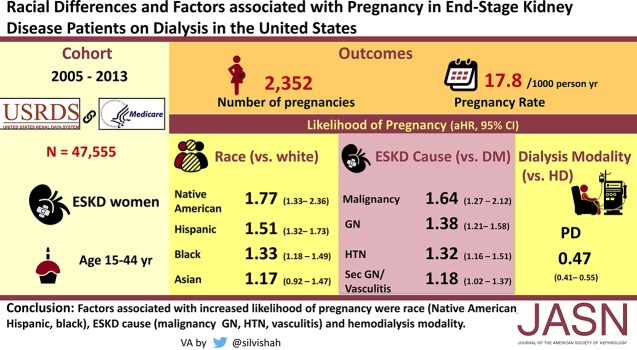
Significance Statement
Pregnancy is not common in women on dialysis due to impaired fertility. Literature is scarce about pregnancy in women on dialysis in the United States. In this retrospective study, the authors examined the pregnancy rates and associated factors in 47,555 US women on dialysis from 2005 to 2013. They identified 2352 pregnancies, for a rate of 17.8 per thousand person years, a higher rate than seen in previous studies. Pregnancy rates were highest in Native American women followed by Hispanics, blacks, Asians, and whites. Younger age, hemodialysis as the dialysis modality, and ESKD caused by GN, vasculitis, neoplasm, and hypertension were associated with the higher likelihood of pregnancy. Patients with diabetes as the cause of ESKD had the lowest pregnancy rates. This study improves our understanding of pregnancy in women on dialysis, and can lead to better counseling and shared decision making.
Keywords: pregnancy, rates, dialysis, race
Visual Abstract
Abstract
Background
Pregnancy in women with ESKD undergoing dialysis is uncommon due to impaired fertility. Data on pregnancy in women on dialysis in the United States is scarce.
Methods
We evaluated a retrospective cohort of 47,555 women aged 15–44 years on dialysis between January 1, 2005 and December 31, 2013 using data from the United States Renal Data System with Medicare as primary payer. We calculated pregnancy rates and identified factors associated with pregnancy.
Results
In 47,555 women on dialysis, 2352 pregnancies were identified. Pregnancy rate was 17.8 per thousand person years (PTPY) with the highest rate in women aged 20–24 (40.9 PTPY). In the adjusted time-to-event analysis, a higher likelihood of pregnancy was seen in Native American (HR, 1.77; 95% CI, 1.33 to 2.36), Hispanic (HR, 1.51; 95% CI, 1.32 to 1.73), and black (HR, 1.33; 95% CI, 1.18 to 1.49) women than in white women. A higher rate of pregnancy was seen in women with ESKD due to malignancy (HR, 1.64; 95% CI, 1.27 to 2.12), GN (HR, 1.38; 95% CI, 1.21 to 1.58), hypertension (HR, 1.32; 95% CI, 1.16 to 1.51), and secondary GN/vasculitis (HR, 1.18; 95% CI, 1.02 to 1.37) than ESKD due to diabetes. A lower likelihood of pregnancy was seen among women on peritoneal dialysis than on hemodialysis (HR, 0.47; 95% CI, 0.41 to 0.55).
Conclusions
The pregnancy rate is higher in women on dialysis than previous reports indicate. A higher likelihood of pregnancy was associated with race/ethnicity, ESKD cause, and dialysis modality.
Pregnancy is uncommon in women with ESKD on dialysis. Uremia leads to dysregulation of the hypothalamic-pituitary-gonadal axis and menstrual cycle irregularities, and fertility in women diminishes with a decline in the GFR.1 Achievement of pregnancy is further compounded by a failure in the surge of both luteinizing hormone and follicle-stimulating hormone, low progesterone during the menstruation phase, and hyperprolactinemia. Most women are anovulatory once they initiate dialysis, even if regular menstruation is present.2 Pregnancy in women undergoing dialysis is a challenging clinical scenario due to significant maternal and fetal morbidity.3,4 The conception rate in women on regular dialysis is low and reported to range from <1% to 7% of women.3,5,6 The exact rates of pregnancy for women with ESKD on dialysis are not known due to inconsistent methods in ascertainment from surveys, single-center studies, or voluntary registry data.7,8
Whereas kidney transplantation provides the best pregnancy outcomes for women with ESKD, pregnancy for women on dialysis is now more feasible and safer due to improvements in both obstetric care and the delivery of dialysis.9–11 Although advances have been made in the management of pregnancies in women receiving dialysis, small numbers of cases preclude a clear understanding of factors associated with pregnancy in women with ESKD on dialysis.5,12 We used the national ESKD registry, the United States Renal Data System (USRDS), to determine the pregnancy rates in women with ESKD who were on dialysis at any time between January 1, 2005 and December 31, 2013. This study involves a large cohort that is not a voluntary registry and examines the pregnancy rates by age, race, dialysis modality, time on dialysis, socioeconomic status, rurality, and cause of ESKD, along with factors associated with pregnancy.
Methods
Data Sources and Study Population
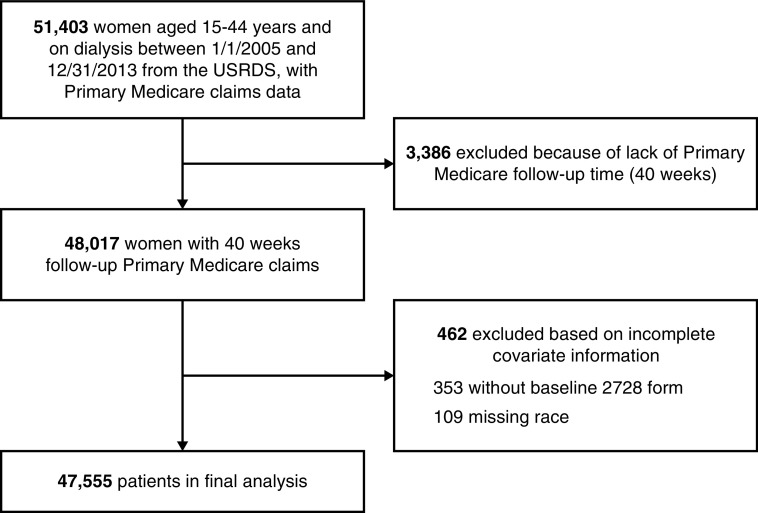
We performed an observational retrospective study to determine the rates of pregnancy in women with ESKD who were on dialysis in the United States. We used the USRDS database, a national registry of patients receiving chronic dialysis which contains information from the ESKD Medical Evidence Form of the Centers for Medicare and Medicaid Services (CMS; form CMS-2728), as well as Medicare Part A institutional claims and Medicare Part B physician/supplier claims.13 We included 47,555 women who were aged 15–44 years and were on peritoneal or hemodialysis at any time between January 1, 2005 and December 31, 2013 from the USRDS, with Medicare as primary payer. The USRDS payer history file was used to obtain information on insurance coverage for the study period. We excluded patients with no baseline CMS-2728 form and those with missing data on race. Patient time at risk was interrupted when a woman turned 45 years old, died, received a kidney transplant, stopped dialysis for other reasons, 40 weeks before the end of primary Medicare coverage, or on December 31, 2013; but this could resume if they went on dialysis again, or resumed primary Medicare coverage after a lapse. Figure 1 illustrates the study cohort derivation. Because the data were de-identified, the University of Cincinnati Institutional Review Board committee deemed the study exempt from review.
Figure 1.
Cohort selection results in 47,555 women aged 15–44 years, on dialysis, with primary Medicare coverage and complete data between 1/1/2005 and 12/31/2013.
Our outcome of interest was the occurrence of pregnancy in women with ESKD who were on dialysis. We searched for discharge diagnoses and medical procedures indicative of pregnancy among all inpatient and outpatient Medicare claims using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes; Current Procedural Terminology, 4th Edition (CPT-4) codes; and diagnostic related group (DRG) codes. The specificity of a code-based method for establishing the occurrence of pregnancy has been validated in prior studies.14,15 Our method used codes, timing, and rules from a validated method. (Parts of the method could not be applied because Medicare lacks some of the required information; this led to more unknown outcomes in our data set than in the prior studies.) We used all codes to construct pregnancy episodes to identify separate pregnancies within the time period without overcounting the pregnancy episodes. Codes that marked the end of pregnancy were considered to be the most accurate in terms of timing and outcome of pregnancy, so they were used first in the following order: ICD-9 diagnosis codes denoting live births, ICD-9 procedure codes indicating end of pregnancy, CPT-4 procedure codes indicating end of pregnancy, DRG codes which showed reasons for hospitalization related to end of pregnancy, and other ICD-9 diagnosis codes denoting end of pregnancy. Additional codes during the identified pregnancies or within 8 weeks after were then used only to update information about outcomes, when possible. The outcome assigned was the most specific indicated by codes within the gestation period, with the following hierarchy: live birth and stillbirth (in twin pregnancies), live birth, ectopic/trophoblastic pregnancy, stillbirth, spontaneous abortion, and therapeutic abortion (Supplemental Material). Outcome-specific estimates of gestational age were used to estimate conception dates as follows: deliveries not identified as stillbirths or multiple, 40 weeks; twins, 36 weeks; triplets, 33 weeks; quadruplets, 31 weeks; stillbirths, 28 weeks; all abortions, 10 weeks; and ectopic/trophoblastic pregnancies, 8 weeks. The conception date for twin pregnancies resulting in both live birth and stillbirth was estimated at 40 weeks. Further codes that demonstrated the presence of a pregnancy, but not the timing or outcome, were then grouped together to identify pregnancies with unknown outcomes. Unidentified early losses that occurred within 6 months of the end of a prior pregnancy other than a live birth were deleted. Finally, remaining pregnancies were examined for consistency of dates. When overlapping pregnancy episodes were identified, a review was conducted to determine whether to delete one or adjust the date of the second pregnancy episode. An interval of at least 24 weeks was required between the ends of two pregnancies resulting in deliveries, 20 weeks between an early loss and a subsequent delivery, 10 weeks between a delivery and a subsequent early loss, and 6 weeks between two early losses. In case of overlap, dates were adjusted to set the start date for the second pregnancy to 1 week after the completion of the first. Start dates for pregnancies that resulted in delivery were adjusted by a maximum of 8 weeks, and start dates for pregnancies that resulted in early loss were adjusted by a maximum of 2 weeks.14,16–18 The final 40 weeks of primary Medicare coverage were used only to identify completion of pregnancies that began during the time at risk. Maternal outcome of cesarean section was also determined using ICD-9 codes, CPT-4 codes, and DRG codes.19
The CMS-2728 form was used to obtain information on demographics including age, incident dialysis modality, ESKD cause, body mass index (BMI), smoking history, ethnicity, and predialysis nephrology care.13 The patient file was used to ascertain information on race and prior transplants. Race and ethnicity were combined into one variable, categorized as Hispanic, non-Hispanic Asian, non-Hispanic black, non-Hispanic Native American, and non-Hispanic white. Incident dialysis modalities were classified into hemodialysis, peritoneal dialysis, and other (uncertain dialysis). Predialysis nephrology care was grouped into none, ≤12 months, >12 months, and unknown. These variables were all gathered at one time point and applied to the entire time period, although the covariate of BMI was set to missing if the CMS-2728 form date was >5 years before study entry.
Other data were available historically and were updated when changes occurred throughout time at risk. The treatment history file was used to obtain information on current dialysis modalities during the study period (henceforth called current dialysis modality) and transplants, and to calculate the current duration of dialysis (<1, 1–3, and >3 years). Current dialysis modalities were classified into hemodialysis and peritoneal dialysis. (Patient time with uncertain dialysis was not included in the analysis, so current modality did not have the category of “other,” but it was included as a category for incident modality.) The residence file was used to obtain information on patients’ zip codes of current residence throughout the study period. These zip codes were combined with zip-code-level data from the US Census Bureau American Community Survey 5-year estimates from 2007 to 2011 to determine neighborhood socioeconomic status, which we defined as the percentage of zip-code residents living below the federal poverty level and grouped similarly to the US Census Bureau literature into five categories: I (<13.8%), II (13.8%–19.9%), III (20.0%–39.9%), IV (40% or more), and unknown.20,21 Rurality of the neighborhood was determined using the rural-urban commuting area (RUCA) code version 2.0 and grouped into four categories: metropolitan (RUCA 1.0–3.9), micropolitan (RUCA 4.0–6.0), rural (RUCA 7.0–10.6), and unknown.21,22
Groups were created based on clinical relevance, with patients with unavailable information on covariates categorized into a “missing” group for that covariate, as shown in Table 1. The covariates for adjusted analyses were chosen based on their known clinical relevance.
Table 1.
Baseline characteristics of women with ESKD on dialysis separated by pregnancy during the follow-up period
| Characteristics | Women Who Conceived (n=2008) | Women Who Did Not Conceive (n=45,547) |
|---|---|---|
| Race/ethnicity | ||
| Asian | 3.7 | 4.2 |
| Black | 53.6 | 45.7 |
| Hispanic | 18.8 | 15.7 |
| Native American | 2.2 | 1.6 |
| White | 21.7 | 32.8 |
| Age at study entry (yr)a | 29 (6) | 34 (7) |
| 15–17 | 2.7 | 2.2 |
| 18–19 | 3.9 | 1.6 |
| 20–24 | 18.5 | 7.1 |
| 25–29 | 24.6 | 12.2 |
| 30–34 | 23.8 | 17.7 |
| 35–39 | 18.7 | 24.5 |
| 40–44 | 8.0 | 34.6 |
| Cause of ESKD | ||
| Diabetes mellitus | 18.7 | 29.2 |
| Hypertension/large vessel disease | 21.0 | 19.5 |
| Malignancy | 3.1 | 3.3 |
| Cystic/hereditary | 4.3 | 5.4 |
| GN | 25.4 | 18.2 |
| Secondary GN/vasculitis | 14.9 | 12.4 |
| Interstitial nephritis/pyelonephritis | 2.7 | 3.6 |
| Others | 9.9 | 8.4 |
| Predialysis nephrology care | ||
| None | 18.7 | 18.6 |
| ≤12 mo | 17.3 | 20.6 |
| >12 mo | 15.0 | 17.4 |
| Unknown | 49.1 | 43.5 |
| BMI (kg/m2)a | 30.0 (9.4) | 29.7 (9.3) |
| <18.5 | 4.1 | 4.8 |
| 18.5–25 | 26.2 | 27.1 |
| 25–30 | 18.7 | 19.6 |
| ≥30 | 38.7 | 37.1 |
| Missing | 12.4 | 11.4 |
| Incident modality | ||
| Hemodialysis | 88.5 | 83.3 |
| Peritoneal dialysis | 10.1 | 14.4 |
| Other/missing | 1.4 | 2.3 |
| Dialysis modality at study entry | ||
| Hemodialysis | 89.8 | 83.3 |
| Peritoneal dialysis | 10.2 | 16.7 |
| Time on dialysis at study entry (yr) | ||
| <1 | 59.4 | 57.2 |
| 1–3 | 19.0 | 22.4 |
| >3 | 21.6 | 20.4 |
| Neighborhood poverty at study entry | ||
| I (<13.8%) | 42.1 | 48.3 |
| II (13.8%–20%) | 21.1 | 20.8 |
| III (20%–40%) | 31.5 | 26.7 |
| IV (>40%) | 3.0 | 2.3 |
| Unknown | 2.3 | 1.9 |
| Neighborhood rurality at study entry | ||
| Metropolitan | 82.1 | 77.9 |
| Micropolitan | 8.9 | 10.4 |
| Rural | 7.6 | 9.9 |
| Unknown | 1.4 | 1.9 |
| Tobacco use | 5.0 | 6.4 |
| First prevalent year in study | ||
| 2005 | 43.2 | 36.8 |
| 2006 | 10.6 | 8.4 |
| 2007 | 11.8 | 7.9 |
| 2008 | 7.9 | 7.9 |
| 2009 | 8.2 | 8.0 |
| 2010 | 6.8 | 8.1 |
| 2011 | 5.9 | 7.8 |
| 2012 | 3.3 | 7.5 |
| 2013 | 2.3 | 7.6 |
| Years in study | 4.9 (2.6) | 2.7 (2.3) |
| Transplants before study entry | 4.4 | 4.2 |
| Censored due to transplantation | 0.7 | 4.4 |
Values reported as group percentages.
Reported in mean (SD).
Statistical Analyses
Summary statistics are presented as percentages for categoric data and mean±SD for continuous variables. Women who ever became pregnant during the study period were compared with women who never became pregnant, and women who ever became pregnant were compared across racial groups. These comparisons did not account for time at risk or for multiple pregnancies, and used characteristics at study entry for the time-dependent covariates of age, current dialysis modality, time on dialysis, socioeconomic status, rurality, prior transplantation, and calendar year of dialysis. We determined the unadjusted rates of pregnancy overall and by race/ethnicity, current age, calendar year of prevalent ESKD, incident type of dialysis modality, current type of dialysis modality, current time on dialysis, socioeconomic status, rurality, and cause of ESKD. Rates are expressed as the number of pregnancies per thousand person years (PTPY). Because rates for each race were based on different age distributions, we also calculated age-standardized rates, directly standardizing to the female United States population according to the 2000 census.
Due to the possibility of multiple pregnancies per woman, we used the Prentice, Williams, and Peterson total time recurrent event time-to-event analysis, with sandwich variance estimators, to determine factors associated with the pregnancy.23 This model stratifies for repeat pregnancies to allow hazards to differ for subsequent events. Due to the small numbers of repeat pregnancies, we used only two strata for initial and repeat pregnancies. In addition, we considered transplantation as a competing risk, combining the total time model with the Fine and Gray subdistribution hazards method; however, the inclusion of time-dependent covariates in the Fine and Gray method can lead to bias.24,25 Multivariable time-to-event models were nonparsimonious, and women’s time under observation was censored at the end of person time at risk. The time-to-event models included the time-independent covariates of race/ethnicity, history of smoking, ESKD cause, predialysis nephrology care, and BMI; and the time-dependent covariates included age, calendar year of prevalent dialysis, current dialysis modality, time on dialysis, prior history of transplant, socioeconomic status, and rurality. The risk estimates were expressed as hazard ratios (HRs) and their 95% confidence intervals (95% CIs). We also used logistic regression analysis on all pregnancies with known outcomes in patients on dialysis to determine the factors associated with live births. This model included the covariates of age, race/ethnicity, ESKD cause, dialysis modality, time on dialysis, socioeconomic status, and rurality, using the values for time-dependent variables associated with the time of beginning of pregnancy. The risk estimates were expressed as odds ratios and their 95% CIs. We analyzed the data using SAS 9.4 (SAS Institute, Cary, NC).
Results
Baseline Demographics and Clinical Characteristics
Overall, 2352 pregnancies were identified in 2008 women. Table 1 shows the characteristics of the women with pregnancies and those who did not become pregnant. Mean age at study entry for women who never became pregnant was 34±7 years, whereas mean age at study entry for women who conceived was 29±6 years. Only 10.1% of the women with pregnancies received peritoneal dialysis as the incident dialysis modality as compared with 14.4% of women who never became pregnant. GN was the most common cause of ESKD (25.4%) among those with pregnancies, whereas diabetes was the most common cause of ESKD among women who did not conceive (29.2%). Women who conceived were from lower socioeconomic status neighborhoods than were women who did not conceive (20%–39.9% poverty, 31.5% did conceive versus 26.7% did not conceive; ≥40% poverty, 3.0% versus 2.3%). Table 2 shows the characteristics of women with pregnancies on dialysis by race/ethnicity. The majority of women who conceived were black (51.6%), followed by those who were white (21.7%) and Hispanic (18.8%). As compared with other races, lower proportions of white and Asian women were from neighborhoods with a lower socioeconomic status (20%–39.9% poverty and >40% poverty). White women had higher rates of peritoneal dialysis as the incident dialysis modality (16.1%) than those from nonwhite racial groups.
Table 2.
Baseline characteristics of pregnancies in women with ESKD on dialysis separated by race/ethnicity
| Characteristics | Asian (n=74) | Black (n=1077) | Hispanic (n=377) | Native American (n=45) | White (n=435) |
|---|---|---|---|---|---|
| Age at study entry (yr)a | 29 (6) | 29 (6) | 29 (6) | 28 (7) | 29 (7) |
| 15–17 | 0.0 | 2.0 | 2.9 | 8.9 | 4.1 |
| 18–19 | 4.1 | 2.6 | 5.0 | 2.2 | 6.2 |
| 20–24 | 21.6 | 18.1 | 17.5 | 17.8 | 19.8 |
| 25–29 | 25.7 | 25.0 | 24.4 | 24.4 | 23.5 |
| 30–34 | 16.2 | 24.9 | 24.9 | 24.4 | 21.2 |
| 35–39 | 27.0 | 20.2 | 16.7 | 15.6 | 15.6 |
| 40–44 | 5.4 | 7.3 | 8.5 | 6.7 | 9.7 |
| Cause of ESKD | |||||
| Diabetes mellitus | 9.5 | 18.6 | 22.6 | 28.9 | 16.1 |
| Hypertension/large vessel disease | 16.2 | 27.3 | 14.3 | 8.9 | 13.1 |
| Malignancy | 0.0 | 2.1 | 4.5 | 0.0 | 5.3 |
| Cystic/hereditary | 1.4 | 2.6 | 5.3 | 2.2 | 8.3 |
| GN | 36.5 | 24.1 | 22.6 | 40.0 | 27.4 |
| Secondary GN/vasculitis | 18.9 | 14.6 | 18.8 | 8.9 | 12.4 |
| Interstitial nephritis/pyelonephritis | 6.8 | 1.1 | 2.4 | 0.0 | 6.7 |
| Others | 10.8 | 9.6 | 9.6 | 11.1 | 10.8 |
| Predialysis nephrology care | |||||
| None | 20.3 | 19.8 | 19.4 | 15.6 | 15.4 |
| ≤12 mo | 16.2 | 17.3 | 17.5 | 13.3 | 17.7 |
| >12 mo | 16.2 | 12.1 | 14.6 | 11.1 | 22.8 |
| Unknown | 47.3 | 50.9 | 48.5 | 60.0 | 44.1 |
| BMI (kg/m2)a | 27.2 (7.7) | 31.3 (9.9) | 28.4 (8.5) | 29.0 (7.7) | 28.7 (8.9) |
| <18.5 | 4.1 | 4.2 | 3.7 | 4.4 | 4.1 |
| 18.5–25 | 32.4 | 21.3 | 33.2 | 22.2 | 31.7 |
| 25–30 | 20.3 | 17.3 | 22.6 | 22.2 | 18.2 |
| ≥30 | 27.0 | 44.0 | 30.8 | 44.4 | 33.8 |
| Missing | 16.2 | 13.3 | 9.8 | 6.7 | 12.2 |
| Incident modality | |||||
| Hemodialysis | 89.2 | 90.8 | 89.4 | 91.1 | 81.6 |
| Peritoneal dialysis | 6.8 | 8.5 | 8.8 | 8.9 | 16.1 |
| Other/missing | 4.1 | 0.7 | 1.9 | 0.0 | 2.3 |
| Dialysis modality at study entry | |||||
| Hemodialysis | 89.2 | 92.3 | 88.6 | 95.6 | 84.4 |
| Peritoneal dialysis | 10.8 | 7.7 | 11.4 | 4.4 | 15.6 |
| Time on dialysis at study entry (yr) | |||||
| <1 | 55.4 | 58.8 | 61.8 | 62.2 | 59.3 |
| 1–3 | 20.3 | 19.6 | 15.7 | 15.6 | 20.7 |
| >3 | 24.3 | 21.6 | 22.6 | 22.2 | 20.0 |
| Neighborhood poverty at study entry | |||||
| I (<13.8%) | 70.3 | 34.1 | 32.9 | 28.9 | 66.4 |
| II (13.8%–20%) | 14.9 | 21.1 | 26.5 | 24.4 | 17.2 |
| III (20%–40%) | 8.1 | 40.0 | 32.6 | 37.8 | 12.9 |
| IV (>40%) | 0.0 | 2.8 | 6.4 | 4.4 | 0.9 |
| Unknown | 6.8 | 2.0 | 1.6 | 4.4 | 2.5 |
| Neighborhood rurality at study entry | |||||
| Metropolitan | 87.8 | 85.3 | 82.0 | 35.6 | 70.6 |
| Micropolitan | 5.4 | 7.2 | 6.9 | 11.1 | 14.0 |
| Rural | 0.0 | 5.2 | 5.0 | 42.2 | 13.1 |
| Unknown | 6.8 | 2.2 | 6.1 | 11.1 | 2.3 |
| Tobacco use | 1.4 | 4.4 | 1.9 | 8.9 | 9.7 |
| First prevalent year in study | |||||
| 2005 | 47.3 | 43.9 | 41.1 | 57.8 | 41.2 |
| 2006 | 9.5 | 11.9 | 9.6 | 6.7 | 9.0 |
| 2007 | 4.1 | 11.4 | 12.7 | 6.7 | 13.6 |
| 2008 | 6.8 | 8.1 | 8.2 | 2.2 | 7.8 |
| 2009 | 12.2 | 8.1 | 7.2 | 11.1 | 8.3 |
| 2010 | 4.1 | 7.0 | 8.0 | 8.9 | 5.8 |
| 2011 | 6.8 | 4.8 | 7.7 | 2.2 | 7.4 |
| 2012 | 8.1 | 3.0 | 2.9 | 0.0 | 3.9 |
| 2013 | 1.4 | 1.9 | 2.7 | 4.4 | 3.2 |
Values reported as group percentages.
Reported in mean (SD).
Unadjusted Rates of Pregnancies
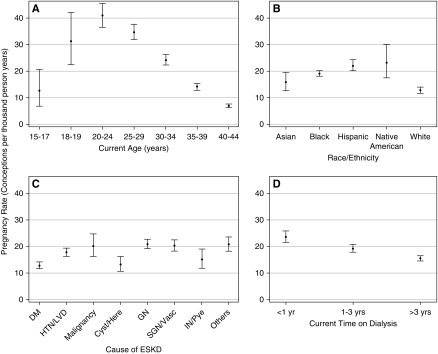
Among women with pregnancies, 86% had a single pregnancy, 12% had two pregnancies, and 2% had three or more pregnancies. Overall, the unadjusted rate of pregnancy was 17.7 PTPY (95% CI, 17.0 to 18.5). Regarding age, the pregnancy rate was highest in women aged 20–24 years (40.9 PTPY; 95% CI, 36.6 to 45.5), and lowest in women aged 40–44 years (6.9 PTPY; 95% CI, 6.2 to 7.7) (Figure 2A). With regards to race/ethnicity, the pregnancy rate was highest in Native American women (23.2 PTPY; 95% CI, 17.5 to 30.2) followed by Hispanic (22.2 PTPY; 95% CI, 20.2 to 24.3), black (19.2 PTPY; 95% CI, 18.1 to 20.3), and Asian (15.8 PTPY; 95% CI, 12.7 to 19.5) women. White women with prevalent ESKD had the lowest pregnancy rates (12.8 PTPY; 95% CI, 11.7 to 14.0) (Figure 2B). Regarding the cause of ESKD, pregnancy rates were higher among women with ESKD due to GN (21.0 PTPY; 95% CI, 19.3 to 22.7), secondary GN/vasculitis (20.4 PTPY; 95% CI, 18.3 to 22.6), malignancy (20.2 PTPY; 95% CI, 16.3 to 24.7), and miscellaneous causes (20.9 PTPY; 95% CI, 18.4 to 23.7), and lower among women with ESKD due to diabetes and cystic/hereditary causes (Figure 2C). Pregnancy rates were higher among women who had been on dialysis <1 year (23.7 PTPY; 95% CI, 21.6 to 26.1) than for those on dialysis for 1–3 years (19.4 PTPY; 95% CI, 18.0 to 20.9) or >3 years (15.6 PTPY; 95% CI, 14.7 to 16.5) (Figure 2D). The rate of pregnancy was higher among women whose incident dialysis modality was hemodialysis (18.8 PTPY; 95% CI, 18.1 to 19.7) than among those initiated with peritoneal dialysis (11.9 PTPY; 95% CI, 10.4 to 13.6) (Figure 3A). Similarly, the rate of pregnancy was higher among women currently getting hemodialysis (19.3 PTPY; 95% CI, 18.5 to 20.2) than among those currently on peritoneal dialysis (9.3 PTPY; 95% CI, 8.0 to 10.7) (Figure 3B). Regarding socioeconomic status, pregnancy rates were higher in neighborhoods with ≥40% poverty (23.0 PTPY; 95% CI, 17.8 to 29.2) and 20.0%–39.9% poverty (19.9 PTPY; 95% CI, 18.5 to 21.4), followed by neighborhoods with 13.8%–19.9% poverty (17.6 PTPY; 95% CI, 16.1 to 19.2) and <13.8% poverty (16.3 PTPY; 95% CI, 15.3 to 17.4) (Figure 3C). Regarding rurality, pregnancy rates were higher for women from metropolitan areas (18.6 PTPY; 95% CI, 17.8 to 19.5) than women from micropolitan (15.6 PTPY; 95% CI, 13.6 to 17.9) or rural (14.1 PTPY; 95% CI, 12.1 to 16.3) areas (Figure 3D). Pregnancy rates were roughly constant for women in the years 2005–2013, with slightly lower rates in 2012–2013 (Figure 3E). Supplemental Table 1 shows the rates of pregnancies in women with ESKD on dialysis. Age-standardized pregnancy rates were as follows: Asian women, 17.2 pregnancies PTPY; black women, 25.3 pregnancies PTPY; Hispanic women, 25.9 pregnancies PTPY; Native American women, 40.7 pregnancies PTPY; and white women, 16.4 pregnancies PTPY. As seen with unadjusted pregnancy rates, Native American women had the highest rates and white women had the lowest rates. All races had higher rates after age adjusting because this was an older cohort than the United States population. However, Native American women showed the largest change in pregnancy rates due to the age adjustment, showing a predominance of older women in their cohort compared with other races.
Figure 2.
Pregnancy rates in women with ESKD demonstrate number of conceptions per thousand person-years by (A) age, (B) race/ethnicity, (C) cause of ESKD, and (D) time on dialysis. DM, diabetes mellitus; HTN/LVD, hypertension/large vessel disease; Cyst/Here, cystic/hereditary; GN, glomerulonephritis; SGN/Vasc, secondary glomerulonephritis/vasculitis; IN/Pye, interstitial nephritis/pyelonephritis.
Figure 3.
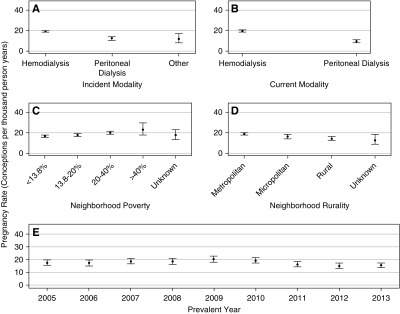
Pregnancy rates in women with ESKD demonstrate number of conceptions per thousand person-years by (A) incident type of dialysis modality, (B) current type of dialysis modality, (C) neighborhood poverty, (D) neighborhood rurality, and (E) prevalent year of dialysis.
Adjusted HRs for Factors Associated with Pregnancies
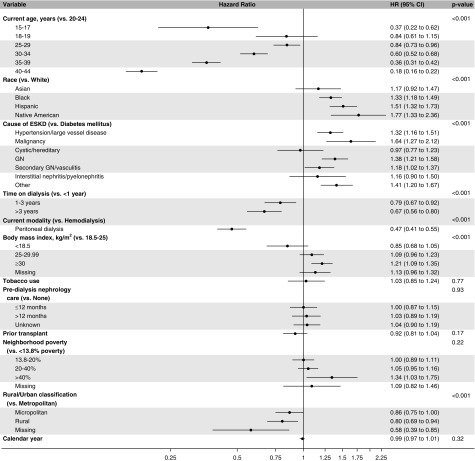
Figure 4 elucidates factors associated with the likelihood of pregnancy among patients on dialysis from the adjusted time-to-event model. Compared with white women, Native American (HR, 1.77; 95% CI, 1.33 to 2.36), Hispanic (HR, 1.51; 95% CI, 1.32 to 1.73), and black (HR, 1.33; 95% CI, 1.18 to 1.49) women had a higher likelihood of pregnancy. Compared with women aged 20–24 years, the likelihood of pregnancy was lower in women aged 15–17 years (HR, 0.37; 95% CI, 0.22 to 0.62), 25–29 years (HR, 0.84; 95% CI, 0.73 to 0.96), 30–34 years (HR, 0.60; 95% CI, 0.52 to 0.68), 35–39 years (HR, 0.36; 95% CI, 0.31 to 0.42), and 40–44 years (HR, 0.18; 95% CI, 0.16 to 0.22). Compared with ESKD due to diabetes, pregnancy was more likely in women with ESKD due to GN (HR, 1.38; 95% CI, 1.21 to 1.58), malignancy (HR, 1.64; 95% CI, 1.27 to 2.12), hypertension/large vessel disease (HR, 1.32; 95% CI 1.16 to 1.51), secondary GN/vasculitis (HR, 1.18; 95% CI, 1.02 to 1.37), and miscellaneous causes (HR, 1.41; 95% CI, 1.20 to 1.67). BMI of >30 kg/m2 was associated with a higher likelihood of pregnancy than BMI 18.5–25 kg/m2 (HR, 1.21; 95% CI, 1.09 to 1.35). Women on peritoneal dialysis had a 53% lower likelihood of pregnancy than did women receiving hemodialysis (HR, 0.47; 95% CI, 0.41 to 0.55). Compared with women who had been on dialysis for <1 year, women who had been on dialysis between 1–3 years (HR, 0.79; 95% CI, 0.67 to 0.92) or >3 years (HR, 0.67; 95% CI, 0.56 to 0.80) had a lower likelihood of pregnancy. Women living in rural areas (HR, 0.80; 95% CI, 0.69 to 0.94) had a lower likelihood of getting pregnant than did women residing in metropolitan areas. Tobacco use, predialysis nephrology care, prior transplant, neighborhood poverty level, and calendar year were not significantly associated with pregnancy among women with ESKD.
Figure 4.
Main effects time-to-event model shows factors associated with pregnancy in patients with ESKD, including hazard ratios, 95% confidence intervals, and P-values from Prentice, Williams, and Peterson total time recurrent event time-to-event analysis, with sandwich variance estimators.
Supplemental Table 2 shows the factors associated with the likelihood of pregnancy among patients on dialysis in the time-to-event model with competing risk to transplantation. Transplantation rates, both before study entry and as a reason for the interruption of person time, were higher for white women (Supplemental Table 3); however, results for the time-independent covariates, including the association of race/ethnicity with the likelihood of pregnancy, were comparable with the time-to-event model.
Pregnancy Outcomes
The percentages of fetal outcomes were as follows: live birth (27.1%, n=637), stillbirth (2.6%, n=60), spontaneous abortion (29.4%, n=691), therapeutic abortion (7.6%, n=178), ectopic/trophoblastic pregnancies (2.7%, n=63), and unknown outcome (31.0%, n=730). Seven deliveries (0.3%) resulted in both live births and stillbirths and were counted in both categories. Supplemental Table 4 shows the rates of fetal outcomes including live births by race and age. With regards to race, relative rates of live birth were highest in women who were Native American (32.7%, n=18) followed by those who were Hispanic (30.4%, n=137), Asian (29.5%, n=26), white (26.9%, n=130), and black (25.6%, n=326). With regards to age, the rates of live birth were highest in women aged 15–17 years (47%, n≤10). In the adjusted regression model, the likelihood of live birth was not associated with age, race, cause of ESKD, dialysis modality, time on dialysis, or socioeconomic status; rurality was significantly associated with the likelihood of live birth (P=0.04) (Supplemental Table 5). Regarding maternal outcomes, the percentage of deliveries that resulted in cesarean section was 35.4% (n=244) (Supplemental Table 4).
Discussion
With 2452 pregnancies, using a large national sample of patients with ESKD, we found that pregnancy rates are substantially higher than in previous reports. Moreover, this study contributes new information by elucidating the racial/ethnic differences in pregnancy rates among women on dialysis. The study found that race, cause of ESKD, dialysis modality, time on dialysis, rurality, BMI, and age are important factors associated with the likelihood of pregnancy in patients with ESKD.
Our study reports higher pregnancy rates (approximately 17.8 pregnancies PTPY) in women with ESKD on dialysis in the United States than have past studies. Additionally, women aged 20–24 years had the highest rates of pregnancy (approximately 40.9 pregnancies PTPY). The overall pregnancy rate was 2.1 PTPY with 49 pregnancies in women on dialysis from 1966 to 2008 as reported in the Australian and New Zealand Dialysis and Transplant Registry (ANZDATA). Although pregnancy rates increased from 0.67 pregnancies PTPY in 1986–1995 to approximately 3.3 pregnancies PTPY in 1996–2008, they were extremely low.7 Bagon et al.5 showed that, in the United States, the incidence of pregnancy going beyond the first trimester was 0.3 per 100 patient years. Okundaye et al.12 surveyed 930 dialysis units and reported that 2.2% of women aged 14–44 years became pregnant from 1992 to 1995. These lower pregnancy rates may be attributed to the use of voluntary registries, typical biases of surveys, and differences in the study methodology. In a literature review, Piccoli et al.4,9 reported an increase in the number of pregnancies from 2000 to 2014 (681 pregnancies in 647 patients), as compared with those in 2000–2008 (90 pregnancies in 78 patients). The higher pregnancy rates in women with ESKD on dialysis is encouraging and is important to health care providers who counsel patients of child-bearing age. In this study, pregnancy rates in the women on dialysis remained fairly constant during the recent decade. Although the higher pregnancy rate than earlier studies is encouraging, physician- and patient-related factors like individual preferences, practice patterns, and education level should be explored further to understand the possible reasons.
This study shows significant racial differences in the occurrence of pregnancy in the women on dialysis. Hispanic women had a 51% higher likelihood of pregnancy, black women had a 33% higher likelihood, Native Americans had a 77% higher likelihood, and Asian women had a 17% higher likelihood of pregnancy than did white women on dialysis. Although there are no studies examining racial/ethnic differences in pregnancy among women on dialysis, in the general population pregnancy rates are highest in black (144.3 PTPY) and Hispanic women (136.9 PTPY) as compared with non-Hispanic white women (87.5 PTPY) (Comparable pregnancy rates were not reported separately for Asians or Native Americans).26 The rates found in our study in the dialysis population were consistent with the national pattern in the general population for these three racial/ethnic groups. Access to transplantation and socioeconomic status can lead to barriers specific to the utilization of medical services and possibly differences across race in pregnancy rates.11,27 Racial disparities exist in access to transplant, with white people being more likely than black or Hispanic people to have an active wait-list status.28 However, racial differences in pregnancy rates were comparable in our model where transplantation was treated as a competing risk instead of a censoring event. Poverty has been associated with higher fertility rates in the general population, especially among young teenagers.29,30 Although pregnancy rates were higher in women of lower socioeconomic status, it was not a significant factor once we adjusted for other confounders. Women residing in rural neighborhoods had a 20% lower likelihood of pregnancy than did women residing in metropolitan neighborhoods. This is in contrast to what has been in reported in the general population: women aged 18–44 living in rural areas were more likely than women living in urban areas to have had any birth (69.7% versus 58.4%).31 Whereas reasons remain unclear for differences among women with ESKD on dialysis, differential access to obstetric care could be a contributing factor.
In our study, pregnancy rates in women on peritoneal dialysis (9.3 PTPY) were lower than for those on hemodialysis (19.3 PTPY). Although the findings were consistent with prior literature, overall rates were much higher than what has been reported. Data from the ANZDATA showed that women on peritoneal dialysis had lower conception rates compared with women on hemodialysis (1.06 pregnancies PTPY versus 2.54 pregnancies PTPY).7 In the largest survey on pregnancy in the ESKD population from the United States, only 1.1% of reproductive-age women on peritoneal dialysis conceived as compared with 2.4% women on hemodialysis.12 The reasons for the lower rates of conception in women on peritoneal dialysis remain unclear, especially because patients with peritoneal dialysis are generally healthier and have higher residual renal function than women on hemodialysis.32 Hypertonic dextrose solutions and the presence of fluid in the peritoneal cavity interfere with ovum transit to the uterus.33 Some of the complications that occur during pregnancy and in the postpartum period are specific to women on peritoneal dialysis, like peritonitis, exit-site infections, catheter-related pain, catheter-related uterine trauma, and hemoperitoneum.34,35 Peritonitis has been shown to be associated with premature rupture of membranes, chorioamnionitis, and postpartum hemorrhage.35 Therefore, it may be reasonable to switch women on peritoneal dialysis to hemodialysis if a pregnancy is desired. There could also be a possibility of reverse causation in which women on peritoneal dialysis may not have chosen to become pregnant out of concerns for complications, and women who elected to start hemodialysis may have chosen against peritoneal dialysis because they knew they wanted to become pregnant.
This study shows that pregnancy rates were higher in women with shorter duration of dialysis. As compared with women on dialysis for <1 year, women on dialysis for 1–3 years had a 21% lower likelihood of pregnancy, and women on dialysis >3 years had a 33% lower likelihood of pregnancy. The findings underscore the importance of better residual renal function, which is associated with higher frequencies of conceptions and more successful pregnancies.36 Giatras et al.37 showed that only six pregnancies were successful in 120 women undergoing dialysis for >10 years, and 47% of the pregnancies occurred in the first of 2 years of dialysis. Women with ESKD conceiving before the initiation of RRT exhibit a 30% higher live-birth rate compared with pregnancies with longer dialysis vintage time.36,38,39 We found no association of duration of dialysis with likelihood of live birth but, because we did not have predialysis data for our cohort, we were unable to compare outcomes with those of women who conceived before the initiation of dialysis. Taken together, these findings could lead health care providers to encourage conception before dialysis in women with of child-bearing age, with a proper dialysis preparation as needed. The live-birth rate in women on dialysis in this study was lower than in the general population (27% versus 62%) and in the prior reports in the ESKD population.40 Whereas the ANZDATA registry from 2001 to 2011 described a live-birth rate of 63% in 53 pregnancies in women who conceived after dialysis initiation, Okundaye et al.12 reported a newborn survival rate of 40.2% in 184 pregnancies in women with ESKD undergoing dialysis, and a national survey in patients on hemodialysis demonstrated a live-birth rate of 50%.5,36 The lower live-birth rate in our study was possibly due to a high percentage of unknown outcomes (31%) and the use of accurate codes to determine outcomes from the administrative data, which did not have the limitation of reporting bias from the surveys and voluntary registries. We used a modification of a validated method for reporting outcomes of pregnancy from the administrative databases to accurately determine and classify the outcomes, but our data were restricted due to unavailability of ambulatory and emergency department records.
A significant strength of our study is that it involves a large number of pregnant women with ESKD on dialysis in the United States, thus providing information about pregnancy rates for a heterogeneous population. Additionally, we have analyzed region-specific rates and identified particular factors that may require consideration while counseling and managing pregnancy in women with ESKD. This will help in making future region-specific guidelines for follow-up and management of pregnancy among women on dialysis. The limitations of our study include the observational design, which precludes the determination of causality. Additionally, variability in the quality and completeness of the data recorded on form 2728, including the high number of missing data for predialysis nephrology care, is associated with its inherent limitations for using USRDS data. Patient-level variables of health literacy, which may affect pregnancy, are not captured on form 2728 and were not available for our analysis. An unknown pregnancy outcome in approximately 30% of the cohort remains another limitation. The study also could not account for differences between intentional and unintentional pregnancies. However, importantly, it accounts for all of the pregnancies, and not just those reported in the voluntary registries, thereby predicting accurate pregnancy rates among women with ESKD.
In conclusion, our study demonstrates higher pregnancy rates in patients on dialysis in the United States than prior studies and significant differences across race/ethnicity with the likelihood of pregnancy. This study improves our understanding of factors associated with pregnancy and live births which would help health care providers in the counseling and management of pregnancy in women with ESKD. Our study shows significantly higher rates of pregnancy than the previous reports, and this information deserves higher emphasis by physicians while discussing pregnancy in patients with ESKD on dialysis in shared decision making.
Disclosures
None.
Funding
Dr. Shah is supported by the intramural funds from the Division of Nephrology, University of Cincinnati, and the Dialysis Clinic Inc. (DCI) grant.
Supplementary Material
Acknowledgments
Dr. Shah initiated the study, designed the study, and wrote the initial manuscript. Ms. Christianson and Dr. Leonard contributed to the study design, analyzed and interpreted the data, contributed to the study figures, and reviewed the manuscript. Mr. Meganathan contributed to the study design, data management, and manuscript review. Dr. Schauer contributed to the study design and manuscript review. Dr. Thakar assisted Dr. Shah with study design and implementation, revision of the manuscript, and did the final approval of the manuscript. All authors reviewed the manuscript.
The results presented in this article have not been published previously in whole or part, except in abstract format. The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Race, Pregnancy, and ESKD,” on pages 2280–2282.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019030234/-/DCSupplemental.
Supplemental Material. Discharge diagnoses and medical procedures indicative of pregnancy.
Supplemental Table 1. Pregnancy rates in women with ESKD on dialysis.
Supplemental Table 2. Main effects time-to-event model with transplantation as a competing risk showing factors associated with pregnancy in patients with ESKD.
Supplemental Table 3. Rates by race of prior transplants and those censored due to transplant.
Supplemental Table 4. Fetal and maternal outcomes by race and age.
Supplemental Table 5. Adjusted regression model showing factors associated with live births in patients with ESKD.
References
- 1.Holley JL, Schmidt RJ, Bender FH, Dumler F, Schiff M: Gynecologic and reproductive issues in women on dialysis. Am J Kidney Dis 29: 685–690, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Lin CT, Liu XN, Xu HL, Sui HY: Menstrual disturbances in premenopausal women with end-stage renal disease: A cross-sectional study. Med Princ Pract 25: 260–265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladunewich M, Hercz AE, Keunen J, Chan C, Pierratos A: Pregnancy in end stage renal disease. Semin Dial 24: 634–639, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Piccoli GB, Conijn A, Consiglio V, Vasario E, Attini R, Deagostini MC, et al.: Pregnancy in dialysis patients: Is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol 5: 62–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagon JA, Vernaeve H, De Muylder X, Lafontaine JJ, Martens J, Van Roost G: Pregnancy and dialysis. Am J Kidney Dis 31: 756–765, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Toma H, Tanabe K, Tokumoto T, Kobayashi C, Yagisawa T: Pregnancy in women receiving renal dialysis or transplantation in Japan: A nationwide survey. Nephrol Dial Transplant 14: 1511–1516, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Shahir AK, Briggs N, Katsoulis J, Levidiotis V: An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology (Carlton) 18: 276–284, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Sachdeva M, Barta V, Thakkar J, Sakhiya V, Miller I: Pregnancy outcomes in women on hemodialysis: A national survey. Clin Kidney J 10: 276–281, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccoli GB, Minelli F, Versino E, Cabiddu G, Attini R, Vigotti FN, et al.: Pregnancy in dialysis patients in the new millennium: A systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant 31: 1915–1934, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Shah S, Venkatesan RL, Gupta A, Sanghavi MK, Welge J, Johansen R, et al.: Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol 20: 24, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S, Verma P: Overview of pregnancy in renal transplant patients. Int J Nephrol 2016: 4539342, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okundaye I, Abrinko P, Hou S: Registry of pregnancy in dialysis patients. Am J Kidney Dis 31: 766–773, 1998 [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System : Researcher’s Guide to the USRDS Database, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 14.Hornbrook MC, Whitlock EP, Berg CJ, Callaghan WM, Bachman DJ, Gold R, et al.: Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res 42: 908–927, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill JS, Zalunardo N, Rose C, Tonelli M: The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant 9: 1541–1549, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML: Births: Final data for 2002. Natl Vital Stat Rep 52: 1–113, 2003 [PubMed] [Google Scholar]
- 17.Copper RL, Goldenberg RL, DuBard MB, Davis RO; Collaborative Group on Preterm Birth Prevention : Risk factors for fetal death in white, black, and Hispanic women. Obstet Gynecol 84: 490–495, 1994 [PubMed] [Google Scholar]
- 18.Wilcox AJ, Treloar AE, Sandler DP: Spontaneous abortion over time: Comparing occurrence in two cohorts of women a generation apart. Am J Epidemiol 114: 548–553, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Camelo Castillo W, Boggess K, Stürmer T, Brookhart MA, Benjamin DK Jr., Jonsson Funk M: Association of adverse pregnancy outcomes with glyburide vs insulin in women with gestational diabetes. JAMA Pediatr 169: 452–458, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Bishaw A: Areas with concentrated poverty: 2006–2010, Suitland, MD, US Census Bureau, 2011. Available at: https://www.census.gov/library/publications/2011/acs/acsbr10-17.html. Accessed July 19, 2019
- 21.Johns TS, Estrella MM, Crews DC, Appel LJ, Anderson CA, Ephraim PL, et al.: Neighborhood socioeconomic status, race, and mortality in young adult dialysis patients. J Am Soc Nephrol 25: 2649–2657, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WWMAI RUCA Rural Health Research Center: ZIP Code RUCA Approximation Methodology, Seattle, WA, WWMAI RUCA Rural Health Research Center, 2006. Available at: http://depts.washington.edu/uwruca/index.php. Accessed July 10, 2019
- 23.Yang W, Jepson C, Xie D, Roy JA, Shou H, Hsu JY, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : Statistical methods for recurrent event analysis in cohort studies of CKD. Clin J Am Soc Nephrol 12: 2066–2073, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 25.Latouche A, Porcher R, Chevret S: A note on including time-dependent covariate in regression model for competing risks data. Biom J 47: 807–814, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ventura SJ, Curtin SC, Abma JC, Henshaw SK: Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep 60: 1–21, 2012 [PubMed] [Google Scholar]
- 27.Kim MK, Lee SM, Bae SH, Kim HJ, Lim NG, Yoon SJ, et al.: Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. Int J Equity Health 17: 2, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulkarni S, Ladin K, Haakinson D, Greene E, Li L, Deng Y: Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation system. JAMA Surg 154: 618–625, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larson CP: Poverty during pregnancy: Its effects on child health outcomes. Paediatr Child Health 12: 673–677, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirby D, Coyle K, Gould JB: Manifestations of poverty and birthrates among young teenagers in California zip code areas. Fam Plann Perspect 33: 63–69, 2001 [PubMed] [Google Scholar]
- 31.Daniels K, Martinez GM, Nugent CN: Urban and rural variation in fertility-related behavior among U.S. Women, 2011-2015. NCHS Data Brief (297): 1–8, 2017 [PubMed]
- 32.Lang SM, Bergner A, Töpfer M, Schiffl H: Preservation of residual renal function in dialysis patients: Effects of dialysis-technique-related factors. Perit Dial Int 21: 52–57, 2001 [PubMed] [Google Scholar]
- 33.Hou S: Pregnancy in women treated with dialysis: Lessons from a large series over 20 years. Am J Kidney Dis 56: 5–6, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Chou CY, Ting IW, Hsieh FJ, Lee CN: Haemoperitoneum in a pregnant woman with peritoneal dialysis. Nephrol Dial Transplant 21: 1454–1455, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Gadallah MF, Ahmad B, Karubian F, Campese VM: Pregnancy in patients on chronic ambulatory peritoneal dialysis. Am J Kidney Dis 20: 407–410, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Jesudason S, Grace BS, McDonald SP: Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol 9: 143–149, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giatras I, Levy DP, Malone FD, Carlson JA, Jungers P: Pregnancy during dialysis: Case report and management guidelines. Nephrol Dial Transplant 13: 3266–3272, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Nakabayashi M, Adachi T, Itoh S, Kobayashi M, Mishina J, Nishida H: Perinatal and infant outcome of pregnant patients undergoing chronic hemodialysis. Nephron 82: 27–31, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Hou SH: Frequency and outcome of pregnancy in women on dialysis. Am J Kidney Dis 23: 60–63, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Martin JA, Hamilton BE, Osterman MJK: Births in the United States, 2016. NCHS Data Brief, No 287, Hyattsville, MD, National Center for Health Statistics, 2017 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.