Movie 2); this was performed manually or using a three-axis manipulator. Next, for cell filling, a mouse pipette was used to aspirate cells into a glass tube. When the glass tube filled with cells was attached to the injector, the three-way stopcock (TS-TL2K; Terumo Corporation) was opened in advance to release the pressure to ensure the cells did not leak from the tip of the needle due to internal pressure at the time of adhesion. After the glass tubule was attached to the three-way stopcock, the cap was gently closed (Supplemental Figures 1C, 2, and 3). The glass needle filled with the cell suspension was then injected (SBP-100G-LL; Takasago Electric; for injector details settings, refer to the Supplemental Methods) under stereomicroscopic visualization (MZ16FA; Leica Microsystems). The dose of the suspended cells administered to the fetus was approximately 0.1 μl (2×104 cells). After transplantation, the uterus was returned to the peritoneal cavity of the mother and the incision was closed with sutures. Pups were delivered by cesarean section on day 19 of pregnancy. When GFP-RPCs were transplanted, fluorescence could be observed from the body surface of the fetus. We then investigated the effects of embryonic growth on cell transplantation by measuring the crown-rump length and weight of E19.5 fetuses.
MN-Derived Suspended RPCs
The details of this technique have been described previously.13 Briefly, E13.5 mouse MN from GFP mice were dissected in α-MEM (Gibco Life Technologies). MNs were collected into 1-ml Accutase tubes (Innovative Cell Technologies) and incubated at 37°C for 5 minutes, with manual pipetting every 5 minutes. MNs were then centrifuged at 300 × g for 5 minutes. Pellets were resuspended in 1 ml PBS (Gibco Life Technologies) and dissociated by vortexing for 10 seconds to generate single-cell suspensions. Cells were passed through a 40-μm cell strainer (BD Falcon) and centrifuged at 700 × g for 3 minutes. The supernatant was removed completely. RPCs are cells of heterogeneous constitution, which contain Six2-positive NPCs (Supplemental Figure 2). These cells were defined as dissociated single cells (Supplemental Movie 3).
In Vivo Imaging Using Fluoro Labeled Dextran
The standard method for in vivo imaging using fluoro labeled dextran has been previously described.15,19 For details regarding the method, refer to the Supplemental Methods.
RPC Transwell Culture
RPCs were cocultured with mouse embryonic spinal cords taken from E13.5 embryos.20,21 For details regarding the method, refer to the Supplemental Methods.
Diphtheria Toxin Injection into Amniotic Fluid
Details for this procedure have been described previously.22 A diphtheria toxin (DT) (50 ng/ml; Wako) stock solution, stored at −80°C until use, was thawed and diluted in PBS. Pregnant female mice were anesthetized and laparotomized; in each case, the uterus was delivered through the incision. Each embryo was microinjected with 25 ng DT in the diluted working solution. Subsequently, in the same manner as described above, the uterine muscle layer was dissected and the cells were transplanted into the retroperitoneal cavity through the amniotic membrane. The uterus was returned to the abdominal cavity and the incision was closed. Embryos were allowed to develop in situ until required for experimental use.
Depositing Fetuses with Foster Mother
The standard method for foster parenting has been previously described.23 For details regarding the method, refer to the Supplement Methods.
Histology and Immunofluorescence
Sectioning and whole-mount immunohistochemistry analyses were performed as previously described.13,15 For details regarding the staining method, see Supplemental Table 1, Supplemental Methods. The labeled slides were visualized under a fluorescence microscope (LSM880 confocal and BZ-9000; Zeiss and Keyence, respectively).
Electron Microscopy
Electron microscopy was performed as previously described.13 For details regarding the method, refer to the Supplemental Methods.
Statistical Analyses
All data are presented as means±SEM. Data were analyzed using the two-tailed Mann–Whitney U test (Figure 1, G–I) and the Kruskal–Wallis test (Figure 2H) was used for comparison. A P value of <0.05 was considered statistically significant. Experimental data were analyzed using GraphPad Prism software, version 7.0 (GraphPad Software), and Microsoft Excel (Microsoft).
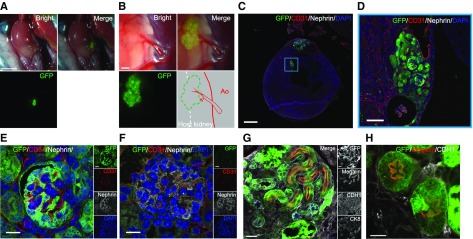
Figure 1.
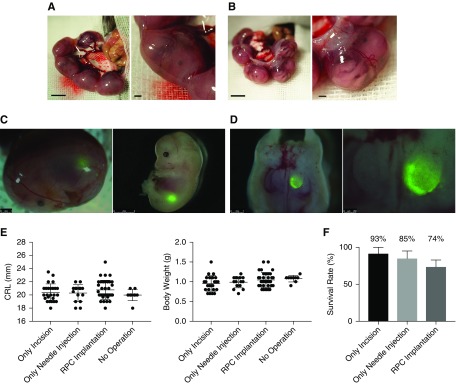
Transplantation of renal progenitor cells into the fetus allows surviving without inhibiting growth. (A) Photomicrographs of a fetus in utero at E13.5. (B) Fetus in utero after cutting the uterine muscle layer. (C and D) Visualization of GFP-expressing transplanted RPCs in the retroperitoneal cavity at E13.5. Images demonstrate a fetus removed after cell injection and dissected for validation of cell transplantation. (E, left) Crown-rump length (CRL) and (right) body weight at E19.5 were compared across the RPC-injected experimental group (n=35), a noninjected (puncture only) group (n=15), and a sham-operated group (n=20). (F) The survival rate was calculated as a percentage of the number of surviving fetuses 6 days after the operation (sham operation, n=14; puncture only, n=20; and RPC injection, n=19). There was no significant difference between groups. Bars represent means±SEM. P<0.05, Mann–Whitney U test. Scale bars, 1 mm in (A), (B), and left panel of (C) and (D); 2 mm in right panel of (C); 250 µm in right panel of (D).
Figure 2.
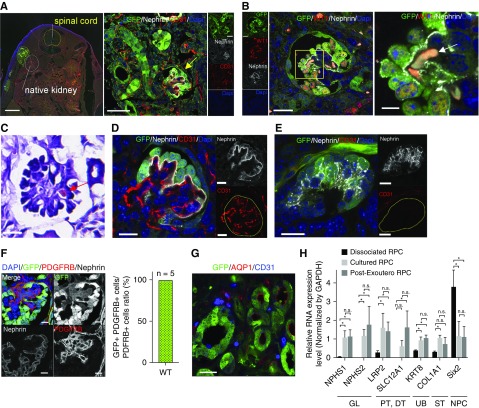
Analysis of nephrons derived from RPC regenerated inside the retroperitoneal cavity of fetus. (A) In immunofluorescence imaging, transplanted RPCs expressing GFP were present outside the host kidney in the retroperitoneum. Mature glomeruli with capillary loops (yellow arrow) derived from transplanted cells can be seen. (B and C) Erythrocytes (white arrow in [B] and red arrow in [C]) and blood flow were identified. (C) Hematoxylin and eosin stain. (D) In vivo glomeruli had capillary loops containing host embryo vascular endothelial cells (CD31+ and GFP–). (E) In vitro glomeruli without vascular endothelial cells (CD31+). (F) PDGFRb-positive cells in GFP-expressing glomeruli. All PDGFRb-positive cells expressed GFP. Refer to Supplemental Methods. (G) Transplanted cells expressing tubule markers AQP1 (red). (H) Quantified PCR analysis of nephron genes. Kruskal–Wallis test was for comparison. Error bars indicate SEM; *P<0.05; n=6. Scale bars, 500 µm in the left panel of (A); 50 µm in the right panel of (A); 20 µm in left panel of (B) and (C); 5 µm in right panel of (B); 20 µm in (D), (E), and (G); 10 µm in (F). DAPI, 4′,6-diamidino-2-phenylindole; DT, distal tubule; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GL, glomerulus; PT, proximal tubule; ST, stromal tissue; WT, wild type.
Results
Effects of Transuterine RPC Implantation on Fetal Growth
By transecting only the uterine muscle layer, the transparent fetal membranes (yolk sac) remained intact, yet allowed the embryonic morphology to be clearly visible (Figure 1, A and B). Using a three-axis manipulator and electric motor–driven injector (as shown in Supplemental Figure 1) allowed for minimally invasive transplantation of mouse E13.5 RPCs (Figure 1, C and D). To analyze the potential for fetal damage, we compared fetal growth in cell-transplanted, sham-injected (needle insertion only), and sham-operated (uterine incision only) groups. No significant differences were observed between groups for either the crown-rump lengths or the body weights of the embryos at E19.5 (6 days postinjection) (Figure 1E). When RPCs were transplanted, 74% of the fetuses survived to E19.5 (Figure 1F, Table 1).
Table 1.
Survival rate of fetus transplanted with cells, retained rate of donor cells, and rate of including donor cells in the host kidney
| Recipient Fetus | Survival Ratea | Rate of Donor Cells Remainingb | Rate of Including Cells inside Host Kidneyc |
|---|---|---|---|
| Wild-type mouse | 14 of 19 (74%) | 11 of 14 (79%) | 2 of 11 (18%) |
| Six2Cre/iDTR mouse | 8 of 10 (80%) | 7 of 8 (88%) | 2 of 7 (29%) |
Living fetus/cells-implanted fetus.
Fluorescence stereomicroscopy confirmed the presence of GFP-positive cells inside the embryos.
Analysis by immunostaining confirmed the donor cells were included inside the host kidney.
Development of Transplanted RPCs into Nephrons In Vivo
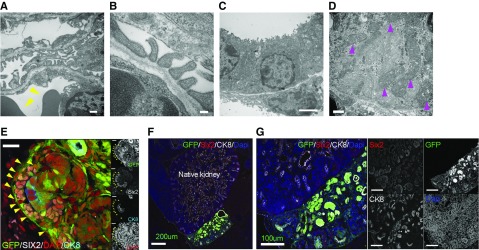
Injected RPCs expressing GFP were allowed to develop in utero for 6 days, but independently from the native embryonic kidney (Figure 2A). However, RPCs differentiated into the more mature glomeruli demonstrated CD31-positive vascular endothelial cells and Wilms tumor 1-positive epithelial cells, indicating glomerular capillaries had formed (Figure 2, A and B). Notably, the CD31-immunoreactive cells were negative for GFP, suggesting the blood vessels in the mature glomeruli were derived from the host (Figure 2, A–D); erythrocytes were observed inside the glomerulus (Figure 2B, white arrow; Figure 2C, red arrow); and blood was found flowing through the glomeruli. In contrast, blood vessels did not form in the immature glomeruli of transwell-cultured RPCs (Figure 2E). In addition, mesangial cells expressing platelet derived growth factor receptor β (PDGFRb),24 which were not observed in the culture experiment, were observed in the glomerulus (Figure 2F, left panel). All PDGFRb-positive cells in the regenerated glomeruli expressed GFP, suggesting they were derived from the transplanted cells (Figure 2F, right graph). Furthermore, the GFP-positive cells expressed the renal tubule markers Aquaporin 1 (AQP1) (Figure 2G). In quantitative PCR analysis, the expression of nephron markers in transplanted RPCs was significantly increased compared with that in RPCs before transplantation (Figure 2H). Moreover, glomerular differentiation in transplanted RPCs was not inferior to that in RPCs cultured in transwell chambers for the same period (6 days). In contrast, NPHS2 (glomerular marker) and SLC12A1 (distal nephron marker) levels tended to be higher in transplanted RPCs (Figure 2H). In detailed analysis using an electron microscope, the transplanted RPCs formed glomeruli that included host-derived capillary loops, as well as podocytes and slit diaphragms (Figure 3, A and B). A brush border was found in the lumen of the proximal tubule derived from transplanted RPCs (Figure 3C), which also demonstrated numerous mitochondria (Figure 3D, magenta triangles). Dissociated RPCs for transplantation included NPCs, UB cells, and SPCs (Supplemental Figure 2).25 NPCs and UB cells in dissociated RPCs self-aggregated to form the cap mesenchyme (Figure 3E). The transplanted UB cells differentiated and self-organized to form collecting ducts but did not integrate with host UB and collecting ducts (Figure 3, F and G). Notably, low-molecular-weight dextran experiments demonstrated the generated glomeruli were filtering the blood (Figure 4, A–D, yellow triangle). Podocytes were observed to form filtration slit structures (Figure 4D, yellow arrow). In addition, dextran was observed in the uriniferous tubule lumen (Figure 4E).
Figure 3.
Nephrons regenerate inside the retroperitoneal cavity are highly differentiated but do not integrate with the host kidney beyond the renal capsule. (A and B) Podocytes and slit diaphragms in glomeruli derived from transplanted cells. Erythrocytes indicated by yellow triangles on the endothelial side. (C) Brush border in the lumen of the proximal tubule. (D) There were abundant mitochondria in the cells (magenta triangles). (E) GFP-positive cells surrounded Six2-positive NPCs and Six2-negative cells (yellow triangle) in the cap mesenchyme. (F and G) Donor UB cells differentiated into UB tip and collecting duct but did not integrate with the host UB. Scale bars, 500 nm in (A) and (D); 100 nm in (B); 2 µm in (C); 20 µm in (E); 200 µm in (F); 100 µm in (G). CK8, Cytokeratin 8; DAPI, 4′,6-diamidino-2-phenylindole.
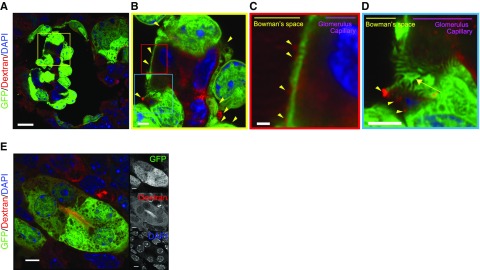
Figure 4.
Generated glomeruli displayed podocytes and filtration function. (A–C) Fluorescence-labeled dextrans in blood were filtered to the Bowman’s space side in the glomerulus (displayed filtered dextran with yellow triangles) derived from RPCs implanted in the wild-type mice. (D) Podocytes formed a filtration slit (yellow arrow). (E) Dextran was seen in the uriniferous tubule cavity. Scale bars, 20 µm in (A); 2 µm in (B) and (D); 1 µm in (C); 5 µm in (E). DAPI, 4′,6-diamidino-2-phenylindole.
Transplanted RPCs are Chimeric in Cap Mesenchyme
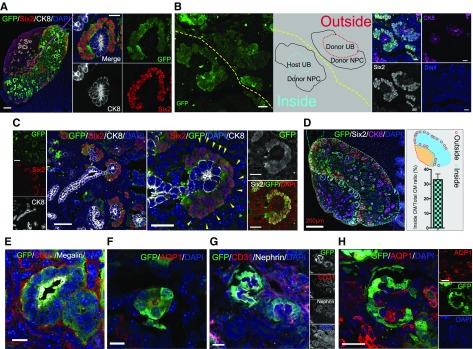
When transplanted into wild-type mice (those with normal embryonic kidney development; Figure 5A), the transplanted GFP-expressing RPCs connected to the host kidney (Figure 5, B and C). RPCs transplanted into the host kidney nephrogenic zone were found to be exogenous cells that invaded the host cap mesenchyme (Figure 5D). These integrated cells (GFP positive) were NPCs as verified by the expression of Six2 (Figure 5, D–F). However, most host UB cells did not show the integration of the donor UB cells (Supplemental Figure 3). These data indicate the NPCs included in transplant RPCs integrated into the developing host nephrons and formed a chimeric kidney.
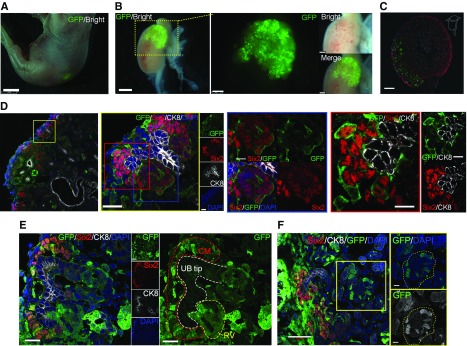
Figure 5.
Transplantation of RPC under the renal capsule enables integration with the host kidney but is a chimeric structure. (A) Fetus at E19.5 with RPCs expressing GFP (day 6 postinjection). (B) GFP-expressing progenitor cells integrated into the host kidney. (C and D) Integration of exogenous NPCs into host cap mesenchyme. Higher magnification of (D) left panel (blue frame panel) showing transplanted NPCs in the host renal vesicle (RV), and (red frame panel) chimerism of NPCs in cap mesenchyme (CM) visualized by GFP. (E) Mosaicism in the RV. (F) Chimeric glomeruli-like mosaics contributed by the host and donor NPCs. The yellow dotted line indicates the glomerulus. Scale bars, 2 mm in (A); 1 mm in left panel of (B); 250 µm in right panel of (B); 200 µm in (C); 20 µm in left panel and yellow-framed panel of (D) and in (E); 10 µm in blue- and red-framed panel of (D); 50 µm in (F). CK8, Cytokeratin 8; DAPI, 4′,6-diamidino-2-phenylindole.
Kidney Regeneration by Transplantation of RPCs into Kidney-Deficient Fetuses
We tested whether transplanted RPCs could regenerate a kidney in Six2Cre/iDTR fetuses with atrophic kidneys produced by ablation with DT. Genotyping data demonstrated the fetus was carrying a progenitor cell replacement system (Figure 6A). When GFP-NPCs were transplanted unilaterally into the renal development area of the kidney-deficient embryos (Figure 6B) and allowed to develop for 6 days (Figure 6C), the transplanted cells had developed throughout the kidney of the transplanted side (Figure 6, D and E). The nontransplanted side was clearly atrophic when compared with the wild-type control kidney (Figure 6F).
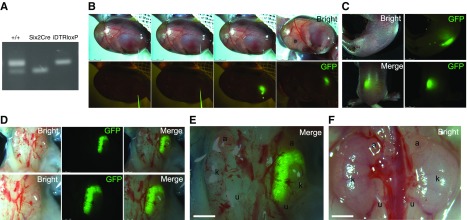
Figure 6.
Transplanted RPC form kidney in the renal-deficient fetus. (A) Genotyping of the fetus (Six2-GFPCre+; iDTR+). (B) GFP-expressing RPCs transplanted into the renal development area of the kidney-deficient embryo (Six2-GFPCre+; iDTR+) at E13.5. (C) Fetuses 6 days after transplantation. (D and E) Fetal kidney 6 days after transplantation. Note the unilateral GFP expression (right side). (F) Kidney of wild-type B6 mouse at E19.5. a, adrenal gland; k, fetal kidney; u, fetal ureter.
Analysis of the embryo demonstrated that dissociated single NPCs had adhered to the UB tip of the host, at which host NPCs were not found, and formed cap mesenchyme (Figure 7, A–C); 30% of the cap mesenchymes generated by implanted RPCs were integrated with the host UB tip (Figure 7D). NPCs and UB cells that did not integrate with the host kidney remained around the host kidney and formed their own cap mesenchyme by self-aggregation and organization (Figure 7B).
Figure 7.
RPC transplanted in a renal-deficient fetus connect with the host ureteric bud to form nephrons. (A) Immunolabeling of transplanted cells integrated into the host kidney. Transplanted NPCs gathered around the UB tip and formed cap mesenchyme (CM). (B) Transplanted cells present outside the kidney self-organized to form CM with GFP-expressing UB and NPCs (outside). The transplanted cells that reached the host nephrogenic zone aggregated around the host UB tip (GFP negative) and formed new CM (inside). (C) GFP-negative cells (yellow triangle) surrounded Six2-positive NPCs in the CM. (D) Overall, 33% of the regenerated cap mesenchyme was integrated with the host UB tip (counted to a total of 119 tips, n=4 slices). Refer to Supplemental Methods. (E–H) Transplanted cells expressing tubule and nephron markers. (G and H) Mature glomeruli with capillary loops derived from transplanted GFP cells. (H) AQP1 is expressed in structures associated with the developing renal vasculature. The expression of AQP1 was observed inside regenerated glomeruli. It was noted as a host-derived tissue because it was GFP negative. Scale bars, 100 µm in (A) and (B); 50 µm in (C); 10 µm in (E) and (F); 20 µm in (G) and (H). CK8, Cytokeratin 8; DAPI, 4′,6-diamidino-2-phenylindole.
GFP-positive cells from transplanted cells showed only Six2-positive NPCs when transplanted into a kidney of an NPC replacement model, despite transplant cells containing SPCs (Figure 7C, right panel, yellow triangle; Supplemental Figures 2 and 4). Six2-negative cells were attached to the circumference of Six2-positive cells but did not express GFP. In addition, we even found the GFP-expressing glomeruli and renal tubules inside the kidney of the kidney-deficient mouse (Figure 7, E–H). The tubule structures expressed Cadherin 1 (distal marker) and megalin/AQP1 (proximal marker) (Figure 7, E and F). Glomerulus structures displayed podocin-immunopositive capillary loops with CD31- and AQP1-positive vascular endothelial cells inside the basement membrane (Figure 7, G and H). AQP1 is expressed in the glomerular capillary system at the developmental stage.26 The glomerular capillary inside the regenerated glomeruli revealed a host-derived tissue because it was GFP negative (Figure 7, G and H).
Donor RPC Implantation and Differentiation Rates
When RPCs were transplanted into wild-type mice, living fetuses were obtained from 74% of animals (Figure 1I, Table 1). Moreover, GFP-positive cells were present inside the living fetuses in approximately 80% of the individuals, as confirmed by fluorescence stereomicroscopy (Table 1). When GFP-positive cells were analyzed by quantitative PCR and immunofluorescence using frozen sections, all specimens showed differentiated nephrons (Table 2). At transplant sites, the proportion of transplanted cells inside the kidney was 18%. In 82% of the cases, the transplanted cells existed outside the kidney in the retroperitoneum (Table 1). Survival rate was 80% in the Six2Cre/iDTR mice (the NPC replacement model), with a GFP-positive cell retention rate of 88%. The rate of integration between the host kidney and the transplanted cells was 29% (Table 1).
Table 2.
Efficiency of the differentiation of transplanted cells into glomeruli
| Experiments | Wild-Type Mouse Differentiated Nephron |
|---|---|
| IF | 11 of 11 (100%)a |
| qPCR | 8 of 8 (100%)b |
IF, imunnofluorescence; qPCR, quantitative PCR.
Imunnofluorescence staining confirmed Nephrin-positive glomerular structure.
Postnatal Development of Transplanted RPCs
Finally, we analyzed embryos for continued kidney development after RPC transplantation by fostering some of the fetuses delivered by cesarean section with C3H dams (Supplemental Movie 4) until postnatal day 14. Examination on postnatal day 14 showed that the neo-nephron derived from transplanted cells had adhered to the native kidney (Figure 8, A and B) and was integrated with the host’s blood vessels (Figure 8, C–E), indicating that, apparently, normal development had continued. Particularly, it showed a regenerative glomerular structure comparable to that of B6 fetal glomeruli at the same time (Figure 8, E and F). It was confirmed that glomeruli-expressing nephrin, proximal renal tubule–expressing megalin, and distal tubule–expressing Cadherin 1 were present and that they differentiated into the nephron (Figure 8, E, G, and H). We were able to raise RPC-transplanted fetuses of B6 mice after birth. However, cell-transplanted, kidney-deficient mice were killed by foster parents on the first day of life.
Figure 8.
Regenerating nephrons inside the retroperitoneal cavity of the Wild-type mouse are able to be maintained after birth. (A and B) Transplanted GFP-RPCs adhered to the native kidney and were connected to the host’s blood vessels. (C) Kidney was collected and immunofluorescence staining was performed. (D) Transplanted GFP-RPCs partially penetrated into the fetal kidney at postnatal day 14 (P14). (E) The transplanted GFP-RPCs differentiated into nephrons with expressed nephrin, and CD31-positive endothelial cells were observed in the glomerulus under high magnification (63× magnification). (F) As control, a normal B6 fetal glomerulus on P14. (G) The transplanted GFP-RPC expressed a proximal tubular marker (Megalin). A site positive for CDH1 (collecting duct and distal tubule marker) and negative for CK8 (collecting duct marker) indicates a distal tubule. (H) Proximal tubule in the high magnification. Scale bars, 2 mm in (A); 250 µm in (B); 500 µm in (C); 20 µm in (D) and (G); 10 µm in (E), (F), and (H). Ao, Aorta; CDH1, Cadherin 1; CK8, Cytokeratin 8; DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
Organ regeneration technologies using animal embryos have great potential for regenerative medicine.27 Hybrid animal techniques use two strategies, either pre- or postimplantation chimerism.31 Postimplantation chimerism is a combination of progenitor cells and mid- to late-stage embryo, whereas organogenesis can avoid generating off-target organs (e.g., germ cells or brain cells).5,11,27,28 Moreover, higher signal homology during organogenesis may overcome cross-species barriers.12,27,29–31 Thus, the strategy of postimplantation chimerism for organ regeneration is drawing attention as a new concept; however, to date, no method has been established to transplant cells into the embryo at the stage of nephrogenesis. Our preliminary experiments led to the hypothesis that the opacity and difficulty in penetrating the uterine muscle layer (myometrium) was one reason for this lack of methodology. By applying the exo utero method, it was possible to accurately confirm the morphology of the fetus from the transparent amniotic membrane; however, even the transparent amniotic membrane, which was thinner than the myometrium, was difficult to puncture for cell transplantation due to its hardness and thickness. We were able to overcome this problem by first forming a small hole using a thin steel needle, which then allowed for a slightly thicker glass needle to enter smoothly for cell transplantation (Supplemental Figure 1A, Supplemental Movie 2).
We transplanted the RPCs into the midstage (during nephrogenesis) embryo and confirmed two findings: (1) it was possible to differentiate RPCs into vascularized glomeruli inside the retroperitoneal cavity of the fetus; and (2) another embryo had formed a chimeric cap mesenchyme and renal vesicle between the host and donor cells within the fetal kidney. In analyzing the former, a one-lump mass of transplanted cells which remained outside the fetal kidney continued to spontaneously differentiate into mature nephrons in the embryonic retroperitoneal area, in a similar manner to the process in natural kidney growth. Almost all transplanted RPCs began to differentiate into nephrons (Table 2). We believed that because the transplanted RPCs were MN-dissociated cells, including multiple renal lineages (i.e., NPCs, UB cells, and SPCs; Supplemental Figure 2), they showed self-organization. RPCs with self-organization ability normally differentiate into immature glomeruli when induced by coculturing with mouse spinal cord cells.20,32 However, the absence of blood flow prevents the formation of glomerular capillary loops in vitro (Figure 2E), which are indispensable for mature kidney regeneration. In this study, injected cells that developed in the retroperitoneal space (around the kidney) integrated with host blood vessels, allowing more mature glomeruli to develop (Figure 2D). The glomeruli exhibited filtration capacity and dextran uptake in the tubular lumen, indicating renal function (Figure 4). In addition, in nonepithelial cells, those cells negative for Six2 and positive for GFP were located adjacent to NPCs outside the regenerated cap mesenchyme (Figure 3E, yellow triangle). The cell location suggests these were stromal cells.33,34 Mesangial cells derived from the transplanted cells were seen in the glomerulus (Figure 2F). Although they differentiated well, the one-lump mass of RPCs did not directly connect to the host kidneys (Figures 2A and 3, F and G).
In analyzing the chimeric cap mesenchyme, we determined the NPCs could enter the host cap mesenchyme when directly transplanted into the nephrogenic zone below the host kidney capsule, where they underwent epithelialization and differentiated into renal vesicles and glomeruli (Figure 5, D–F). However, donor RPCs contributed little to the host UB (Supplemental Figure 3). The host UB is a structure with tight junctions between cells35; thus it is likely that there is no space for exogenous cells to integrate. These result are consistent with those of previous ex vivo studies.13,15 Two findings showed that glomerular capillaries differentiated from RPCs can include host blood vessels and that if donor NPCs can deliver to the host nephrogenic zone, they can connect with the host UB. Therefore, we tried to transplant into a previously reported, conditional-eliminating NPC model to generate a nephron comprising only transplanted cells connected with host kidney.
Remarkably, the transplanted NPCs not only adhered to the host UB tips and completely replaced the (ablated) host-derived NPCs (Figure 7, A–C), they also differentiated into nephrons (Figure 7, E–H). Of the cap mesenchyme generated from exogenous RPCs, approximately 30% were found to integrate with the host UB tip and 70% were found to differentiate into self-organizing tissue without integrating into the host kidney (Figure 7D). Such tissue failed to connect to the host kidneys because the UBs of the transplanted cells attracted the transplanted NPCs (Figure 7B, cap mesenchyme outside kidney). To increase the efficiency of NPC replacement in the host cap mesenchyme, the transplanted cells may have to be sorted to eliminate the UB component. Furthermore, we validated the embryonic retroperitoneal cell transplantation technique in this study, but the efficiency of transplantation was not yet high enough. The rate of direct fetal implantation of RPCs into the nephrogenic zone was 18% in the wild-type mouse transplants and 29% in NPC replacement models (Table 1). Thus the technique needs further improvement. For example, a technique for transplanting cells more extensively into the nephrogenic zone is required. We believe the penetration depth of the injection needle is important. Deep-needle penetration has been assumed to be related to fetal lethality. It is necessary to visualize the kidney from the outside to more accurately grasp the distance to the kidney and nephrogenic zone. For example, fluorescence-expressed kidney by a gene modification may also be used. However, our Six2CreEGFP mice showed weak fluorescence and were thus not suitable for the observation of the kidney from outside the body via a transparent embryonic membrane. However, our transplantation technique using a conditionally eliminating NPC model opens up new horizons for organ regenerative medicine because this study serves as a proof of concept that kidney regeneration from exogenous cells is possible within the developing fetus.
To assess the potential utility of this technique, we also analyzed the long-term survival of the transplanted cells and their nascent kidneys. The fetus of the nonkidney-deficient model was able to grow while containing the transplanted cells and differentiated nephron. Regarding the fetus of the kidney-deficient model, neonates that underwent cell transplantation have so far always been killed by the foster mother on the first day of life. There is a possibility that only one-sided cell transplantation may be insufficient for survival. Also, the possibility that foster mothers will eliminate fetuses increases when newborns are weakened, so temporary artificial nursing may be required for the debilitated fetus. This is the first study representing the use of orthotopic and isochronic RPCs transplants for in vivo kidney regeneration. Future studies will include determining whether transplanting RPCs can rescue the kidney-deficient mouse fetus and using RPCs derived from pluripotent stem cells.
If the embryo-injection technique is applied to immunodeficient mice, human RPCs can likely be transplanted without immune rejection as with a chimera-formation assay for human pluripotent stem cells. Moreover, because glomerular differentiation can be induced in vitro by coculture with a mouse-dissociated kidney cell,36,37 renal epithelialization may be induced even between a human and mouse. However, to regenerate the kidney integrated with the urologic system of the host animal, it is necessary to combine the immunodeficiency and NPC replacement systems using genetic engineering. In addition, transplant rejection and cross-species infection issues must also be addressed if it is to be used as a transplantable human organ.38–40 Meanwhile, regenerating a complete three-dimensional organ only by an induction experiment in a dish remains challenging and we anticipate that our approach could serve as a bridging technology for kidney regeneration.
In conclusion, we generated nascent organs from organ progenitor cells transplanted into later-stage embryos. Chimeric organs and organ building blocks were able to grow after birth. However, we could not achieve kidney-deficient mouse model survival using regenerative kidney derived from transplanted cells in this study. We hope that this concept and the newly developed transplantation techniques will greatly contribute to the progress of organ regeneration.
Disclosures
None.
Funding
This work was supported by grants from Japan Agency for Medical Research and Development, grant number 17ek0310006h0002; Japan Society for the Promotion of Science, KAKENHI grant numbers 19K17756, 17K16102, and 16H03175; the Yukiko Ishibashi Foundation; and the Uehara Memorial Foundation.
Supplementary Material
Acknowledgments
We thank Ms. H. Gotoh, Ms. M. Ishida, and Ms. M. Tamatsukuri for experimental/technical assistance. Six2Cremice were gifted from Prof. R. Nishinakamura (The Kumamoto University, Institute of Molecular Embryology and Genetics).
Dr. Yamanaka designed the study, analyzed the data, made the figures, and drafted and revised the paper; all authors approved the final version of the manuscript; Dr. Yamanaka, Dr. Saito, Dr. Fujimoto, Dr. Tajiri, and Dr. Takamura carried out experiments; Dr. Matsumoto and Prof. Yokoo supervised the study.
This work received technical support from The Jikei University School of Medicine.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related Perspective, “Prioritizing Functional Goals as We Rebuild the Kidney,” on pages 2287–2288.
bQuantitative PCR confirmed NPHS1, NPHS2, LRP2, and SLC12A1 expressionSupplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020148/-/DCSupplemental.
Supplemental Figure 1. Figure of entering the embryonic membrane using steel puncture needle before using injector device.
Supplemental Figure 2. Dissociated renal progenitor cells characterized using flow cytometry.
Supplemental Figure 3. Integration of donor UB cells into the host kidney.
Supplemental Figure 4. Non-epithelial cells of generated cap mesenchyme (CM) in the kidney deficient model.
Supplemental Movie 1. Movie of fetus myometrium dissection.
Supplemental Movie 2. Movie of fetus transplantation of renal progenitor cells.
Supplemental Movie 3. Dissociated single renal progenitor cells.
Supplemental Movie 4. 14th day after birth of the fetus after cell transplantation.
References
- 1.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al.: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Yokoo T, Ohashi T, Shen JS, Sakurai K, Miyazaki Y, Utsunomiya Y, et al.: Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A 102: 3296–3300, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoo T, Fukui A, Ohashi T, Miyazaki Y, Utsunomiya Y, Kawamura T, et al.: Xenobiotic kidney organogenesis from human mesenchymal stem cells using a growing rodent embryo. J Am Soc Nephrol 17: 1026–1034, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, et al. : Interspecies chimerism with mammalian pluripotent stem cells. Cell 168: 473–486.e15, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J, Greely HT, Jaenisch R, Nakauchi H, Rossant J, Belmonte JC: Stem cells and interspecies chimaeras. Nature 540: 51–59, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H: Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol 180: 2417–2426, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Takasato M, Little MH: The origin of the mammalian kidney: Implications for recreating the kidney in vitro. Development 142: 1937–1947, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al.: Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanigawa S, Taguchi A, Sharma N, Perantoni AO, Nishinakamura R: Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell Reports 15: 801–813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Izpisua Belmonte JC: Dynamic pluripotent stem cell states and their applications. Cell Stem Cell 17: 509–525, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Cohen MA, Markoulaki S, Jaenisch R: Matched developmental timing of donor cells with the host is crucial for chimera formation. Stem Cell Reports 10: 1445–1452, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka S, Tajiri S, Fujimoto T, Matsumoto K, Fukunaga S, Kim BS, et al.: Generation of interspecies limited chimeric nephrons using a conditional nephron progenitor cell replacement system. Nat Commun 8: 1719, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little MH: Improving our resolution of kidney morphogenesis across time and space. Curr Opin Genet Dev 32: 135–143, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto T, Yamanaka S, Tajiri S, Takamura T, Saito Y, Matsumoto K, et al.: In vivo regeneration of interspecies chimeric kidneys using a nephron progenitor cell replacement system. Sci Rep 9: 6965, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatta T, Matsumoto A, Otani H: Application of the mouse exo utero development system in the study of developmental biology and teratology. Congenit Anom (Kyoto) 44: 2–8, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al.: A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419–426, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al.: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Araoka T, Wu J, Liao HK, Li M, Lazo M, et al.: 3D culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19: 516–529, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junttila S, Saarela U, Halt K, Manninen A, Pärssinen H, Lecca MR, et al.: Functional genetic targeting of embryonic kidney progenitor cells ex vivo. J Am Soc Nephrol 26: 1126–1137, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tajiri S, Yamanaka S, Fujimoto T, Matsumoto K, Taguchi A, Nishinakamura R, et al.: Regenerative potential of induced pluripotent stem cells derived from patients undergoing haemodialysis in kidney regeneration. Sci Rep 8: 14919, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukunaga S, Yamanaka S, Fujimoto T, Tajiri S, Uchiyama T, Matsumoto K, et al.: Optimal route of diphtheria toxin administration to eliminate native nephron progenitor cells in vivo for kidney regeneration. Biochem Biophys Res Commun 496: 1176–1182, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Yamada M, Hatta T, Otani H: Mouse exo utero development system: Protocol and troubleshooting. Congenit Anom (Kyoto) 48: 183–187, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Qiao J, Cohen D, Herzlinger D: The metanephric blastema differentiates into collecting system and nephron epithelia in vitro. Development 121: 3207–3214, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, et al.: Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol 130: 927–942, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Suchy F, Yamaguchi T, Nakauchi H: iPSC-derived organs in vivo: Challenges and promise. Cell Stem Cell 22: 21–24, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Suchy F, Nakauchi H: Interspecies chimeras. Curr Opin Genet Dev 52: 36–41, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Rashid T, Kobayashi T, Nakauchi H: Revisiting the flight of Icarus: Making human organs from PSCs with large animal chimeras. Cell Stem Cell 15: 406–409, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Irie N: Remaining questions related to the hourglass model in vertebrate evolution. Curr Opin Genet Dev 45: 103–107, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Slack JM, Holland PW, Graham CF: The zootype and the phylotypic stage. Nature 361: 490–492, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Kispert A, Vainio S, McMahon AP: Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: 4225–4234, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Daniel E, Azizoglu DB, Ryan AR, Walji TA, Chaney CP, Sutton GI, et al.: Spatiotemporal heterogeneity and patterning of developing renal blood vessels. Angiogenesis 21: 617–634, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindström NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, et al. : Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell 45: 651–660.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, et al.: Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332: 273–286, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, et al.: Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16: 118–126, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Taguchi A, Nishinakamura R: Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21: 730–746.e6, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, et al.: Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357: 1303–1307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, et al.: Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564: 430–433, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Itoh M, Mukae Y, Kitsuka T, Arai K, Nakamura A, Uchihashi K, et al.: Development of an immunodeficient pig model allowing long-term accommodation of artificial human vascular tubes [published correction appears in Nat Commun 10: 3625, 2019]. Nat Commun 10: 2244, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.