Abstract
PURPOSE
Stratum 1 of ACNS1123 (ClinicalTrials.gov identifier: NCT01602666), a Children’s Oncology Group phase II trial, evaluated efficacy of reduced-dose and volume of radiotherapy (RT) in children and adolescents with localized nongerminomatous germ cell tumors (NGGCTs). The primary objective was to evaluate the impact of reduced RT on progression-free survival (PFS) with a goal of preserving neurocognitive function.
PATIENTS AND METHODS
Patients received six cycles of chemotherapy with carboplatin and etoposide alternating with ifosfamide and etoposide, as used in the Children’s Oncology Group predecessor study (ACNS0122; ClinicalTrials.gov identifier: NCT00047320). Patients who achieved a complete response (CR) or partial response (PR) with or without second-look surgery were eligible for reduced RT, defined as 30.6 Gy whole ventricular field and 54 Gy tumor-bed boost, compared with 36 Gy craniospinal irradiation plus 54 Gy tumor-bed boost used in ACNS0122.
RESULTS
A total of 107 eligible patients were enrolled. Median age was 10.98 years (range, 3.68 to 21.63) and 75% were male. Sixty-six of 107 (61.7%) achieved a CR or PR and proceeded to reduced RT. The 3-year PFS and overall survival and standard error values were 87.8% ± 4.04% and 92.4% ± 3.3% compared with 92% and 94.1%, respectively, in ACNS0122. There were 10 recurrences, prompting early study closure; however, after a retrospective central review, only disease in eight of 66 (12.1%) patients eligible for reduced RT subsequently progressed; six patients had distant spinal relapse alone and two had disease with combined local plus distant relapse. Serum and CSF α-fetoprotein and β-human chorionic gonadotropin levels were not associated with PFS.
CONCLUSION
Patients with localized NGGCT who achieved a CR or PR to chemotherapy and received reduced RT had encouraging PFS similar to patients in ACNS0122 who received full-dose craniospinal irradiation. However, the patterns of failure were distinct, with all patients having treatment failure in the spine.
INTRODUCTION
Germ cell tumors (GCTs) of the CNS are a heterogeneous group of tumors most commonly located in the suprasellar and pineal regions in children and adolescents.1,2 Classically, CNS GCTs are divided into two categories: germinoma and nongerminomatous germ cell tumors (NGGCTs). Whereas germinomas are histologically more homogenous, NGGCTs can be classified as embryonal carcinoma, endodermal sinus tumor, choriocarcinoma, immature teratoma, teratoma with malignant transformation, and mixed histology tumors.2-4
Patients with CNS NGGCT historically have had worse outcomes compared with patients with germinoma. Five-year overall survival (OS) in patients with NGGCT treated with full-dose craniospinal (CSI) radiotherapy (RT) alone or chemotherapy alone ranges from 20% to 40%.5-9 In addition, full-dose CSI in children often leads to deleterious late effects, such as increased risk of secondary malignancy, endocrinopathies, reduced spinal growth, ototoxicity, and neuropsychological dysfunction.10-12
Recent international and Children’s Oncology Group (COG) trials combining chemotherapy and RT have reported improved survival, supporting the possibility of reducing therapy in a subgroup of patients.13,14 In the SIOP CNS GCT 96 (SIOP 96) trial (ClinicalTrials.gov NCT00293358), patients with NGGCT received four courses of cisplatin, etoposide, and ifosfamide. Patients with localized disease received involved-field RT to 54 Gy, whereas those with metastatic disease received 30 Gy CSI with a boost to a total dose of 54 Gy to the primary tumor and sites of macroscopic metastases.13 Patients with localized tumors had a 5-year progression-free survival (PFS) and OS (± standard deviation [SD]) of 72% ± 4% and 82% ± 4%, respectively, whereas patients with metastatic disease had 5-year PFS and OS of 68% ± 9% and 75% ± 8%, respectively.13 COG ACNS0122 used six cycles of chemotherapy with carboplatin/etoposide alternating with etoposide/ifosfamide before 36 Gy CSI and 54 Gy to the primary tumor bed.14 Five-year event-free survival (EFS) and OS rates (± SD) for all patients were 84% ± 4% and 93% ± 3%, respectively, which are the best published outcomes to date.14 Seventy-nine patients (77.5%) had localized tumors, including bifocal lesions. Disease in 49 of 79 patients (62%) achieved a CR (40.5%) or PR (21.5%) to chemotherapy. Three-year EFS rates were 92% and 94.1% for patients with localized disease that achieved a CR or PR to chemotherapy, respectively.14 On the basis of these data, we hypothesized it would be safe to reduce irradiation without affecting survival outcomes in patients with localized NGGCT and a CR or PR to chemotherapy.
PATIENTS AND METHODS
Study Objectives
The primary objective was to determine, on the basis of 3-year PFS rates, whether dose and volume of RT could be safely reduced to 30.6 Gy whole-ventricular irradiation from 36 Gy CSI (as prescribed in ACNS0122), followed by involved-field focal boost to 54 Gy, henceforth referred to as reduced RT, in children with localized NGGCT and a CR or PR to chemotherapy with or without second-look surgery. A coprimary objective was to use the COG ALTE07C1 study (ClinicalTrials.gov identifier: NCT00772200) to prospectively and longitudinally model cognitive, social, and behavioral functioning in children treated with reduced RT.15 These data will be published separately. A secondary objective was to estimate the PFS and OS distributions of patients treated with reduced RT. The ACNS1123 study also included a germinoma stratum that will be reported separately. The present study was approved by all local institutional review boards and all patients or their legal guardians provided written informed consent.
Patients
Patients between 3 and 21 years of age with localized CNS NGGCT and a histologic diagnosis of endodermal sinus tumor, embryonal carcinoma, choriocarcinoma, immature teratoma, teratoma with malignant transformation, or mixed germ cell tumors were eligible to be enrolled in this study. In the absence of a histologic diagnosis, patients were eligible if imaging was suggestive of a CNS GCT with the following tumor marker elevations: serum and/or CSF β-human chorionic gonadotropin (β-hCG) level greater than 100 IU/L and serum and/or CSF α-fetoprotein (AFP) level greater than 10 ng/mL or above the local institutional normal values. Patients whose tumor had histology consistent with germinoma and tumor markers that met these criteria were also eligible. Patients must not have received any previous therapy other than surgery and/or corticosteroids. Patients with metastatic disease were excluded, including disease noted on magnetic resonance imaging (MRI) or a positive lumbar CSF cytology.
Tests and Examinations
Evaluations included MRI of the brain and spine with and without contrast at baseline, postoperatively, and after cycles 2, 4, and 6 of chemotherapy. Serum and CSF AFP and β-hCG levels, as well as lumbar CSF cytology, were required at baseline unless clinically contraindicated. Subsequent serum tumor markers were obtained before every chemotherapy cycle; CSF tumor marker and lumbar cytology measurements were repeated after cycle 6 of chemotherapy.
Treatment
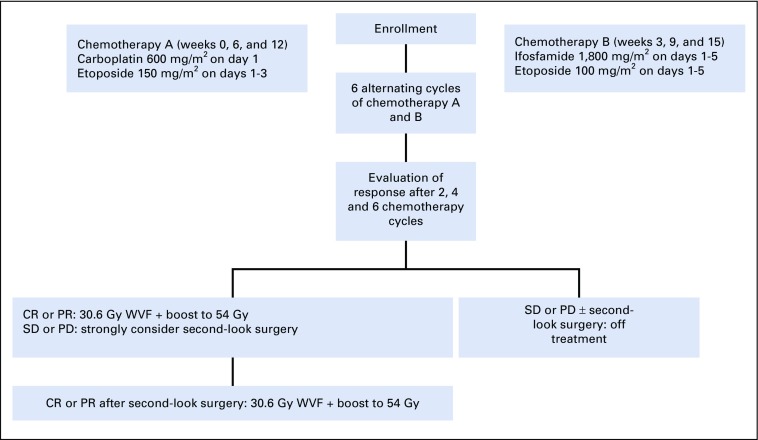
Chemotherapy consisted of carboplatin on day 1 and etoposide on days 1 through 3, alternating at 21-day intervals with cycles of ifosfamide/etoposide on days 1 through 5, which closely mirrored the COG ACNS0122 chemotherapy timing. Patients whose disease achieved a CR or PR were eligible to receive reduced RT. In patients whose disease did not achieve a CR or PR, second-look surgery was strongly recommended. If second-look surgery resulted in a CR or PR and confirmed mature teratoma or scar or fibrosis, the patient was eligible to receive reduced RT. Patients whose disease did not achieve a CR or PR or whose second-look surgery histology revealed viable malignant elements no longer received protocol treatment (Fig 1).
FIG 1.
Treatment schema. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; WVF, whole ventricular field.
Response Definitions
Responses were defined on the basis of MRI and tumor markers and were the same as those used in the COG ACNS0122 trial. CR was defined as a complete disappearance of disease on imaging, allowing for minimal residual disease or enhancement of not more than 0.5 cm in the suprasellar region or not more than 1 cm in the pineal region. PR was defined as greater than 0.5-cm residual in the suprasellar region or greater than 1 cm in the pineal region and at least a 65% decrease in the sum of the products of the three perpendicular diameters of the localized target lesion. The CR and PR definitions mandated normalization of serum and CSF AFP and β-HCG levels. Stable disease was defined as neither sufficient decrease to qualify for PR nor increase to qualify for progressive disease (PD). PD was defined as a 40% or more increase of the lesion, the appearance of new lesions, and/or increased tumor markers.
Statistical Design
All eligible and evaluable patients who received reduced RT were included in the primary analysis. The study benchmarks were based on the observed outcome of patients with localized disease treated while the patients were participating in the ACNS0122 trial, in which all observed treatment failures among patients who experienced CR or PR occurred within the first 3 years.14 The 3-year PFS estimate for the 32 patients who experienced CR and the 17 who experienced PR at the time of the ACNS1123 trial design was 90.6% (95% CI, 73.7% to 96.9%) and 94.1% (95% CI, 65.0% to 99.2%), respectively. Thus the 3-year PFS rates for these two cohorts of patients were both excellent and similar, supporting the decision to combine these groups. The observed 3-year PFS rate for the combined cohort of 49 patients was 91.8% (95% CI, 80.4% to 97.7%). Using a one-sample exact binomial test with a baseline 3-year PFS of 93% and a noninferiority margin of 11% (null hypothesis P ≤ .82) led to a sample size of 77 patients to show noninferiority with 90% power and 5% type 1 error. This design would reject the null hypothesis if at least 69 of 77 patients were free of disease progression after 3 years.
No interim analyses were planned, because of the expected accrual rate, high PFS rate, and long observation window; however, if nine or more patients were documented to have experienced disease recurrence before 3 years of follow-up and fewer than 77 patients eligible for the primary analysis had begun treatment, accrual would be suspended. The analyses were conducted per protocol and Kaplan-Meier estimates were used to estimate PFS, which was calculated from the date the patient began study participation to the date of disease progression, date of death, or date of last follow-up, if the patient was censored. Log-log transformation method was used to calculate CIs for PFS.
RESULTS
Patient Characteristics
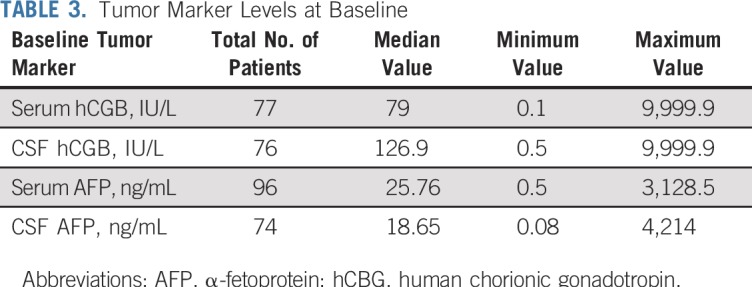
Between May 2012 and November 2016, 111 patients were enrolled; four were ineligible (physician choice [n = 1], incorrect histology [n = 1], and did not meet eligibility timing requirements [n = 2]). Eighty of the 107 eligible patients (74.8%) were male, and the median age was 10.98 (range, 3.68 to 21.63) years (Table 1). The most common tumor locations were pineal in 58 (54.2%) and suprasellar in 37 (34.6%) of the 107 patients. Six patients (5.6%) had ventricular tumors and an additional six (5.6%) had bifocal tumors. The most common histology was a mixed histology in 49 of the 107 tumors (45.8%; Table 2). Histology was unknown in 22 of 107 patients (20.6%), and these patients were enrolled on the basis of tumor marker elevation, as described in Patients and Methods (Table 3).
TABLE 1.
Patient Demographic Data
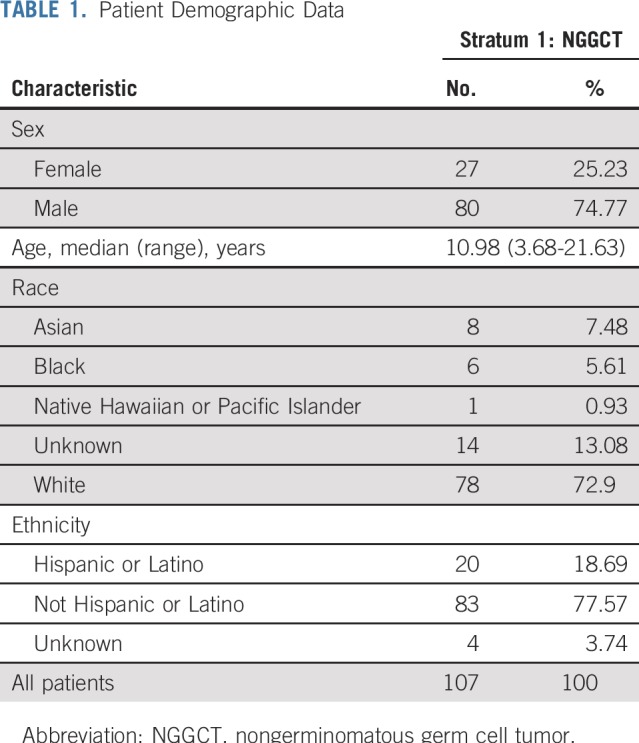
TABLE 2.
Tumor Characteristics
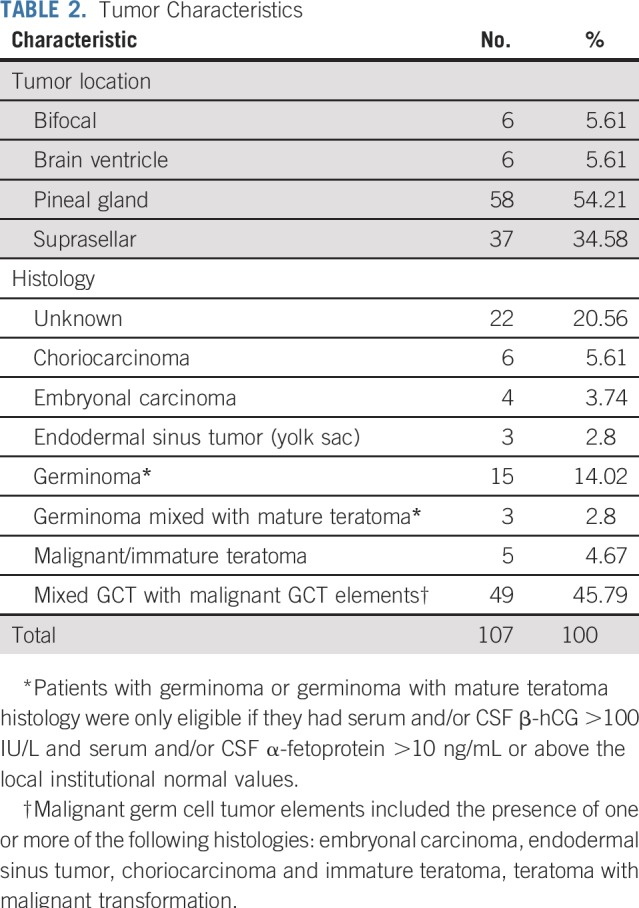
TABLE 3.
Tumor Marker Levels at Baseline
Responses
Disease in 66 of 107 eligible patients (61.7%) achieved a CR or PR after chemotherapy and received reduced RT per protocol. Twenty-four patients underwent second-look surgery; 17 (70.8%) achieved CR or PR, had mature teratoma or residual scar or fibrosis, and were included in the total 66 of patients whose disease achieved CR or PR and received RT. Seven of the 24 patients (29.2%) who underwent second-look surgery did not receive reduced RT for the following reasons: less than a PR (n = 2), viable tumor at second-look surgery (n = 2), abnormal tumor marker (n = 1), delayed RT timing (n = 1), and physician choice (n = 1).
Survival
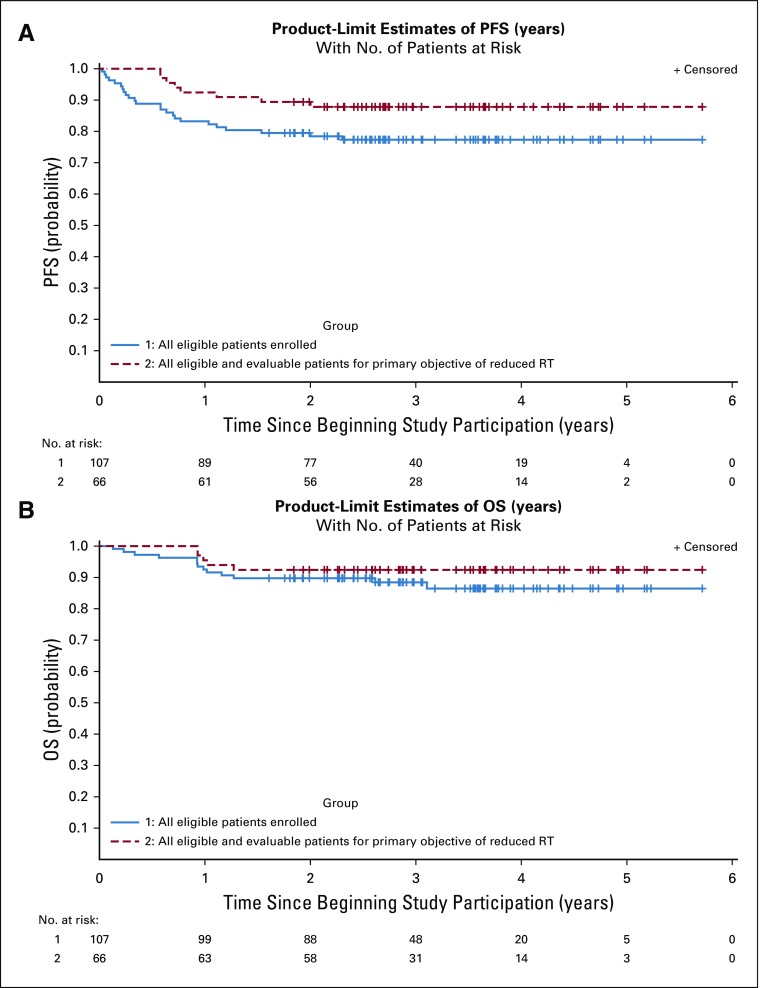
The 3-year PFS and OS rates (± standard error) for all 107 eligible patients were 77.3% ± 4.1% and 88.4% ± 3.2%, respectively (Fig 2). Neither serum nor CSF AFP and β-HCG levels nor tumor location or size were associated with response. The 3-year PFS and OS rates (± standard error) of the 66 patients eligible and evaluable for the primary objective (ie, reduced RT) were 87.80% ± 4.0% and 92.4% ± 3.3%, respectively (Fig 2).
FIG 2.
Kaplan-Meier curves of all eligible patients (N = 107) and all eligible and evaluable patients for the primary objective of reduced radiotherapy (RT) (n = 66). (A) progression-free survival (PFS) and (B) overall survival (OS).
Treatment Failures and Recurrences
Disease in 41 of 107 patients (38.3%) did not achieve a CR or PR and thus these patients did not qualify for reduced RT. Among these 41, 15 had stable disease and nine had PD after chemotherapy. One patient did not have an end-of-chemotherapy evaluation documented. In addition, there were 16 patients whose disease had a CR or PR on imaging but who did not receive reduced RT (physician discretion [n = 6], positive tumor markers [n = 7], patient refusal [n = 2], exceeded protocol timing for RT initiation [n = 1], and death [n = 1). The 3-year PFS and OS rates (± SD) for these 41 patients were 60.2% ± 7.8% and 81.7% ± 6.4%, respectively.
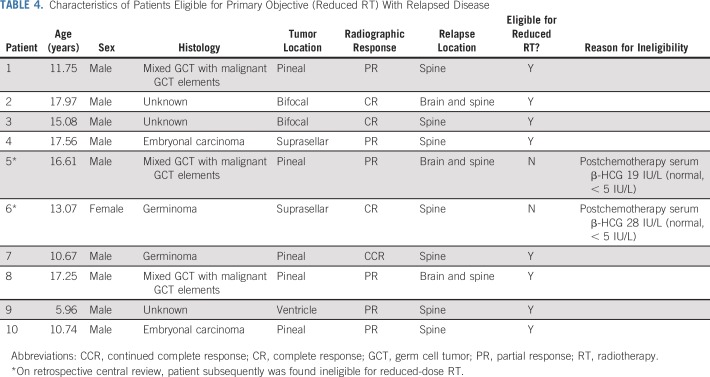
At a median follow-up of 2.97 (range, 1.84 to 5.72) years, there have been eight recurrences among the 66 eligible patients who received reduced RT. Six of these recurrences were distant relapses (in the spine) alone and two were a combination (distant and local; Table 4; Fig 3). There were two additional recurrences, one distant and one combined, that subsequently were found ineligible for reduced RT on retrospective central review. Upon learning of the 10 total recurrences, we closed the study to accrual in September 2016 because the stopping rules had been met.
TABLE 4.
Characteristics of Patients Eligible for Primary Objective (Reduced RT) With Relapsed Disease
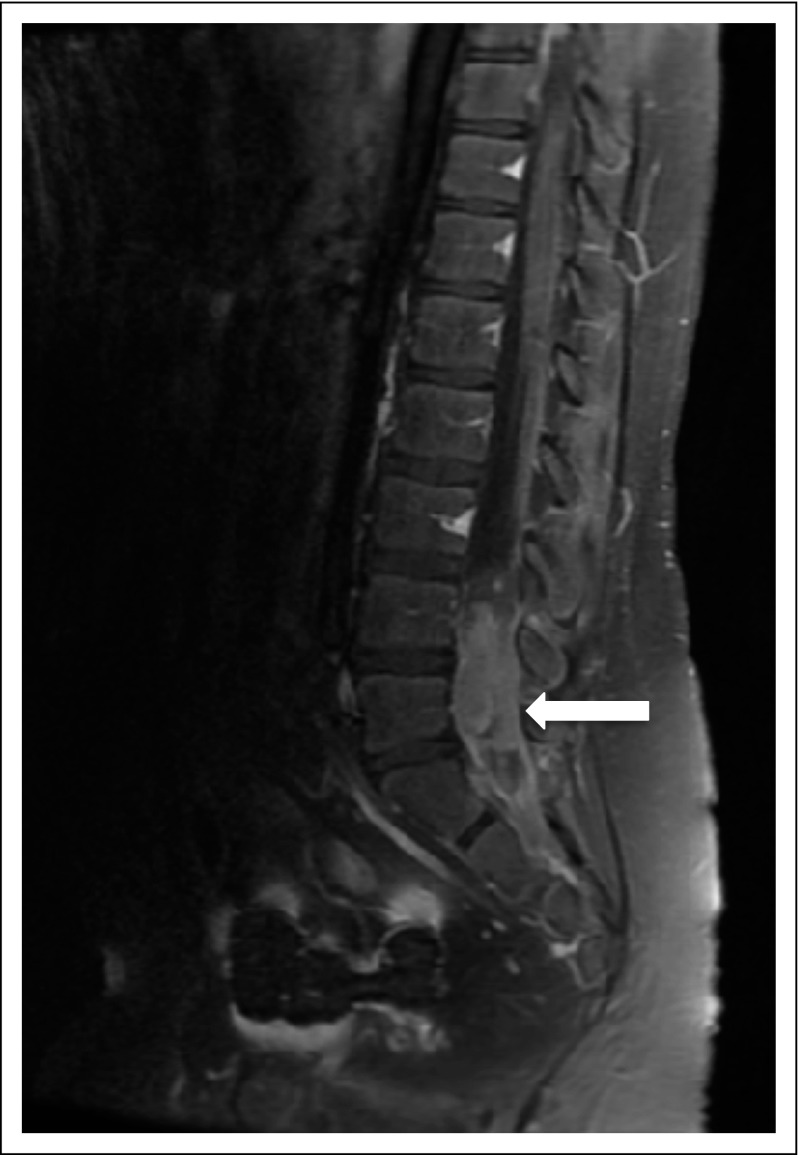
FIG 3.
Example of spinal recurrence (white arrow) in a patient with a localized nongerminomatous germ cell tumor who received reduced radiotherapy.
The study team initiated a retrospective central review of all patients enrolled, including baseline eligibility, imaging, tumor markers, response evaluations, and RT plans. This review revealed two patients who were inevaluable for the primary objective. Both patients had elevated serum β-HCG levels at the end of chemotherapy and should not have received reduced RT during the study (Table 4). One had a level of 19 IU/L and the other had a level of 28 IU/L (normal β-HCG level, < 5 IU/L). Therefore, disease recurred in only eight evaluable patients who met all eligibility criteria for the primary objective and the stopping rules, in fact, were not met. However, the study team and COG leadership made the decision to permanently close the NGGCT stratum to avoid enrollment bias and inform the scientific community of the unique pattern of spinal relapse.
Toxicity
There were no unexpected toxicities. The most common grade 3 or higher toxicities were anemia, febrile neutropenia, lymphopenia, neutropenia, thrombocytopenia, leucopenia, hypernatremia, and hyponatremia.
DISCUSSION
A combination of chemotherapy and RT has resulted in significantly improved outcomes for children with CNS NGGCT, and response to chemotherapy has consistently proven prognostic.13,14,16 In the ACNS0122 trial, patients whose disease had a CR or PR to chemotherapy had excellent survival.14 In the present study, we asked whether reduced RT in patients with localized NGGCT with a CR or PR to chemotherapy would maintain the ACNS0122 survivals while potentially minimizing late effects of CSI.
In COG ACNS0122, all recurrences were within 3 years of diagnosis, except one late recurrence, which occurred at 68 months.14 The median follow-up of the 58 patients in the present study whose disease has not progressed is 2.97 (range, 1.84 to 5.72) years, suggesting most recurrences may have already happened. It is yet unclear, however, if the timing of relapses also may have been altered and whether there will be late relapses. In ACNS0122, 5-year PFS and OS rates (± standard error) for patients with localized disease were 91.5% ± 4.1% and 97.9% ± 2.1%, respectively. In the present study, 3-year PFS and OS rates (± standard error) were 87.8% ± 4.0% and 92.4% ± 3.3%, respectively. ACNS1123 was not powered to compare resulting PFS with that of ACNS0122; therefore, there is no P value to report; however, at 3 years, the outcomes seem similar. This is noteworthy given that the treatments were almost identical with the exception of RT. The patients whose disease has not recurred have been spared full-dose CSI. Although the longitudinal neurocognitive and behavioral data are forthcoming, one can hypothesize that less than the full CSI dose will reduce these patients’ risk of late effects and potentially improve their quality of life.
Disease in eight of 66 patients (12.1%) who received reduced RT subsequently recurred; all eight recurrences involved the spine. In the SIOP 96 trial, 27 of 106 patients with localized disease had disease recurrence—14 locoregional, six combined, and seven distant.13 In the ACNS0122 trial, 16 of 102 patients (15.7%) experienced disease recurrence—10 local, one combined, three distant, and two with tumor markers alone.14 The preponderance of distant relapses in our study is concerning and most likely attributed to the elimination of spinal irradiation. Interestingly, the SIOP 96 data using focal RT did not show this same pattern of relapse. We hypothesize the difference may be the chemotherapy. The SIOP 96 trial’s chemotherapy regimen was more dose and time intense as compared with that of the present study. Although the survival outcomes are similar, a direct statistical comparison with SIOP 96 is difficult, given the differences in eligibility, staging, and treatment. One potential strategy that may minimize distant relapses is to intensify induction chemotherapy, similar to the SIOP strategy.
While COG ACNS1123 was underpowered for a statistical comparison to COG ACNS0122, a comparison of survival outcomes was intended. Although the pattern of recurrence is different between the two studies, approximately the same fraction of patients experienced disease recurrence. One hypothesis is that the lack of spinal RT has simply changed the natural history of recurrence, specifically location, in patients with biologically aggressive disease that would have recurred regardless of the volume or dose of RT. Another concern is that a lack of staging could have contributed to missed cytologic metastatic disease; however, only one of eight patients with recurrence did not undergo baseline lumbar CSF cytology (because it was medically contraindicated), suggesting this was not a major contributing factor. In fact, among the remaining 58 patients whose disease has not recurred, only five patients (8.6%) did not have baseline lumbar CSF cytology because it was medically contraindicated. Although exceedingly unlikely, the spinal recurrences could also be due to chance alone, because no direct statistical comparison can be made to ACNS0122.
Advances in CNS GCT biology may provide insight into why certain histologically identical tumors behave clinically differently. For example, distinct mRNA profiles correlate with GCT histologic differentiation and prognosis.17 The current accepted COG strategy is to diagnose many of these patients without tissue since markers alone can make a diagnosis; therefore, understanding the biology of high-risk patients may require a paradigm shift. One possibility is to begin obtaining tissue from all patients at diagnosis in an effort to identify and then validate molecular markers for future prognostication. Unfortunately, tissue samples are not available from the present study cohort to assess molecular differences. Alternatively, if novel biologic serum or CSF makers are identified and validated, these could potentially help prognostication.
Although our data and that of the ACNS0122 trial show good response rates to chemotherapy, in both trials, approximately 40% of patients did not achieve a CR or PR.14 In the ACNS0122 trial, these patients were to receive myeloablative consolidation therapy followed by autologous hematopoietic cell rescue and CSI. Only two patients went on to receive this therapy in the study, and these two patients’ disease achieved a CR between 39 and 53 months after therapy, suggesting this is an alternative strategy for patients with a poor response to induction chemotherapy14. Another consideration for patients whose disease does not achieve a CR or PR is an intensification of chemotherapy up front; however, currently, there are no reliable biomarkers that can identify the group of patients who are less likely to respond to chemotherapy, making this strategy difficult.
Unfortunately, our study enrollment, based on initial statistical design, was not completed: Only 66 of 77 patients enrolled counted toward the primary objective. If the same statistical hypothesis is considered with 5% type-1 error, enrollment of 66 patients would attain 82% power. In that scenario, the rejection boundary would be seven treatment failures. Although this technically was achieved in the 66 eligible patients, accrual was not completed to the target goal, making it difficult to predict if the threshold would have been met on the basis of the initial statistical design.
Although a thorough retrospective central review was undertaken, one limitation of the present study was the lack of a prospective central review in real time, which may have avoided the two patients who were ineligible for reduced RT continuing in the trial. Other limitations include the rarity of this tumor type, which precluded prospective randomized studies, highlighting the need for continued international collaboration.18 These small numbers also limit the power to explore other prognostic factors, such as tumor size, location, and tumor markers, as they relate to response. Another important limitation is that little information was collected regarding treatment strategies after disease recurrence in this study; hence, we do not know the salvageability of patients with spinal recurrence after reduced RT beyond the OS outcome. Finally, the follow-up is currently relatively short and thus longer-term outcomes are not yet available.
To our knowledge, these data still represent one of the largest prospective cohorts in this disease. We report encouraging survival data in children with localized NGGCT whose disease achieves a CR or PR to chemotherapy followed by reduced RT; however, the preponderance of spinal failures is concerning. We hypothesize we may have simply altered the pattern of recurrence in those patients with biologically aggressive disease. It is also noteworthy that patients without recurrence have been spared full-dose CSI. By publishing these data now, we hope to provide pediatric oncologists the most current data for comparison between reduced RT versus full-dose CSI when discussing risks and benefits to make the best informed treatment decisions with their patients and patients’ families.
AUTHOR CONTRIBUTIONS
Conception and design: Jason Fangusaro, Shannon MacDonald, Ute Bartels, Hsiao-Ming Lu, David Morris, Ashok Panigrahy, Arzu Onar-Thomas, Maryam Fouladi, Amar Gajjar, Girish Dhall
Provision of study material or patients: Amar Gajjar, Girish Dhall
Collection and assembly of data: Jason Fangusaro, Shengjie Wu, Arzu Onar-Thomas, Girish Dhall
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Response-Based Radiation Therapy for Patients With Localized CNS Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jason Fangusaro
Consulting or Advisory Role: Celgene
Mark Souweidane
Consulting or Advisory Role: Aesculap
Travel, Accommodations, Expenses: Aesculap
Hsiao-Ming Lu
Employment: Mevion Medical Systems (I)
David Morris
Employment: IQVIA (I)
Stock and Other Ownership Interests: St Louis Cyberknife
Other Relationship: St Louis Cyberknife
Arzu Onar-Thomas
Honoraria: Eli Lilly
Research Funding: Novartis (Inst), Apexigen (Inst), Pfizer (Inst), Celgene (Inst), Novartis (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eli Lilly
Amar Gajjar
Research Funding: Genentech (Inst), Kazia Pharmaceutical (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.McCarthy BJ, Shibui S, Kayama T, et al. Primary CNS germ cell tumors in Japan and the United States: An analysis of 4 tumor registries. Neuro-oncol. 2012;14:1194–1200. doi: 10.1093/neuonc/nos155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford JR, Santi MR, Vezina G, et al. CNS germ cell tumor (CNSGCT) of childhood: Presentation and delayed diagnosis. Neurology. 2007;68:1668–1673. doi: 10.1212/01.wnl.0000261908.36803.ac. [DOI] [PubMed] [Google Scholar]
- 3.Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: A review. Oncologist. 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2007. [Google Scholar]
- 5.Fujimaki T. Central nervous system germ cell tumors: Classification, clinical features, and treatment with a historical overview. J Child Neurol. 2009;24:1439–1445. doi: 10.1177/0883073809342127. [DOI] [PubMed] [Google Scholar]
- 6.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics. 1994;25:26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 7.Kellie SJ, Boyce H, Dunkel IJ, et al. Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: Failure of a primary chemotherapy approach. Pediatr Blood Cancer. 2004;43:126–133. doi: 10.1002/pbc.20026. [DOI] [PubMed] [Google Scholar]
- 8.Balmaceda C, Finlay J. Current advances in the diagnosis and management of intracranial germ cell tumors. Curr Neurol Neurosci Rep. 2004;4:253–262. doi: 10.1007/s11910-004-0046-0. [DOI] [PubMed] [Google Scholar]
- 9.da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: Results of the Third International CNS Germ Cell Tumor Study. Pediatr Blood Cancer. 2010;54:377–383. doi: 10.1002/pbc.22381. [DOI] [PubMed] [Google Scholar]
- 10.Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: From the First International CNS Germ-Cell Tumor Study. Neuro-oncol. 2001;3:174–183. doi: 10.1093/neuonc/3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avizonis VN, Fuller DB, Thomson JW, et al. Late effects following central nervous system radiation in a pediatric population. Neuropediatrics. 1992;23:228–234. doi: 10.1055/s-2008-1071348. [DOI] [PubMed] [Google Scholar]
- 12.Arain A, Herman T, Matthiesen C. Late effects of radiation therapy in pediatric cancer survivors. J Okla State Med Assoc. 2015;108:129–135. [PubMed] [Google Scholar]
- 13.Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro-oncol. 2017;19:1661–1672. doi: 10.1093/neuonc/nox122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman S, Bouffet E, Fisher PG, et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: A Children’s Oncology Group Study. J Clin Oncol. 2015;33:2464–2471. doi: 10.1200/JCO.2014.59.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Embry L, Annett RD, Kunin-Batson A, et al. Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: Can it be done? Pediatr Blood Cancer. 2012;59:536–539. doi: 10.1002/pbc.24139. [DOI] [PubMed] [Google Scholar]
- 16.Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation--a novel approach for newly diagnosed CNS germ cell tumors: Results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996;14:2908–2915. doi: 10.1200/JCO.1996.14.11.2908. [DOI] [PubMed] [Google Scholar]
- 17.Wang HW, Wu YH, Hsieh JY, et al. Pediatric primary central nervous system germ cell tumors of different prognosis groups show characteristic miRNome traits and chromosome copy number variations. BMC Genomics. 2010;11:132. doi: 10.1186/1471-2164-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray MJ, Bartels U, Nishikawa R, et al. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16:e470–e477. doi: 10.1016/S1470-2045(15)00244-2. [DOI] [PubMed] [Google Scholar]