Abstract
PURPOSE
Regular physical activity is associated with reduced risk of recurrence and mortality in patients with nonmetastatic colorectal cancer. Its influence on patients with advanced/metastatic colorectal cancer (mCRC) has been largely unexplored.
PATIENTS AND METHODS
We conducted a prospective cohort study nested in Cancer and Leukemia Group B (Alliance)/SWOG 80405 (ClinicalTrials.gov identifier: NCT00265850), a National Cancer Institute–sponsored phase III trial of systemic therapy for mCRC. Within 1 month after therapy initiation, patients were invited to complete a validated questionnaire that reported average physical activity over the previous 2 months. On the basis of responses, we calculated metabolic equivalent task (MET) hours per week to quantify physical activity. The primary end point of the clinical trial and this companion study was overall survival (OS). Secondary end points included progression-free survival (PFS) and first grade 3 or greater treatment-related adverse events. To minimize confounding by poor and declining health, we excluded patients who experienced progression or died within 60 days of activity assessment and used Cox proportional hazards regression analysis to adjust for known prognostic factors, comorbidities, and weight loss.
RESULTS
The final cohort included 1,218 patients. Compared with patients engaged in less than 3 MET hours per week of physical activity, patients engaged in 18 or more MET hours per week experienced an adjusted hazard ratio for OS of 0.85 (95% CI, 0.71 to 1.02; PTrend = .06) and for PFS of 0.83 (95% CI, 0.70 to 0.99; PTrend = .01). Compared with patients engaging in less than 9 MET hours per week, patients engaging in 9 or more MET hours per week experienced an adjusted hazard ratio for grade 3 or greater treatment-related adverse events of 0.73 (95% CI, 0.62 to 0.86; PTrend < .001).
CONCLUSION
Among patients with mCRC in Cancer and Leukemia Group B (Alliance)/SWOG 80405, association of physical activity with OS was not statistically significant. Greater physical activity was associated with longer PFS and lower adjusted risk for first grade 3 or greater treatment-related adverse events.
INTRODUCTION
Sedentary lifestyle is associated with increased colorectal cancer (CRC) incidence,1-5 and the International Agency for Research on Cancer considers inactivity a causal CRC risk factor.6 Beyond risk, sedentary lifestyle is associated with increased recurrence and mortality in CRC without distant metastases.7-14
The influence of physical activity on advanced/metastatic CRC (mCRC), however, has been largely unexplored. Although several observational studies have investigated the relationship between physical activity and mCRC survival, these studies were limited to small, secondary, subgroup analyses with conflicting results.11,15-18 Small trials show exercise interventions to be feasible in advanced cancer, including mCRC19-21; therefore, understanding the impact of physical activity on mCRC may translate into improved outcomes.
In the current study, we examined associations of physical activity with survival, cancer progression, and treatment-related toxicities in a large National Cancer Institute (NCI) –sponsored trial of therapy for mCRC. We prospectively collected data on physical activity near the time of chemotherapy initiation. Moreover, data on disease, treatment, and patient characteristics were carefully captured, which allowed for adjustment for potential confounding.
METHODS
Study Population
Patients were participants in a previously published NCI-sponsored phase III trial who received as initial treatment of mCRC irinotecan, fluorouracil, and leucovorin; or oxaliplatin, fluorouracil, and leucovorin combined with either cetuximab, bevacizumab, or both cetuximab and bevacizumab (Cancer and Leukemia Group B [CALGB, now part of the Alliance for Clinical Trials in Oncology]/SWOG 80405; ClinicalTrials.gov identifier: NCT00265850).22 During enrollment, the trial underwent major design changes in treatment and KRAS inclusion criteria as a result of evolving science.22-26 Our cohort includes patients who participated throughout the trial history. To account for this, we adjusted for treatment regimen and KRAS status.
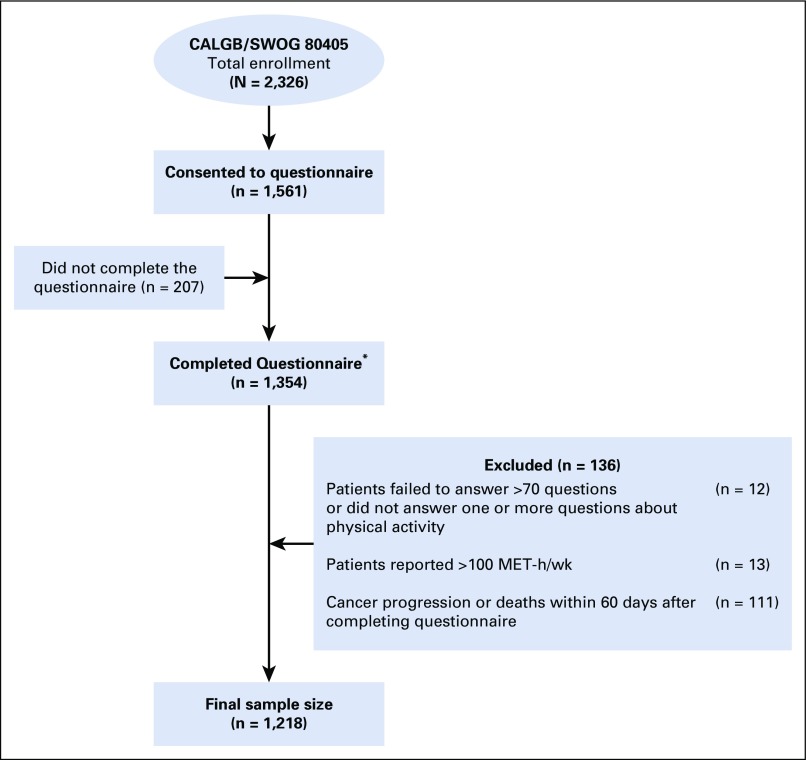
Patients had the option to participate in this companion study by completing a diet/lifestyle questionnaire. Ultimately, 67% of patients consented to the companion study, of which 87% returned the questionnaire. Compared with others in the trial, patients who completed the questionnaire were more likely to be white, to have better performance status, and were less likely to have indeterminate or missing KRAS status, but did not differ in other baseline characteristics (Data Supplement). Figure 1 shows the cohort’s derivation.
FIG 1.
Derivation of the study cohort. Median follow-up from questionnaire completion was 6.18 years. During follow-up, 1,056 of the 1,218 patients included in the analysis experienced cancer progression and 945 of these patients subsequently died. An additional 89 patients died without documented disease progression. Of patients, 795 experienced one or more grade 3 or greater treatment-related adverse events. (*) Physical activity was collected by voluntary questionnaire administered within one month after initiating chemotherapy for metastatic disease. CALGB, Cancer and Leukemia Group B (now Alliance); MET, metabolic equivalent task.
Trial eligibility and, thus, this companion study required a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 and adequate bone marrow, renal, and hepatic function.27 Thirteen patients who reported unrealistic levels of activity were excluded (> 100 metabolic equivalent task [MET] -hours per week). Considering the potential for declining health to bias physical activity assessment, we excluded patients (n = 111) with disease progression or mortality within 60 days after completing the questionnaire. In sensitivity analyses, we extended this restriction to 90 days. All patients signed informed consent approved by each site’s institutional review board.
Assessment of Physical Activity
Assessment of physical activity has been described and extensively validated previously.10,16,28-30 The paper questionnaire for activity assessment was given to patients within 1 month after initiating chemotherapy before any documented cancer progression. Participants were asked, “During the past 2 months, what was your average time per week spent at each of the following recreational activities?” regarding nine leisure-time activities (ranging from 0 to ≥ 11 hours per week), as well as normal walking pace and number of stair flights per day. Each activity was assigned a MET score, consistent with validated calculations.10,31 One MET is equivalent to the energy expenditure of sitting quietly for 1 hour. Total MET hours per week were derived by summing MET scores from each activity multiplied by total hours per week.
For analyses of total physical activity and survival, we categorized study participants by total MET hours per week, consistent with previous studies.9,32 We defined vigorous activity a priori as any activity requiring 6 or more METs—for example, running, bicycling, tennis, and aerobic exercises, such as skiing or lap swimming—consistent with physical activity guidelines and previous studies.33-35 Other activities, such as walking, climbing stairs, or yoga, were defined as nonvigorous. We also classified individuals according to normal walking pace and duration, consistent with a previous study.34 In analyses of treatment-related toxicities, physical activity was divided into two categories (< 9 v ≥ 9 MET hours per week) to conserve statistical power.
Study End Points
The primary end point of the clinical trial and this companion study was overall survival (OS), defined as the time from questionnaire completion to death from any cause. We also assessed progression-free survival (PFS), defined as the time from questionnaire completion to death from any cause or progression of disease, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.36 First-ever adverse events were recorded if grade 3 or greater (grade 2 or greater for neuropathy) and possibly, probably, or definitely related to trial therapy. We excluded adverse events that occurred before physical activity measurement.
Statistical Analyses
We used Cox proportional hazards regression analysis to examine associations of physical activity with patient outcome, adjusting for age (continuous years), sex (female or male), ECOG performance status (0 v 1 to 2), planned chemotherapy (irinotecan, fluorouracil, and leucovorin; or oxaliplatin, fluorouracil, and leucovorin), prior adjuvant chemotherapy (yes or no), prior radiation therapy (yes or no), assigned treatment arm (bevacizumab, cetuximab, bevacizumab plus cetuximab), body mass index (< 21, 21 to 24.9, 25 to 29.9, 30 to 34.9, ≥ 35 kg/m2), primary tumor location (right/transverse colon, left colon, multiple/missing), and KRAS tumor status (wild type, mutant, indeterminate/missing).37 Considering the potential for declining health to bias physical activity assessment, we further adjusted for weight change (loss ≥ 5%, change < 5%, gain ≥ 5%) and comorbidities (none v any) as measured by the questionnaire. On the questionnaire, patients were asked their weight at that time and 6 months prior and if they had a history of heart attack (myocardial infarction), angina pectoris, coronary bypass surgery, angioplasty or cardiac stent, congestive heart failure, peripheral arterial disease, peripheral artery angioplasty or bypass, high cholesterol, stroke, atrial fibrillation, transient ischemic attack, carotid surgery or endarterectomy, deep venous thrombosis, pulmonary embolus, asthma or chronic obstructive lung disease, diabetes mellitus, and inflammatory bowel disease. Missing covariates were replaced with the median or most frequent category, except for covariates with missing data from more than 5% of patients (19.7% missing KRAS status; 7.4% missing primary tumor location) wherein missing covariates were coded with indicator variables.
Predefined categories of physical activity, walking pace, walking duration, vigorous activity, and nonvigorous activity were included in unadjusted and multivariable models. We tested for linear trends across categories by assigning each participant the median value for her or his category and modeling this value as a continuous variable, consistent with prior studies.38-40 To better characterize associations of physical activity with patient outcomes, we generated smoothing splines that depicted the log of hazards for OS and PFS versus the log of total MET hours per week. We conducted subgroup exploratory analyses to explore associations of physical activity across strata of covariates, dividing patients into two categories of less than 9 MET hours per week or 9 or more MET hours per week to conserve statistical power. Secondary analyses also examined associations of physical activity with treatment-related toxicities, assessed using the NCI Common Toxicity Criteria version 3.0. The proportionality of hazards assumption was tested and satisfied using time-dependent covariates in the model. Data collection was conducted by the Alliance Statistics and Data Center. Data analyses were performed using SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC) on a data set locked on January 18, 2018. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. P < .05 was considered statistically significant. P values are two sided and not adjusted for multiple comparisons.
RESULTS
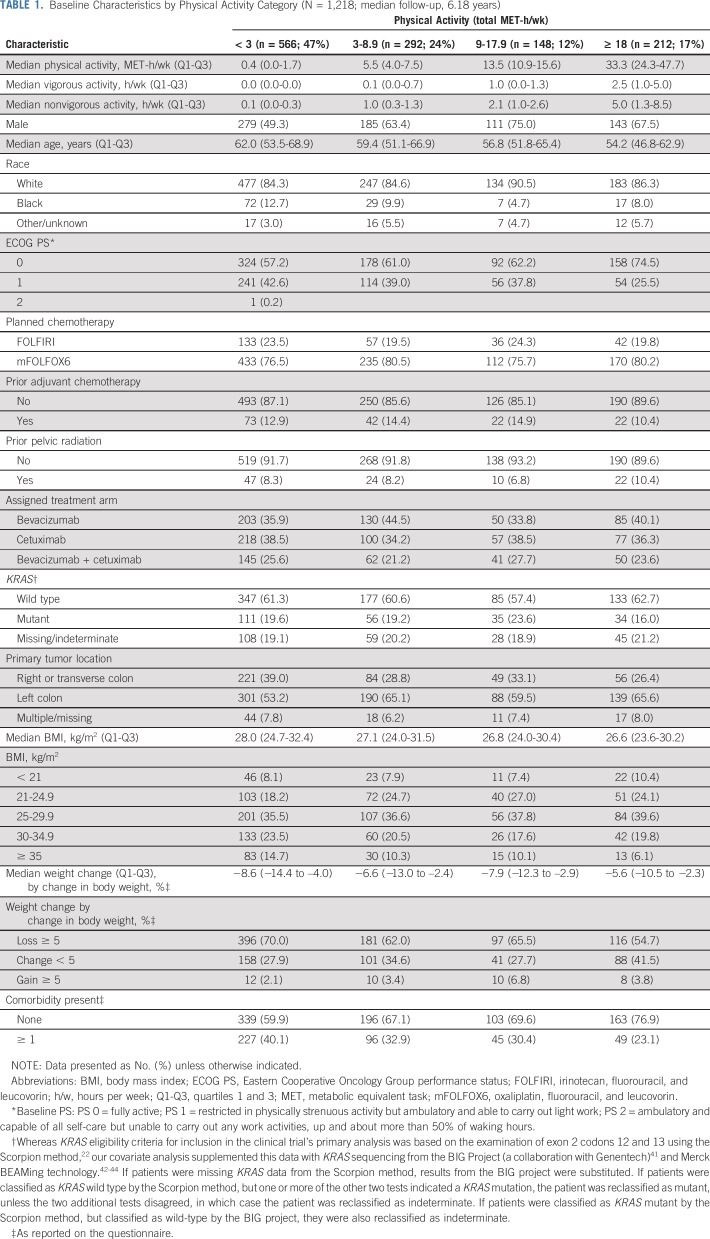
Table 1 displays baseline characteristics by physical activity level. Physically active individuals were younger and more likely to be male and have left-sided primary tumors, less likely to have comorbidities and weight loss, and tended to have better performance status and lower body mass index. Patients included in this analysis did not differ significantly in clinical characteristics from the remainder of trial patients, with the exception of a greater frequency of white race, better average performance status, and a lower rate of primary tumor resection (Data Supplement); 71.6% completed the questionnaire within 14 days after initiating trial therapy. The distribution of physical activity in our cohort of patients with metastatic cancer was similar to a prior cohort of patients with stage III colon cancer after surgical resection, but with a shift toward greater inactivity (prior cohort with 33% patients < 3 MET hours/week, 22% 3 to 8.9, 16% 9 to 17.9, and 28% ≥ 18; our cohort featured 47% patients < 3 MET hours/week, 24% 3 to 8.9, 12% 9 to 17.9, and 17% ≥ 18).10
TABLE 1.
Baseline Characteristics by Physical Activity Category (N = 1,218; median follow-up, 6.18 years)
Associations of Total Physical Activity With Survival and Cancer Progression
Median follow-up was 6.18 years. During follow-up, 1,056 of the 1,218 patients included in the analysis experienced cancer progression and 945 subsequently died. An additional 89 patients died without documented progression. Of patients, 795 experienced grade 3 or greater adverse events.
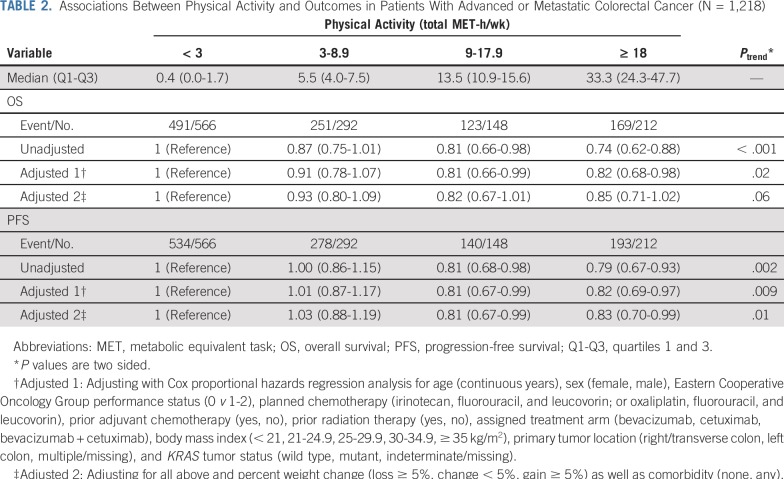
Whereas greater physical activity was associated with longer OS in the unadjusted model, the association became nonsignificant after adjusting for other potential predictors of patient outcome, including weight loss and comorbidities (Table 2). Compared with individuals with less than 3 MET hours per week, individuals with 18 or more MET hours per week experienced a fully adjusted hazard ratio (HR) for OS of 0.85 (95% CI, 0.71 to 1.02; PTrend = .06). However, greater physical activity was associated with significantly longer PFS. Compared with individuals with less than 3 MET hours per week, individuals with 18 or more MET hours per week experienced a fully adjusted HR for PFS of 0.83 (95% CI, 0.70 to 0.99; PTrend = .01). These analyses excluded patients who experienced disease progression or mortality within 60 days after questionnaire completion, given the potential for declining health as a result of occult disease progression to reduce physical activity. When we extended this restriction to 90 days, the association between greater physical activity and longer PFS persisted (HR, 0.84; 95% CI, 0.71 to 1.00; PTrend = .01).
TABLE 2.
Associations Between Physical Activity and Outcomes in Patients With Advanced or Metastatic Colorectal Cancer (N = 1,218)
Of note, 156 patients completed the physical activity questionnaire more than 30 days after trial registration, and 32 patients did not return the questionnaire until more than 60 days after. After excluding the latter 32 in a sensitivity analysis, our results were largely unchanged (OS: adjusted HR, 0.83; 95% CI, 0.69 to 1.01; PTrend = .05; PFS: adjusted HR, 0.84; 95% CI, 0.70 to 0.999; PTrend = .02). After excluding all 156 patients, HR point estimates were similar, though power was reduced (OS: adjusted HR, 0.85; 95% CI, 0.69 to 1.03; PTrend = .07; PFS: adjusted HR, 0.87; 95% CI, 0.72 to 1.05; PTrend = .05).
The Data Supplement displays smoothing splines that characterize associations of total MET hours per week with OS and PFS. The splines suggest longer PFS and OS with increasing physical activity.
In exploratory subgroup analyses, we examined associations of total physical activity with OS and PFS across strata of other potential predictors of patient outcome after adjustment for covariates (Data Supplement). In these analyses, the test for interaction between total physical activity and performance status as a predictor of OS was significant (PInteraction = .02), wherein the association of activity with longer OS was more robust among patients with ECOG performance status of 1 or 2 versus 0. Subgroup analyses also revealed a marginal significant interaction between physical activity and KRAS status as a predictor of PFS (PInteraction = .05), wherein greater activity was associated with improved PFS in patients with KRAS wild-type tumors (PTrend = .001), but not KRAS mutant (PTrend = .68).
Associations of Walking With Survival and Cancer Progression
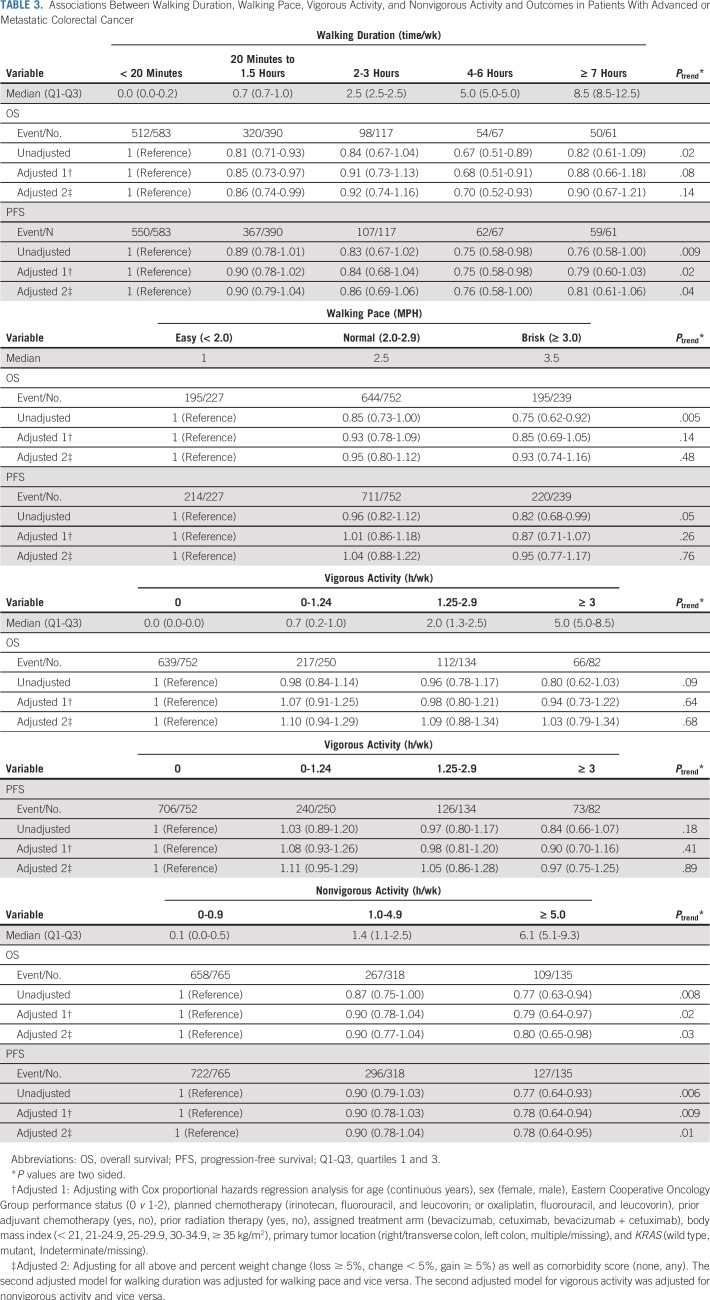
There was no significant association between walking duration and risk of all-cause mortality after adjusting for other predictors of patient outcome (PTrend = .14; Table 3). However, greater walking duration was associated with longer PFS in the unadjusted (PTrend = .009) and adjusted models (PTrend = .04). Faster walking pace was associated with longer OS in the unadjusted model (PTrend = .005), but not after adjusting for potential confounders (PTrend = .48). Walking pace was not significantly associated with PFS in unadjusted or adjusted models.
TABLE 3.
Associations Between Walking Duration, Walking Pace, Vigorous Activity, and Nonvigorous Activity and Outcomes in Patients With Advanced or Metastatic Colorectal Cancer
Associations of Vigorous and Nonvigorous Activity With Survival and Cancer Progression
Greater nonvigorous activity was associated with longer OS and PFS even after adjusting for potential confounders (Table 3). Compared with individuals with less than 1 hour per week of nonvigorous activity, individuals with 5 or more hours per week of nonvigorous activity experienced an adjusted HR for all-cause mortality of 0.80 (95% CI, 0.65 to 0.98; PTrend = .03) and an adjusted HR for PFS of 0.78 (95% CI, 0.64 to 0.95; PTrend = .01). In contrast, vigorous activity was not significantly associated with patient outcome.
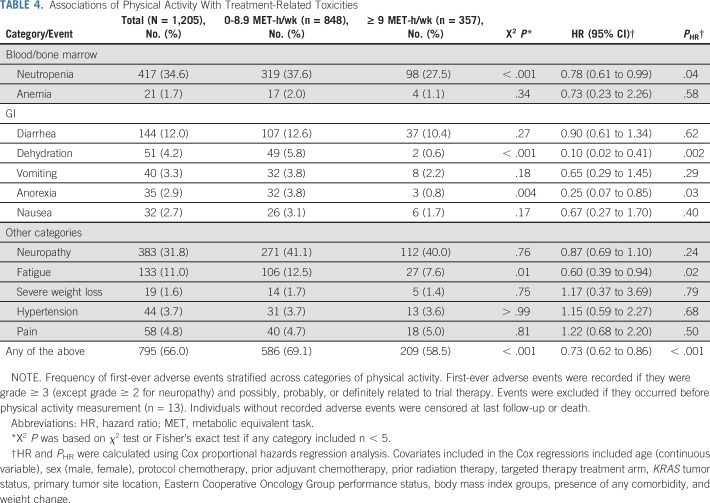
Associations of Physical Activity With Treatment-Related Toxicities
Physical activity was associated with a significantly lower rate of treatment-related adverse events (Table 4 and Fig 2). Patients participating in 9 or more MET hours per week experienced an adjusted HR for treatment-related adverse events of 0.73 (95% CI, 0.62 to 0.86; P < .001) compared with patients participating in less than 9 MET hours per week. This result remained statistically significant after adjusting for time between therapy initiation and physical activity questionnaire completion (HR, 0.73; 95% CI, 0.62 to 0.86; P < .001), as well as exclusion of patients who completed the questionnaire more than 14 days after chemotherapy initiation (HR, 0.80; 95% CI, 0.66 to 0.97; P = .02). Associations of physical activity with individual types of adverse events are also listed in Table 4.
TABLE 4.
Associations of Physical Activity With Treatment-Related Toxicities
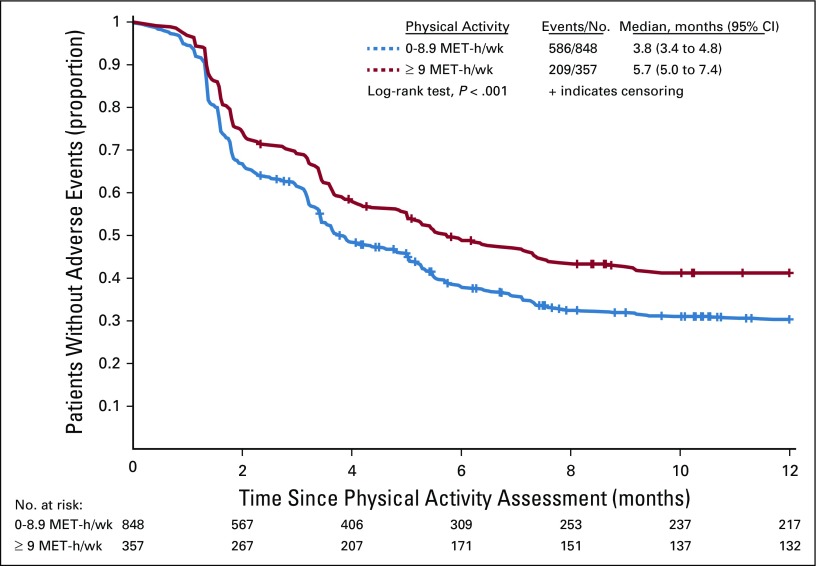
FIG 2.
Kaplan-Meier curve for any first-time adverse event stratified by physical activity level. The blue curve represents individuals who reported activity of 0-8.9 metabolic equivalent task (MET) hours per week. The red curve represents individuals who reported activity of 9 or more MET hours per week. Below the x-axis is displayed the number of patients at risk at each 2-month interval, categorized by baseline physical activity level (0-8.9 MET-h/wk v ≥ 9 MET-h/wk).
DISCUSSION
In this prospective study of patients with mCRC enrolled in an NCI-sponsored, randomized trial of systemic therapy, greater total physical activity was associated with longer PFS and reduced incidence of treatment-related toxicities; however, there was no association with OS. Greater nonvigorous activity and walking duration were associated with longer PFS and greater nonvigorous activity was associated with longer OS. Walking pace and vigorous activity were not associated with patient outcome.
Activity may improve cancer outcomes by reducing hyperinsulinemia,45-52 oxidative damage,53 inflammation,54-56 or treatment-related toxicities.57,58 Prospective studies have demonstrated an association between greater physical activity and reduced mortality8 and disease recurrence10 in CRC without distant metastases. Examination of activity and outcome in patients with CRC with metastases has been limited to small subgroup analyses.11,15-17 These have not demonstrated a relationship between prediagnosis activity and mCRC outcome.11,15-17 A prior study that included patients with mCRC found postdiagnosis activity to be inversely associated with mortality.18 However, the patient subgroup with metastatic disease was relatively small (n = 234).18 To our knowledge, our study, which features comprehensive data on patient characteristics, tumor characteristics, and clinical follow-up, features the largest prospective cohort to examine physical activity in mCRC and is the first mCRC investigation of activity to adjust for comorbidities and weight loss to reduce confounding by poor or rapidly declining health.
Our study is also unique in its investigation of vigorous and nonvigorous activity and walking. Although exercise intensity has been studied in relation to CRC risk,59 we are not aware of previous literature on vigorous or nonvigorous activity in relation to CRC outcome. One CRC cohort study found walking duration to be inversely associated with mortality, but did not specifically investigate this association in metastatic disease.18 Our findings suggest that patients with mCRC may benefit from nonvigorous activity which is achievable for many receiving chemotherapy.
To our knowledge, our study is also the first to demonstrate an association in mCRC between greater activity and lower incidence of treatment-related toxicities. Our questionnaire assessed physical activity averaged over the 2 months preceding its administration and was administered around the time of chemotherapy initiation. However, nearly 30% of patients completed the questionnaire more than 14 days after chemotherapy initiation, which raises the possibility of reverse causation in our toxicity analysis. Nonetheless, the association of greater physical activity with reduced risk of grade 3 or greater toxicities remained largely unchanged after excluding patients who completed the questionnaire more than 14 days after therapy initiation and after adjusting our model for time between treatment initiation and questionnaire completion. Although an association between greater activity and reduced treatment toxicity is novel in the context of mCRC, it is consistent with studies in nonmetastatic malignancies, including randomized trials of exercise in patients with breast cancer that decreased nausea, pain, and improved chemotherapy completion rates.57,58
Conducting a prospective cohort study nested within an NCI-sponsored clinical trial offers several advantages. First, patients had confirmed metastatic disease at baseline, which reduces heterogeneity by disease stage. Second, treatment and follow-up were standardized, which allowed for disease progression and mortality to be recorded prospectively and accurately. Finally, detailed information on prognostic variables was collected at baseline, allowing adjustment for potential confounders.
A potential criticism of our study is that reduced physical activity may simply be a marker of poor health, resulting in spurious association between inactivity and mortality. Given our study’s observational design, we cannot completely exclude residual confounding by poor health; however, our findings are supported by the fact that patients had normal or near-normal performance status. Moreover, all analyses were adjusted for performance status. To minimize the influence of deteriorating health on activity, we also excluded patients with cancer progression or death in the 60 days after questionnaire completion. When extended to 90 days, we continued to observe a beneficial association between activity and PFS. Finally, our findings remained statistically significant after adjusting for weight loss and comorbidities. Nonetheless, randomized clinical trials of physical activity interventions are needed to confirm our findings.
Our study has notable limitations. First, patients in clinical trials may differ from the general population. Such patients must satisfy eligibility criteria, be selected for the study, and be motivated to participate; however, this cohort, which was drawn from a large NCI-sponsored trial, included patients from community and academic centers throughout North America. Trial participants who voluntarily complete questionnaires may also differ from other participants, and only 58% of the trial’s participants completed our questionnaire. These patients were more likely to be white and have good performance status; however, they did not significantly differ in most measured characteristics. Our study is also subject to limitations that are inherent to self-reported physical activity, although our activity questionnaire has been extensively used and validated.10,16,28-30 Given trial exclusion criteria, our findings may not be generalizable to patients with poor performance status. Such patients, however, have limited ability to exercise regardless of potential benefits.
In summary, this prospective study of patients with mCRC, embedded in a randomized, phase III trial, demonstrated longer PFS and lower risk of treatment-related toxicities with greater total physical activity. Greater nonvigorous activity was associated with longer OS and PFS, and greater walking duration with longer PFS. Although our observational study does not offer evidence for causality, it builds on mounting evidence that demonstrates improved CRC outcomes with greater physical activity and extends this association to CRC with metastases. Although additional studies are needed to confirm these results, our findings support the discussion and recommendation of physical activity in the management of mCRC.
ACKNOWLEDGMENT
The authors thank Sherry Breaux and Paul Novotny, MD, of the Alliance for Clinical Trials in Oncology. Additional acknowledgments can be found at https://acknowledgments.alliancefound.org.
Footnotes
Presented at the 2017 Gastrointestinal Cancer Symposium, San Francisco, CA, January 19-21, 2017.
Supported by the National Institutes of Health Grants No. U10-CA180821 (Alliance Chairman’s Grant to the Alliance for Clinical Trials in Oncology) and U10-CA180882 (Alliance Statistics and Data Center Grant to the Alliance for Clinical Trials in Oncology); U10-CA180791, U10-CA180795, U10-CA180826, U10-CA180836, U10-CA180838, U10-CA180850, U10-CA180867, UG1-CA189858, and UG1-CA189869; U10-CA180820 (ECOG-ACRIN); U10-CA180888 and U10-CA180830 (SWOG); R01-CA149222, R01-CA169141, R01-CA118553, K07-CA197077, R01-CA205406, and CA180820; and the Stand-Up-to-Cancer Colorectal Dream Team Grant. Supported in part by funds from Bristol-Myers Squibb, Genentech, Eli Lily, Pfizer, and Sanofi. Supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, and the George Stone Family Foundation (J.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The National Cancer Institute helped design the study and participated in review of the manuscript. Nongovernment sponsors of the study played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
AUTHOR CONTRIBUTIONS
Conception and design: Donna Niedzwiecki, Robert J. Mayer, Charles S. Fuchs, Jeffrey A. Meyerhardt
Financial support: Jeffrey A. Meyerhardt
Administrative support: Richard M. Goldberg, Charles S. Fuchs
Provision of study materials or patients: Alan P. Venook, Heinz-Josef Lenz, Bert H. O’Neil, Blase N. Polite, Howard S. Hochster, Robert J. Mayer, Charles S. Fuchs, Jeffrey A. Meyerhardt
Collection and assembly of data: Sui Zhang, Alan P. Venook, Donna Niedzwiecki, Heinz-Josef Lenz, Federico Innocenti, Bert H. O’Neil, James E. Shaw, Blase N. Polite, Howard S. Hochster, Richard M. Goldberg, Jeffrey A. Meyerhardt
Data analysis and interpretation: Brendan J. Guercio, Sui Zhang, Fang-Shu Ou, Alan P. Venook, Federico Innocenti, Bert H. O’Neil, Howard S. Hochster, James N. Atkins, Richard M. Goldberg, Kaori Sato, Kimmie Ng, Erin Van Blarigan, Robert J. Mayer, Charles D. Blanke, Eileen M. O’Reilly, Charles S. Fuchs, Jeffrey A. Meyerhardt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Associations of Physical Activity With Survival and Progression in Metastatic Colorectal Cancer: Results From Cancer and Leukemia Group B (Alliance)/SWOG 80405
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Brendan J. Guercio
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Eli Lily (Inst), Pfizer (Inst), Sanofi (Inst)
Alan P. Venook
Consulting or Advisory Role: Taiho Pharmaceutical, Bayer, Halozyme, Eisai
Research Funding: Genentech (Inst), Bristol-Myers Squibb (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Now-UptoDate for authoring and maintaining two chapters
Travel, Accommodations, Expenses: Genentech, Roche, Halozyme, Bayer
Heinz-Josef Lenz
Honoraria: Merck Serono, Roche, Bayer, Boehringer Ingelheim
Consulting or Advisory Role: Merck Serono, Roche, Bayer, Pfizer
Travel, Accommodations, Expenses: Merck Serono, Bayer, Roche
Bert H. O'Neil
Employment: Eli Lilly
Consulting or Advisory Role: Bristol-Myers Squibb, Merck
Blase N. Polite
Research Funding: Merck
Travel, Accommodations, Expenses: Tapestry Pharmaceuticals
Other Relationship: Gerson Lehrman Group
Howard S. Hochster
Consulting or Advisory Role: Bayer, Genentech, Amgen, Exelixis
Richard M. Goldberg
Honoraria: Amgen
Consulting or Advisory Role: Merck, Taiho Pharmaceutical, Merck, Novartis
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Merck, Merck, Amgen
Kimmie Ng
Consulting or Advisory Role: Genentech, Eli Lilly, Tarrex Biopharma, Bayer, Seattle Genetics
Research Funding: Genentech (Inst), Pharmavite (Inst), Gilead Sciences, Trovagene, Celgene
Eileen M. O’Reilly
Consulting or Advisory Role: Celgene, Astellas Pharma (I), Celsion (I), Sanofi (I), Silenseed, Gilead Sciences, Merrimack Pharmaceuticals, Bristol-Myers Squibb (I), Bristol-Myers Squibb, Agios (I), ASLAN Pharmaceuticals (I), Bayer (I), Boston Scientific (I), CASI Pharmaceuticals (I), Delcath Systems (I), Eisai (I), Halozyme (I), Ipsen (I), Merck (I), Onxeo (I), Servier (I), Sillajen (I), Sirtex Medical (I), AstraZeneca (I), Antengene (I), Aptus Clinical, CytomX Therapeutics, Debiopharm Group (I), Daiichhi (I), Exelixis (I), Inovio Pharmaceuticals (I), PCI Biotech (I), Sanofi, Yakult (I), Amgen (I), Aptus Clinical (I), Carsgen Therapeutics (I), Celgene (I), Eli Lilly (I), Halozyme, Targovax, twoXAR, twoXAR (I), VAXIMM, Vicus (I), Yakult (I), Yiviva (I), Tekmira (I), SOBI, RedHill Biopharma (I), QED (I), Pieris Pharmaceuticals, Pharmacyte Biotech, Pharmacyclics, Pfizer, Novella Clinical (I), Newlink Genetics, Mina (I), Loxo, LAM Therapeutics (I), Kyowa Hakko Kirin (I), Jazz Pharmaceuticals (I), Janssen Pharmaceuticals, Hengrui Medicine (I), Genoscience Pharma (I), Cipla (I), Bridgebio (I), BiolineRx, BeiGene (I), Alignmed (I), 3DMedcare (I)
Research Funding: Celgene (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), MedImmune (Inst), Mabvax (Inst), Roche (Inst), Acta Biologica (Inst), Genentech (Inst), Halozyme (Inst), OncoQuest (Inst)
Charles S. Fuchs
Leadership: CytomX Therapeutics
Stock and Other Ownership Interests: CytomX Therapeutics, Entrinsic Health
Consulting or Advisory Role: Eli Lilly, Sanofi, Merck, Entrinsic Health, Agios, Merrimack Pharmaceuticals, Taiho Pharmaceutical, Genentech, CytomX Therapeutics, Unum Therapeutics, Bain Capital, Bayer, Gilead Sciences, Dicerna, Five Prime Therapeutics, KEW, Celgene, Pfizer
Jeffrey A. Meyerhardt
Honoraria: Ignyta, Cota Healthcare, Taiho Pharmaceutical
Consulting or Advisory Role: Array BioPharma
Research Funding: Boston Biomedical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Martínez ME, Giovannucci E, Spiegelman D, et al. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 3.Wolin KY, Yan Y, Colditz GA, et al. Physical activity and colon cancer prevention: A meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Murphy TK, Calle EE, Rodriguez C, et al. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000;152:847–854. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 6.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: International evaluation of the evidence. Eur J Cancer Prev. 2002;11(suppl 2):S94–S100. [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Je Y, Jeon JY, Giovannucci EL, et al. Association between physical activity and mortality in colorectal cancer: A meta-analysis of prospective cohort studies. Int J Cancer. 2013;133:1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 9.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 11.Haydon AM, Macinnis RJ, English DR, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baade PD, Meng X, Youl PH, et al. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20:1410–1420. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 13.Des Guetz G, Uzzan B, Bouillet T, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013;2013:340851. doi: 10.1155/2013/340851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 15.Boyle T, Fritschi L, Platell C, et al. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109:814–822. doi: 10.1038/bjc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon J, Sato K, Niedzwiecki D, et al. Impact of physical activity after cancer diagnosis on survival in patients with recurrent colon cancer: Findings from CALGB 89803/Alliance. Clin Colorectal Cancer. 2013;12:233–238. doi: 10.1016/j.clcc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter V, Jansen L, Knebel P, et al. Physical activity and survival of colorectal cancer patients: Population-based study from Germany. Int J Cancer. 2017;140:1985–1997. doi: 10.1002/ijc.30619. [DOI] [PubMed] [Google Scholar]
- 18.Ratjen I, Schafmayer C, di Giuseppe R, et al. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: A prospective cohort study. BMC Cancer. 2017;17:701. doi: 10.1186/s12885-017-3697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: A randomized controlled trial. J Pain Symptom Manage. 2013;45:811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark W, Siegel EM, Chen YA, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216:1070–1081. doi: 10.1016/j.jamcollsurg.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: A randomized controlled trial. J Clin Oncol. 2006;24:635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 22.Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: A randomized clinical trial. JAMA. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 25.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 26.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 27.Zubrod CG, Schneiderman M, Frei E, III, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 28.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 29.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 31.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper JG, Phipps AI, Neuhouser ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23:1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Health and Human Services Physical activity advisory committee report, 2008. https://health.gov/paguidelines/2008/report/pdf/committeereport.pdf
- 34.Richman EL, Kenfield SA, Stampfer MJ, et al. Physical activity after diagnosis and risk of prostate cancer progression: Data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889–3895. doi: 10.1158/0008-5472.CAN-10-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Cox DR. Regression models and life tables (with discussion) J R Stat Soc Ser B (Method) 1972;34:187–220. [Google Scholar]
- 38.Fuchs MA, Sato K, Niedzwiecki D, et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance) PLoS One. 2014;9:e99816. doi: 10.1371/journal.pone.0099816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–764. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 40.Meyerhardt JA, Sato K, Niedzwiecki D, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Natl Cancer Inst. 2012;104:1702–1711. doi: 10.1093/jnci/djs399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleifman EB, Tam R, Patel R, et al. Next generation MUT-MAP, a high-sensitivity high-throughput microfluidics chip-based mutation analysis panel. PLoS One. 2014;9:e90761. doi: 10.1371/journal.pone.0090761. [Erratum: PLoS One 9:e96019, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgins MJ, Jelovac D, Barnathan E, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–3469. doi: 10.1158/1078-0432.CCR-11-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehl F, Li M, He Y, et al. BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3:551–559. doi: 10.1038/nmeth898. [DOI] [PubMed] [Google Scholar]
- 44.Li M, Diehl F, Dressman D, et al. BEAMing up for detection and quantification of rare sequence variants. Nat Methods. 2006;3:95–97. doi: 10.1038/nmeth850. [DOI] [PubMed] [Google Scholar]
- 45.Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 47.Ligibel JA, Campbell N, Partridge A, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 48.Chu SH, Park JH, Lee MK, et al. The association between pentraxin 3 and insulin resistance in obese children at baseline and after physical activity intervention. Clin Chim Acta. 2012;413:1430–1437. doi: 10.1016/j.cca.2012.06.002. [Erratum: Clin Chim Acta 414:26, 2012] [DOI] [PubMed] [Google Scholar]
- 49.Kim ES, Im JA, Kim KC, et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring) 2007;15:3023–3030. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 50.Frank LL, Sorensen BE, Yasui Y, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: A randomized clinical trial. Obes Res. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 51.Ross R, Dagnone D, Jones PJ, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 52.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes Res. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 53.Allgayer H, Owen RW, Nair J, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008;43:971–978. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- 54.Il’yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 55.Hamer M, Sabia S, Batty GD, et al. Physical activity and inflammatory markers over 10 years: Follow-up in men and women from the Whitehall II cohort study. Circulation. 2012;126:928–933. doi: 10.1161/CIRCULATIONAHA.112.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Waart H, Stuiver MM, van Harten WH, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: Results of the PACES randomized clinical trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 58.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 59.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–252. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]