Abstract
Introduction
As malaria transmission declines, understanding the differential impact of intensified control on Plasmodium falciparum relative to Plasmodium vivax and identifying key drivers of ongoing transmission is essential to guide future interventions.
Methods
Three longitudinal child cohorts were conducted in Papua New Guinea before (2006/2007), during (2008) and after scale-up of control interventions (2013). In each cohort, children aged 1–5 years were actively monitored for infection and illness. Incidence of malaria episodes, molecular force of blood-stage infections (molFOB) and population-averaged prevalence of infections were compared across the cohorts to investigate the impact of intensified control in young children and the key risk factors for malaria infection and illness in 2013.
Results
Between 2006 and 2008, P. falciparum infection prevalence, molFOB, and clinical malaria episodes reduced by 47%, 59% and 69%, respectively, and a further 49%, 29% and 75% from 2008 to 2013 (prevalence 41.6% to 22.1% to 11.2%; molFOB: 3.4 to 1.4 to 1.0 clones/child/year; clinical episodes incidence rate (IR) 2.6 to 0.8 to IR 0.2 episodes/child/year). P. vivax clinical episodes declined at rates comparable to P. falciparum between 2006, 2008 and 2013 (IR 2.5 to 1.1 to 0.2), while P. vivax molFOB (2006, 9.8; 2008, 12.1) and prevalence (2006, 59.6%; 2008, 65.0%) remained high in 2008. However, in 2013, P. vivax molFOB (1.2) and prevalence (19.7%) had also substantially declined. In 2013, 89% of P. falciparum and 93% of P. vivax infections were asymptomatic, 62% and 47%, respectively, were sub-microscopic. Area of residence was the major determinant of malaria infection and illness.
Conclusion
Intensified vector control and routine case management had a differential impact on rates of P. falciparum and P. vivax infections but not clinical malaria episodes in young children. This suggests comparable reductions in new mosquito-derived infections but a delayed impact on P. vivax relapsing infections due to a previously acquired reservoir of hypnozoites. This demonstrates the need to strengthen implementation of P. vivax radical cure to maximise impact of control in co-endemic areas. The high heterogeneity of malaria in 2013 highlights the importance of surveillance and targeted interventions to accelerate towards elimination.
Keywords: P. falciparum, P. vivax, Papua New Guinea, Epidemiology, Malaria control, Incidence, Prevalence
Background
Intensification of malaria control measures has been associated with marked reductions in transmission and infection and illness burden in many endemic areas [1]. In the Americas [1, 2] and some parts of Asia-Pacific [3, 4], these reductions have been associated with a marked shift to the predominance of Plasmodium vivax as the primary source of Plasmodium spp. infections. In parallel, the proportion of low-density, asymptomatic infections has been observed to increase [5–8] and transmission becomes more heterogeneous [9–11].
The reasons underlying these shifts are likely to be multifactorial. A major factor for the relative increase in P. vivax is the poor uptake and/or adherence of anti-hypnozoite therapy [12, 13]. As a result, P. vivax hypnozoites are able to cause repeated bouts of blood-stage parasitaemia and are responsible for up to 80% of all P. vivax blood-stage infections [14]. Even in low and very low transmission settings, most P. vivax infections are asymptomatic [15, 16] and often of very low density [16] but almost all carry detectable gametocytaemia [6, 17, 18]. These infections are thus not detected and treated by the health systems and can sustain transmission. P. vivax is also considered more easily transmissible given the rapid maturation and thus early presence of its gametocytes [19] and faster development cycle in its mosquito host [20]. Lastly, it has also been observed that P. vivax-infected mosquitoes may be younger and more likely to bite early and outdoors [21, 22]. All of these factors may render P. vivax transmission less susceptible to vector control and routine case management interventions.
The highly heterogeneous nature of malaria transmission across countries, between neighbouring villages and within the same village has long been recognised [23–25] and is driven by an interplay of host, vector and environmental factors [23, 26, 27]. As transmission declines, there is a tendency for malaria infections to become increasingly clustered in high-risk populations and high-risk areas [11, 28] and it becomes more important to be able to identify these clusters since they may be responsible for sustaining transmission [11]. There is growing evidence that despite achieving overall reductions in malaria transmission through improved malaria control, infections and illness burden in many hyperendemic areas remain unaltered [29–31] and that more targeted interventions may be necessary for elimination [11].
In the early 2000s, the overall burden of malaria in Papua New Guinea (PNG) was amongst the highest in the Asia-Pacific region, albeit with intensity of transmission geographically highly variable across the country [27, 32, 33]. Plasmodium falciparum and P. vivax are the two predominant species that account for most of the burden of malaria infections and illness in PNG [32, 34].
Beginning in 2004, with the support of Global Fund to Fight AIDs, Tuberculosis and Malaria, PNG scaled up its malaria control interventions through scheduled 3-yearly nationwide distribution of long-lasting insecticide treated nets (LLINs), introduction of a test-and-treat approach and a switch to artemether-lumefantrine (AL) as first-line treatment [35, 36]. Subsequent surveys revealed a substantial decline in the overall burden of malaria [6, 33], with the nationwide infection prevalence by light microscopy (LM) declining from 11.1% in 2009 to 0.9% in 2014 [33, 37]. Entomological studies also revealed a large decline in human biting rates from 83 bites/person/night to 31 bites/person/night [37, 38]. As elsewhere, these reductions in PNG have gone hand-in-hand with an increase in the proportion of asymptomatic and sub-microscopic infections [6] and a pronounced heterogeneity of residual transmission [39]. Although the prevalence of PCR-detectable P. vivax infections in community surveys has not declined to the same extent as P. falciparum infection [6], the shift towards P. vivax predominance has not yet been as pronounced as in neighbouring SE Asia- and SW Pacific countries [7].
To better understand the relationship between changing transmission and the risk profile of malaria infections and disease, it is vital to gain insight into the impact that control measures have on the two main species, P. falciparum and P. vivax. Using three consecutive longitudinal child cohorts (1–5-year-old children) conducted in the same study area, prior [40], during [41] and following 5 years of intensification (2013 cohort), we investigated the impact of improved malaria control on the breadth of metrics including clinical incidence, incidence of newly acquired infections (i.e. the molecular force of blood-stage infection, molFOB) [42, 43] and infection prevalence to better understand changing P. falciparum and P. vivax epidemiology in the context of rapid reductions in transmission. In order to guide continued reductions in transmission, we also investigated the key drivers of infection and illness in young children during the period of low transmission in 2013.
Methods
Study design and sites
Three longitudinal cohort studies of 1–5-year-old children were conducted in the same study area in the Ilahita area of Maprik District, East Sepik Province in 2006, 2008 and 2013. A detailed description of the study area is given elsewhere [40]. Briefly, the study area is located in northern PNG where malaria transmission is considered hyperendemic [34, 44] and all human malaria species are endemic [40, 41, 45, 46]. Health services are provided solely by the church-run Ilahita Health Centre with inconsistent services from a government aid post. The cohorts were conducted at three different time-points before and during the scale-up of malaria control interventions in the study area (Fig. 1).
Fig. 1.
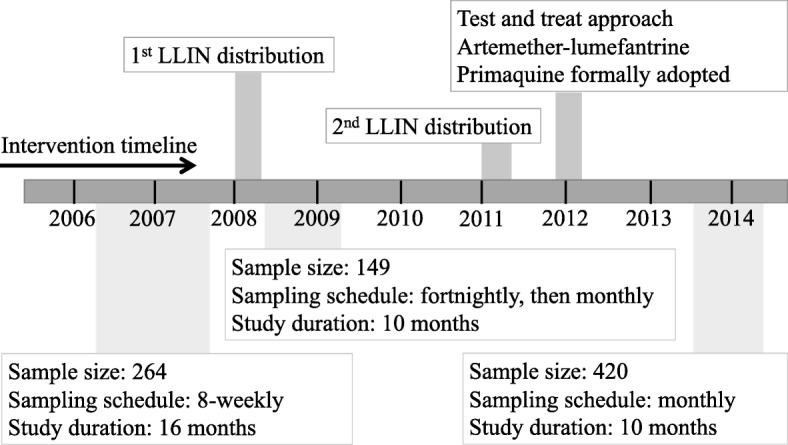
Study and intervention timeline. Legend: The timeline shows the time-points when the three cohorts were conducted in reference to malaria control interventions that occurred in the study area
Cohorts
2006 cohort (pre-intensification)
Children aged 1–3 years were enrolled into the study and actively followed up for malaria infection and illness every 8 weeks for a total of 16 months from March 2006 to August 2007 [40, 42, 43]. Passive case detection at Ilahita Health Centre was maintained throughout the study for detection of clinical episodes. All rapid diagnostic test (RDT) or LM confirmed febrile illness episodes were treated with AL (Coartem®, Novartis) (if treated by study staff) or amodiaquine plus sulphadoxine-pyrimethamine as per the PNG standard treatment for common illnesses in children [47] (if receiving treatment from a non-study source). Children with P. vivax episodes were not treated with primaquine as it had not yet been introduced into PNG standard treatment guidelines [47]. Full details of the study methodology are published [40, 42, 43].
2008 cohort (during early intensification)
Children 1–5 years of age were enrolled into this randomised controlled trial in April 2008, a month after the first population-wide distribution of LLIN into the study area [41]. Analysis was restricted to the control arm to allow comparability to the other two observational studies. Children were actively checked for malaria infection and illness fortnightly for the first 3 months and monthly thereafter for another 7 months. All RDT or LM confirmed febrile illness episodes were treated with AL (Coartem®, Novartis) (if treated by study staff) or Amodiaquine plus sulphadoxine-pyrimethamine as per the PNG standard treatment guidelines [47] (if receiving treatment from a non-study source). Children with P. vivax episodes were not treated with primaquine as it had not yet been introduced into PNG standard treatment guidelines [47]. Full details of the study methodology are published [41].
2013 cohort (5 years after sustained control)
This cohort was conducted after 5 years of sustained malaria control in the study area (Fig. 1) A total of 465 children aged 1–5 years at enrolment from 12 villages (Ilahita 1–7, Kamanokor, Sunuhu 1 and 2, Balanga and Balif) in Ilahita area were enrolled from July to September, 2013, and followed for 12 months. Of these, 45 children were excluded post hoc (11 withdrawals, 26 lost to follow-up, 8 with erratic attendance), resulting in a final sample size of 420 children (90% retention rate). All 420 children ranging in age from 0.9–6.4 years during the study period were included in the analysis investigating the key drivers of infection and illness in 2013. A subset (n = 371) aged ≤ 55 months were age-matched to earlier two cohorts to investigate the changing burden of malaria across the intervention time-points.
At enrolment, demographic and clinical data on recent illness and medications, bednet use and current state of health were recorded. Axillary temperatures were measured using an electronic digital thermometer. A 5-ml (ml) venous blood sample and two blood slides were collected. Haemoglobin level was measured using a portable HemoCue machine (HemoCue, Angholm, Sweden). The location of each child’s residence was recorded using a Garmin eTrex®.
Following enrolment, children were actively followed up fortnightly for morbidity surveillance and monthly for blood sampling (250 μl finger prick sample, two blood slides and haemoglobin measurement). If a child had a febrile illness at a morbidity surveillance visit, a finger prick sample of 250 μL blood and 2 blood slides were collected. RDT for malaria was performed and, if positive, children were treated with AL (Coartem®, Novartis) and occasionally AL plus primaquine for RDT positive P. vivax, as per PNG standard treatment guidelines [48]. Over the course of the study, 9 children were documented as receiving primaquine, suggesting that primaquine was inconsistently administered by health workers. Anaemic children with haemoglobin < 7.5 g/dL were given an anthelminthic drug (albendazole) and iron supplementations while other ailments were treated according to PNG standard treatment [48].
Plasmodium spp. infections were detected by real-time quantitative PCR assay (qPCR), as previously described [40–43, 49] and LM. Briefly, parasite DNA was extracted from cell pellets (equivalent to 200 μL whole blood) using a Favorgen 96-well Genomic DNA Extraction Kit following the manufacturer’s instructions and eluted in 200 μL elution buffer. The presence of P. falciparum, P. vivax, P. malariae and P. ovale infections were determined using two multiplex 2-species qPCR assays [49]. Infections with P. falciparum and P. vivax were further genotyped for Pfmsp2, Pvmsp1F3 and PvMS16 to identify individual parasite clones. All blood slides positive by first read and/or by Plasmodium screening qPCR [50], as well as 10% of the negatives, were independently examined by a second microscopist. Any discrepancies between the first and the second reads were then re-read by a third expert-level microscopist (WHO Level 1 certified). The final density was calculated by taking the geometric mean of the two concordant reads.
Statistical analysis
Analysis for this paper occurred in two parts and focussed on the two predominant species, P. falciparum and P. vivax. In the first part “Analysis of changing burden of malaria infections and illness: 2006 – 2013”, we aimed to compare the prevalence, molFOB and clinical incidence across the three cohorts to determine patterns of decline for P. falciparum relative to P. vivax across the intervention time-points. In the second part, “Analysis of key determinants of malaria infection and illness during the time of low transmission 2013”, the objective was to explore the full dataset of the 2013 cohort to identity factors that were key predictors of infection and illness during the period of low transmission in 2013. In both analyses, a clinical malaria episode was defined as history of febrile illness during the preceding 48 h and/or measured temperature ≥ 37.5 °C in the presence of a microscopically detectable infection of any density. The molFOB (number of genetically unique blood-stage infections) was calculated from the number of new infections acquired during the intervals between sampling time-points by counting all new msp2 alleles for P. falciparum and msp1F3 and MS16 alleles for P. vivax per unit time that were not present in the preceding intervals.
Analysis of changing burden of malaria infections and illness: 2006–2013
Data from each cohort were analysed separately due to the differences in the sampling schedules and the length of follow-up between the studies. However, to allow direct comparison, we used the full dataset of the 2006 cohort as the baseline while age-matched subsets of the 2008 and 2013 cohorts were used.
The population-averaged prevalence (referred to as prevalence) of P. falciparum and P. vivax infections in the three cohorts was estimated using generalised estimating equations (GEE) with a logit link and an exchangeable working correlation matrix, to account for the dependency between observations from the same child. Robust standard errors were also used to correct for working correlation matrix misspecification. Incidence rates (IR) for clinical episodes were calculated from the total number of clinical episodes experienced by each child over the study period and was modelled using negative binomial regression for the 2006 and 2013 cohorts and Poisson regression for the 2008 cohort. The relative percentage change in the prevalence and incidence was calculated using the formula: percentage change = ((current estimate − previous estimate)/previous estimate) × 100. Both the frequency of sampling and duration of blood-stage infections [51] are important factors influencing the molFOB variable. Due to the differences in the frequency of sampling in the 2006, 2008 and 2013 cohorts, it was necessary to censor any sampling time-points that were not available across all three cohorts in order to be able to directly compare the molFOB estimate across the cohorts. Incidence of new clones was defined as the sum of all new clones over the study period and derived using negative binomial regression, adjusting for individual time of exposure.
Analysis of key determinants of malaria infection and illness during the time of low transmission 2013
Risk factors of infection and malaria episode investigated in 2013 included the child’s age (years), timing of active detection of infection visits, area of residence, bednet use in the previous night, history of febrile illness in the past 2 weeks, presence of febrile illness, which is defined as the 2-day history of fever ± axillary temperature ≥ 37. 5 °C, and haemoglobin levels.
For all risk factor analyses, both univariable and multivariable regression models including all risk factors were examined. The association between prevalence of infections at monthly time-points and the risk factors was estimated using GEEs with a logit link and exchangeable working correlation matrix. Incidence of new blood-stage infections was estimated using GEE with negative binomial regression and an exchangeable working correlation matrix. Due to a very low number of clinical episodes observed in 2013, we used the total number of clinical episodes for each child across the follow-up period to assess the association between incidence of clinical infections and the risk factors. This was estimated using negative binomial regression. The risk factors were summarised across the study period for each child as follows: age at enrolment, residence (assumed not to vary across follow-up), mean haemoglobin level and molFOB. Two multivariable models of the incidence of clinical infections, one including all aggregated risk factors and molFOB (molFOB-adjusted model) and the other excluding molFOB (base model) were examined.
Due to reduced levels of transmission in 2013, several villages had few P. falciparum or P. vivax infections detected, no clinical P. falciparum or P. vivax episodes and very few new blood-stage clones. Therefore, villages were grouped into 4 areas with geographically similar characteristics (1 = Ilahita 1, 2, 3, 4, 6 and 7; 2 = Balanga and Balif; 3 = Kamanokor and Ilahita 5; and 4 = Sunuhu 1 and 2). Due to the universally high bednet use, analyses of their association with incidence of new blood-stage infections and clinical episodes did not converge and bednet use was excluded from both analyses. The associations are expressed as odds ratio (OR) and incidence rate ratios (IRR) and were considered to be statistically significant if the Wald test p value was below the nominal level of significance of 0.05.
The analyses were conducted using Stata 12.0 (StataCorp, USA) and R v2.12 (2011) [2006 cohort molFOB analysis] and v3.4.0 (2017) [2008 cohort analyses] (R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
Changing burden of malaria infections and illness: 2006–2013
The prevalence of infection, molFOB and incidence of clinical malaria were compared across three independent age-matched child cohorts conducted before (cohort 1, n = 264) and during (cohort 2, n = 149; cohort 3, n = 371) the intensification of malaria control activities. The overall prevalence of all Plasmodium spp. infections by PCR was 79.4% (CI95 76.7–81.9%) in 2006, 77.0% (CI95 73.4–80.3%) in 2008 and 25.6% (CI95 22.5–29.0%) in 2013, with P. vivax the predominant species across all time-points.
In 2006, 2 years prior to the scale-up of control activities in the study area, prevalence of P. falciparum and P. vivax was 41.6% (CI95 38.4–44.9%) and 59.6% (CI95 56.6–62.4%) by PCR and 24.8% (CI95 21.9–27.6%) and 45.3% (CI95 42.3–48.3%) by LM, respectively (Fig. 2a, b). Two years later and within several months of the first population-wide distribution of LLIN by the National Malaria Control Program, the prevalence of P. falciparum almost halved [PCR 22.1% (CI95 7.7–27.3%); LM 12.8% (CI95 10.0–16.2%)), Fig. 2a, b], with little observed impact on P. vivax prevalence [PCR 65.0% (CI95 61.4–68.4%); LM 49.4% (CI95 45.4–53.5%), Fig. 2a, b]. However, after 5 years of sustained control in the area, the prevalence of P. vivax had also substantially declined (PCR 19.6% (CI95 16.9–22.6%); LM 11.4% (CI95 9.5–13.6%), Fig. 2a, b), and P. falciparum prevalence had continued to decline further to 11.2% (CI95 9.2–13.0%) by PCR and 4.5% (CI95 3.5–5.8%) by LM in 2013 (Fig. 2a, b). Infections due to P. malariae [2006 (7.9%), 2008 (4.1%), 2013 (0.3%)] and P. ovale [2006 (3.5%), 2008 (3.0%), 2013 (0.2%)] were only occasionally detected by PCR and also declined from 2006 to 2013.
Fig. 2.
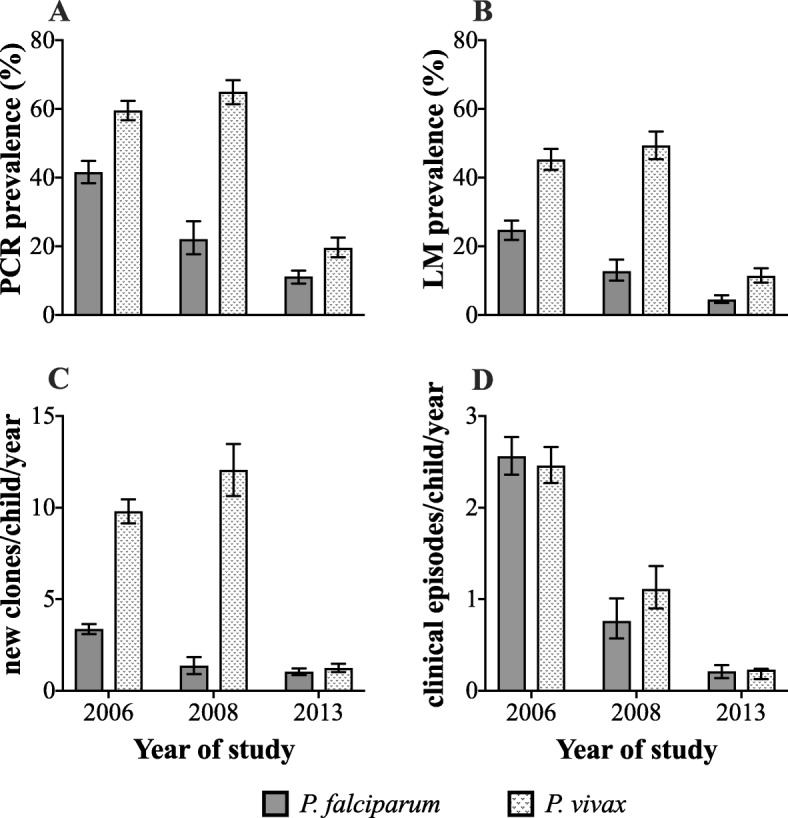
Changing burden of malaria infections and illness across the different time-points of malaria control intensification in the study area. Legend: Impact of improved malaria control on prevalence of infections detectable by a polymerase chain reaction assay (PCR), b light microscopy (LM), c incidence of new blood-stage infections (molFOB) and d incidence of clinical malaria episodes. Error bars are 95% confidence intervals
As observed with the prevalence of infections, the incidence of P. falciparum genetically distinct blood-stage infections substantially declined following the first LLIN distribution. In contrast, P. vivax molFOB did not change over the same interval. P. falciparum molFOB decreased from 3.4 clones/child/year-at-risk (CI95 3.1–3.6) in 2006 to 1.4 clones/child/year-at-risk (CI95 0.9–1.8) in 2008, which further declined to 1.0 clones/child/year-at-risk (CI95 0.9–1.2) in 2013 (Fig. 2c). In contrast, P. vivax molFOB was observed to increase from 9.8 clones/child/year-at-risk (CI95 9.1–10.5) in 2006 to 12.1 clones/child/year-at-risk (CI95 10.6–13.5) in 2008, before declining to 1.2 clones/child/year-at-risk (CI95 1.0–1.5%) in 2013 (Fig. 2c).
Interestingly, a different pattern was observed for the incidence of clinical P. vivax episodes (Fig. 2d). In spite of the persistence of a relatively high P. vivax prevalence and molFOB following the first LLIN distribution, the incidence of clinical P. vivax declined by 55% in 2008 (2006, 2.46 episodes/child/year-at-risk (CI95 2.27–2.66); 2008, 1.11 episodes/child/year-at-risk (CI95 0.90–1.36)), before further declining to 0.23 episodes/child/year-at-risk (CI95 0.13–0.24) in 2013. This corresponded to an overall reduction of 91% between 2006 and 2013. The incidence of clinical P. falciparum exhibited a similar pattern to that of the prevalence and molFOB, with a continuous decline (2006, 2.56 episodes/child/year-at-risk (CI95 2.36–2.77); 2008, 0.76 episodes/child/year-at-risk (CI95 0.57–1.01); 2013, 0.21 episodes/child/year-at-risk (CI95 0.14–0.28)), corresponding to an overall reduction of 92% between 2006 and 2013 (Fig. 2d).
Key determinants of malaria infection and illness during the time of low transmission 2013
Demographic characteristics of enrolled participants
Of the 465 children enrolled into the 2013 cohort, data from 420 were available for analyses (retention rate 90%). These children ranged in age from 0.9 to 6.4 years (mean 3.3), 53.8% were male and 93% reported sleeping under a bednet the previous night. On average, the children attended 8 out of the 10 [range 1–10] active detection of infection visits.
Prevalence of infections during follow-up
Throughout the follow-up period, 47% children had at least one P. falciparum infection and 48% had at least one P. vivax infection (detected by PCR). Overall, the averaged prevalence of P. vivax was 19.9% by PCR and 10.8% by LM, while P. falciparum prevalence was 11.0% by PCR and 4.2% by LM. Sub-microscopic infections accounted for 64% of P. falciparum and 47% of P. vivax infections.
The prevalence of PCR-detectable infections varied markedly across the different areas (Pf: range 4.5–28.8%, Pv: range 6.0–45.2%; Table 1) with significantly higher risk of infection observed amongst children living in Sunuhu 1 and 2 compared to Ilahita 1, 2, 3, 4, 6 and 7 (Pf crude OR 8.49 (CI95 6.14–11.8) p < 0.001, Pv 12.6 (CI95 8.11–19.6) p value < 0.001); Additional file 1). Whereas the prevalence and the risk of P. falciparum infections also varied significantly over time (range 7.1–32.2%, p < 0.0001), P. vivax prevalence and risk was more stable over time (range 17.8–23.2%, p = 0.1777; see Table 1 and Additional file 1). The risk of both P. falciparum and P. vivax infections was higher in children experiencing a febrile illness in the last 2 weeks (Pf: crude OR 2.97 (CI95 1.57–5.63) p = 0.001, Pv 1.68 (CI95 1.06–2.66) p = 0.028), as well as those with an enlarged spleen (Pf: crude OR 2.25 (CI95 1.23–4.11) p = 0.009, Pv 1.82 (CI95 1.07–3.11) p = 0.028); see Additional file 1). The prevalence and risk of P. falciparum infections was also increased in children experiencing a concurrent febrile illness (crude OR 2.28 (CI95 1.66–3.15) p = 0.001), increased linearly with age (crude OR 1.24 (CI95 1.09–1.41) p = 0.001) but declined for every 1 g/dL increase in haemoglobin level (crude OR 0.72 (CI95 0.64–0.80) p < 0.001; Additional file 1). Bednet use was associated with a reduced prevalence of infections for both species (Pf crude OR 0.58 (CI95 0.27–1.29) p = 0.182, Pv 0.80 (CI95 0.45–1.40) p = 0.431), but the very low number of non-users results in insufficient power. Having received recent antimalarial treatment was associated with a decrease in P. vivax (crude OR 0.36 (CI95 0.15–0.85) p = 0.021; Additional file 1) prevalence and risk.
Table 1.
Key predictors of infections due to P. falciparum and P. vivax as detected by qPCR in 2013
| P. falciparum | P. vivax | |||||||
|---|---|---|---|---|---|---|---|---|
| Observed positive (%; n = 4363) | OR | CI95 | p | Observed positive (%; n = 4363) | OR | CI95 | p | |
| Areas of residence | ||||||||
| Ilahita 1–4, 6, 7 | 4.5 | Reference group | 6.1 | Reference group | ||||
| Balanga and Balif | 4.6 | 1.01 | 0.59–1.72 | 0.969 | 6.0 | 0.98 | 0.51–1.88 | 0.946 |
| Kamanokor and Ilahita 5 | 12.9 | 2.29 | 1.38–3.80 | 0.001 | 38.0 | 9.22 | 5.55–15.3 | < 0.001 |
| Sunuhu 1 and 2 | 28.8 | 7.63 | 5.34–10.9 | < 0.001 | 45.2 | 13.7 | 8.81–21.3 | < 0.001 |
| p < 0.0001a | p < 0.0001a | |||||||
| Age | ||||||||
| Linear | 2.91 | 1.26–6.70 | 0.012 | 1.32 | 1.16–1.51 | < 0.001 | ||
| Quadratic | 0.88 | 0.78–0.99 | 0.032 | |||||
| ADI visit | ||||||||
| Enrolment | 18.4 | Reference group | 23.2 | Reference group | ||||
| Week 4 | 8.7 | 0.40 | 0.27–0.58 | < 0.001 | 21.4 | 0.80 | 0.61–1.05 | 0.105 |
| Week 8 | 8.7 | 0.39 | 0.19–0.46 | < 0.001 | 21.3 | 0.85 | 0.65–1.11 | 0.238 |
| Week 12 | 7.1 | 0.30 | 0.19–0.46 | < 0.001 | 19.1 | 0.71 | 0.53–0.96 | 0.024 |
| Week 16 | 8.1 | 0.34 | 0.22–0.53 | < 0.001 | 17.8 | 0.65 | 0.49–0.88 | 0.004 |
| Week 20 | 9.9 | 0.47 | 0.27–0.79 | 0.004 | 20.3 | 0.66 | 0.45–0.98 | 0.04 |
| Week 24 | 7.6 | 0.31 | 0.19–0.52 | < 0.001 | 21.8 | 0.83 | 0.59–1.19 | 0.312 |
| Week 28 | 7.2 | 0.36 | 0.22–0.60 | < 0.001 | 17.9 | 0.64 | 0.43–0.94 | 0.024 |
| Week 32 | 8.8 | 0.40 | 0.25–0.65 | < 0.001 | 18.1 | 0.57 | 0.40–0.83 | 0.004 |
| Week 36 | 10.8 | 0.55 | 0.35–0.85 | 0.007 | 18.0 | 0.53 | 0.37–0.76 | 0.001 |
| Week 40 | 32.2 | 3.20 | 2.15–4.74 | < 0.001 | 22.9 | 0.82 | 0.56–1.19 | 0.298 |
| p < 0.0001a | p = 0.0129a | |||||||
| Haemoglobin | 0.65 | 0.57–0.74 | < 0.001 | |||||
| Recent antimalarial | ||||||||
| No | 11.5 | 20.3 | ||||||
| Yes | 21.3 | 15.0 | 0.34 | 0.17–0.71 | 0.004 | |||
| Enlarged spleen | ||||||||
| No | 10.8 | 19.2 | ||||||
| Yes | 38.6 | 54.6 | 1.66 | 0.98–2.79 | 0.059 | |||
| Febrile illness | ||||||||
| No | 10.9 | 19.7 | ||||||
| Yes | 25.0 | 1.84 | 1.30–2.62 | 0.001 | 29.2 | |||
| b2 weeks history of febrile illness | ||||||||
| No | 11.5 | 20.0 | ||||||
| Yes | 28.6 | 2.24 | 0.93–5.38 | 0.073 | 38.1 | 1.84 | 1.02–3.32 | 0.042 |
Multivariate GEE model-based estimates of risk of infection detected at each monthly active case detection visit time-point via backward selection of significant risk factors. OR multivariate adjusted odds ratio, CI95 95% confidence interval, p p value, ADI active detection of infection. aOverall significance level for the variable estimated using Wald chi-square test. bExcluding febrile illness at the time of visit
In multivariate analyses, area of residence, time of visit, age, haemoglobin level and the presence of a concurrent febrile illness remained independently associated with the presence of a P. falciparum infection (Table 1). Area of residence, time of visit, recent antimalarial use, age and having an episode of febrile illness in the previous 2 weeks were all associated with the risk of carrying a P. vivax infection (Table 1). Risk factors of LM-detectable infections were similar (see Additional file 2).
Molecular force of blood-stage infections in monthly intervals
Incidence of new blood-stage infections was determined for a total of 303.4 person-years of follow-up with each child at risk of acquiring new blood-stage infections for an average of 0.73 years during the cohort. The mean molFOB for P. falciparum was 1.6 (CI95 1.4–1.9) new infections per child per year-at-risk and 2.2 (CI95 1.9–2.6) infections/child/year-at-risk for P. vivax.
The rate of acquiring new P. falciparum clones was higher in Sunuhu 1 and 2 compared to Ilahita 1, 2, 3, 4, 6 and 7 (Pf IRR 3.10 (CI95 2.08–4.63) p value < 0.001) and also in those with recent antimalarial use (IRR 10.4 (CI95 5.92–18.2) p value < 0.001, Table 2). Age was not associated with P. falciparum molFOB in multivariate analysis despite the significant linear association observed in the crude analysis. The P. vivax molFOB was increased in both Sunuhu 1 and 2 and Kamanokor and Ilahita 5 compared to Ilahita 1, 2, 3, 4, 6 and 7 (IRR 8.16 (CI95 5.38–12.4) p value < 0.001 and 6.66 (CI95 4.24–10.5) p value < 0.001, respectively), and also increased linearly with age (IRR 1.26 (CI95 1.13–1.40) p value < 0.001, Table 2). Both P. falciparum and P. vivax incidence varied markedly over the follow-up time period (both p < 0.0001, Table 2).
Table 2.
Multivariate predictors of molecularly determined new P. falciparum and P. vivax blood-stage infections in 2013
| P. falciparum | P. vivax | |||||||
|---|---|---|---|---|---|---|---|---|
| IR | IRR | CI95 | p | IR | IRR | CI95 | p | |
| Areas of residence | ||||||||
| Ilahita 1–4, 6,7 | 1.09 | Reference group | 0.65 | Reference group | ||||
| Balanga and Balif | 1.25 | 1.22 | 0.70–2.12 | 0.485 | 0.94 | 1.46 | 0.81–2.64 | 0.213 |
| Kamanokor and Ilahita 5 | 1.82 | 1.61 | 0.92–2.80 | 0.096 | 4.83 | 6.66 | 4.24–10.5 | < 0.001 |
| Sunuhu 1 and 2 | 3.57 | 3.10 | 2.08–4.63 | < 0.001 | 5.37 | 8.16 | 5.38–12.4 | < 0.001 |
| p < 0.0001a | p < 0.0001a | |||||||
| Age | 1.26 | 1.13–1.40 | < 0.001 | |||||
| ADI visit interval | ||||||||
| Enrolment–week 4 | 1.23 | Reference group | 3.20 | Reference group | ||||
| Week 4–week 8 | 0.85 | 0.58 | 0.27–1.23 | 0.153 | 2.62 | 0.71 | 0.52–0.98 | 0.035 |
| Week 8–week 12 | 0.22 | 0.15 | 0.06–0.38 | < 0.001 | 1.60 | 0.44 | 0.30–0.63 | < 0.001 |
| Week 12–week 16 | 0.77 | 0.50 | 0.23–1.09 | 0.081 | 2.30 | 0.59 | 0.43–0.82 | < 0.001 |
| Week 16–week 20 | 1.23 | 3.20 | 0.87 | 0.60–1.25 | 0.446 | |||
| Week 20–week 24 | 2.58 | 1.99 | 1.00–3.96 | 0.049 | 3.60 | |||
| Week 24–week 28 | 1.17 | 0.83 | 0.43–1.61 | 0.583 | 2.20 | 0.57 | 0.41–0.79 | < 0.001 |
| Week 28–week 32 | 1.13 | 0.84 | 0.43–1.64 | 0.602 | 1.98 | 0.48 | 0.34–0.69 | < 0.001 |
| Week 32–week 36 | 1.28 | 0.89 | 0.49–1.64 | 0.719 | 2.46 | 0.58 | 0.42–0.80 | < 0.001 |
| Week 36–week 40 | 7.19 | 5.55 | 3.33–9.25 | < 0.001 | 2.26 | 0.56 | 0.39–0.79 | < 0.001 |
| p < 0.0001a | p < 0.0001a | |||||||
| Recent antimalarial useb | 8.50 | 10.4 | 5.92–18.2 | < 0.001 | 2.79 | |||
| Febrile illness | 2.05 | 3.02 | ||||||
| 2 weeks history of febrile illnessc | 2.11 | 2.31 | ||||||
| Haemoglobin | ||||||||
| ≥ 10 g/dL | 1.60 | 2.15 | ||||||
| 9–9.9 g/dL | 1.88 | 2.48 | ||||||
| ≤ 9 g/dL | 2.06 | 2.87 | ||||||
Estimates from a multivariate negative binomial regression with GEE model predicting risk of acquiring new species-specific clones for P. falciparum and P. vivax in a 4-week interval when the child was considered at risk. A backward selection approach was used with the best fitting model consisting of the significant associations. IR incidence rate, IRR incidence rate ratio, CI95 95% confidence interval, p p value, g/dL grams/decilitre, ADI active detection of infections. aOverall significance level for the variable estimated using Wald chi-square test. bAntimalarial treatment within 28 days before the start of the interval. cExcluding febrile illness at the time of visit
Predictors of clinical malaria episodes
Over the 10 months of follow-up, a total of 366 febrile illness episodes were observed, of which 109 (30%) were associated with microscopically confirmed infections (IR, 0.36/child/year), with 51 P. vivax (any density: IR, 0.19) and 49 P. falciparum (any density: IR, 0.18) episodes. Another 7 were P. falciparum and P. vivax mixed infections (any density: IR 0.02), 2 were P. malariae (any density: IR, 0.07). Clinical episodes with high-density parasitaemia (≥ 2500 for P. falciparum and ≥ 500 for non-falciparum infections) accounted for 63.3% (35 Pf, 27 Pv, 7 PfPv mixed) of all the clinical episodes. There were no P. ovale clinical episodes observed.
The incidence of clinical P. falciparum episodes was significantly higher in Kamanokor, Ilahita 5 and Sunuhu 1/2 compared to Ilahita 1, 2, 3, 4, 6 and 7 (IRR 4.30 (CI95 1.59–11.6) p value 0.004 and 8.15 (CI95 3.40–19.6) p value < 0.001, respectively; Table 3). Each 1 g/dL increase in haemoglobin was associated with a 48% reduction in the incidence of clinical P. falciparum (CI95 0.35–0.77, p value: 0.001, Table 3), and each 1-year increase in age was associated with a 38% increase in the rate of clinical P. falciparum (CI95 1.10–1.73, p value: 0.006, Table 3). After adjustment for molFOB, all remained associated with the rate of clinical P. falciparum episodes, and a unit increase in molFOB (i.e. one new P. falciparum infection per child per year-at-risk) was associated with a 10% (CI95 1.02–1.18, p value 0.008) increase in the rate of clinical P. falciparum infections (Table 3).
Table 3.
Key predictors of clinical malaria episodes due to P. falciparum and P. vivax in 2013
| P. falciparum | P. vivax | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Base model | molFOB adjusted | Base model | molFOB adjusted | |||||||
| IR | IRR (CI95) | p | IRR (CI95) | p | IR | IRR (CI95) | p | IRR (CI95) | p | |
| Areas of residence | ||||||||||
| Ilahita 1–4, 6, 7 | 0.05 | Reference group | 0.06 | Reference group | ||||||
| Balanga & Balif | 0.03 | 0.63 (0.13–3.14) | 0.575 | 0.56 (0.11–2.81) | 0.485 | 0.06 | 1.15 (0.32–4.06) | 0.833 | 1.08 (0.30–3.91) | 0.909 |
| Kamanokor & Ilahita 5 | 0.25 | 4.30 (1.59–11.6) | 0.004 | 3.96 (1.46–10.8) | 0.007 | 0.52 | 8.01 (3.23–19.9) | < 0.001 | 3.86 (1.44–10.3) | 0.007 |
| Sunuhu 1&2 | 0.50 | 8.15 (3.40–19.6) | < 0.001 | 6.48 (2.65–15.8) | < 0.001 | 0.33 | 3.71 (1.53–8.99) | 0.004 | 2.00 (0.77–5.17) | 0.152 |
| p < 0.0001a | p < 0.0001a | p < 0.0001a | p < 0.0234a | |||||||
| Age | 1.38 (1.10–1.73) | 0.006 | 1.30 (1.03–1.64) | 0.026 | ||||||
| Haemoglobin | 0.52 (0.35–0.77) | 0.001 | 0.61 (0.40–0.92) | 0.017 | 0.31 (0.19–0.48) | < 0.001 | 0.38 (0.24–0.59) | < 0.001 | ||
| FOBb | 1.10 (1.02–1.18) | 0.008 | 1.17 (1.09–1.25) | < 0.001 | ||||||
Multivariate negative binomial regression model-based estimates predicting risk of clinical P. falciparum and P. vivax. Backward selection approach was used to derive significant associations. Base models included all variables except molFOB. Incidence is based on aggregated clinical data for entire 10-month study period thus precluding analysis of recent antimalarial treatment as a covariate. Bednet use was not analysed due to non-converge of data when included into models. aOverall significance level for the variable estimated using wald chi-square test; molFOB: molecular force of blood-stage infections; bmolFOB was included as a rate; IR: Incidence rate; IRR: Incidence rate ratio. CI95 95% confidence interval; p p value
The rate of clinical P. vivax episodes was also significantly higher in Kamanokor, Ilahita 5 and Sunuhu 1/2 compared to Ilahita 1, 2, 3, 4, 6 and 7 (IRR 8.01 (CI95 3.23–19.9) p value < 0.001 and 3.71 (CI95 1.53–8.99) p value 0.004, respectively; Table 3). Each 1 g/dL increase in haemoglobin was associated with a 69% reduction in the rate of clinical P. vivax (CI95 0.19–0.48, p value < 0.001). After adjustment for molFOB, only area of residence and haemoglobin remained associated with the rate of clinical P. vivax episodes (Table 3). A unit increase in molFOB (i.e. one new P. vivax infection per child per year-at-risk) was associated with a 17% (CI95 1.09–1.25, p value < 0.001) increase in the rate of clinical P. vivax infections. Age was not associated with the rate of clinical P. vivax episodes, either before or after adjustment for molFOB.
Discussion
This is the first study in a P. falciparum/P. vivax co-endemic area and amongst very few studies globally [52] to examine the impact of improved malaria control on the epidemiology of malaria in young children using longitudinal cohorts rather than the widely used nationwide and community household surveys and routine health information systems [6, 33, 37]. Longitudinal cohort studies allow for a detailed investigation into the dynamics of infection, and illness, as well as the rate of acquiring new infections (molFOB) and clinical illness over time.
By analysing these metrics in three consecutive longitudinal cohorts in young PNG children, we demonstrate a differential impact of control interventions on P. vivax compared to P. falciparum that may be overlooked in routine surveillance. Following the first LLIN distribution, the prevalence of P. falciparum infection and both P. falciparum and P. vivax clinical episodes declined immediately and continuously across the time period of the three cohorts. Contrastingly, the prevalence and force of P. vivax blood-stage infections did not decline, remaining initially relatively high with a substantial decline only evident in the most recent cohort that was conducted 5 years after commencement of intensified control in the area. These observations confirm that key biological differences between the two species render them differentially susceptible to standard control tools such as LLINs and case management, highlighting the need for P. vivax-focused interventions in co-endemic regions.
Notably, the relationship between transmission and molFOB differs for P. falciparum and P. vivax. P. falciparum metrics are directly linked to blood-stage infections, which are always mosquito-derived, hence closely reflecting current levels of transmission. The reductions in P. falciparum molFOB observed across these three cohorts confirm reductions in P. falciparum prevalence and EIR observed through monitoring and evaluation of the national programme [37, 38]. Due to the biological ability of P. vivax to remain dormant in liver cells as hypnozoites and to serve as a continuing source of relapsing infections, P. vivax metrics are not able to differentiate between mosquito-derived and relapsing infections and therefore do not reflect active transmission as closely as P. falciparum metrics. This is particularly relevant in PNG, where P. vivax is the predominant species detectable in young children and relapses account for more than 50–80% of P. vivax infections in pre-school and primary school children [14, 41]. As a consequence, the P. vivax molFOB is a composite measure reflecting the joint burden of new, mosquito-derived and relapsing infections [42, 43]. This metric therefore reveals a high burden of persisting low-density relapsing infections in young children, contrasting results of nationwide surveys that showed a comparable decline in P. falciparum and P. vivax prevalence detectable by LM in both children under 5 years and the general population [37].
Given the persistence of a high burden of P. vivax infections following the initial LLIN distribution, the observation that the burden of clinical P. vivax dropped and continued to decline over the years of intensification marked a striking difference. Clinical immunity to P. vivax is acquired rapidly, even under relatively low transmission [15]. In malaria therapy patients, only few mild febrile symptoms were observed when they were re-infected with a homologous infection [53]. As relapsing infections are either genetically identical or meiotic siblings of the primary infection [54, 55], it is generally thought that clinical episodes are more likely to be caused by new mosquito-bite-acquired infections. Considering that reduction in transmission results in the acquisition of fewer new mosquito-derived infections, the observation that the immediate impact of LLIN was exclusively on incidence of P. vivax clinical episodes and not on risk of infection strongly suggests that the majority of clinical episodes due to P. vivax may indeed be associated with mosquito-derived rather than relapsing infections.
The observation of a delayed impact of LLIN scale-up on P. vivax compared to P. falciparum blood-stage infections in co-endemic areas is important evidence for control programmes. It suggests that the large reservoir of hypnozoites acquired when transmission is high (prior to scale-up of control) gives rise to a sufficient burden of relapsing infections that may be transmissible, although often not symptomatic, such that minimal impact may be observed on P. vivax prevalence in the years immediately following scale-up even though transmission is being reduced. This highlights the importance of strengthening the implementation of radical cure of P. vivax in order to accelerate reduction in the burden of P. vivax [56]. Reluctance to prescribe primaquine without G6PD testing and poor adherence to the 14-day regime are major issues limiting the effectiveness of P. vivax radical cure in many settings, including PNG.
The observed impact on clinical incidence and the comparable longer-term reduction in P. vivax and P. falciparum burden of infections does however provide reassurance that vector control with LLINs can reduce the burden of P. vivax, at least in countries where malaria transmission is largely peri-domestic [57], even if coverage needs to be maintained for a longer period of time before the full effectiveness is observed. Interestingly, in many countries in Asia and the Americas where dramatic shifts to P. vivax predominance have been observed, programmes rely upon clinical case management (often with poor coverage of anti-hypnozoite therapy) as their primary malaria control strategy [2, 58] and/or have highly exophilic vectors with transmission occurring mainly in forested areas where LLIN and other traditional vector control tools such as indoor-residual spraying have limited efficacy [59–61].
During the period of reduced transmission in 2013, the individual level of exposure to new blood-stage infections (molFOB) and the geographical location of the child’s residence were the two key determinants of infection and illness. In the previous 2006 and 2008 cohorts, age-dependent decreases in the incidence of clinical P. vivax were observed [40, 41], suggestive of rapid acquisition of clinical immunity due to high P. vivax molFOB during those periods. Conversely, we did not observe any age association in 2013, which may be explained by the substantial decline in the force of P. vivax infection.
As documented in other settings, declining transmission leads to increasing transmission heterogeneity [60, 62] and an increasing proportion of asymptomatic low-density infections [6–8]. In 2013, over two thirds all PCR-detected infections were sub-microscopic and the risk of clinical malaria was highly dependent on where the child lived, with higher risk of clinical illness observed in areas with higher force of infection. This pronounced spatial heterogeneity in the risk of infections and malaria illness has also been observed in the two previous cohorts [40–43] indicating that despite the declining transmission between 2006 and 2013, the high burden areas remained stable. In particular, we observed marked geographical clustering of infections and illness in two areas, Sunuhu 1/2 and Kamanokor/Ilahita 5 in 2013, the same geographical locations that were identified as highest burden areas before [40, 42, 43] and during scale-up of interventions [41]. The persistence of high-burden areas such as these despite the ongoing implementation of control interventions is supported by observations made elsewhere [29, 30] and strengthens the rationale for surveillance strategies that target interventions to these potential transmission hotspots in order to accelerate control. Such strategies will clearly need to identify the characteristics of hotspots that fuel sustained transmission and address the diagnostic challenge imposed by asymptomatic, low-density infections [5, 63–65].
A limitation of this study is the differences in the study designs, sampling schedules and the length of follow-up as well as the non-uniform structuring of the individual datasets. Consequently, each cohort was analysed separately and the calculated burden of malaria infection and disease were compared between the cohorts to determine the patterns of decline for P. falciparum and P. vivax across the intervention time-points. As such, we did not statistically test the differential patterns of decline exhibited by P. falciparum and P. vivax across the intervention time-points. However, confidence intervals of the prevalence, molFOB and clinical incidence across the three cohorts are provided illustrating when differences are statistically significant. It should also be noted that the cohorts were conducted in the same study area with a stable population and the cohorts were age-matched hence minimising variation between the cohorts.
Lastly, the impact of malaria control interventions on transmission are a function of diverse social and ecological settings leading to differences in mosquito abundance, mosquito behaviour and human-mosquito interaction. While improvements in the quality of housing have occurred over the past decade in many urban areas of PNG, housing for PNG’s rural majority remains largely dependent on bush material. Higher quality of housing and socio-economic status was associated with reduced risk of malaria in previous studies in PNG [37]; however, such data is not available from the child cohorts analysed. The association between weather patterns and long-term malaria trends in PNG have also been investigated using site-specific satellite weather variables and did not explain variations in observed malaria incidence over time (Rodríguez-Rodríguez & Hetzel, unpublished data). Further work understanding the dynamic, complex and responsive ecological niches driving ongoing malaria transmission in certain areas of PNG will inform the development of targeted control and elimination efforts.
Conclusions
Scale-up of standard malaria control interventions in PNG substantially reduced the burden of malaria infection and disease in the most vulnerable 1–5-year-old age group. Data presented here suggests comparable reductions in new mosquito-derived infections for both P. falciparum and P. vivax but a delayed impact on P. vivax relapsing infections due to the previously acquired reservoir of hypnozoites. We confirm the effectiveness of sustained implementation of LLINs and case management in reducing transmission of both species in PNG but highlight the critical need to strengthen case management, radical cure, surveillance and targeted intervention strategies in order to accelerate control of malaria in co-endemic settings.
Supplementary information
Additional file 1: Table S1. Bivariate associations between risk factors and the prevalence, molFOB and clinical incidence. Estimates of bivariate associations calculated via generalised estimating equation (GEE) models for prevalence and molecular force of blood-stage (molFOB) infections and negative binomial regression model used for clinical malaria episodes. Recent antimalarial use was not tested in the model for clinical malaria episodes due to aggregated clinical data. aAge in years at enrolment was used for clinical malaria incidence while at the start of interval was used for molFOB. bComparison group; For multilevel variables, comparison group estimates are presented as odds or incidence rate. PCR: Polymerase chain reaction assay; LM: light microscopy; OR: odds ratio; CI95: 95% confidence interval; IRR: incidence rate ratio.
Additional file 2: Table S2. Key predictors of the prevalence of infections due to P. falciparum and P. vivax as diagnosed by light microscopy. Multivariate generalised estimating equation (GEE) model-based estimates of the risk of infection detected at each monthly active detection of infection visits via backward selection of significant risk factors. aOverall significance level for the variable estimated using wald chi-squared test. AOR: multivariate adjusted odds ratio. CI95: 95% confidence interval; bExcluding febrile illness at the time of visit; Data for observed positive are %.
Acknowledgements
We would like to sincerely thank the participating children and their parents/guardians for their time and participation, Ilahita community and health facilities. Specific thank you to Brenda Wingi, Magarina Bakandu, Kolsen Ganba and all field staff for field work; Naomi Sambale, late Nandao Taronka, Lina Lorry, Charles Kongs and Jessica Brewster for assistance with sample preparation and microscopy; Thomas Adiguma, Wilson Phillip and the team for data management; and Bethuel Kosoaleng, Anna Samuel and Immaculata Yandimawi for project management.
Abbreviations
- AL
Artemether-lumefantrine
- CI95
95% confidence interval
- DNA
Deoxyribonucleic acid
- GEE
Generalised estimating equations
- GPS
Global Positioning System
- IR
Incidence rate
- IRR
Incidence rate ratio
- LLIN
Long-lasting insecticide treated nets
- molFOB
Molecular force of blood-stage infection
- OR
Odds ratio
- p
p value
- PCR
Polymerase chain reaction
- Pf
P. falciparum
- PNG
Papua New Guinea
- Pv
P. vivax
- qPCR
Quantitative polymerase chain reaction
- RDT
Rapid diagnostic test
- spp.
Species
Authors’ contributions
LJR, IM and JK conceived and designed the study; MOK, LJR, MS and DM supervised enrolment and follow-up of participants; MOK, LJR, IM, SZ and TO analysed and interpreted the data: MOK, LJR, SZ and IM wrote the first draft of the manuscript. All authors critically revised the manuscript and read and approved the final draft.
Funding
The 2006 cohort was funded in part by National Institutes of Health (AI063135, AI-46919, and TW007872), the Swiss National Science Foundation (grant no. 31003A-112196), the Australian Agency for International Development (AusAID) and the National Health and Medical Research Council (Grant no. 516735). The 2008 cohort received funding support from the Cellex Foundation, Barcelona, Spain. The 2013 cohort was funded by National Institute of Allergy and Infectious Diseases through Southwest Pacific International Centre of Excellence in Malaria Research (grant U19 AI089686) and Bill and Melinda Gates Foundation through the TransEPI consortium. MOK is supported by an Australian Awards DFAT Scholarship through University of Melbourne. LJR was supported by NHMRC Early Career Research Fellowship (GNT1016443) to conduct the 2013 cohort and is currently supported by NHMRC Career Development Fellowship Level 2 (GNT1161627). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
Anonymised data is available upon reasonable request by contacting the PNG Medical Research Advisory Committee and the PNG Institute of Medical Research IRB. The contact is Dr. William Pomat, secretary PNGIMR IRB: William.Pomat@pngimr.org.pg.
Ethics approval and consent to participate
Ethical approvals for the three cohort studies were granted by Papua New Guinea Institute of Medical Research Institutional Review Board (2006, 09.24: 2008, 07.20; 2013, 11.16) and PNG Medical Research and Advisory Committee [2006, 05.19; 2008, 07.34; 2013, 11.21]. Voluntary written informed consent was obtained from the parents or guardians of the children following community awareness and individual study information sessions.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Ome-Kaius, Email: marai.kaius@gmail.com.
Johanna Helena Kattenberg, Email: ekattenberg@itg.be.
Sophie Zaloumis, Email: sophiez@unimelb.edu.au.
Matthew Siba, Email: matthewpsiba@gmail.com.
Benson Kiniboro, Email: b.kiniboro@gmail.com.
Shadrach Jally, Email: shadrachjally@gmail.com.
Zahra Razook, Email: razook.z@wehi.edu.au.
Daisy Mantila, Email: dmantila86@gmail.com.
Desmond Sui, Email: sui.desmond0@gmail.com.
Jason Ginny, Email: j274yagaum@gmail.com.
Anna Rosanas-Urgell, Email: arosanas@itg.be.
Stephan Karl, stephanunkarl@googlemail.com.
Thomas Obadia, Email: thomas.obadia@pasteur.fr.
Alyssa Barry, Email: alyssa.barry@wehi.edu.au.
Stephen J. Rogerson, Email: sroger@unimelb.edu.au
Moses Laman, Email: drmlaman@yahoo.com.
Daniel Tisch, Email: Daniel.tisch@case.edu.
Ingrid Felger, Email: Ingrid.Felger@unibas.ch.
James W. Kazura, Email: jxk14@case.edu
Ivo Mueller, Email: mueller@wehi.edu.au.
Leanne J. Robinson, Email: leanne.robinson@burnet.edu.au
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12916-019-1456-9.
References
- 1.W.H.O. World Malaria Report 2018. Geneva: World Health Organization; [https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf?ua=1. Accessed: 01 May 2019.
- 2.Coura JR, Suarez-Mutis M, Ladeia-Andrade S. A new challenge for malaria control in Brazil: asymptomatic Plasmodium infection--a review. Mem Inst Oswaldo Cruz. 2006;101(3):229–237. doi: 10.1590/S0074-02762006000300001. [DOI] [PubMed] [Google Scholar]
- 3.Nguitragool W, Karl S, White M, Koepfli C, Felger I, Singhasivanon P, et al. Highly heterogeneous residual malaria risk in western Thailand. Int J Parasitol. 2019;49(6):455–462. doi: 10.1016/j.ijpara.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.W.H.O. W.P.R.O. Meeting Report: Malaria Programme Managers Meeting to review progress on implementation of the regional action framework for malaria control and elimmination in the Western Pacific 2016–2020 [https://iris.wpro.who.int/bitstream/handle/10665.1/14326/RS-2018-GE-31-PHL-eng.pdf. Accessed: 02 May 2019.
- 5.Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, et al. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:254. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koepfli C, Ome-Kaius M, Jally S, Malau E, Maripal S, Ginny J, et al. Sustained malaria control over an 8-year period in Papua New Guinea: the challenge of low-density asymptomatic Plasmodium infections. J Infect Dis. 2017;216(11):1434–1443. doi: 10.1093/infdis/jix507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, et al. High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9(5):e0003758. doi: 10.1371/journal.pntd.0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattabongkot J, Suansomjit C, Nguitragool W, Sirichaisinthop J, Warit S, Tiensuwan M, et al. Prevalence of asymptomatic Plasmodium infections with sub-microscopic parasite densities in the northwestern border of Thailand: a potential threat to malaria elimination. Malar J. 2018;17(1):329. doi: 10.1186/s12936-018-2476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201(11):1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- 11.Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, et al. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9(1):e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khantikul N, Butraporn P, Kim HS, Leemingsawat S, Tempongko MA, Suwonkerd W. Adherence to antimalarial drug therapy among vivax malaria patients in northern Thailand. J Health Popul Nutr. 2009;27(1):4–13. doi: 10.3329/jhpn.v27i1.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi R, Lawpoolsri S, Imwong M, Kobayashi J, Kaewkungwal J, Pukrittayakamee S, et al. Directly-observed therapy (DOT) for the radical 14-day primaquine treatment of Plasmodium vivax malaria on the Thai-Myanmar border. Malar J. 2010;9:308. doi: 10.1186/1475-2875-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laman M, Aipit S, Bona C, Siba PM, Robinson LJ, Manning L, et al. Ultrasonographic assessment of splenic volume at presentation and after anti-malarial therapy in children with malarial anaemia. Malar J. 2015;14:219. doi: 10.1186/s12936-015-0741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller I, Galinski MR, Tsuboi T, Arevalo-Herrera M, Collins WE, King CL. Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. Adv Parasitol. 2013;81:77–131. doi: 10.1016/B978-0-12-407826-0.00003-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9(1):e3413. doi: 10.1371/journal.pntd.0003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis. 2018;18(10):1108–1116. doi: 10.1016/S1473-3099(18)30411-0. [DOI] [PubMed] [Google Scholar]
- 18.Koepfli C, Schoepflin S, Bretscher M, Lin E, Kiniboro B, Zimmerman PA, et al. How much remains undetected? Probability of molecular detection of human plasmodia in the field. PLoS One. 2011;6(4):e19010. doi: 10.1371/journal.pone.0019010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenzie FE, Jeffery GM, Collins WE. Plasmodium vivax blood-stage dynamics. J Parasitol. 2002;88(3):521–535. doi: 10.1645/0022-3395(2002)088[0521:PVBSD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffery GM. The infection of mosquitoes by Plasmodium vivax (Chesson strain) during the early primary parasitemias. Am J Trop Med Hyg. 1952;1(4):612–617. doi: 10.4269/ajtmh.1952.1.612. [DOI] [PubMed] [Google Scholar]
- 21.Bockarie MJ, Alexander N, Bockarie F, Ibam E, Barnish G, Alpers M. The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans R Soc Trop Med Hyg. 1996;90(1):23–25. doi: 10.1016/S0035-9203(96)90465-4. [DOI] [PubMed] [Google Scholar]
- 22.Bockarie MJ, Dagoro H. Are insecticide-treated bednets more protective against Plasmodium falciparum than Plasmodium vivax-infected mosquitoes? Malar J. 2006;5:15. doi: 10.1186/1475-2875-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenwood BM. The microepidemiology of malaria and its importance to malaria control. Trans R Soc Trop Med Hyg. 1989;83(Suppl):25–29. doi: 10.1016/0035-9203(89)90599-3. [DOI] [PubMed] [Google Scholar]
- 24.Strickland GT, Zafar-Latif A, Fox E, Khaliq AA, Chowdhry MA. Endemic malaria in four villages of the Pakistani province of Punjab. Trans R Soc Trop Med Hyg. 1987;81(1):36–41. doi: 10.1016/0035-9203(87)90274-4. [DOI] [PubMed] [Google Scholar]
- 25.Burkot TR, Graves PM, Paru R, Wirtz RA, Heywood PF. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am J Trop Med Hyg. 1988;39(2):135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- 26.Hii JL, Smith T, Mai A, Mellor S, Lewis D, Alexander N, et al. Spatial and temporal variation in abundance of Anopheles (Diptera:Culicidae) in a malaria endemic area in Papua New Guinea. J Med Entomol. 1997;34(2):193–205. doi: 10.1093/jmedent/34.2.193. [DOI] [PubMed] [Google Scholar]
- 27.Muller I, Bockarie M, Alpers M, Smith T. The epidemiology of malaria in Papua New Guinea. Trends Parasitol. 2003;19(6):253–259. doi: 10.1016/S1471-4922(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 28.Mwesigwa J, Okebe J, Affara M, Di Tanna GL, Nwakanma D, Janha O, et al. On-going malaria transmission in the Gambia despite high coverage of control interventions: a nationwide cross-sectional survey. Malar J. 2015;14:314. doi: 10.1186/s12936-015-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roca-Feltrer A, Kwizombe CJ, Sanjoaquin MA, Sesay SS, Faragher B, Harrison J, et al. Lack of decline in childhood malaria, Malawi, 2001-2010. Emerg Infect Dis. 2012;18(2):272–278. doi: 10.3201/eid1802.111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bautista CT, Chan AS, Ryan JR, Calampa C, Roper MH, Hightower AW, et al. Epidemiology and spatial analysis of malaria in the Northern Peruvian Amazon. Am J Trop Med Hyg. 2006;75(6):1216–1222. doi: 10.4269/ajtmh.2006.75.1216. [DOI] [PubMed] [Google Scholar]
- 31.Jagannathan P, Muhindo MK, Kakuru A, Arinaitwe E, Greenhouse B, Tappero J, et al. Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo. Uganda Malar J. 2012;11:435. doi: 10.1186/1475-2875-11-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazura James W., Siba Peter M., Betuela Inoni, Mueller Ivo. Research challenges and gaps in malaria knowledge in Papua New Guinea. Acta Tropica. 2012;121(3):274–280. doi: 10.1016/j.actatropica.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Tropical Med Int Health. 2015;20(12):1745–1755. doi: 10.1111/tmi.12616. [DOI] [PubMed] [Google Scholar]
- 34.Genton B., Al-Yaman F., Beck H.-P., Hii J., Mellor S., Narara A., Gibson N., Smith T., Alpers M. P. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Annals of Tropical Medicine & Parasitology. 1995;89(4):359–376. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 35.Hetzel MW, Choudhury AA, Pulford J, Ura Y, Whittaker M, Siba PM, et al. Progress in mosquito net coverage in Papua New Guinea. Malar J. 2014;13:242. doi: 10.1186/1475-2875-13-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetzel MW, Gideon G, Lote N, Makita L, Siba PM, Mueller I. Ownership and usage of mosquito nets after four years of large-scale free distribution in Papua New Guinea. Malar J. 2012;11:192. doi: 10.1186/1475-2875-11-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hetzel MW, Pulford J, Ura Y, Jamea-Maiasa S, Tandrapah A, Tarongka N, et al. Insecticide-treated nets and malaria prevalence, Papua New Guinea, 2008-2014. Bull World Health Organ. 2017;95(10):695–705B. doi: 10.2471/BLT.16.189902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimer LJ, Thomsen EK, Koimbu G, Keven JB, Mueller I, Siba PM, et al. Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea. Malar J. 2016;15:25. doi: 10.1186/s12936-015-1067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofmann NE, Karl S, Wampfler R, Kiniboro B, Teliki A, Iga J, et al. The complex relationship of exposure to new Plasmodium infections and incidence of clinical malaria in Papua New Guinea. Elife. 2017;6:e23708. [DOI] [PMC free article] [PubMed]
- 40.Lin E, Kiniboro B, Gray L, Dobbie S, Robinson L, Laumaea A, et al. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS One. 2010;5(2):e9047. doi: 10.1371/journal.pone.0009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Betuela I, Rosanas-Urgell A, Kiniboro B, Stanisic DI, Samol L, de Lazzari E, et al. Relapses contribute significantly to the risk of Plasmodium vivax infection and disease in Papua New Guinean children 1-5 years of age. J Infect Dis. 2012;206(11):1771–1780. doi: 10.1093/infdis/jis580. [DOI] [PubMed] [Google Scholar]
- 42.Koepfli C, Colborn KL, Kiniboro B, Lin E, Speed TP, Siba PM, et al. A high force of plasmodium vivax blood-stage infection drives the rapid acquisition of immunity in Papua new guinean children. PLoS Negl Trop Dis. 2013;7(9):e2403. doi: 10.1371/journal.pntd.0002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller I, Schoepflin S, Smith TA, Benton KL, Bretscher MT, Lin E, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A. 2012;109(25):10030–10035. doi: 10.1073/pnas.1200841109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6(3):e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller I, Widmer S, Michel D, Maraga S, McNamara DT, Kiniboro B, et al. High sensitivity detection of Plasmodium species reveals positive correlations between infections of different species, shifts in age distribution and reduced local variation in Papua New Guinea. Malar J. 2009;8:41. doi: 10.1186/1475-2875-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, et al. Random distribution of mixed species malaria infections in Papua New Guinea. Am J Trop Med Hyg. 2000;62(2):225–231. doi: 10.4269/ajtmh.2000.62.225. [DOI] [PubMed] [Google Scholar]
- 47.Paediatric society of PNG. Standard treatment for common illness of children in Papua New Guinea: a manual for nurses, community health workers, health extension officers, and doctors. 8th ed.2006.
- 48.Paediatric society of PNG. Standard treatment for common illness of children in Papua New Guinea: a manual for nurses, community health workers, health extension officers, and doctors 9th ed.2011.
- 49.Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, et al. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8(9):e76316. doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White MT, Karl S, Koepfli C, Longley RJ, Hofmann NE, Wampfler R, et al. Plasmodium vivax and Plasmodium falciparum infection dynamics: re-infections, recrudescences and relapses. Malar J. 2018;17(1):170. doi: 10.1186/s12936-018-2318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamya MR, Arinaitwe E, Wanzira H, Katureebe A, Barusya C, Kigozi SP, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 2015;92(5):903–912. doi: 10.4269/ajtmh.14-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Collins WE, Jeffery GM, Roberts JM. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2004;70(6):642–644. doi: 10.4269/ajtmh.2004.70.642. [DOI] [PubMed] [Google Scholar]
- 54.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen N, Auliff A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195(7):934–941. doi: 10.1086/512242. [DOI] [PubMed] [Google Scholar]
- 56.Wells TN, Burrows JN, Baird JK. Targeting the hypnozoite reservoir of Plasmodium vivax: the hidden obstacle to malaria elimination. Trends Parasitol. 2010;26(3):145–151. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Cooper RD, Waterson DG, Frances SP, Beebe NW, Sweeney AW. Speciation and distribution of the members of the Anopheles punctulatus (Diptera: Culicidae) group in Papua New Guinea. J Med Entomol. 2002;39(1):16–27. doi: 10.1603/0022-2585-39.1.16. [DOI] [PubMed] [Google Scholar]
- 58.Alves FP, Gil LH, Marrelli MT, Ribolla PE, Camargo EP, Da Silva LH. Asymptomatic carriers of Plasmodium spp. as infection source for malaria vector mosquitoes in the Brazilian Amazon. J Med Entomol. 2005;42(5):777–779. doi: 10.1603/0022-2585(2005)042[0777:ACOPSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Somboon P, Lines J, Aramrattana A, Chitprarop U, Prajakwong S, Khamboonrua C. Entomological evaluation of community-wide use of lambdacyhalothrine-impregnated bed nets against malaria in a border area of north-west Thailand. Trans R Soc Trop Med Hyg. 1995;89:248–254. doi: 10.1016/0035-9203(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 60.Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, et al. Malaria in the greater Mekong subregion: heterogeneity and complexity. Acta Trop. 2012;121(3):227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cook J, Grignard L, Al-Eryani S, Al-Selwei M, Mnzava A, Al-Yarie H, et al. High heterogeneity of malaria transmission and a large sub-patent and diverse reservoir of infection in Wusab as Safil district, Republic of Yemen. Malar J. 2016;15:193. doi: 10.1186/s12936-016-1249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200(10):1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 64.Okell LC, Bousema T, Griffin JT, Ouedraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti-Infect Ther. 2013;11(6):623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Bivariate associations between risk factors and the prevalence, molFOB and clinical incidence. Estimates of bivariate associations calculated via generalised estimating equation (GEE) models for prevalence and molecular force of blood-stage (molFOB) infections and negative binomial regression model used for clinical malaria episodes. Recent antimalarial use was not tested in the model for clinical malaria episodes due to aggregated clinical data. aAge in years at enrolment was used for clinical malaria incidence while at the start of interval was used for molFOB. bComparison group; For multilevel variables, comparison group estimates are presented as odds or incidence rate. PCR: Polymerase chain reaction assay; LM: light microscopy; OR: odds ratio; CI95: 95% confidence interval; IRR: incidence rate ratio.
Additional file 2: Table S2. Key predictors of the prevalence of infections due to P. falciparum and P. vivax as diagnosed by light microscopy. Multivariate generalised estimating equation (GEE) model-based estimates of the risk of infection detected at each monthly active detection of infection visits via backward selection of significant risk factors. aOverall significance level for the variable estimated using wald chi-squared test. AOR: multivariate adjusted odds ratio. CI95: 95% confidence interval; bExcluding febrile illness at the time of visit; Data for observed positive are %.
Data Availability Statement
Anonymised data is available upon reasonable request by contacting the PNG Medical Research Advisory Committee and the PNG Institute of Medical Research IRB. The contact is Dr. William Pomat, secretary PNGIMR IRB: William.Pomat@pngimr.org.pg.


