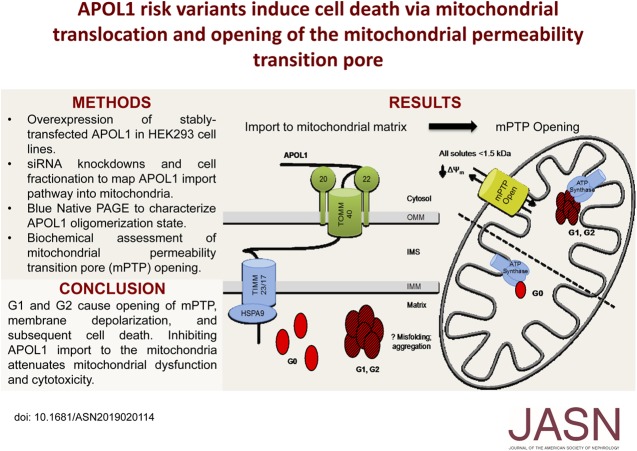
Significance Statement
Some variants in APOL1 are associated with high CKD rates in African Americans, but the molecular mechanism of disease remains elusive. Previous studies demonstrated that expression of APOL1 risk variants is associated with mitochondrial dysfunction. In this study, the authors show that import of APOL1 protein into mitochondria is essential for risk variant–mediated cytotoxicity, and map the APOL1 import pathway. They found that whereas APOL1 is mostly monomeric, risk variant APOL1 can form large oligomers and cause opening of the mitochondrial permeability transition pore, ultimately leading to cell death. This difference in propensity of different variants to oligomerize could help explain APOL1 risk variants’ gain-of-function biology despite a recessive mode of inheritance. Understanding APOL1 trafficking and interactions could help inform new therapeutic approaches.
Keywords: APOL1, mitochondria, chronic kidney disease, focal segmental glomerulosclerosis, genetic renal disease
Visual Abstract
Abstract
Background
Genetic Variants in Apolipoprotein L1 (APOL1) are associated with large increases in CKD rates among African Americans. Experiments in cell and mouse models suggest that these risk-related polymorphisms are toxic gain-of-function variants that cause kidney dysfunction, following a recessive mode of inheritance. Recent data in trypanosomes and in human cells indicate that such variants may cause toxicity through their effects on mitochondria.
Methods
To examine the molecular mechanisms underlying APOL1 risk variant–induced mitochondrial dysfunction, we generated tetracycline-inducible HEK293 T-REx cells stably expressing the APOL1 nonrisk G0 variant or APOL1 risk variants. Using these cells, we mapped the molecular pathway from mitochondrial import of APOL1 protein to APOL1-induced cell death with small interfering RNA knockdowns, pharmacologic inhibitors, blue native PAGE, mass spectrometry, and assessment of mitochondrial permeability transition pore function.
Results
We found that the APOL1 G0 and risk variant proteins shared the same import pathway into the mitochondrial matrix. Once inside, G0 remained monomeric, whereas risk variant proteins were prone to forming higher-order oligomers. Both nonrisk G0 and risk variant proteins bound components of the mitochondrial permeability transition pore, but only risk variant proteins activated pore opening. Blocking mitochondrial import of APOL1 risk variants largely eliminated oligomer formation and also rescued toxicity.
Conclusions
Our study illuminates important differences in the molecular behavior of APOL1 nonrisk and risk variants, and our observations suggest a mechanism that may explain the very different functional effects of these variants, despite the lack of consistently observed differences in trafficking patterns, intracellular localization, or binding partners. Variant-dependent differences in oligomerization pattern may underlie APOL1’s recessive, gain-of-function biology.
African Americans develop ESKD much more frequently that other groups. A large fraction of this disparity is due to genetic variants in the APOL1 gene. Inheriting two copies of APOL1 risk variants (RVs), known as G1 and G2, causes high rates of FSGS, HIV-associated nephropathy and hypertension-associated ESKD.1–4
Of the six members of the APOL gene family, APOL1 is the sole member with a signal peptide permitting cellular export.5,6 Circulating non-risk APOL1 (G0) confers resistance to trypanosome Trypanosoma brucei brucei through ion channel formation and trypanolysis, but cannot protect against the two subspecies Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense.7,8 These highly pathogenic subspecies have evolved multiple mechanisms to inactivate APOL1-mediated lysis.9,10 G1 (two amino acid substitutions: S342G and I384M) and G2 (a two-amino-acid deletion: del388N389Y) mutations originated in sub-Saharan Africa and rose to high frequency because they provided a selective advantage in regions with pathogenic trypanosomes.1,11–13 Although inheriting a single copy of G1 or G2 is sufficient to enhance protection against resistant trypanosomes, inheriting two copies increases risk of developing kidney disease.
Not all individuals with two copies of the RVs develop kidney disease, suggesting a second hit is required for disease to develop in high-risk individuals. APOL1 RVs increase risk in a recessive manner, but unlike most variants that cause a recessive mode of inheritance, evidence suggests that APOL1 RVs are toxic, gain-of-function mutations.14 This hypothesis is supported by the fact that most mammals and even some primates have no APOL1 gene, and at least one human is completely null for APOL1 while having no apparent kidney dysfunction.6,15 Although the increased risk of kidney disease due to APOL1 RVs has been well established, it remains uncertain how the APOL1 RVs promote kidney injury.
Our goal was to address several important questions about APOL1 biology. First, the nature of APOL1 gain-of-function toxicity has remained elusive despite many well designed studies. Evidence supporting many different cell death mechanisms—apoptosis, autophagy, necrosis, pyroptosis, and necroptosis—has been put forth.16–32 Second, the reason why two RVs are required for kidney disease, if the variants are truly gain-of-function mutations, is not yet understood. The recessive mode of inheritance rather than an additive effect of APOL1 RVs has been proposed to involve oligomer formation or threshold effects, but there is little direct evidence for these theories to date.33 Third, definitive differences in behavior between G0 and RV APOL1 relating to subcellular localization and/or affinity for binding partners have not been consistently demonstrated.34,35 We explore a model that may begin to unify these questions.
In this study, we build on the observation that APOL1 RVs cause mitochondrial dysfunction27,32,36 by (1) mapping an APOL1 mitochondrial import pathway, (2) demonstrating enhanced APOL1 RV self-aggregation that occurs after transport to the mitochondria, (3) defining a set of inner mitochondrial membrane (IMM) binding partners of APOL1 that include several major mitochondrial permeability transition pore (mPTP) constituents and modulators, and (4) activating the mPTP, triggering first mitochondrial dysfunction and then cell death. Our data on differential oligomer formation may ultimately help illuminate why both alleles must be RVs for disease to occur, and our findings regarding mPTP activation could explain why multiple modes of cell death have been observed in association with APOL1 overexpression.
Methods
Cells and Antibodies
HEK293 cells expressing tetracycline-inducible APOL1 integrated at a single locus were generated using the T-REx system (Thermo Fisher Scientific). APOL1 sequences used for each genotype are shown in Supplemental Table 1. Cells were cultured at 37°C with 5% CO2 in DMEM (Corning) supplemented with 10% tetracycline-free FBS (Atlanta Biologicals). Cells were validated to be free of any Mycoplasma contamination. Anti-APOL1 rabbit polyclonal antibody (HPA018885, lot #E114503) and anti-FLAG (F1804) mouse monoclonal antibody were from Sigma. Anti-ACSL4 (SC-365230), anti-TOMM20 (SC-17764), anti-TOMM22 (SC-101286), anti-TOMM70 (SC-390545), anti-TIMM23 (SC-514463), anti-TIMM17 (SC-271152), anti-HSPA9 (SC-133137), anti-HSP60 (SC-13115), anti-ATP5A (SC-136178), anti-ATP5B (SC-55597) mouse mAb, and anti-APOL1 (SC-18759) goat polyclonal antibodies were from Santa Cruz Biotechnology. Anti-TIMM22 (14927–1-AP) rabbit polyclonal antibody was from Proteintech. Anti-SLC25A5 (14671S) anti-calreticulin (12338S), anti-GM130 (12480S), anti-Rab7 (9367S), anti-LC3 (12741S), anti-myc (2278S) rabbit polyclonal antibodies were from Cell Signaling Technologies.
Preparation of Mitochondria-Enriched Fractions
Mitochondrial enrichment protocol was adapted from Jastroch et al.37 Briefly, cells were scraped in PBS and centrifuged at 500×g for 5 minutes. Cell pellets were resuspended in STE medium (250 mM sucrose, 5 mM Tris, 2 mM EGTA; pH 7.4) and plasma membranes were disrupted using a dounce homogenizer. The whole homogenate was centrifuged for 10 minutes at 1000×g and the supernatant was saved in a fresh tube. To increase yields, the cell pellet was resuspended again in STE, dounced, and centrifuged at 1000×g for 10 minutes. The two supernatants were pooled and filtered through a 5 µM syringe filter. An aliquot of filtered lysate was saved as loading control and denoted on figures as “total” protein. The remaining lysate was spun at 10,000×g for 10 minutes at 4°C. The “supernatant/cytosolic” fraction was transferred to a fresh tube. The resulting mitochondria-enriched pellet was washed twice and then resuspended in STE medium.
Proteinase K Protection Assay
The protocol was adapted from Ryan et al.38 Mitochondrial-enriched pellet obtained after differential centrifugation was resuspended in STE buffer or hypotonic solution (5 mM Tris, pH 7.4) and divided into three. Samples were incubated on ice with only STE or 25 µg/ml Proteinase K or 25 µg/ml Proteinase K with 0.1% Triton X-100 for 1 hour; 5 mM PMSF was added to stop the reaction. After 10 minutes incubation on ice, samples were boiled in SDS loading buffer for immunoblotting.
Small Interfering RNA Transfections
Silencer select small interfering RNA (siRNA) (Ambion) were used at a final concentration of 40 nM with RNAiMAX (Invitrogen) for knockdown experiments. All experiments were performed via reverse transfections (siRNA/OptiMEM/RNAiMAX mix was added to each well before overlaying the cells). siRNAs used were: siNT (Negative Control #2), TOMM20 (s18950), TOMM22 (s32550), TOMM70 (s19107), TIMM22 (s26725), TIMM23 (s223113), TIMM17 (s20424, s20425), HSPA9 (s6989, s6990), SLC25A3 (s10428), SLC25A4 (s223817), SLC25A5 (s1375), CYPD (s19662), and APOL1 (s16255).
Mitochondria Functional (Seahorse) Assay
HEK293 empty vector (EV), G0, G1, and G2 cells were reverse transfected with 40 nM Non-Target or TOMM20 siRNA in a V3-PS cell culture plate (Agilent Technologies) for 48 hours. Cells were then induced with 50 ng/ml tetracycline for 8 hours. Media was replaced with Agilent Seahorse XF base medium supplemented with 10 mM glucose, 1 mM sodium pyruvate, and 2 mM L-glutamine, at pH 7.4. Oxygen consumption rate was measured using the XFe96 Extracellular Flux Analyzer (Agilent Technologies). Next, 1 µM oligomycin, 0.25 µM FCCP, or 0.5 µM mix of antimycin A and rotenone were serially injected to measure ATP production, maximal respiratory capacity, and nonmitochondrial respiration, respectively. After obtaining all measurements, media was aspirated and protein concentrations were measured using a bicinchoninic acid (BCA) assay. Oxygen consumption rate was normalized to protein concentration in each well.
Cytotoxicity Assays
For siRNA knockdown experiments cells were reverse transfected with 40 nM of respective siRNA in a 96-well, black-walled, clear-bottomed plate, allowed to incubate for 48 hours, and then induced with 50 ng/ml tetracycline for 18 hours. For cyclosporin A (CsA) and NIM811 rescue experiments, cells were pretreated with respective dose of drug for 2 hours and then induced with 50 ng/ml tetracycline for 18 hours. Cytotoxicity/viability was measured using the MultiTox-Fluor Multiplex Cytotoxicity Assay kit (Promega).
Blue Native PAGE and SDS-PAGE
For blue native PAGE, cell pellets were solubilized with 50 mM imidazole/HCl (pH 7), 50 mM NaCl, 1% digitonin lysis buffer supplemented with Protease Inhibitor Cocktail (Sigma), and PhosSTOP tablets (Sigma). After 30 minutes of incubation, lysates were centrifuged at 15,000×g for 10 minutes at 4°C and cell pellets were discarded. For blue native PAGE and SDS-PAGE after differential centrifugation, each fraction was solubilized in 1% digitonin in STE buffer. Protein concentrations were measured using DC Assay Kit (Bio-Rad). For blue native PAGE, 4× NativePAGE Sample Buffer and 5% G-250 Additive (final 0.25%) were added to the lysates and were run on 3%–12% Bis-Tris protein gels (Thermo Fisher Scientific). For SDS-PAGE, cells were lysed in RIPA buffer and the lysate centrifuged at 17,000×g. Supernatants were boiled in reducing SDS sample buffer at 95°C for 5 minutes, separated on 4%–20% Criterion TGX gels, and then transferred onto PVDF membranes (Millipore). After blocking, membranes were incubated overnight in primary antibody, washed, incubated for 1 hour in HRP-linked secondary antibody, and washed again. Blots were imaged with film after brief incubation in SuperSignal HRP substrate solution (Thermo Fisher Scientific).
Preornithine Transcarbamylase Mitochondrial Import Assay
Flag-tagged human ornithine transcarbamylase precursor plasmid (NM_000531; Origene) was in vitro transcribed and translated in vitro (TNT quick coupled transcription/translation system, L1170; Promega). Mitochondria-enriched pellets isolated from HEK293 EV, G0, G1, and G2 cells induced with 50 ng/ml tetracycline for 6 hours were resuspended in import buffer: 0.1% (w/v) BSA, 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 2 mM KH2PO4, and 10 mM MOPS-KOH, at pH 7.4. Final import reactions contained 2 mM ATP, 2 mM NADH, 5 mM succinate, mitochondria, and 3% (v/v) in vitro translated preornithine transcarbamylase (pOTC). pOTC import was performed at 30°C for 30 minutes. Mitochondria were reisolated by spinning at 10,000×g for 10 minutes before subjecting to SDS-PAGE.
Immunoprecipitation Mass Spectrometry
HEK293 EV, G0, G1, and G2 cells were induced with 50 ng/ml tetracycline for 8 hours. Cells were lysed in 50 mM Tris/HCL (pH 7.5), 150 mM NaCl, 0.5% NP-40, and 0.3% CHAPS lysis buffer supplemented with Protease Inhibitor Cocktail and PhosSTOP tablets. Lysates were incubated overnight with anti-APOL1 goat polyclonal antibody crosslinked Protein G Dynabeads. Beads were washed three times with ice-cold lysis buffer and proteins were eluted with nonreducing Laemmli sample buffer. Samples were separated on a 10% Criterion gel. After gel staining with colloidal blue (Thermo Fisher Scientific), the gel was cut into several small bands and the gel fragments sent for protein identification by liquid chromatography with tandem mass spectrometry (LC/MS/MS). The mitochondrial protein list was generated by performing gene ontology enrichment analysis followed by manual curation using UniProtKB data. A minimum of five total peptides cutoff was applied after subtracting background from EV lanes.
Live Cell Imaging
HEK293 EV, G0, G1, and G2 cells were induced with 50 ng/ml tetracycline for 6 hours. mPTP opening was assessed using the Image-IT live mitochondrial transition pore assay kit (Thermo Fisher Scientific). Briefly, cells were stained with 1 µM calcein acetoxymethyl ester (calcein-AM), 200 nM Mitotracker Red CMXRos, 1 µM Hoechst 33342±4 mM CoCl2 for 15 minutes. Images were then acquired as z-stacks. The raw integrated density of each optical slice was added and then divided by the number of cells in that field, using ImageJ. Mitochondrial membrane potential was measured in cells were stained for 30 minutes with 50 nM tetramethylrhodamine (TMRM; Invitrogen). All images were acquired with the Zeiss LSM live-cell confocal system.
Statistical Analyses
All data shown are presented as means±SEM unless stated otherwise. Data sets were analyzed for statistical significance by ANOVA with the Dunnett post-test, using GraphPad Prism.
Results
G0 and RV APOL1 Translocate to the Mitochondrial Matrix
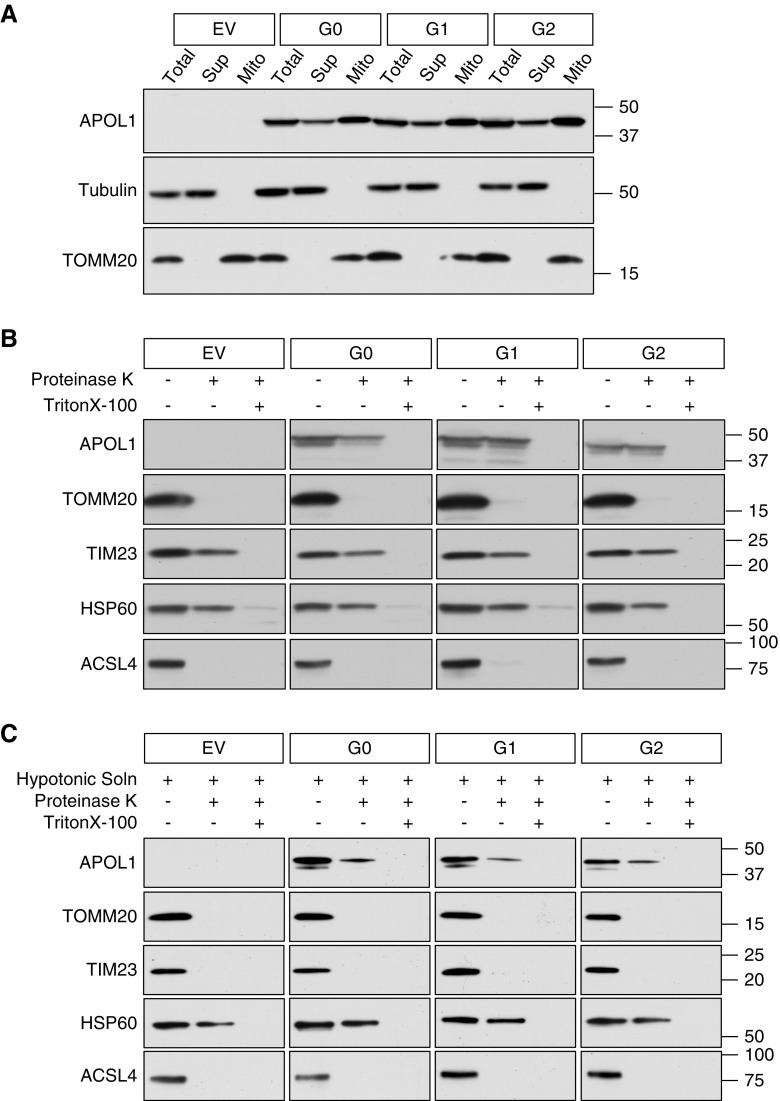
APOL1 RVs have been shown to impair maximum respiratory capacity, whereas G0 either had no effect or enhanced mitochondrial respiratory function.27,32 APOL1 protein has been shown to localize to mitochondria by immunostaining in several studies, but it is unclear from imaging alone whether APOL1 gets imported into mitochondria or is instead in close proximity (e.g., apposed to the outer mitochondrial membrane [OMM] or located in mitochondrial-associated membranes).27,32,39 We sought biochemical confirmation of APOL1 mitochondrial localization and identification of the mechanism of APOL1 trafficking into mitochondria. HEK293 cells stably transfected with APOL1 (G0 or RVs) were induced with 50 ng/ml tetracycline for 6 hours and then fractionated using a differential centrifugation protocol.37 Total cell lysates, cytosolic fraction, and mitochondrial-enriched fraction were separated by SDS-PAGE. Tubulin served as a cytosolic marker and TOMM20 as a mitochondrial marker. APOL1 G0, G1, and G2 each cofractionated with the TOMM20-positive, mitochondria-enriched fraction (Figure 1A).
Figure 1.
APOL1 translocates into the mitochondrial matrix. (A) APOL1 cofractionates with the mitochondrial marker TOMM20, indicating mitochondrial association. HEK293 cells stably transfected with APOL1 (EV, G0, G1, or G2) were induced with tetracycline for 6 hours. The cell lysate was separated by differential centrifugation into a mitochondria-enriched fraction (TOMM20 positive) and a supernatant fraction (tubulin positive). (B) APOL1 is translocated into mitochondria. Isolated mitochondria were subjected to Proteinase K digestion to degrade proteins bound to the OMM and MAM-associated proteins. Proteinase K digests the OMM protein TOMM20 and the MAM protein ACSL4 but not the IMM protein TIM23 or matrix protein HSP60. Continued presence of APOL1 after protease digestion indicates prior mitochondrial internalization. (C) APOL1 translocates to mitochondrial matrix. Isolated mitochondria were first incubated in hypotonic solution to lyse the OMM, then subjected to Proteinase K digestion. OMM (TOMM20), IMM (TIM23), and MAM (ACSL4) proteins were digested, but matrix proteins HSP60 and APOL1 are protected. Addition of TritonX-100 digestion solubilized lipids of the IMM and rendered APOL1 in the mitochondrial matrix susceptible to digestion by Proteinase K.
To determine the submitochondrial localization of APOL1, we performed Proteinase K protection assays on mitochondria-enriched fractions. TOMM20 was used as an OMM marker, TIMM23 as an inner mitochondrial membrane (IMM) marker, HSP60 as a mitochondrial matrix marker, and ACSL4 as a marker of mitochondria-associated endoplasmic reticulum membrane (MAM). Proteinase K treatment fully degraded MAM and OMM proteins but most APOL1 remained protected (Figure 1B), suggesting that APOL1 was present either in the IMM, the intermembrane space, or within the mitochondrial matrix.38 To refine APOL1 localization, mitochondria-enriched fraction was resuspended in hypotonic medium to disrupt the OMM, then subjected to Proteinase K treatment to digest IMM-spanning proteins.38 Hypotonicity followed by Proteinase K digestion degraded MAM, OMM, and IMM proteins, whereas HSP60 and APOL1 remained protected (Figure 1C), indicating that a fraction of APOL1 is translocated into the mitochondrial matrix despite its lack of a canonical N-terminal mitochondrial matrix targeting signal sequence, on the basis of sequence prediction programs.40 The purity of the mitochondria-enriched fraction was immunoblot-validated by probing for markers of endoplasmic reticulum, Golgi, plasma membrane, endosomes, and autophagosomes (Supplemental Figure 1).
Specific OMM and IMM Proteins Are Required for APOL1 Translocation to Mitochondrial Matrix and for APOL1-Induced Cytotoxicity
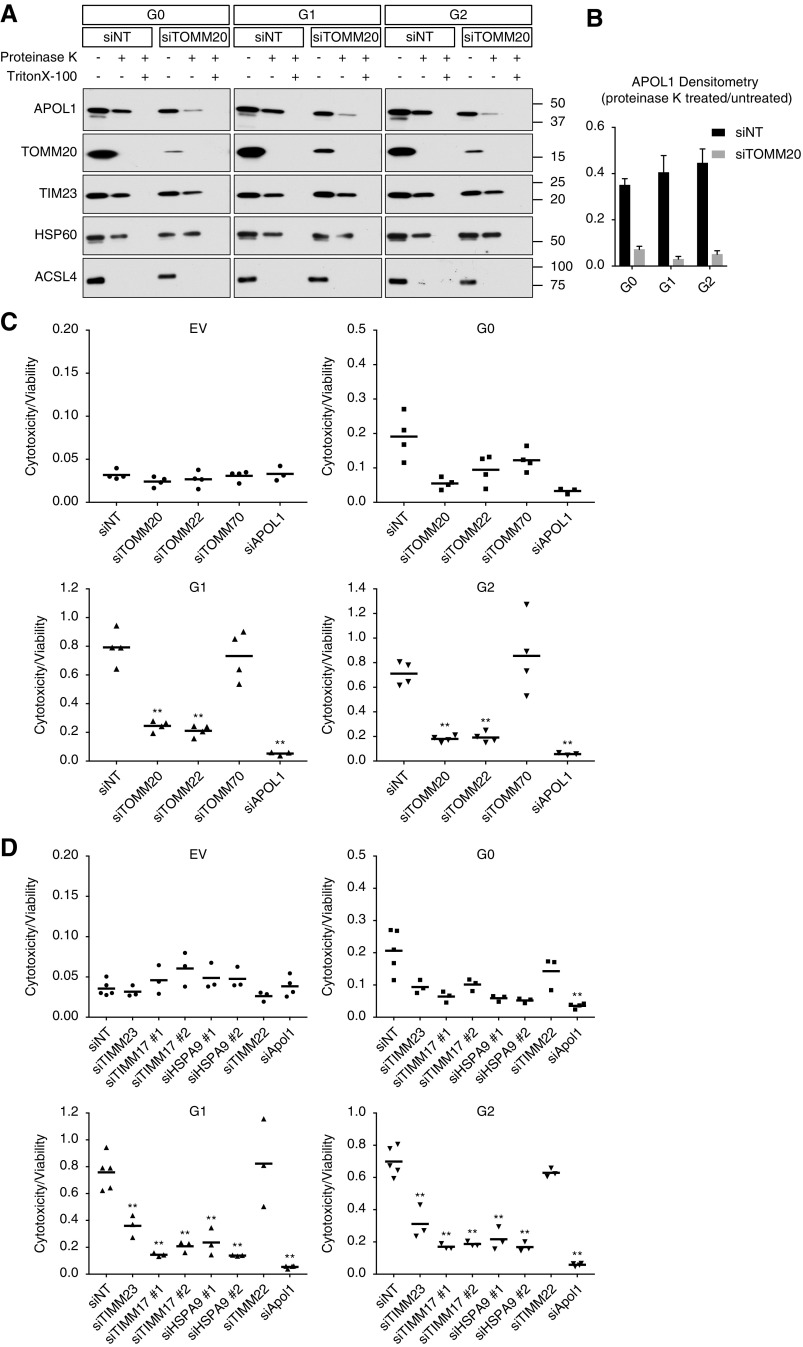
Multiple trafficking pathways have been defined for import of mitochondrial proteins.41,42 Most matrix-targeted proteins contain a presequence that is sequentially recognized by TOMM20, TOMM22, and TOMM40, and then directed to the matrix via the TIMM23/TIMM17 complex. To test whether TOMM20 was essential for APOL1 localization, we knocked down TOMM20 in stably inducible APOL1-expressing HEK293 cells, then induced APOL1 expression. Six hours after induction, mitochondria were isolated by differential centrifugation as above, then subjected to Proteinase K treatment. Eliminating TOMM20 rendered APOL1 susceptible to Proteinase K degradation (Figure 2, A and B), suggesting APOL1 was no longer protected inside the matrix. We also tried doing the same experiment after knocking down TOMM70, an essential component of carrier pathway for protein insertion into IMM but not matrix import. In contrast, elimination of TOMM70 did not sensitize APOL1 to Proteinase K digestion (Supplemental Figure 2).
Figure 2.
APOL1 translocation to mitochondrial matrix is dependent on IMM and OMM translocase machinery. (A and B) Knockdown of TOMM20 reduces mitochondrial APOL1. Nontarget siRNA (siNT) does not affect APOL1 translocation, as demonstrated by persistence of intact APOL1 in the presence of Proteinase K. Knockdown of TOMM20 (siTOMM20) substantially decreased mitochondrial APOL1 content after Proteinase K treatment. (C) Knockdown of TOMM20 and TOMM22 in matrix protein translocation pathway reduces RV APOL1-induced cell death. In contrast, knockdown of TOMM70, an OMM protein involved in targeting proteins to the IMM in the carrier pathway, does not affect RV APOL1-induced cell death. TOMM70 knockdown efficiency is shown in Supplemental Figure 6B. (D) Knockdown of TIMM17 and TIMM23 in the matrix protein translocation pathway and knockdown of mitochondrial matrix translocation chaperone HSPA9 independently reduced APOL1-induced cell death. In contrast, knockdown of the carrier pathway protein TIMM22 that shuttles incoming proteins into the IMM does not affect APOL1-induced cell death. TIMM22 knockdown efficiency is shown in Supplemental Figure 6C. (A–D) Stably transfected HEK293 cells were treated with 50 ng/ml of tetracycline for 6 (A and B) or 18 (C and D) hours. (B) Densitometry was performed on three independent experiments. (C and D) Each dot represents an independent experiment. **P<0.001 comparing each siRNA to siNT-treated cells.
We next asked if blocking APOL1 translocation to the mitochondria via TOMM20 knockdown could reverse APOL1 effects on mitochondrial metabolism. Seahorse analysis on cells in which TOMM20 was knocked down showed partial rescue of basal and maximum respiration capacity in G1 and G2 cells (Supplemental Figure 3). We also asked whether knocking down various components of the inner and outer mitochondrial translocase machinery could reduce G1 and G2 cytotoxicity. Knockdown of TOMM20 or TOMM22 rescued cell death associated with induction of G1 or G2, but knockdown of TOMM70 did not rescue cell death (Figure 2C).
Similarly, knockdown of key components of the IMM and matrix machinery required for most matrix translocation of proteins with classic mitochondrial presequences (TIMM23, TIMM17, and HSPA9) all rescued cell death associated with induction of G1 or G2. Conversely, knockdown of TIMM22, an essential component of the carrier pathway for IMM-directed proteins, had no effect on APOL1 RV–associated cell death (Figure 2D; see Supplemental Figure 4 for validation of siRNA knockdown efficiencies). Our data suggest that mitochondrial matrix delivery of APOL1 is needed for APOL1-induced cytotoxicity.
G1 and G2 Form Oligomers after Translocation to the Mitochondrial Matrix
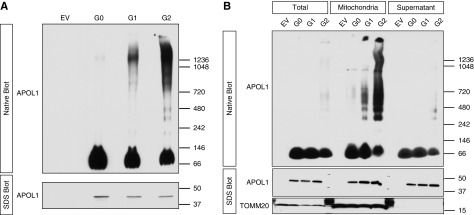
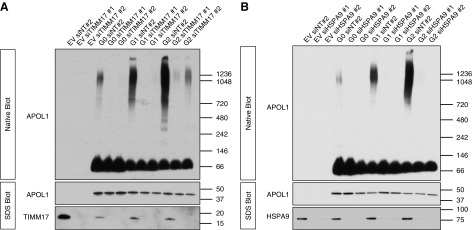
Investigators have hypothesized that APOL1 multimerization may be central to the explanation of why two risk alleles are required to increase kidney disease risk.33 We documented by coexpressing Myc- and FLAG-tagged APOL1 constructs in HEK293 cells, followed by pulldown with FLAG beads and immunoblot for Myc, that APOL1 undergoes homomerization (Supplemental Figure 5). We tested APOL1 behavior in our stable APOL1-expressing cell lines using nonreducing, nondenaturing blue native PAGE. We found that G1 and G2 formed a range of higher-ordered oligomers, whereas G0 appeared predominantly monomeric (Figure 3A). Because APOL1 translocation to the mitochondria is important for G1- and G2-induced toxicity, we examined the cytosolic versus mitochondrial-enriched fractions on native gels. Most of the G1 and G2 higher-ordered oligomers were located in the mitochondria-enriched fraction (Figure 3B).
Figure 3.
APOL1 RVs form higher-ordered oligomers that are enriched in the mitochondria. (A) Soluble fractions from digitonin lysates of APOL1-expressing cells were subjected to native PAGE, demonstrating that RV APOL1 forms a range of higher-ordered oligomers. (B) Differential centrifugation with mitochondrial isolation followed by digitonin lysis and native PAGE demonstrate that higher-order RV APOL1 is present primarily in the mitochondrial enriched fraction. Discrete oligomers appear at up to 1.2 Md; larger aggregates are retained in the well at top. (A and B) Stably transfected HEK293 cells expressing APOL1 were induced for 15 hours with (A) 25 ng/ml tetracycline or (B) 6 hours with 50 ng/ml tetracycline before lysis.
To distinguish whether G1- and G2-containing oligomers form before or after mitochondrial translocation, cells knocked down for TOMM20 were induced and subjected to blue native PAGE (Supplemental Figure 6A). There was a marked reduction in the RV APOL1 oligomerization after TOMM20 knockdown, indicating that oligomers are forming inside the mitochondria. Knockdown of the carrier pathway protein TOMM70 had no effect on multimerization (Supplemental Figure 6B), suggesting the destination of APOL1 monomers was not directly to the IMM but rather the matrix via the TOMM20-dependent pathway. To confirm translocation to the mitochondrial matrix was necessary for oligomerization, native gels were run on cell lysates after knocking down TIMM17 (Figure 4A) and HSPA9 (Figure 4B), proteins required for both APOL1 matrix translocation and cytotoxicity. Blocking APOL1 translocation into the mitochondrial matrix largely prevented G1 and G2 oligomerization, again suggesting oligomerization was occurring inside the mitochondria. Conversely, knockdown of TIMM22 (responsible for import of proteins directly into the IMM) that does not alter APOL1 RV–associated cytotoxicity (Figure 2D) also failed to inhibit G1 or G2 oligomerization (Supplemental Figure 6C). These data show that G1 and G2 oligomerization occurs after translocation into mitochondrial matrix, and that blocking mitochondrial import of APOL1 RVs prevents both APOL1 oligomerization and cell death.
Figure 4.
APOL1 transport to mitochondrial matrix is required for formation of higher-order oligomers. (A and B) Knockdown of TIMM17 (a key component of the IMM protein translocation machinery) or HSPA9 (a mitochondrial matrix translocation chaperone needed to guide proteins through the translocation machinery) prevents APOL1 higher-order oligomer formation (in addition to preventing cell death: see Figure 2D). Conversely, knockdown of TIMM22 affects neither cell death nor APOL1 oligomer formation (see Supplemental Figure 6C). Stable APOL1-transfected cells were treated for 48 hours with siRNA prior to inducing APOL1 with 50 ng/ml tetracycline. Cells were lysed with digitonin after 15 hours and were fractionated by native PAGE (large top panels) or SDS page (lower narrow panels). The signal from the monomeric fraction is saturated in these images.
We tested whether APOL1 import into mitochondria could be indirectly causing mitochondrial dysfunction by blocking import of other mitochondrial proteins. We performed an import assay using in vitro translated pOTC and mitochondria isolated from EV, G0-, G1-, and G2-expressing cells. pOTC has a mitochondrial matrix targeting presequence, which is cleaved by mitochondrial peptidases after import, producing mature ornithine transcarbamylase. pOTC cleavage was observed at similar levels in mitochondria expressing all genotypes of APOL1, demonstrating that general mitochondrial import of proteins remained intact (Supplemental Figure 7).
APOL1 G0 and RVs Bind mPTP Components, but Only RVs Activate Pore Opening
To explore how mitochondrial APOL1 alters mitochondrial function and drives cell death, we performed immunoprecipitation mass spectrometry to identify potential mitochondrial APOL1-binding partners. We observed that APOL1 pulled down nearly all putative components or modulators of the mPTP, an IMM complex that forms a pore between the matrix and intermembrane space, and several mPTP components were among the most enriched mitochondrial proteins (Table 1; full list in Supplemental Table 2).43–45 We validated several of these candidate proteins by immunoprecipitating with antibodies to ATP5A, ATP5B, and SLC25A5/ANT2, and then immunoblotting with anti-APOL1 antibody. We found that the association of these mitochondrial proteins with RVs generally exceeded their association with G0 (Supplemental Figure 8).
Table 1.
APOL1 immunoprecipitate contains multiple mPTP-associated proteins
| Protein | Total Peptides | ||
|---|---|---|---|
| G0 | G1 | G2 | |
| ATP5B | 56 | 72 | 40 |
| ATP5A1 | 51 | 51 | 62 |
| ATP5C1 | 9 | 16 | 12 |
| ATP5F1 | 5 | 3 | 1 |
| ATP5O | 3 | 5 | 7 |
| SLC25A3 | 28 | 20 | 27 |
| SLC25A4 | 17 | 25 | 29 |
| SLC25A5 | 14 | 25 | 17 |
| SLC25A6 | 5 | 6 | 5 |
APOL1 was immunoprecipitated from HEK293 cells stably expressing G0, G1, or G2 and mass spectrometry was performed to identify potential APOL1 binding partners. Identified candidate binding partners were highly enriched for mitochondrial proteins. Total peptide numbers are shown for the MPTP-associated proteins among them. The criteria for assignment of mitochondrial proteins is described in the Methods and the full list of mitochondrial proteins identified by mass spectrometry is listed in Supplemental Table 2.
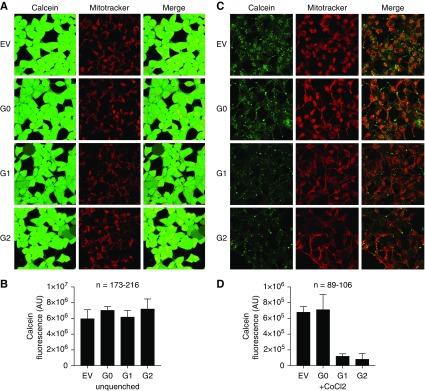
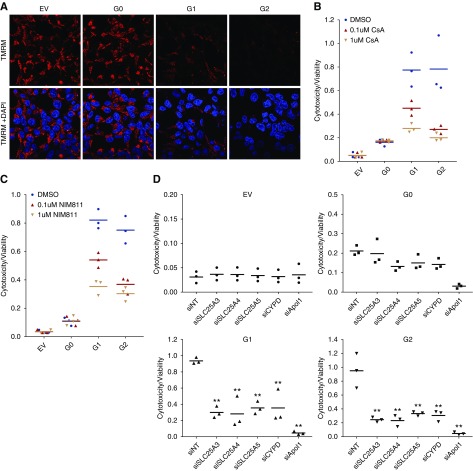
To test whether APOL1 expression leads to mPTP opening, we used a CoCl2-calcein fluorescence quenching assay.46 Nonfluorescent calcein-AM freely diffuses throughout the cells. The acetoxymethyl ester group is then cleaved by intracellular proteases, yielding hydrophilic, fluorescent calcein. CoCl2, which can quench calcein fluorescence, enters the cell but is restricted from the mitochondria unless the mPTP is open. In the absence of CoCl2, calcein fluorescence was similar in cells expressing either G0 or RV APOL1. (Figure 5, A and B). However, after addition of CoCl2, calcein fluorescence was reduced in G1- and G2-expressing cells but not in G0-expressing cells (Figure 5, C and D), indicating increased mitochondrial permeability to CoCl2 and suggesting increased mPTP opening in G1 and G2 cells only. As mPTP activation should also lead to rapid mitochondrial depolarization, we measured mitochondrial membrane potential using TMRM dye.46 Consistent with the CoCl2 quench data, G1 and G2 cells showed reduced TMRM staining, whereas fluorescence remained strong in EV and G0 cells, indicating intact mitochondrial membrane potential in EV and G0 cells (Figure 6A).
Figure 5.
APOL1 RVs induce MPTP opening (A). Calcein staining before CoCl2 quench was not different between EV, G0, G1 and G2 cells. (B) Quantification of calcein fluorescence before addition of cobalt chloride. Data expressed as mean±SD, for “n” cells subjected to quantification. (C) Calcein-AM staining with CoCl2 quenching indicates that APOL1 RVs cause opening of the mPTP. Calcein-AM dye diffuses into and throughout the cell and is activated upon esterase cleavage of the acetoxymethyl ester group in live cells (green staining). CoCl2 enters the cell but, under normal conditions, cannot enter the mitochondria. mPTP activation leads to pore formation and allows cobalt chloride to enter the mitochondria. Confocal imaging demonstrates decreased calcein fluorescence in G1- and G2-expressing cells after CoCl2 quenching, indicating MPTP pore opening. Images shown are maximum intensity projections of a representative z-stack. (D) Quantification of mPTP pore induction as a function of APOL1 genotype, as measured by calcein fluorescence after CoCl2 as described in (B) above. Data expressed as mean±SD, for “n” cells subjected to quantification. Original magnification, X630.
Figure 6.
APOL1 RVs depolarize mitochondrial potential and induce cytotoxicity, reversible with pharmacologic inhibition or knockdown of mPTP components. (A) TMRM dye accumulates in the mitochondrial matrix on the basis of the hyperpolarized mitochondrial transmembrane potential. Compared with EV and G0 cells, TMRM fluorescence is much lower in APOL1 RV-expressing cells, indicating reduced mitochondrial transmembrane potential in RV cells. Original magnification, X630. (B) CYPD inhibitor CsA blocks mPTP formation and reduces APOL1 RV–induced cell death. Each dot represents an independent experiment. For both G1 and G2 cell lines, DMSO versus 0.1 µM CsA and DMSO versus 1 µM CsA; P<0.001. (C) NIM811, a CsA analog that can inhibit MPTP by binding to CYPD but cannot bind to calcineurin, blocks APOL1 RV induced cell death. Each dot represents an independent experiment. For both G1 and G2 cell lines, DMSO versus 0.1 µM NIM811 and DMSO versus 1 µM NIM811; P<0.001. (D) Knockdown of individual MPTP pore components or modulators blocks APOL1-induced cell death. Supplemental Figure 8 includes immunoprecipitation Western blots demonstrating interaction between APOL1 and MPTP components. Each dot represents an independent experiment. **P<0.001 comparing each siRNA to siNT-treated cells.
We tested the effect on APOL1-associated cytotoxicity of CsA, an mPTP inhibitor that binds CYPD and prevents mPTP opening.47 We observed that CsA rescued G1- and G2- mediated cytotoxicity in a dose-dependent manner (Figure 6B) without affecting APOL1 protein levels (Supplemental Figure 9A). To ensure CsA rescue was attributable to CYPD binding and not to calcineurin inhibition, we treated cells with NIM811, a CsA analog that potently inhibits CYPD without binding calcineurin. NIM811 also rescued G1 and G2 mediated cytotoxicity in a dose-dependent manner (Figure 6C) without effect on APOL1 protein levels (Supplemental Figure 9B). We then tested cytotoxicity after knockdown of several mPTP components or modulators (Figure 6D) that were identified by APOL1 by immunoprecipitation mass spectrometry assay (and that the cell was most likely to tolerate). Knockdown of SLC25A3, A4, and A5 all markedly reduced APOL1 RV–induced cell death. Similarly, knockdown of CYPD (the target of CsA and a well established activator of the mPTP) also rescued APOL1 RV-expressing cells from death. These data indicate that both G0 and RV APOL1 can interact with the mPTP, but mPTP is preferentially activated by RV APOL1.
Discussion
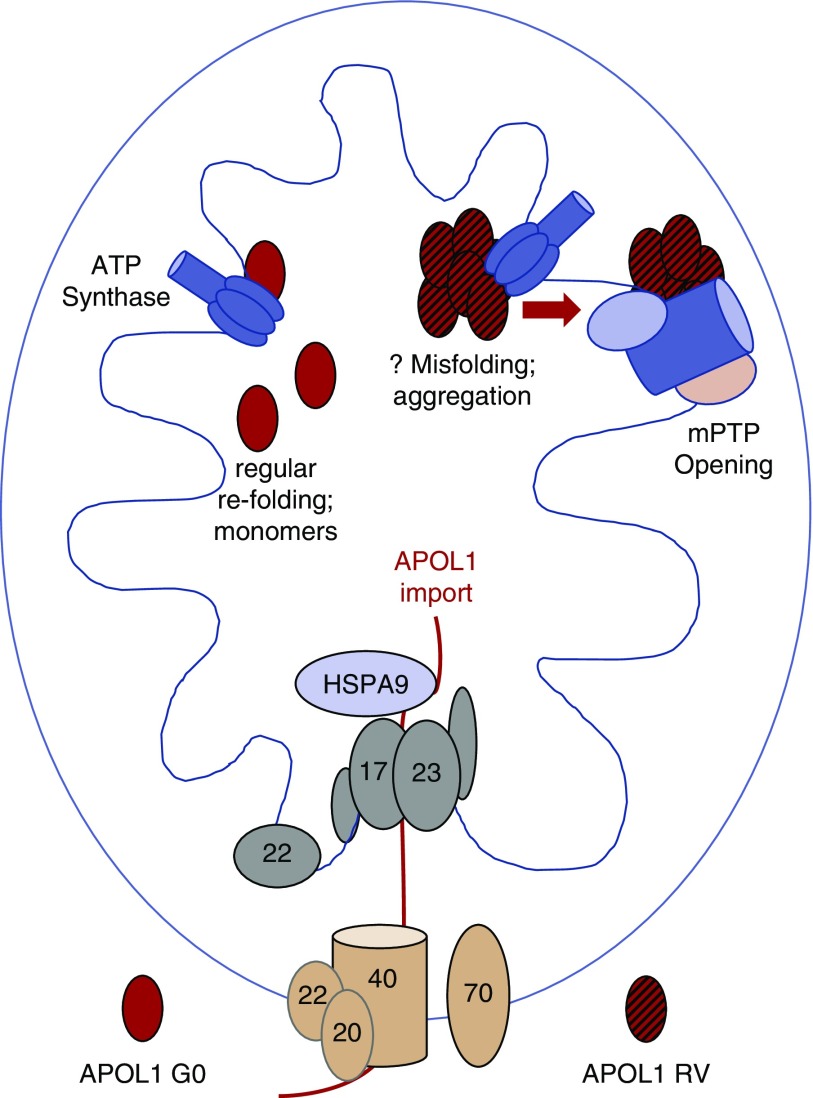
Our data demonstrate a pathway that begins with mitochondrial APOL1 import and leads to binding of an IMM complex related to the mPTP (Figure 7). APOL1 mitochondrial import alters mitochondrial metabolism, and APOL1 RVs eventually activate pore opening, leading to cell death. These data directly address several important, unanswered questions related to APOL1 biology.
Figure 7.
Model for differential toxicity of G0 and RV APOL1. APOL1 G0 and RVs enter the mitochondrial matrix via the same import machinery. Although they remain mostly monomeric, RVs tend to form higher-order oligomers after import. Whereas all APOL1 variants appear to bind components or regulators of the mPTP, the binding of aggregated RVs (likely in oligomeric form) activates pore opening, mitochondrial dysfunction, and cell death. Because proteins generally unfold before mitochondrial import, one possibility is that G0 refolds normally after import and remains monomeric whereas RVs refold abnormally and have a propensity to aggregate.
First, it has been surprising that investigators have not identified clear, consensus differences between APOL1 G0 and RVs subcellular localization using a range of microscopy techniques. Similarly, although candidate proteins have been proposed, investigators have not identified APOL1 protein binding partners that consistently bind only (or with major affinity differences) to G0 versus RVs across studies.28,48 We show here that both non-risk and RV APOL1 appear to follow similar mitochondrial import pathways. However, once inside the mitochondria, behavior diverges. G0 remains mostly monomeric whereas RVs appear to form a range of higher-order oligomers. Blocking mitochondrial APOL1 import and aggregation also prevents APOL1-induced cell death. These findings may explain how the lack of differences in subcellular localization of G0 versus RVs and the lack of identified proteins uniquely binding G0 or RVs can be compatible with marked differences in cytotoxicity of G0 and RVs expression: both G0 and RVs are imported into the matrix and both associate with mPTP machinery in the IMM, with different consequences.
Second, the differing propensities of G0 versus RV APOL1 to oligomerize point toward potential mechanisms that may eventually illuminate recessive gain-of-function toxicity. Although much additional experimentation will be required to understand APOL1-APOL1 interactions in their full complexity, the ability of G0 to alter RVs self-interaction may be central to why RV toxicity from one allele could be inhibited by G0 expression from the other allele, and why humans heterozygous for APOL1 RVs do not develop rates of disease intermediate between G0 and RV homozygotes. Our native PAGE gels reveal APOL1-containing oligomeric complexes of varying sizes (up to about 1.2 MDa) as well as aggregates too large to escape from the stacking gel. It will be important in the future to directly demonstrate that APOL1 oligomerization is necessary and sufficient to cause cell death, and to isolate the toxic APOL1 species. Neurologic diseases caused by aggregate formation present several models to consider. For example, the theory that large molecules consisting of amyloid fibrils cause disease is giving way to the idea that toxic prefibrillar oligomers may be the pathogenic entity in Alzheimer, Parkinson, and amyotrophic lateral sclerosis disease.49,50 These prefibrillar oligomers can form pores and drive neuron death, suggesting avenues to explore regarding the effect of APOL1 on podocytes, a cell type with many similarities to neurons.
Third, our data appear to have important implications for the perplexing effects of APOL1 on mitochondrial respiration. We have replicated the interesting observation that G1 and G2 impairs mitochondrial respiration, whereas G0 may in some cases enhance it, and we at least partially reversed these effects by blocking new APOL1 mitochondrial import.27,32 In a nonhypothesis-based, mass spectrometry experiment we saw that APOL1 of all genotypes pull down the fundamental ATP-generating complex of the cell, the mitochondrial ATP synthase, a finding we verify with immunoprecipitation Western blots performed in the reverse direction. Direct interaction of both G0 and RVs with the ATP synthase, but in different oligomeric states, provides a potential basis for opposing changes in mitochondrial respiration. A remarkable precedent is the recent demonstration that α-synuclein also binds to the ATP synthase.51 Whereas monomeric α-synuclein binding to ATP synthase improves the efficiency of ATP synthesis, binding of α-synuclein oligomers impairs ATP generation and ultimately leads to activation of the mPTP, an event that precedes neuronal cell death. Parallel mechanisms involving self-aggregation in two types of complex, terminally differentiated cells with limited capacity for repair or regeneration suggest that terminally differentiated cells may lack intrinsic resistance to this type of injury.
Finally, the convergence of APOL1 at the mPTP provides a candidate early mechanism for triggering cell death that may, in part, explain the wide range of downstream cell death mechanisms that have been attributed to APOL1. Although the exact nature and subunit composition of the mPTP have been subjects of contention for decades, evidence strongly supports a model in which the ATP synthase, SLC25A3, SLC25A4, and SLC25A5 all have important roles, either as components of the pore itself or as key modulators.43–45 Not only did we observe direct binding of APOL1 to these mPTP complex components, but we found that individual knockdown of several (for which knockdown was expected to be compatible with cell survival) blocked APOL1-induced cell death. Lending additional support, we found that knockdown of the matrix protein CYPD, a well validated mPTP regulator, also blocked cell death, as did the CYPD inhibitor CsA, a drug known to be useful clinically in a subset of patients with FSGS.52 Our CoCl2 calcein fluorescence quench experiments provide functional evidence that the mPTP is activated in the setting of RV APOL1 expression. We find it intriguing that aggregated proteins have been proposed to play a role in mPTP function long before the demonstration of α-synuclein multimers binding to ATP synthase.53 Because mPTP opening leads to Ca2+ efflux from mitochondria into cytosol, it may trigger a range of downstream cell death effector pathways and provide a unifying candidate mechanism driving the several modes of APOL1-associated cell death observed in previous reports.
Our data add strong mechanistic support at the molecular level to the key observation that mitochondrial dysfunction is among the earliest detectable cellular disturbances temporally after APOL1 RV expression.32 The very early occurrence of mitochondrial dysfunction, our demonstration that cell death depends on mitochondrial APOL1 import and mPTP activation, and the marked differences in APOL1 variant aggregation that appear to occur inside mitochondria together support the idea that mitochondrial injury could be the primary cytotoxic event triggered by the APOL1 RVs. After mitochondrial APOL1 RVs initiate global cellular events such as calcium release through the mPTP, many processes may become dysregulated and downstream cell death effector mechanisms activated (e.g., ion fluxes, kinases, autophagy, and apoptosis, just to name a few). Because APOL1-mediated disturbances in other organelles could also, in theory, cause ATP-depletion and altered mitochondrial function as a secondary event, the exact relationships between APOL1-induced mitochondrial injury and alteration of membrane currents, kinase activation, and regulation of processes such as autophagy remain to be fully worked out. The different clinical phenotypes observed in various APOL1 nephropathies—rapid versus indolent, proteinuric versus nonproteinuric, with a variety of histologic patterns of injury in the glomerulus—leave open the possibility of more than one mechanism in more than one target cell type.
The experiments here present a basic molecular model for APOL1 kidney disease but leave numerous follow-up questions that need to be answered. The critical APOL1 mitochondrial localization signal sequence is not yet known and identifying it will be important evidence to support any mitochondria-centric APOL1 injury model. Identifying the proteins or lipids that ferry APOL1 to the mitochondria, and reversing the cell death phenotype by blocking their transport function, will also help support mitochondrial initiation of cellular injury. A deeper understanding of the nature of the APOL1-APOL1 interactions is a high priority, including defining the relevant binding domains, the relative affinities between APOL1 protein of different genotypes, and other proteins that may be part of the higher-order APOL1 complexes. Determining the precise molecular events governing the interaction between APOL1 (both monomeric and oligomeric) and the ATP synthase will be required to establish with certainty that oligomeric RV-containing complexes directly cause mPTP activation and cell death. Other APOL1 mitochondrial binding partners identified in our mass spectrometry experiments may also be substantive factors in causing mitochondrial dysfunction, and excluding them or defining their contribution is needed for a more complete understanding of APOL1 behavior in mitochondria.
We will also ultimately have to demonstrate that similar processes are occurring in humans with APOL1-mediated kidney disease and answer a range of related questions: why do APOL1 RVs only seem to have deleterious effects on kidney cells? Does low APOL1 glomerular expression in kidney biopsies from some patients with APOL1-related diseases reflect podocyte death due to high APOL1 expression (gain-of-function) or does it suggest that there may be loss-of-function effects of RVs as well? Does APOL1 cause kidney disease in podocytes or other kidney cell types by suppressing mitochondrial function, by activating pore opening when it binds to the mPTP, or by other processes? What is the second hit that initiates the APOL1 kidney disease process in individuals with two RV? Additional experiments in other cell systems, animal models, and human tissue represent important next steps in understanding the effects of APOL1 on mitochondrial function and its importance in the different phenotypes driven by APOL1 RVs.
Disclosures
Dr. Friedman and Dr. Pollak are co-inventors of patents related to APOL1 diagnostics and therapeutics, own equity in Apolo1Bio, and have research funding from and consulted for Vertex.
Funding
This work was supported by funds from the US Department of Defense (W81XWH-14-1-0333), National Institutes of Health (MD007898), the NephCure Foundation, Vertex Pharmaceuticals, and the Ellison Foundation.
Supplementary Material
Acknowledgments
Shah and Friedman conceived the project, designed the experiments, analyzed results, and wrote the manuscript. Shah, Lannon, Dias, and Zhang performed the experiments. Dias, Lannon, Alper, and Pollak proposed experiments, provided scientific insight and analysis, offered critical review, and edited the manuscript.
We would like to thank Lay-Hong Ang at the Beth Israel Deaconess Medical Center (BIDMC) Imaging Core, Xiaowen Liu at the BIDMC Metabolism and Mitochondrial Research Core, and Ross Tomaino at the Harvard Medical School Taplin Mass Spectrometry Facility for guidance with our experiments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019020114/-/DCSupplemental.
Supplemental Table 1. APOL1 Sequences used to make Tet-inducible HEK293 cells.
Supplemental Table 2. List of mitochondrial proteins pulled down after APOL1 immunoprecipitation mass spectrometry.
Supplemental Figure 1. Western blot analysis of organelle markers in the cytosolic and mitochondrial-enriched fractions.
Supplemental Figure 2. APOL1 translocation to mitochondrial matrix is independent of carrier pathway OMM protein TOMM70.
Supplemental Figure 3. TOMM20 knockdown can partially rescue APOL1 RV–mediated mitochondrial dysfunction.
Supplemental Figure 4. siRNA Validation blots for knockdown efficiencies not shown in main figures.
Supplemental Figure 5. APOL1 can bind to other APOL1 molecules.
Supplemental Figure 6. TOMM20 knockdown reduces APOL1 oligomerization, whereas TOMM70 and TIMM22 knockdown does not alter APOL1 oligomerization.
Supplemental Figure 7. G1 and G2 do no inhibit import of newly synthesized protein into the mitochondria.
Supplemental Figure 8. APOL1 interacts with multiple MPTP components.
Supplemental Figure 9. CsA and NIM811 treatment does not affect APOL1 protein levels.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al.: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al.: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al.: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR: Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 22: 2098–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchateau PN, Pullinger CR, Cho MH, Eng C, Kane JP: Apolipoprotein L gene family: Tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 42: 620–630, 2001 [PubMed] [Google Scholar]
- 6.Smith EE, Malik HS: The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res 19: 850–858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina-Portela Mdel P, Lugli EB, Recio-Pinto E, Raper J: Trypanosome lytic factor, a subclass of high-density lipoprotein, forms cation-selective pores in membranes. Mol Biochem Parasitol 144: 218–226, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homblé F, et al.: Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Science 309: 469–472, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Xong HV, Vanhamme L, Chamekh M, Chimfwembe CE, Van Den Abbeele J, Pays A, et al.: A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell 95: 839–846, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Uzureau P, Uzureau S, Lecordier L, Fontaine F, Tebabi P, Homblé F, et al.: Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature 501: 430–434, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Friedman DJ, Pollak MR: Genetics of kidney failure and the evolving story of APOL1. J Clin Invest 121: 3367–3374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson R, Genovese G, Canon C, Kovacsics D, Higgins MK, Carrington M, et al.: Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A 111: E2130–E2139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper A, Ilboudo H, Alibu VP, Ravel S, Enyaru J, Weir W, et al.: APOL1 renal risk variants have contrasting resistance and susceptibility associations with African trypanosomiasis. Elife 6: e25461, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman DJ, Pollak MR: Apolipoprotein L1 and kidney disease in African Americans. Trends Endocrinol Metab 27: 204–215, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnstone DB, Shegokar V, Nihalani D, Rathore YS, Mallik L, Ashish, et al.: APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PLoS One 7: e51546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA: Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem 283: 21540–21549, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA: ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy 4: 1079–1082, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, et al.: Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res 56: 1583–1593, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, et al. : APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan X, Wen H, Lederman R, Malhotra A, Mikulak J, Popik W, et al.: Protein domains of APOL1 and its risk variants. Exp Mol Pathol 99: 139–144, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan X, Wen H, Saleem MA, Mikulak J, Malhotra A, Skorecki K, et al.: Vascular smooth muscle cells contribute to APOL1-induced podocyte injury in HIV milieu. Exp Mol Pathol 98: 491–501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khatua AK, Cheatham AM, Kruzel ED, Singhal PC, Skorecki K, Popik W: Exon 4-encoded sequence is a major determinant of cytotoxicity of apolipoprotein L1. Am J Physiol Cell Physiol 309: C22–C37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, et al.: APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson R, Finkelstein A: Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: Relevance to trypanosome lysis. Proc Natl Acad Sci U S A 112: 2894–2899, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen H, Kumar V, Lan X, Shoshtari SSM, Eng JM, Zhou X, et al.: APOL1 risk variants cause podocytes injury through enhancing endoplasmic reticulum stress. Biosci Rep 38: BSR20171713, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Toole JF, Schilling W, Kunze D, Madhavan SM, Konieczkowski M, Gu Y, et al.: ApoL1 overexpression drives variant-independent cytotoxicity. J Am Soc Nephrol 29: 869–879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granado D, Müller D, Krausel V, Kruzel-Davila E, Schuberth C, Eschborn M, et al.: Intracellular APOL1 risk variants cause cytotoxicity accompanied by energy depletion. J Am Soc Nephrol 28: 3227–3238, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al.: A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat Med 23: 945–953, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, et al.: Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruzel-Davila E, Shemer R, Ofir A, Bavli-Kertselli I, Darlyuk-Saadon I, Oren-Giladi P, et al.: APOL1-mediated cell injury involves disruption of conserved trafficking processes. J Am Soc Nephrol 28: 1117–1130, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, Zhu JY, Richman A, Zhang Y, Xie X, Das JR, et al.: APOL1-G1 in nephrocytes induces hypertrophy and accelerates cell death. J Am Soc Nephrol 28: 1106–1116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L, Chou JW, Snipes JA, Bharadwaj MS, Craddock AL, Cheng D, et al.: APOL1 renal-risk variants induce mitochondrial dysfunction. J Am Soc Nephrol 28: 1093–1105, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limou S, Dummer PD, Nelson GW, Kopp JB, Winkler CA: APOL1 toxin, innate immunity, and kidney injury. Kidney Int 88: 28–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, et al.: Localization of APOL1 protein and mRNA in the human kidney: Nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanwalleghem G, Fontaine F, Lecordier L, Tebabi P, Klewe K, Nolan DP, et al.: Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1. Nat Commun 6: 8078, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jastroch M, Hirschberg V, Klingenspor M: Functional characterization of UCP1 in mammalian HEK293 cells excludes mitochondrial uncoupling artefacts and reveals no contribution to basal proton leak. Biochim Biophys Acta 1817: 1660–1670, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Ryan MT, Voos W, Pfanner N: Assaying protein import into mitochondria. Methods Cell Biol 65: 189–215, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Chun J, Zhang JY, Wilkins MS, Subramanian B, Riella C, Magraner JM, et al.: Recruitment of APOL1 kidney disease risk variants to lipid droplets attenuates cell toxicity. Proc Natl Acad Sci U S A 116: 3712–3721, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukasawa Y, Tsuji J, Fu SC, Tomii K, Horton P, Imai K: MitoFates: Improved prediction of mitochondrial targeting sequences and their cleavage sites. Mol Cell Proteomics 14: 1113–1126, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiedemann N, Pfanner N: Mitochondrial machineries for protein import and assembly. Annu Rev Biochem 86: 685–714, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N: Importing mitochondrial proteins: Machineries and mechanisms. Cell 138: 628–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baines CP, Gutiérrez-Aguilar M: The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium 73: 121–130, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardi P, Rasola A, Forte M, Lippe G: The mitochondrial permeability transition pore: Channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev 95: 1111–1155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung AW, Halestrap AP: Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta 1777: 946–952, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Bonora M, Morganti C, Morciano G, Giorgi C, Wieckowski MR, Pinton P: Comprehensive analysis of mitochondrial permeability transition pore activity in living cells using fluorescence-imaging-based techniques. Nat Protoc 11: 1067–1080, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM: Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174: 167–172, 1997 [PubMed] [Google Scholar]
- 48.Madhavan SM, O’Toole JF, Konieczkowski M, Barisoni L, Thomas DB, Ganesan S, et al.: APOL1 variants change C-terminal conformational dynamics and binding to SNARE protein VAMP8. JCI Insight 2: 92581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenberg D, Jucker M: The amyloid state of proteins in human diseases. Cell 148: 1188–1203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glabe CG: Structural classification of toxic amyloid oligomers. J Biol Chem 283: 29639–29643, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, et al.: α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun 9: 2293, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 53.He L, Lemasters JJ: Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett 512: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.