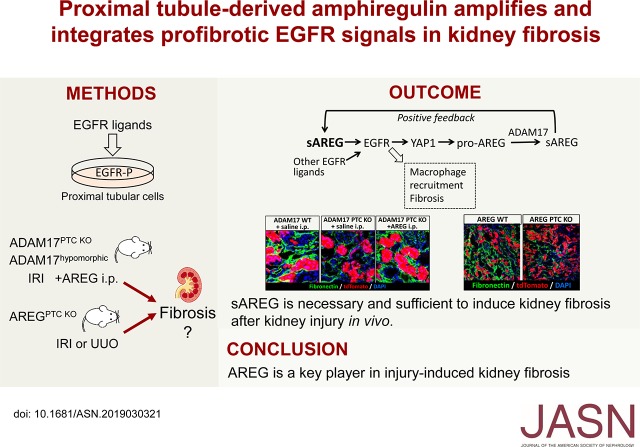
Significance Statement
Sustained activation of EGF receptor (EGFR) in proximal tubule cells (PTCs) is a hallmark of fibrotic CKD, but the molecular mechanism(s) involved are unknown. Here, we show that the injury-upregulated, low-affinity EGFR ligand amphiregulin (AREG) amplifies its own signaling in PTCs and integrates signals of other moderately injury-upregulated EGFR ligands by inducing the transcriptional regulator Yes-associated protein 1 (YAP1). In vivo, AREG is necessary and sufficient to induce kidney fibrosis after injury, as AREG PTC-knockout mice exhibit protection and soluble AREG injection suffices to reverse protection in mice that cannot release EGFR ligands from PTCs. Our results identify AREG as a critical driver of the transition of kidney injury to fibrosis.
Keywords: chronic kidney disease, fibrosis, epidermal growth factor, proximal tubule
Visual Abstract
Abstract
Background
Sustained activation of EGF receptor (EGFR) in proximal tubule cells is a hallmark of progressive kidney fibrosis after AKI and in CKD. However, the molecular mechanisms and particular EGFR ligands involved are unknown.
Methods
We studied EGFR activation in proximal tubule cells and primary tubular cells isolated from injured kidneys in vitro. To determine in vivo the role of amphiregulin, a low-affinity EGFR ligand that is highly upregulated with injury, we used ischemia-reperfusion injury or unilateral ureteral obstruction in mice with proximal tubule cell–specific knockout of amphiregulin. We also injected soluble amphiregulin into knockout mice with proximal tubule cell–specific deletion of amphiregulin’s releasing enzyme, the transmembrane cell-surface metalloprotease, a disintegrin and metalloprotease-17 (ADAM17), and into ADAM17 hypomorphic mice.
Results
Yes-associated protein 1 (YAP1)–dependent upregulation of amphiregulin transcript and protein amplifies amphiregulin signaling in a positive feedback loop. YAP1 also integrates signals of other moderately injury-upregulated, low-affinity EGFR ligands (epiregulin, epigen, TGFα), which also require soluble amphiregulin and YAP1 to induce sustained EGFR activation in proximal tubule cells in vitro. In vivo, soluble amphiregulin injection sufficed to reverse protection from fibrosis after ischemia-reperfusion injury in ADAM17 hypomorphic mice; injected soluble amphiregulin also reversed the corresponding protective proximal tubule cell phenotype in injured proximal tubule cell–specific ADAM17 knockout mice. Moreover, the finding that proximal tubule cell–specific amphiregulin knockout mice were protected from fibrosis after ischemia-reperfusion injury or unilateral ureteral obstruction demonstrates that amphiregulin was necessary for the development of fibrosis.
Conclusions
Our results identify amphiregulin as a key player in injury-induced kidney fibrosis and suggest therapeutic or diagnostic applications of soluble amphiregulin in kidney disease.
AKI is a significant risk factor for the development of CKD.1,2 CKD is a major public health problem affecting 10% of the world’s population and is the strongest risk factor for cardiovascular disease.3–5 Therapies to prevent AKI-induced CKD are lacking and available therapies to slow CKD progression are limited and only moderately effective. Sustained chronic EGFR activation in proximal tubule cells (PTCs) is a hallmark of the fibrotic response after AKI and in CKD and can be detected in mouse and human kidneys.6–8 It is unknown which mechanism(s) drive sustained profibrotic EGF receptor (EGFR) activation in the kidney and which EGFR ligands are involved.
We have shown that proximal tubule ADAM17, a transmembrane cell-surface metalloprotease, releases the active soluble EGFR ligand ectodomains from proforms upregulated in injured PTCs in vivo.7 The low-affinity EGFR ligand amphiregulin (AREG) is most prominently upregulated by AKI in response to ischemia-reperfusion injury (IRI) or unilateral ureteral obstruction (UUO), and also in human tubule cells in CKD biopsy specimens. Its active soluble form, soluble amphiregulin (sAREG), is very significantly elevated in urine of patients with AKI and moderately elevated in patients with CKD, as compared with controls. Other ADAM17 substrate EGFR ligands, such as the low-affinity EGFR ligands TGFα, epiregulin, and epigen, as well as the high-affinity EGFR ligand heparin-binding EGF (HB-EGF), are only moderately upregulated by AKI in mice or, as far as tested (TGFα, HB-EGF), by AKI and CKD in humans.7
Here, we report that sAREG acts as a key driver, amplifier, and integrator of profibrotic signals in PTCs in vitro, and is necessary and sufficient for injury-induced fibrosis in mice in vivo. We show that sustained EGFR activation in response to all injury-upregulated, soluble, low-affinity EGFR ligands, including sAREG, requires Yes-associated protein 1 (YAP1)–dependent upregulation of AREG transcript and ADAM17-dependent release of sAREG on the PTC cell surface, sustaining profibrotic EGFR activation in a positive forward loop. We show that sAREG injection into ADAM17 PTC knockout (KO) mice or ADAM17 hypomorph mice suffices to induce fibrosis, and that AREG PTC-KO mice are protected from injury-induced fibrosis.
Methods
Animal Experiments
For all studies, adult (8–12 weeks old) male mice were used in accordance with the animal care and use protocol approved by the Institutional Animal Care and Use Committee of Washington University School of Medicine. ADAM17ex/ex and ADAM17 PTC-KO mice have been described.7,9 R26tdTomato mice were obtained from The Jackson Laboratory. AREG floxed mice were a gift from Dr. Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY) and Dr. Arpaia (Columbia University Irving Medical Center).10 For induction of the SLC34a1-driven Cre, mice were injected with three doses of tamoxifen 3 mg (every other day) and we allowed 1 week for tamoxifen washout before performing any procedure.
Ischemia for 23 minutes (ADAM17ex/ex and wild-type [wt] littermates) or 21 minutes (ADAM17 PTC-KO and wt littermates) at 37°C was induced in both kidneys using the flank approach as previously reported.11 Sham operations were performed with exposure of both kidneys, but without induction of ischemia. A group of AREG PTC-KO mice and wt littermates was subjected to unilateral ischemia for 23 minutes.
UUO was executed as described previously.11 Briefly, after flank incision, the left ureter was tied off at the level of the lower pole with two 3.0 silk ties. Sham-operated mice underwent the same surgical procedure except for the ureter ligation.
For sAREG injection experiments, 18.3 ng per gram body wt sAREG (from R&D Systems) in saline or vehicle (0.2 ml saline) were intraperitoneally (i.p.) injected daily.
Renal Histology
Kidney histology was examined in formalin-fixed sections. For each staining, ten images per kidney throughout the tissue were collected for blinded quantification. The degree of tubulointerstitial damage was scored semiquantitatively using periodic acid–Schiff stained sections (scale 1–5) according to the percentage of the cortical area affected by tubular atrophy, tubular dilation and loss of brush border, as previously described.12 Fibrosis severity was quantified in kidney cortex by measuring the Picrosirius red–stained area using ImageJ software.13
Cell Isolation and Culture
Primary kidney tubular cells from ADAM17 PTC-KO or wt littermates were isolated using established methods.12 In brief, kidneys were removed at the specified time postischemia from PBS perfused mice, minced into 1 mm3 pieces on ice and incubated for 30 minutes in PBS containing 1 mg/ml Liberase TL (Roche). The reaction was terminated by adding FBS to 3% (v/v) and single cell suspension was obtained by pipetting the tissue using a 5 ml pipette and straining through a 70 µm strainer, wash with PBS, resuspension in PBS, and subsequent straining through a 40 µm strainer. TdTomato-positive PTCs were separated by FACS (Sony SY3200 Synergy), using isolated PTCs that do not express TdTomato as negative controls.
The human proximal tubular cell (HPTC) line (HPTC-0514) used was maintained as previously described.7 We confirmed by RNA-sequencing that this cell line expresses many proximal tubular cell markers, as they were identified by recent single-cell RNA expression analysis15 (http://humphreyslab.com/SingleCell/) (Supplemental Table 1).
Cell Treatments
All experiments in HPTCs were performed using subconfluent cells that were serum-deprived overnight. Equimolar (17 pM) or increasing (85 fM–170 pM) amounts of different soluble EGFR ligands (all from R&D Systems) were added to the cells for different time points as noted. When AREG neutralization antibody was used, cells were treated with the ligands for 4 hours, quickly washed with warm PBS. and exposed to warm medium containing 5 ∝g/ml anti-AREG, 5 ∝g/ml anti-TGFα, or 5 ∝g/ml anti–HB-EGF antibody (#AF262, #AF259, and #AF239; all from R&D Systems, respectively) for the remaining 20 hours. For BB94 (batimastat; Tocris Bioscience) treatments cells were pretreated with the inhibitor for 30 minutes and then stimulated with the different ligands for 24 hours in the presence of the inhibitor. For all ELISA measurements, cells were treated with the respective ligand for 4 hours, washed with warm PBS, and then fresh medium (without any ligand) was added for the remaining 20 hours.
For YAP1 knockdown experiments, control or human YAP1 targeting small interfering RNA (siRNA) (esiRNA; Mission) and Mission transfection reagent (#S1452) (all from Sigma) were used according to the manufacturer’s instructions. At 48 hours after siRNA transfection, cells were serum-deprived and stimulated with different ligands for 24 hours.
Quantitative RT-PCR
Total RNA was isolated from mouse kidneys or cultured cells using the QIAzol reagent (Qiagen) or from FACS-sorted cells using RNeasy mini kit (Qiagen), following the manufacturer’s instructions. Total RNA was reverse-transcribed using the QuantiTect RT Kit (Qiagen) and real-time PCR was performed with Fast SYBR Green (Qiagen). Primer sequences are provided in Supplemental Table 2. GAPDH or RPLP0 were used as housekeeping genes. Data were analyzed using the 2–∆∆Ct method.
Protein Sample Preparation and Western Blotting
Whole kidney lysates, cell lysates, and cytoplasmic/nuclear fractions were prepared as previously described.7,16 In brief, kidneys were homogenized on ice in lysis buffer (50 mM Tris/HCL, pH 7.4, 50 mM NaCl, 0.1 mM EDTA, 0.5% Triton X-100, NaPP, NaF, Na3VO4, and protease inhibitors [cOmplete Protease Inhibitor Cocktail; Roche]). Homogenates were centrifuged at 10,000×g for 30 minutes at 4°C and supernatants were collected. Protein concentration was quantified by BSA protein assay (Pierce). Cell lysates were prepared in the above-described lysis buffer after two washes with ice-cold PBS and after pelleting insoluble materials at 10,000×g for 30 minutes at 4°C. For fractionation experiments, cells were harvested in Buffer A (10 mM HEPES, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA, 1.5 mM MgCl2, 10 mM NaF, 1 mM Na3VO4, 1 mM DDT, and protease inhibitor cocktail) and incubated on ice for 15 minutes. Samples were centrifuged (1400×g for 5 minutes at 4°C), the supernatants containing the cytosolic fraction were collected and the pellets were washed with Buffer A containing 0.6% (v/v) Nonidet P40 and centrifuged (1400×g for 5 minutes at 4°C). Pellets were resuspended in Buffer B (20 mM HEPES, 0.4 M NaCl, 1 mM EGTA, 0.1 mM EDTA, 1.5 mM MgCl2, 10 mM NaF, 1 mM Na3VO4, and protease inhibitor cocktail) and incubated under rotation for 1 hour at 4°C. After centrifugation (11,000×g at 10 minutes for 4°C), the supernatants containing the nuclear protein were collected. Tissue and cell samples were analyzed by standard Western blotting techniques. The primary antibodies used were from Cell Signaling Technology (pEGFR Y1068 #2234, total EGFR #4267, pERK1/2 #9101, phospho-YAP1 #13008, and total YAP1 #14074) or from Abcam (fibronectin #ab2413, histone H2A.× #ab124781, and tubulin #ab7291).
Immunofluorescence Staining
Immunofluorescence staining of the kidney was performed on frozen sections following standard protocols. In brief, cryosections of tissue in Optimal cutting temperature compound or HPTC seeded on coverslips were washed with PBST (0.05% (v/v) Tween-20 in PBS) for 5 minutes at room temperature and permeabilized with 1% SDS in PBS for 5 minutes, followed by three 5 minutes washes with PBST. After 30 minutes incubation in blocking buffer (5% BSA and 5% goat serum in PBS), sections were incubated with primary antibodies overnight at 4°C, followed by three 5-minute washes with PBST, 1 hour incubation with secondary antibodies (where appropriate), three 5-minute washes with PBST, and mounting in DAPI-containing medium (Vector Laboratories). The primary antibodies included αSMA (CY3- or FITC-conjugated, #C6198, #F3777; Sigma-Aldrich), fibronectin (#ab2413; Abcam), F4/80 (#ab6640; Abcam), and EGFR (#4267; Cell Signaling Technology). For each kidney staining, ten images per kidney throughout the tissue were collected for blinded quantification. Images were analyzed using the ImageJ software.
Flow Cytometric Analysis
Surface EGFR levels were measured in HPTCs after stimulation with equimolar amounts of ligands for the time points noted. Cells were washed twice with PBS, incubated for 30 minutes in blocking buffer (5% BSA in PBS), stained for 1 hour with phycoerythrin-conjugated anti-EGFR antibody or isotype control (#BD555997 and #BD555743; both from BD Biosciences, respectively) and washed twice with PBS before flow cytometric analysis (all staining procedures were performed on ice). Data were acquired using the BD LSRFortessa cell analyzer and results were analyzed using the FlowJo software.
ELISA
Human soluble TGFα (sTGFα), soluble HB-EGF (sHB-EGF), and sAREG were measured in HPTC culture media samples and sAREG was measured in human serum samples using microsphere-based Luminex Technology and ELISA kits (DY239, DY259B, and DY262, respectively; all from R&D Systems) as previously described.7,17
Statistical Analyses
All results are reported as the mean±SD. Comparison of two groups was performed using an unpaired, two-tailed t test or a Pearson correlation analysis where appropriate. Comparison of three groups was performed via ANOVA and Tukey post hoc test. Statistical analyses were performed using GraphPad Prism, version 6.0 (GraphPad Software Inc.). A P value of <0.05 was considered significant.
Study Approval
Use of mice in this study was reviewed and approved by the Institutional Animal Care and Use Committee at Washington University School of Medicine, in adherence to standards set in the Guide for the Care and Use of Laboratory Animals.
Results
Low-Affinity EGFR Ligands Induce Sustained EGFR Activation by Inducing Reactivation of EGFR in HPTCs
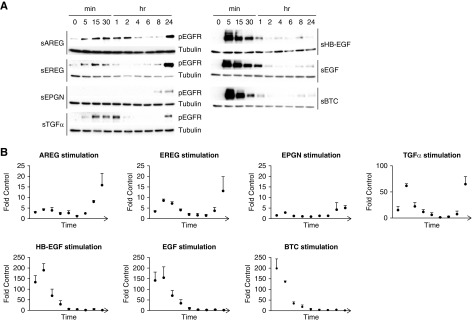
One mechanism by which different soluble EGFR ligands can elicit different cellular responses in the same cell depends on their affinity to EGFR.18 We confirmed that sAREG, soluble epigen (sEPGN), soluble epiregulin (sEREG), and sTGFα act as low-affinity EGFR ligands and sHB-EGF, soluble EGF, and soluble betacellulin act as high-affinity EGFR ligands in dose-response experiments in HPTCs (Supplemental Figure 1). To establish which ligands could induce sustained EGFR activation, we performed time-course experiments in response to equimolar amounts of each ligand. All low-affinity ligands cause a biphasic EGFR activation in HPTCs, with minimal to moderate activation at early time points (5 minutes to 2 hours) and strong sustained EGFR reactivation at 8 hours, lasting at least 24 hours (Figure 1A, left Western blot panels, quantifications of three independent experiments shown in Figure 1B). In contrast, high-affinity EGFR ligands cause very strong and short-lived EGFR phosphorylation, but do not cause sustained EGFR (re)activation (Figure 1A, right Western blot panels), suggesting that injury-upregulated, low-affinity EGFR ligands could be responsible for sustained profibrotic EGFR activation in vivo after kidney injury.
Figure 1.
Low-affinity EGFR ligands induce sustained EGFR phosphorylation in HPTCs. Equimolar amounts (17 pM) of each ligand were used to stimulate HPTCs for the time points indicated. EGFR phosphorylation was examined by (A) Western blot and (B) densitometric analysis was performed after normalization to nonstimulated cells. Tubulin was used for normalization (loading control). Western blots and quantifications shown are representative of three independent experiments.
Sustained EGFR Activation in Response to All Low-Affinity EGFR Ligands Requires Release of Endogenous sAREG
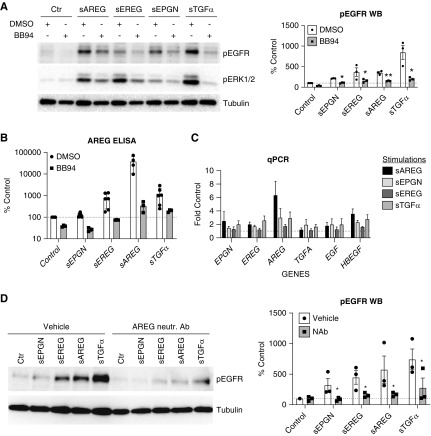
To test whether the observed sustained reactivation of EGFR by low-affinity ligands requires the release of an endogenous EGFR ligand, we incubated HPTCs with the broad-spectrum metalloprotease inhibitor batimastat (BB94). BB94 blocks sustained EGFR reactivation in response to all low-affinity EGFR ligands, sAREG, sEREG, sEPGN, and sTGFα (Figure 2A), and also lowers the basal EGFR phosphorylation, suggesting cleavage-release of an endogenous EGFR ligand (Figure 2A). To identify the responsible endogenous ligand(s), we stimulated HPTCs with exogenous, low-affinity soluble EGFR ligands for 4 hours (the time point by which initial EGFR activation in response to low-affinity ligands has reached baseline levels, Figure 1C), and then replaced medium with fresh serum-free medium for the remaining 20 hours. sAREG is the only EGFR ligand significantly released into HPTC supernatant after stimulation with any low-affinity EGFR ligand and this upregulation can be blocked by BB94 (Figure 2B). In contrast, sHB-EGF and sTGFα proteins are minimally released, although both are expressed in HPTCs (data not shown). Consistent with this, AREG mRNA transcript is upregulated in response to all low-affinity EGFR ligands, with sAREG itself being by far the most effective (Figure 2C). Neutralization of sAREG starting at 4 hours after initial low-affinity ligand stimulation results in almost complete inhibition of EGFR reactivation at 24 hours in response to all low-affinity EGFR ligands (Figure 2D), whereas neutralization of sHB-EGF or sTGFα does not (data not shown). These results suggest that sAREG represents the key driver of sustained EGFR activation in HPTCs, acts as an amplifier of its own action, and integrates signals relayed by other (moderately kidney injury-upregulated) low-affinity EGFR ligands at the level of EGFR.
Figure 2.
Sustained EGFR reactivation requires transcription and release of endogenous AREG. (A and B) HPTCs were pretreated with DMSO or BB94 (10 ∝M) for 30 minutes and then treated with different low-affinity EGFR ligands for 24 hours. (A) EGFR phosphorylation and downstream ERK1/2 phosphorylation was quantified by Western blot (left panel) followed by densitometric analysis (graph) after normalization to control-stimulated cells (% Control). (B) Endogenous sAREG released in cell culture medium was measured by ELISA and presented as percentile of sAREG concentration in control-stimulated cell medium (% Control). (C) HPTCs were treated with different EGFR ligands (shown in the legend) for 24 hours and mRNA expression of endogenous EGFR ligands (shown in x-axis) was tested by quantitative PCR. Results are presented after normalization to non-stimulated cells (fold control). (D) HPTCs were treated with different EGFR ligands for 4 hours and then culture medium was changed to fresh medium containing AREG-neutralization antibody or vehicle for 20 hours. EGFR phosphorylation was quantified by Western blot (left panel) and by densitometric analysis (right column graph) after normalization to control-stimulated/vehicle-treated cells (% Control). Tubulin was used as loading control in (A and D). For all experiments, n=3–6. *P<0.05; **P<0.01.
Interestingly, although all high-affinity EGFR ligands (soluble EGF, sHB-EGF, soluble betacellulin) do not induce significant sustained EGFR reactivation (Figure 1A), they are principally able to induce AREG transcript and release of sAREG (Supplemental Figure 2, A and B). This can be explained by different effects on EGFR cell-surface levels. sAREG does not significantly affect EGFR surface levels in HPTCs over 24 hours, whereas equimolar amounts of the high-affinity ligand sHB-EGF cause strong downregulation of cell-surface EGFR, starting at 15 minutes after stimulation and remaining low for at least 24 hours poststimulation (Supplemental Figure 2, C and D). Total EGFR protein levels over the same time frame are not significantly changed by either ligand (Supplemental Figure 2E) or any other EGFR ligand tested (Supplemental Figure 2, F and G); however, there is a nonsignificant trend for lower total EGFR levels at late time points after high-affinity ligand treatment. Thus, although sHB-EGF and other high-affinity ligands are principally able to induce AREG transcript and sAREG release in HPTCs, strong downregulation of EGFR from the cell surface prevents sustained EGFR reactivation at 24 hours.
AREG Transcriptional Upregulation and Sustained EGFR Activation Require YAP1
In search for transcriptional regulators of AREG in PTCs, we examined the role of the YAP1, a component of the Hippo pathway. When the Hippo pathway is inactive, YAP1 is dephosphorylated and can translocate to the nucleus, where it regulates transcription of genes,19 including AREG.20
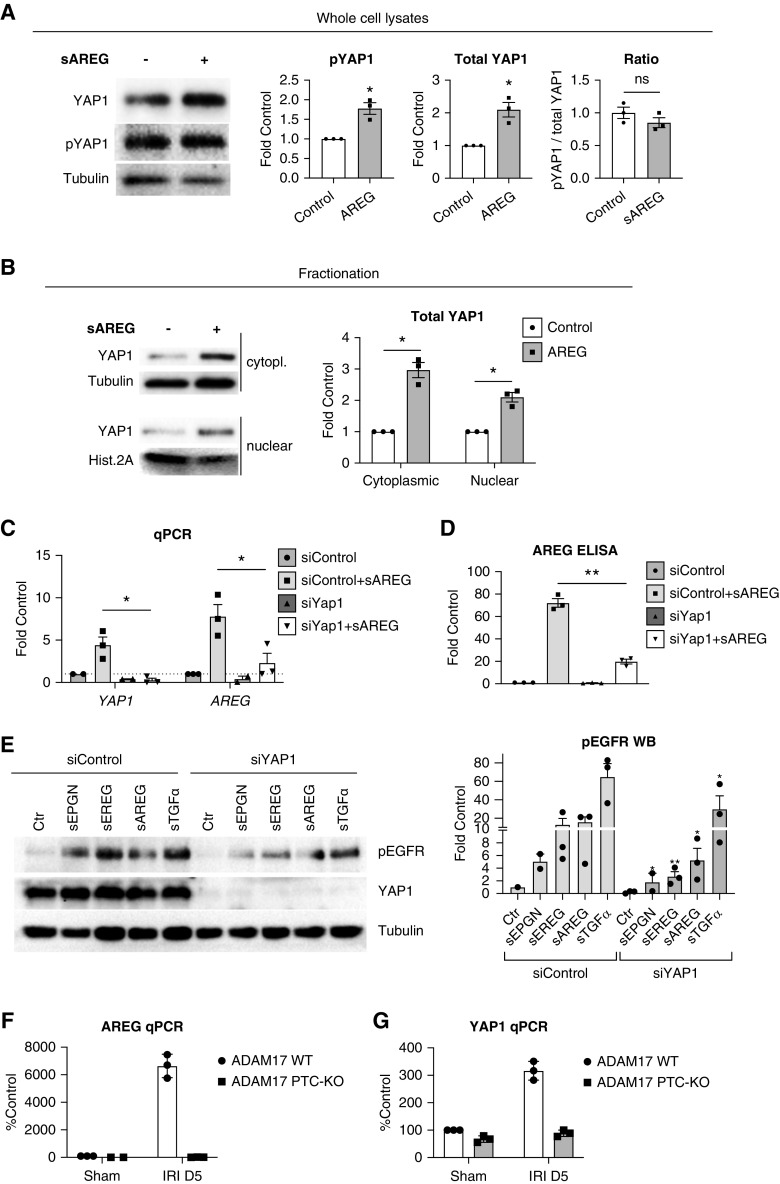
sAREG stimulation upregulates YAP1 protein levels in HPTCs, but does not lead to significant dephosphorylation of YAP1 (Figure 3A). Nonetheless, YAP1 levels in the nucleus are increased, as shown by cell fractionation (Figure 3B), suggesting sAREG regulates overall protein levels of YAP1 rather than acting on YAP1 via the canonical Hippo pathway. Similar results have been reported in a mouse model of diabetic nephropathy.21 To determine whether YAP1 is indeed involved in AREG transcript upregulation and release of sAREG in HPTCs, we used siRNA against YAP1. YAP1 knockdown completely blocks transcriptional upregulation of AREG and YAP1 after sAREG stimulation (Figure 3C) and reduces the subsequent release of endogenous sAREG (Figure 3D). In fact, knockdown of YAP1 significantly reduces sustained EGFR reactivation in response to all low-affinity EGFR ligands, sAREG, sEREG, sEPGN, and sTGFα, with sTGFα less affected than all others (Figure 3E).
Figure 3.
AREG transcriptional upregulation and sustained EGFR reactivation require YAP1. (A) Cells were control-treated or treated with sAREG for 24 hours and total YAP1 or phospho-YAP1 (pYAP1) levels were examined by Western blot in whole cell lysates (left panel). Quantification of phospho-YAP1 and total YAP1 levels, as well as the ratio of phospho-to-total YAP1, is presented in the graphs after densitometric analysis (right panels). Tubulin was used as loading control. (B) HPTCs were control-treated or treated with sAREG for 24 hours and preparations of nuclear and cytoplasmic fractions were analyzed by Western blot (left panels). Tubulin was used as loading control for cytoplasmic fractions and histone 2A was used as loading control for nuclear fractions. Quantification of total YAP1 levels is presented after densitometric analysis (right panel graph). (C and D) HPTCs were transfected with control siRNA (siControl) or siRNA against YAP1 (siYAP1) and at 48 hours post-transfection, serum-starved cells were control-treated or treated with sAREG for 24 hours. Endogenous AREG and YAP1 expression was tested by quantitative PCR ([C], results presented as fold of siControl/control-treated cells) and release of endogenous sAREG to the cell culture medium was tested by ELISA ([D], presented as percent siControl/control-treated cells). Results are presented after normalization for siControl transfected/control-stimulated cells. (E) Control or siYAP1 transfected cells were treated at 48 hours post-transfection with different EGFR ligands for 24 hours and EGFR phosphorylation was tested by Western blot (left panel). Densitometric analysis is presented after normalization for siControl transfected/control-stimulated cells (right panel graph, stars denote significant difference from the respective ligand-treated siControl sample). (F and G) ADAM17 PTC-KO mice or their ADAM17 WT littermates were subjected to IRI or sham surgery and after 5 days their kidneys were collected and tdTomato+ PTCs were sorted by FACS for subsequent mRNA extraction and quantitative PCR analysis of (F) AREG and (G) YAP1 expression. Results are presented after normalization to PTCs from ADAM17 WT/sham surgery mice (% Control). n=3–4; *P<0.05, **P<0.01.
To examine the relevance of the sAREG-YAP1 link in vivo, we generated ADAM17 PTC-KO/tdTomato+ mice, in which ADAM17 PTC-KO blocks the release of all injury-upregulated EGFR ligands from PTCs and tdTomato labeling allows the isolation of PTCs by FACS (tdTomato is driven by the same PTC-Cre as used for the ADAM17 KO Slc34a1GCE+/−7,22). We subjected ADAM17 PTC-KO/tdTomato+ mice and their wt littermates (abbreviated ADAM17 PTC-KO and ADAM17 WT in Figures) to IRI and collected kidneys and their PTCs at day 5 postischemia for analysis. PTCs isolated from injured ADAM17 PTC-KO/tdTomato+ mice fail to upregulate AREG (Figure 3F) and YAP1 (Figure 3G), as compared with PTCs isolated from wt mice. This suggests that the ADAM17-dependent positive feedback loop that affects AREG and YAP1 expression in vitro in HPTCs is also recapitulated in kidney injury in vivo.
sAREG Reverses Protection from Fibrosis in ADAM17 PTC-KO and ADAM17 Hypomorphic Mice
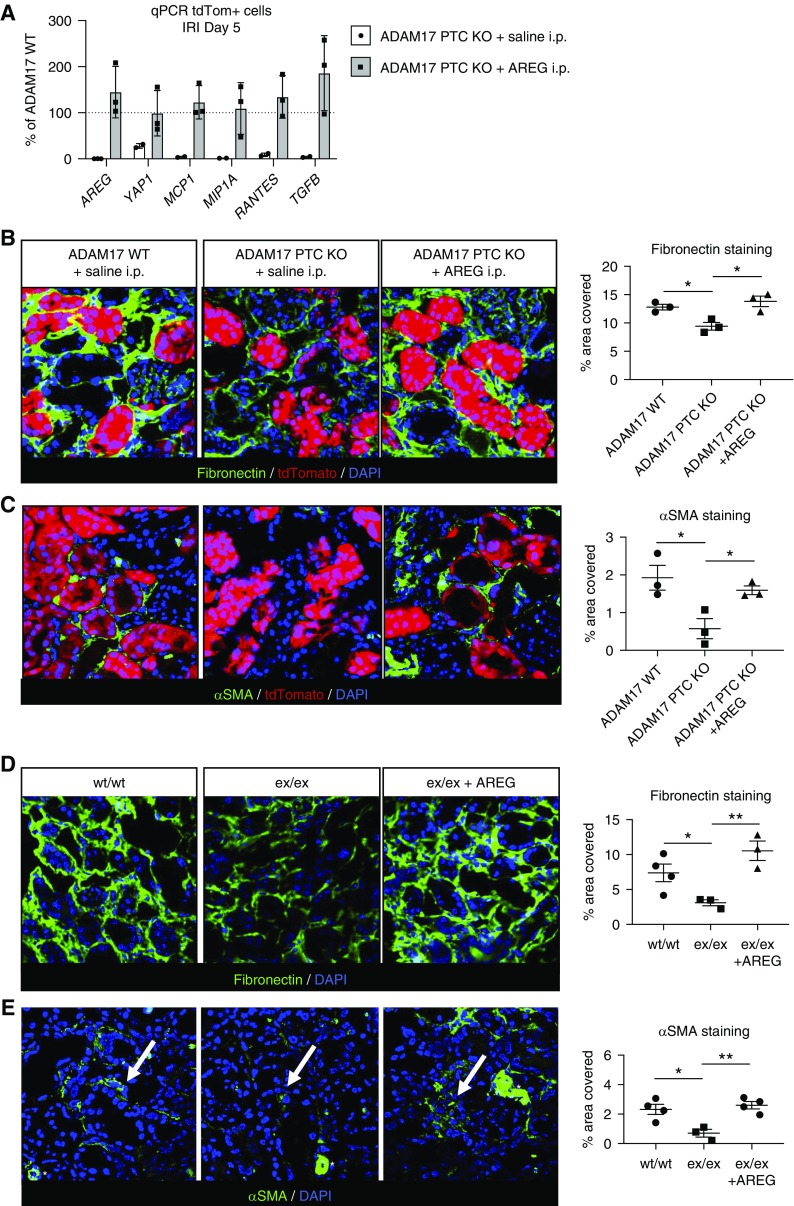
We have previously shown, using whole kidney lysates, that proinflammatory and profibrotic cytokine induction by IRI is significantly reduced in the kidney of ADAM17 PTC-KO mice as compared with wt.7 To test whether sAREG alone would suffice to reinduce these signals in the injured ADAM17 PTC-KO kidney, we used sAREG i.p. injection into injured ADAM17 PTC-KO/tdTomato+ mice. Consistent with our results in whole kidney extracts, tdTomato+ PTCs isolated from ADAM17 PTC-KO/tdTomato+ mice exhibit strongly reduced expression of proinflammatory and profibrotic cytokines (MCP1, MIP1A, RANTES, TGFα) and of AREG, as compared with PTCs isolated from their wt littermates (Figure 4A, white bars). In IRI-injured ADAM17 PTC-KO/tdTomato+ mice, daily i.p. injections of sAREG for 5 days elevate these cytokines and AREG in PTCs (Figure 4A, gray bars) and induce expression of the profibrotic markers αSMA and fibronectin to the levels of wt (or higher) (Figure 4, B and C). The latter results were confirmed in ADAM17ex/ex hypomorphic mice which are also protected from injury-induced fibrosis after IRI or UUO7 (Figure 4, D and E). sAREG is thus sufficient to promote a profibrotic PTC and kidney phenotype after kidney injury.
Figure 4.
sAREG injection reverses protection from fibrosis in ADAM17 PTC-KO and ADAM17ex/ex hypomorph mice. ADAM17 PTC-KO mice or their ADAM17 WT littermates, or ADAM17 hypomorphic mice (ex/ex) or their wt littermates (wt/wt), were subjected to IRI and received daily i.p. injection of saline or sAREG (18.3 ng/gr body wt) for 4 days, as indicated. Kidneys were collected at day 5. (A) TdTomato-positive PTCs were isolated by FACS and the expression of AREG, YAP1, MCP1, MIP1A, RANTES, and TGFβ was examined by quantitative PCR. Results are presented after normalization to ADAM17 WT mice. (B–E) The profibrotic markers fibronectin and αSMA were examined by IF staining (left panels) and stained area for each protein (% area covered) was quantified (right panels, graphs). White stars in (E) denote αSMA-positive blood vessels that were excluded from the quantification. n=3–4; *P<0.05; **P<0.01.
PTC-Derived sAREG Drives Kidney Fibrosis after Injury
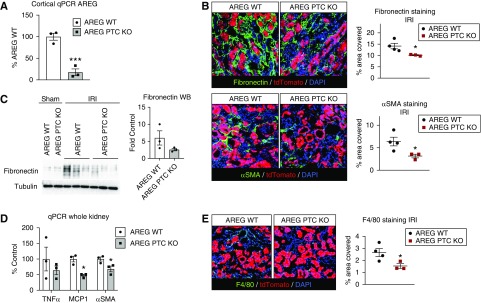
To determine whether PTC-derived sAREG is necessary for injury-induced fibrosis, we created AREG PTC-KO/tdTomato+ mice and wt littermate controls (abbreviated AREG PTC-KO and AREG WT in Figures) using the same Cre as for ADAM17 PTC-KO. KO was validated by quantitative PCR (Figure 5A). AREG PTC-KO/tdTomato+ mice show reduced IRI-induced upregulation of αSMA and fibronectin at day 5 after IRI (Figure 5, B and C), as well as reduced upregulation of MCP1 transcript (Figure 5D), which we showed is upregulated by sAREG injection in vivo (Figure 4A). MCP1 is important for macrophage recruitment to the kidney and other organs after injury, where macrophages have critical functions in injury-induced fibrosis.23 Consistent with reduced MCP1, we observed significantly reduced F4/80+ macrophage numbers in AREG PTC-KO/tdTomato+ mice at day 5, as compared with their wt littermates (Figure 5E).
Figure 5.
AREG PTC-KO reduces kidney proinflammatory and profibrotic markers after ischemic injury. (A) The efficiency of AREG PTC-KO was tested in KO mice and control littermates (AREG WT) by quantitative PCR for AREG after mRNA isolation from cortical samples. (B) Mice were subjected to IRI and kidneys were collected 5 days postinjury. The profibrotic markers fibronectin and αSMA were examined by immunofluorescence staining (left panel) and stained area for each (% area covered) were quantified (right column graph). (C) The levels of fibronectin are examined in whole kidney lysates by western blot (left panels, tubulin is used as loading control) and quantified by densitometric analysis (right panel graph). (D) The expression levels of TNFα, MCP1, and αSMA were examined by quantitative PCR using whole kidney mRNA extracts. (E) Macrophage infiltration was examined by F4/80 staining at day 5 post IRI ([E], left panel) and stained area (% area covered) was quantified ([E], right panel graph). n=3–4; *P<0.05; ***P<0.001.
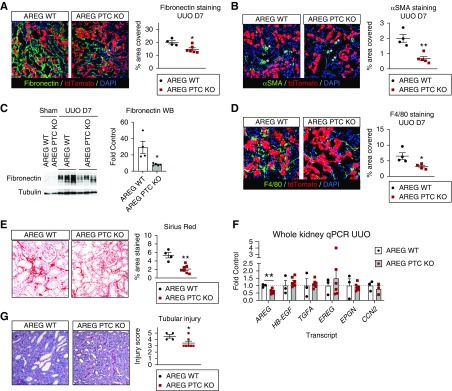
We confirmed our findings in a classic fibrosis model, UUO. Identical to IRI, AREG PTC-KO/tdTomato+ mice show reduced upregulation of the profibrotic markers αSMA and fibronectin (Figure 6, A–C), and reduced F4/80+ macrophage accumulation (Figure 6D) at day 7 after UUO. Actual deposition of collagen fibers is also reduced, as detected by Sirius red staining (Figure 6E). These results establish that PTC-derived sAREG is necessary for injury-induced fibrosis and induces macrophage accumulation in the kidney.
Figure 6.
AREG PTC-KO reduces kidney fibrosis after UUO. AREG PTC-KO mice or AREG WT littermates were subjected to UUO and kidneys were collected after 7 days. (A and B) The profibrotic markers fibronectin and αSMA were examined by immunofluorescence staining (left panels) and the levels of the stained area per field for each marker (% area covered, right panel graphs) were quantified. (C) The levels of fibronectin are examined in whole kidney lysates by Western blot (left panels, tubulin is used as loading control) and quantified by densitometric analysis (right panel graph). (D) Macrophage infiltration was examined by F4/80 staining (left panels) and the levels of the stained area per field (% area covered, right panel graphs). (E) Collagen deposition was quantified after Sirius red staining. (F) Whole kidney mRNA was extracted and the expression of AREG, HB-EGF, TGFA, epiregulin, epigen, and CCN2 was tested by quantitative PCR and presented after normalization to AREG WT mice subjected to UUO (fold control). (G) Tubular injury was scored in kidney sections after periodic acid–Schiff staining as described under Methods. Representative images ([G], left panel) and scoring results ([G], right panel) are shown. n=4–7; *P<0.05; **P<0.01.
To determine whether AREG PTC-KO affects the expression levels of other EGFR ligands (including CTGF/CCN2, which was recently reported to bind to EGFR and is known to contribute to kidney fibrosis,24,25), we examined their transcripts by quantitative PCR. Only AREG transcript is significantly downregulated (to about 50% of wt) in whole kidney extracts of AREG PTC-KO/tdTomato+ mice, and there is no significant change in the injury-induced upregulation of other EGFR ligands (Figure 6F), including all low-affinity EGFR ligands and HB-EGF. This assigns sAREG a dominant role in kidney fibrosis and suggests that other injury-upregulated EGFR ligands might also act through sAREG in vivo, as suggested by our in vitro experiments in HPTCs.
Because EGFR activation has been linked to tubular cell repair after injury,26 and YAP1 PTC-KO can negatively affect early recovery after AKI in IRI,27 we tested this possibility in AREG PTC-KO/tdTomato+ mice. AREG PTC-KO/tdTomato+ mice show decreased tubular injury compared with wt littermates at day 7 of UUO, as determined by tubular injury scoring using periodic acid–Schiff staining (Figure 6G), suggesting AREG does not have a dominant role in structural recovery after kidney injury, at least in this model, and/or that YAP1 PTC-KO affects other targets genes besides AREG that are important in early structural recovery after injury.
Discussion
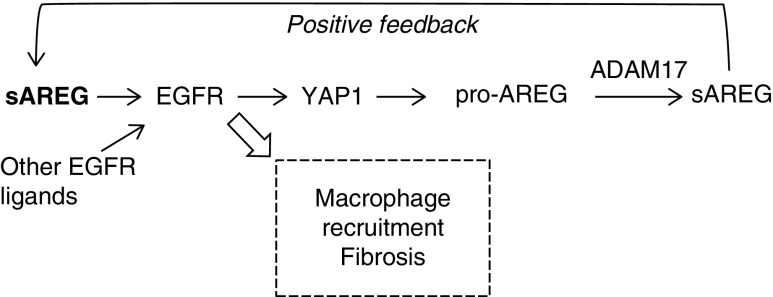
In this study, we identified PTC-derived sAREG as a major driver of kidney fibrosis that sustains profibrotic EGFR activation via signal integration and amplification (positive feedback, Figure 7). PTC-KO of AREG reduces proinflammatory and profibrotic markers after IRI and reduces fibrosis in the classic fibrosis model, UUO. In mice that cannot release any injury-upregulated EGFR ligands globally (ADAM17 hypomorphic mice) or specifically from their PTCs (ADAM17 PTC-KO), injected sAREG is sufficient to reverse their protection from fibrosis after injury. Conversely, sAREG is necessary for kidney fibrosis, as AREG PTC-KO mice were protected from injury-induced fibrosis. Building on our previously published results that AREG is upregulated and correlates with fibrosis in CKD biopsy specimens,7 our study establishes AREG/sAREG as a potential novel drug target or functional biomarker in kidney fibrosis in humans.
Figure 7.
PTC-derived AREG sustains profibrotic EGFR signals in PTC by amplification of its own signaling and integration of other EGFR ligand signals via a YAP1-dependent positive feedback loop.
AREG is able to sustain EGFR activation via a positive feedback loop that includes its YAP1-dependent transcriptional upregulation. AREG was originally identified as a transcriptional target of YAP1 in cancer cells, where its induction contributed to YAP1-mediated cell proliferation.20 Regulation of cellular proliferation by sAREG may be important in surviving tubule cells early after AKI, which are known to proliferate and repair the damaged tubule.28 However, our results did not corroborate findings of delayed early tubular recovery reported in YAP1 PTC-KO mice,27 but rather suggested that AREG PTC-KO protected against tubular injury as compared with wt littermates. It is thus likely that YAP1 PTC-KO affects other gene targets beyond AREG that are important in functional and structural recovery from AKI.
Our collective results are compatible with the notion that sAREG is induced to promote tissue repair, but that its sustained upregulation can lead to fibrosis. Consistent with this, AREG has been linked to fibrosis in the heart, liver, and lung.29–31 It is already known that EGFR expressed in the PTC can drive kidney fibrosis after injury.32,33 Therefore, it is likely that the profibrotic effects of PTC-derived AREG in vivo are mediated to a large degree by EGFR in PTCs, in an autocrine or paracrine response that maintains and amplifies PTC EGFR activation. However, PTC-derived AREG could also act on other kidney cell types. Later stages of tissue injury/repair are known to involve type 2 immunity cells, such as alternatively activated tissue repair macrophages. Exaggerated activation of type 2 immunity drives the development of fibrosis after tissue injury in various organs, including the kidney.34 AREG has indeed been identified as a critical regulator and effector of such type 2 immunity cells, in particular macrophages, ILC2 cells, and Th2 cells,34 all of which have also been implicated in tissue injury repair and the development of fibrosis in the kidney.23,35,36 It is therefore reasonable to assume that PTC-derived sAREG has effects on immune cells involved in tissue repair. Our experiments indeed show that lack of PTC-derived AREG significantly reduced MCP1 expression in PTCs in vivo, a critical monocyte attracting cytokine, as well as the number of macrophages in the injured kidney. It is thus possible that continued upregulation and release of sAREG by PTCs and possibly by type 2 immunity cells, including macrophages, amplifies the effects of AREG and causes an exaggerated type 2 immune response and fibrosis. As an example, it was recently shown that macrophage-derived AREG induces TGF-β activation and the differentiation of pericytes into collagen-producing myofibroblasts after lung injury.37 AREG PTC-KO mice show reduced levels of the myofibroblast marker αSMA compared with their wt littermates and reduced actual fibrosis. Whether sAREG has direct effects on myofibroblasts in vivo in the kidney analogous to the lung is unknown to date.
We have previously shown that different EGFR ligands can induce upregulation of proinflammatory and profibrotic cytokines in PTCs to different degrees, with sAREG being the most efficient in this regard.7 It is intriguing that, although EGFR ligands bind to and activate the same receptor, different EGFR ligands can induce distinct responses in the same cell.38,39 The affinity of ligands to EGFR is one level of regulation that dictates differential responses. The affinity differences we found for all seven different EGFR ligands in HPTCs, correspond to reported findings by others.18,40,41 Regulation of EGFR cell surface levels represents another important level of regulation. We showed that in HPTCs sAREG maintains cells surface levels of EGFR after activation, unlike sHB-EGF, which induces downregulation of EGFR from the cell surface. Our findings are consistent with reports that show that recycling of EGFR to the cell surface after ligand activation represents a feature of low-affinity EGFR ligands such as sAREG, whereas high-affinity EGFR ligands such as sHB-EGF are reported to induce ubiquitination, internalization, and lysosomal breakdown of EGFR18,38,39; findings that have been corroborated in vivo using transgenic mice expressing GFP-tagged EGFR.42 These findings make it reasonable to assume that sAREG and high-affinity EGFR ligands, such as sHB-EGF, regulate EGFR cell surface levels similarly in PTCs after kidney injury in vivo.
Exogenous administration of sAREG in mice that cannot release any injury-induced EGFR ligand(s) (ADAM17 hypomorphic or ADAM17 PTC-KO mice) was able to reverse protection from kidney injury-induced fibrosis. These results suggest that sAREG alone is sufficient to drive upregulation of proinflammatory and profibrotic cytokines and eventually kidney fibrosis after injury. sAREG injection into ADAM17 PTC-KO mice also restored the expression of its own transcript as well as that of YAP1 in isolated PTCs, confirming the presence of the positive AREG-YAP1 feedback loop identified in HPTCs in vitro also in kidneys in vivo. In a report from the Harris group, EGFR PTC-KO mice failed to upregulate YAP1 and AREG protein after IRI in PTCs in vivo, lending further support to our hypothesis that AREG drives a YAP1-dependent feedback loop in vivo in PTCs.43 Whether nonphysiologic exogenous sAREG administration acts via induction of additional EGFR ligands is not known. On the basis of our in vitro results, it is theoretically possible that injection of another EGFR ligand also reverses protection from fibrosis in ADAM17 PTC-KO mice. However, under physiologic conditions, AREG PTC-KO mice did not show significant changes in mRNA expression of any other EGFR ligand except for AREG in total kidney lysates, as compared with wt littermates (Figure 6E), suggesting that their protection from fibrosis was related to AREG and not expression changes in other EGFR ligands.
The fact that sAREG is required for any low-affinity EGFR ligand to sustain EGFR activation in HPTCs is a novel finding. It implies that other only moderately injury-upregulated, low-affinity EGFR ligands, such as TGFα, might also contribute to kidney fibrosis. Global TGFα-KO has been shown to protect against fibrosis in a CKD mouse model of chronic (nonphysiologic) angiotensin II infusion.44 To determine whether sAREG was the actual effector molecule downstream of sTGFα, as our experiments might suggest, would require additional KO mouse models or testing of the angiotensin II infusion model in our AREG PTC-KO/tdTomato+ mice. We further found that high-affinity EGFR ligands, such as HB-EGF, can also induce significant AREG upregulation and sAREG release, but fail to reactivate EGFR because they reduce its cell-surface levels. Interestingly, a recent report showed that transgenic overexpression of HB-EGF in PTCs leads to concentric tubular fibrosis.8 It could be speculated that nonphysiologic HB-EGF overexpression and sHB-EGF release might induce enough sAREG release over longer time frames to amplify sAREG signaling enough to shift the balance to sAREG-induced sustained EGFR activation and fibrosis. Crossing our AREG PTC-KO/tdTomato+ mice to the HB-EGF overexpression mouse or injecting neutralizing sAREG antibody into HB-EGF overexpression mice could help answer these questions. Alternatively, sTGFα and sHB-EGF could use other sAREG-independent mechanisms to induce fibrosis in vivo.
In summary, sAREG may represent a novel fibrosis marker that is directly connected to the injurious process, a fact that might give sAREG serum/urine levels improved diagnostic power over routine kidney function parameters. Further, our findings could stimulate evaluation of sAREG as a therapeutic or diagnostic target in kidney injury and fibrosis.
Disclosures
Dr. Waikar reports grants and personal fees from Allena, and personal fees from Cerus, CVS, GSK, Harvard Clinical Research Institute, Janssen, Mass Medical International, Strataca, Takeda, Venbio, and Wolters Kluwer outside the submitted work; and expert witness consultation for litigation related to Granuflo, Omniscan, statins, cisplatin nephrotoxicity, and mercury exposure. All other authors have nothing to disclose.
Funding
Dr. Herrlich and Dr. Kefaloyianni were supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01DK108947).
Supplementary Material
Acknowledgments
Dr. Herrlich and Dr. Waikar conceived and coordinated the study. Dr. Herrlich and Dr. Kefaloyianni wrote the manuscript. Dr. Kefaloyianni designed and performed experiments and analyzed all the data. Mr. Raja Keerthi Raja performed part of the experiments and analyzed part of the data. Dr. Schumacher performed ELISA multiplex assays. Mrs. Muthu and Mrs. Krishnadoss performed part of the experiments. The authors declare no financial interests.
We thank Dr. Rudensky (Memorial Sloan Kettering Cancer Center, New York, NY) and Dr. Arpaia (Columbia University Irving Medical Center) for provision of AREG floxed mice.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019030321/-/DCSupplemental.
Supplemental Table 1. RNA-sequencing identification of proximal tubule marker expression in HPTCs.
Supplemental Table 2. Quantitative PCR primers list.
Supplemental Figure 1. EGFR ligand dose response in HPTC.
Supplemental Figure 2. sAREG and sHB-EGF differentially regulate EGFR cell-surface levels in HPTCs.
References
- 1.Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Parr SK, Siew ED: Delayed consequences of acute kidney injury. Adv Chronic Kidney Dis 23: 186–194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al.: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al.: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Liu N, Zhuang S: Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int 83: 804–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kefaloyianni E, Muthu ML, Kaeppler J, Sun X, Sabbisetti V, Chalaris A, et al.: ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1: 87023, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overstreet JM, Wang Y, Wang X, Niu A, Gewin LS, Yao B, et al.: Selective activation of epidermal growth factor receptor in renal proximal tubule induces tubulointerstitial fibrosis. FASEB J 31: 4407–4421, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, et al.: Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med 207: 1617–1624, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, et al.: A distinct function of regulatory T cells in tissue protection. Cell 162: 1078–1089, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 1p following 143, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, Hsiao LL, et al.: KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 125: 1620–1636, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW: NIH image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orosz DE, Woost PG, Kolb RJ, Finesilver MB, Jin W, Frisa PS, et al.: Growth, immortalization, and differentiation potential of normal adult human proximal tubule cells. In Vitro Cell Dev Biol Anim 40: 22–34, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, et al.: Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kefaloyianni E, Gaitanaki C, Beis I: ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal 18: 2238–2251, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Doulamis IP, Konstantopoulos P, Tzani A, Antoranz A, Minia A, Daskalopoulou A, et al.: Visceral white adipose tissue and serum proteomic alternations in metabolically healthy obese patients undergoing bariatric surgery. Cytokine 115: 76–83, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Henriksen L, Grandal MV, Knudsen SL, van Deurs B, Grøvdal LM: Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS One 8: e58148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng Z, Moroishi T, Guan KL: Mechanisms of Hippo pathway regulation. Genes Dev 30: 1–17, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, et al.: YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 11: 1444–1450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Harris RC: Interaction of the EGF receptor and the hippo pathway in the diabetic kidney. J Am Soc Nephrol 27: 1689–1700, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD: Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A 111: 1527–1532, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Q, Harris DC, Wang Y: Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 30: 183–194, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Rayego-Mateos S, Rodrigues-Díez R, Morgado-Pascual JL, Rodrigues Díez RR, Mas S, Lavoz C, et al.: Connective tissue growth factor is a new ligand of epidermal growth factor receptor. J Mol Cell Biol 5: 323–335, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Phanish MK, Winn SK, Dockrell MEC: Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron, Exp Nephrol 114: e83–e92, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Chen JK, Harris RC: Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney Int 82: 45–52, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, You H, Li Y, Xu Y, He Q, Harris RC: EGF receptor-dependent YAP activation is important for renal recovery from AKI. J Am Soc Nephrol 29: 2372–2385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramann R, Kusaba T, Humphreys BD: Who regenerates the kidney tubule? Nephrol Dial Transplant 30: 903–910, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, et al.: Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology 59: 1577–1590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Lee JY, Lee CM, Cho WK, Kang MJ, Koff JL, et al.: Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-β-induced pulmonary fibrosis. J Biol Chem 287: 41991–42000, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perugorria MJ, Latasa MU, Nicou A, Cartagena-Lirola H, Castillo J, Goñi S, et al.: The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology 48: 1251–1261, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, et al.: EGFR signaling promotes TGFβ-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, et al.: Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. J Am Soc Nephrol 23: 854–867, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaiss DMW, Gause WC, Osborne LC, Artis D: Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 42: 216–226, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao Q, Wang Y, Niu Z, Wang C, Wang R, Zhang Z, et al.: Potentiating tissue-resident Type 2 innate lymphoid cells by IL-33 to prevent renal ischemia-reperfusion injury. J Am Soc Nephrol 29: 961–976, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braga TT, Correa-Costa M, Guise YF, Castoldi A, de Oliveira CD, Hyane MI, et al.: MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med 18: 1231–1239, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minutti CM, Modak RV, Macdonald F, Li F, Smyth DJ, Dorward DA, et al.: A macrophage-pericyte axis directs tissue restoration via amphiregulin-induced transforming growth factor beta activation. Immunity 50: 645–654.e6, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson KJ, Mill C, Lambert S, Buchman J, Wilson TR, Hernandez-Gordillo V, et al.: EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling. Growth Factors 30: 107–116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grøvdal L, et al.: Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 10: 1115–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones JT, Akita RW, Sliwkowski MX: Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett 447: 227–231, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Macdonald-Obermann JL, Pike LJ: Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J Biol Chem 289: 26178–26188, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YP, Ma H, Starchenko A, Huh WJ, Li W, Hickman FE, et al.: A chimeric egfr protein reporter mouse reveals egfr localization and trafficking in vivo. Cell Reports 19: 1257–1267, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, You H, Li Y, Xu Y, He Q, Harris RC: EGF receptor-dependent YAP activation is important for renal recovery from AKI. J Am Soc Nephrol 29: 2372–2385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, et al.: Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.