Abstract
Glaucoma is the leading cause of irreversible blindness worldwide. Recently, estrogen deficiencies caused by early menopause, alterations in estrogen signaling via mutations in estrogen receptors, and polymorphisms along estrogen metabolic pathways have all been linked to an increased risk of developing glaucoma. Here, we examined how menopause and age impact visual function and retinal structure in an experimental model of glaucoma. Young (3–4 months) and aged (9–10 months) female Brown Norway rats were divided into pre- and post-menopausal cohorts by surgically inducing menopause via ovariectomy (OVX). After six weeks, ocular hypertension (OHT) was induced unilaterally for a period of eight weeks. Four cohorts were successfully followed to eight weeks: young sham (n = 8), young OVX (n =9), aged sham (n =10), and aged OVX (n =11) animals. Intraocular pressure (IOP) was monitored weekly in all groups. Prior to inducing OHT (baseline) and at four and eight weeks after inducing OHT, we assessed visual acuity via the optomotor response (OMR) and retinal structure using optical coherence tomography (OCT). OHT decreased the OMR in all cohorts. We found that spatial frequency thresholds decreased by 54% in OVX animals after OHT compared to sham animals after OHT, regardless of age (p < 0.001). We also found thinning of the retinal nerve fiber layer (RNFL) and loss of total retinal thickness after induction of OHT. Aged animals had more thinning of the RNFL and loss of total retinal thickness compared to young animals (p < 0.001). Overall, OHT caused significant changes in visual function and retinal structure. Observing that OVX in young and aged animals further decreased spatial frequency thresholds after OHT suggests that an estrogen deficiency may intensify visual impairment after OHT.
Keywords: Glaucoma, Menopause, Estrogen, Visual function
1. Introduction
Glaucoma is the second leading cause of blindness worldwide, estimated to affect 80 million people by 2020, (Quigley and Broman, 2006) with an annual economic burden of $2.9 billion in the United States alone (Quigley and Broman, 2006; Morrison and Pollack, 2003). Glaucomatous damage is characterized by distinctive patterns of visual field and retinal ganglion cell (RGC) axon loss (Morrison and Pollack, 2003). While gender is not usually considered a risk factor for developing glaucoma, women represent 59% of the glaucomatous population, and the number of women suffering from glaucoma is thought to be underreported (Vajaranant and Pasquale, 2012). Recent evidence suggests that estrogen deficiencies or alterations in the estrogen pathway are linked to developing glaucoma in both genders (Mabuchi et al., 2010; Pasquale et al., 2013; de Voogd et al., 2008).
Menopause marks a decline in circulating hormones produced by the ovaries preventing menstruation. Early menopause (< 45 years of age), estimated to occur in 15.2 million women, may increase a woman’s risk for developing glaucoma by 2 to 3-fold (Vajaranant and Pasquale, 2012; Mabuchi et al., 2010). In addition, mutations in estrogen receptors or polymorphisms along estrogen metabolic pathways are also linked to an elevated risk of developing glaucoma in men and women (Mabuchi et al., 2010; Pasquale et al., 2013; de Voogd et al., 2008). Thus, it is important to understand how an estrogen deficiency or menopause may be related to glaucomatous damage and the subsequent visual impairment.
To study the impact of estrogen loss and model menopause, researchers frequently use ovariectomy (OVX), the surgical removal of the ovaries. While ovaries are not solely responsible for estrogen production, this surgical approach is well-established and causes a rapid decline in the systemic release of estrogen and progesterone (Moalli et al., 2008; Urbankova et al., 2018; Koebele and Bimonte-Nelson, 2016). In a diabetic rodent model, OVX led to worse rod and cone function measured on full-field electroretinogram (ERG) compared to control animals, (Yamashita et al., 2010) suggesting interaction between OVX and diabetes on retinal function.
Surgical menopause has been related to worse outcomes in animal models of glaucoma. In DBA/2J mice, which exhibit age-related ocular hypertension (OHT) and loss of RGCs, OVX led to a significantly greater loss of RGCs compared to non-OVX controls (Zhou et al., 2007). This study also showed elevated levels of the pro-inflammatory mediator interleukin-18 in OVX mice compared to non-OVX controls. The onset of OHT in DBA/2J mice occurs spontaneously. Therefore, studying the interaction of age and OVX in these animals is challenging due to the variable onset of OHT.
Taken together, these findings demonstrate a knowledge gap as to how menopause directly impacts visual function in glaucoma and highlight the need for a better understanding of the role of both age and estrogen in visual function in glaucoma models. Since early menopause elevates the risk of developing glaucoma in the clinical population, (Vajaranant and Pasquale, 2012; Hulsman et al., 2001) we believe it is also important to understand how the timing of menopause affects visual function in glaucoma. Thus, our goal was to study how menopause, modeled using OVX, and age together impact the progression of vision loss in an experimental model of glaucoma in which OVX and OHT can be induced at well-defined times. We hypothesized that menopause in younger animals would lead to greater visual impairment in animals exposed to OHT compared to older menopausal animals.
2. Methods
2.1. Animals and experimental design
We used young (3–4 months) and aged (9–10 months) female Brown Norway rats (Charles River, Wilmington, MA) as of the date of OVX. Animals were divided into sham surgery and OVX groups, resulting in the following four groups: 1) young sham, 2) young OVX, 3) aged sham, and 4) aged OVX. Experiments were performed on two separate cohorts of animals consisting of eight animals per group resulting in a total of 16 animals per group. We chose OVX because it is a well-established and well-characterized model of menopause, (Moalli et al., 2008; Urbankova et al., 2018) known to reliably cause a rapid decline in systemic estrogen and progesterone levels (Koebele and Bimonte-Nelson, 2016). This model allows us to induce a post-menopausal state in animals at a specific time point, which gives us an effective animal model to study the consequences of menopause on visual function in an experimental model of glaucoma. In addition, we chose these ages because rats typically enter estropause, analogous to menopause in humans, around 15 months of age (Sengupta, 2013). These ages allowed us to conduct our study prior to estropause in all cohorts.
Animals were housed under standard conditions (12:12hr light:dark cycle in 450–650 lux) with chow and water provided ad libitum. Three weeks after OVX or sham surgery, animals were moved from the standard lighting conditions into a constant low-level light environment with lights on 24 h a day at 40–90 lux to minimize the diurnal influence on the circadian fluctuations in intraocular pressure (IOP) (Morrison et al., 2005). Animals were given two weeks to acclimate to the constant light environment before all baseline measurements were performed.
We then performed baseline IOP, optomotor response (OMR), and spectral domain – optical coherence tomography (SD-OCT) assessments on all groups. A week after baseline measurements, animals underwent surgery to induce experimental glaucoma (described below). IOP was measured weekly, while additional OMR and SD-OCT assessments were made at four and eight weeks after successfully inducing experimental glaucoma. A timeline of the experimental design is displayed in Fig. 1. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Atlanta VA Medical Center (VAMC) and conformed to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. At the termination of experimental procedures, animals were euthanized via slow inhalation of CO2 and confirmed by using an approved secondary method in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association (AVMA).
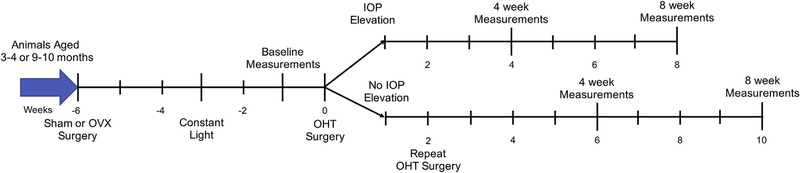
Fig. 1. Timeline of experimental design.
Each tick mark represents one week of time. Animals were aged to either 3–4 months or 9–10 months before being divided to undergo sham or OVX surgery. Three weeks post-OVX surgery, animals were moved into constant light conditions to minimize fluctuations in IOP. After a two week habitualization period, baseline IOP, OMR, and retinal structural measurements were recorded. The following week, we performed OHT surgery to induce experimental glaucoma. If no elevation in IOP was observed within two weeks, animals underwent a second hypertonic saline injection (lower line). Four and eight weeks after successful OHT surgery, we performed OMR and OCT measurements. IOP was measured weekly throughout the experiment.
2.2. Surgical menopause
All surgeries were performed at Charles River by in-house technicians prior to shipment to the Atlanta VAMC. For OVX and sham surgeries, animals were sedated using isoflurane (2–4%) and placed on a heating pad. A small dorsal incision was made, and the fallopian tubes and ovaries were individually isolated. The fallopian tubes were cauterized, and the ovaries were removed. The skin incision was closed with wound clips, and the animals received buprenorphine (0.05 mg/kg) as an analgesic postoperatively. Eyes were kept moist via saline throughout the duration of the surgery. For sham surgery, i.e. control for the effect of OVX surgery, animals underwent the same procedure to expose the fallopian tubes and ovaries, but no tissues were removed. At the time of euthanasia, we confirmed a successful OVX or sham surgery by visual inspection. In brief, pelvic floor tissues were dissected and the absence or presence of ovaries was noted. If the ovaries were not successfully removed the animal was removed from the study.
2.3. IOP measurements
IOPs were measured using a TonoLab (Icare Finland Oy, Vantaa, Finland) in awake animals, i.e. without general or topical anesthesia. We independently calibrated the tonometer on a cannulated eye, in which IOP was externally set to pressures from 5 to 50 mmHg by an external reservoir (data not shown). Prior to recording baseline IOPs, rats were trained over three separate training days spaced one day apart. During IOP measurements the animals were gently restrained by hand, and eight machine-generated readings were recorded on each eye. The lowest and highest IOP values were removed, and the remaining six measurements were averaged to estimate the animal’s IOP. We took IOP measurements weekly throughout the study, always between 9:00–11:00 a.m. local time.
2.4. Experimental glaucoma model
Our experimental model of glaucoma was modified from the established Morrison model of OHT, which is described elsewhere (Morrison et al., 1997). In brief, rats were anesthetized with a mixture of ketamine (60 mg/kg) and xylazine (7.5 mg/kg) followed by topical tetracaine (1%) eye drops applied to the cornea. Rats were placed onto a heating pad and one eye (randomized between OD and OS) was proptosed to inject a randomly selected episcleral vein with 50 μL of a sterile hypertonic saline (1.75 M) solution under a surgical microscope. After injection, the needle was held in place for thirty seconds to limit drainage of the hypertonic saline solution. The hypertonic saline induces an inflammatory response and damages the outflow pathway, causing a unilateral IOP elevation (Morrison et al., 1997). The contralateral eye served as a non-operated control, and during the procedure the control eye was kept moist with 1% carboxymethylcellulose sodium (Refresh Celluvisc Allergan Parsippany, NJ).
After the injection, eyes were treated with topical neomycin antibiotic (Certi-Sporyn, Fisher Scientific, Waltham, MA), and the animals remained on a heating pad until fully recovered from the anesthesia. Animals were given a minimum of five days to recover before any assessments were performed. We monitored IOP over two weeks to determine if we induced OHT. We defined successful induction of OHT as greater than a 5 mm Hg rise in IOP compared to the contralateral eye and baseline measurements of the operated eye. Only animals in which we did not observe an IOP elevation after two weeks underwent a second injection in the same eye, using a second, previously uninjected episcleral vein. If no elevation occurred after the second injection, the animal was removed from the study.
2.5. Visual function assessment
We used the rodent optomotor response (OMR) to assess visual function (OptoMotry®; Cerebral-Mechanics, Lethbridge, AB, Canada) (Douglas et al., 2005; Prusky et al., 2004). The OMR was assessed by a masked observer. The awake rat was placed on a platform in the middle of a virtual reality chamber consisting of four flat screen computer monitors displaying vertical sinewave gratings that virtually rotated at a speed of 12 deg/s. We observed the rat through a video camera mounted above the platform and monitored for a positive or negative reflexive head movement in response to the rotating gratings moving in a clockwise or counter clockwise direction, representing the responses of the left and right eyes, respectively (Douglas et al., 2005; Prusky et al., 2004). Spatial frequency and contrast sensitivity thresholds were calculated using the OMR software. To determine an animal’s spatial frequency threshold, the vertical bands were displayed at 100% contrast starting at 0.042 cycles/degree, and the spatial frequency was adjusted (between 0 and 0.68 cycles/degree) until the rat no longer demonstrated an OMR. During the contrast sensitivity assessment, the spatial frequency was held constant at 0.064 cycles/degree, while the contrast was decreased from 100% until the animal no longer displayed a reflexive response. We reported contrast sensitivity as the reciprocal of the Michelson contrast from the screen’s luminance, as previously described (Prusky et al., 2004).
2.6. Optic nerve head imaging
A SD-OCT system (Bioptigen 4300, Leica Microsystems, Buffalo Grove, IL) was used to evaluate the structure of the tissues adjacent to the optic nerve head. Animals were anesthetized using a combination of ketamine and xylazine as described above, each eye was given topical anesthesia (tetracaine 1%), and the pupils were dilated (tropicamide 1%). We then acquired a 3-mm radial scan (1000 A-scans per B-scan) centered at the optic nerve head in both OHT and control eyes. Our imaging protocol acquired four B-scans consisting of 24 individual frames per scan; however, we limited our analysis to the superior-inferior and nasal-temporal scans.
B-scans of all eyes were assessed manually by a trained technician blinded to treatment group using a customized MATLAB program (MATLAB R2017a, Mathworks, Natick, MA). Retinal nerve fiber layer (RNFL) thickness (inner limiting membrane to the boundary of the inner plexiform layer) and total retinal thickness (inner limiting membrane to the retinal pigment epithelium) were measured at a point located 0.5 mm radially outward from the center of the optic nerve head at four locations (superior, inferior, nasal, or temporal). Measurements were taken at locations avoiding and local vasculature, and these individual values from each quadrant were averaged together.
2.7. Statistics
Data in all figures are represented as boxplots with the median and interquartile range (25th to 75th quartiles). The whiskers or error bars represent the data ranging from the 10th to 90th percentiles, while differences are displayed as the percent difference between groups (95% confidence interval). We used a repeated mixed ANOVA design to determine if age or OVX affected visual function or retinal structure after inducing OHT (SPSS v22, IBM Analytics, Armond, NY). We then performed post-hoc analysis at specific time points and assessed group differences as well as a linear trend test using linear combinations of the appropriate coefficients in the model. We defined differences as being statistically significant at p < 0.05.
We performed a Pearson correlation to understand how changes in the OMR (e.g. spatial frequency threshold and contrast sensitivity) related to changes in RNFL thickness and total retinal thickness in OHT eyes. We used a Bonferroni correction to account for multiple comparisons (α = 0.05/4 = 0.0125). Further, we defined three levels of correlation using Pearson’s correlation coefficient: weak (r < 0.39), moderate (0.40 < r < 0.59) and strong (r > 0.60) (Evans, 1996). Data are represented as r with a subscript denoting the degrees of freedom.
3. Results
3.1. Impact of experimental glaucoma on visual function and retinal structure
The overall success rate after two injections to induce OHT was 60% across all four groups: young sham (8/16), young OVX (9/16), aged sham (10/16), and aged OVX (11/16). This success rate is similar to previously published values using this model of OHT (Morrison et al., 1997). We observed a significant increase in IOP due to OHT induction compared to the contralateral control eyes (F8, 629 = 10.41, p < 0.001) within one week after a successful surgery (Fig. 2A). We did not find any significant impacts of age (F8, 612 = 1.76, p = 0.08) or menopausal status (F8, 612 = 0.8, p = 0.6) on IOP across our groups (Supplementary Fig. 1).
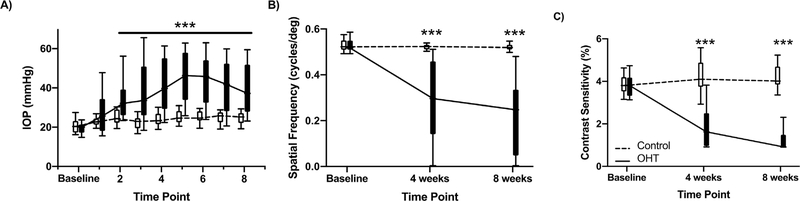
Fig. 2. Characterization of OHT Model.
Results display the impact of OHT across all groups (n = 38 animals) between OHT eyes and contralateral eyes (Control). A) IOP for OHT and contralateral control eyes. We found a significant increase in IOP one week after successfully inducing OHT, and IOP remained elevated for eight weeks. B) After inducing OHT, there was a decrease in spatial frequency threshold at four and eight weeks. C) We also found a decline in contrast sensitivity after four and eight weeks of OHT. Data are presented as boxplots with whiskers displaying the 10th-90th percentile. *** denotes p < 0.001 for multiple comparisons.
After inducing OHT, we found a decline in both spatial frequency threshold (F2, 202 = 62.81, p < 0.001) and contrast sensitivity (F2, 200 = 46.88, p < 0.001) that worsened over eight weeks (Fig. 2B and C). The spatial frequency threshold in OHT eyes decreased by 55% (CI: 43%–65%) and 72% (CI: 63%–79%) after four and eight weeks, respectively. Contrast sensitivity thresholds in OHT eyes also declined after inducing OHT with a 54% (CI: 45%–61%) decrease at four weeks and a 71% (CI: 68%–73%) decrease after eight weeks.
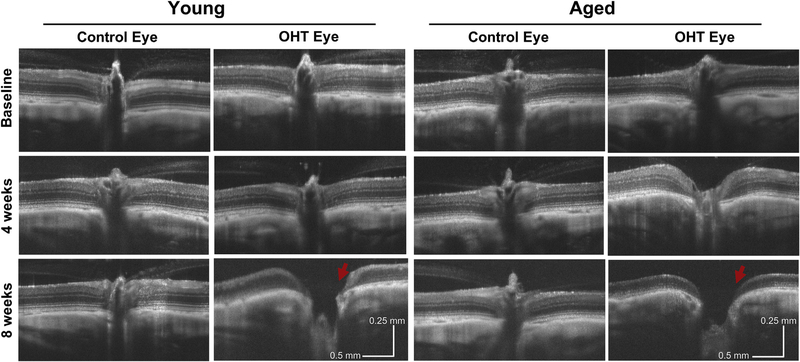
Similar to previous reports, we observed structural changes in SD-OCT images of the retina and optic nerve head from both young and aged animals after inducing OHT, with progressive cupping of the optic nerve head in both groups (Fig. 3). Characteristic of glaucoma, we found that OHT caused thinning of the RNFL (F2, 163 = 24.95, p < 0.001) and the total retina (F2, 164 = 25.25, p < 0.001; Fig. 4A and B).
Fig. 3. Structural changes at the optic nerve head in young and aged rats after OHT.
Representative SD-OCT images of the optic nerve head of Sham-operated young (left) and aged (right) animals. Images are taken from the control and OHT eye of one young and aged animal at each time point in the study. In both age groups, the control eye remained unchanged over eight weeks, however, the OHT eye in both groups underwent remodeling. Both groups typically displayed cupping (red arrows) after eight weeks. This was consistent with changes in the RNFL and total retinal thicknesses (Fig. 4A and B). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
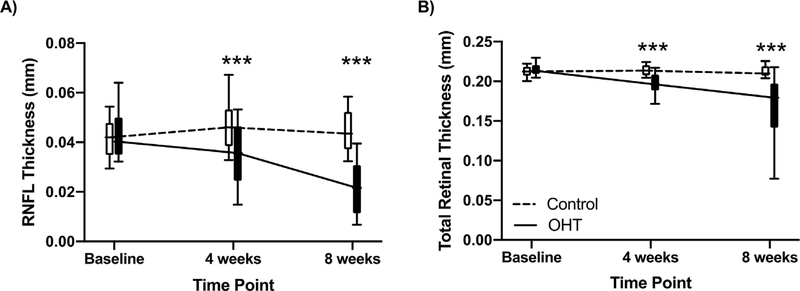
Fig. 4. Retinal Structural Changes after OHT.
Data shown are for all animals (n = 38) comparing OHT eyes and contralateral eyes (Control). We found thinning of the RNFL (A) and total retina (B) after inducing OHT. Data are represented as boxplots with bars representing the 10th and 90th percentile. *** denotes p < 0.001 for multiple comparisons.
3.2. Effect of menopausal status and age on visual function
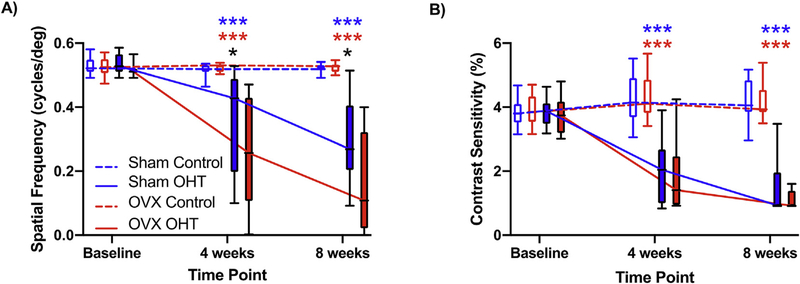
We found that spatial frequency thresholds across all age groups were significantly dependent on menopausal status, OHT, and time point after OHT (F2, 197 = 3.54, p = 0.031; Fig. 5A). We found that OHT eyes in OVX animals had a 54% (CI: 22%–73%) lower spatial frequency threshold at eight weeks compared to the OHT eyes of sham-operated animals. However, we did not observe a significant interaction related to age (F2, 198 = 1, p = 0.37), since OVX animals from both young and aged cohorts had a comparable decline in spatial frequency thresholds after OHT compared to sham-operated animals (Supplementary Fig. 2).
Fig. 5. OVX potentiated the decrease in spatial frequency threshold due to OHT.
Data are shown from Sham (n = 18) and OVX (n = 20) animals across both young and aged cohorts. A) Both Sham and OVX animals had a lower spatial frequency threshold after OHT (***p < 0.001) compared to their respective controls, but this effect was more pronounced in OVX animals vs. Sham-operated animals (*p < 0.05). B) Contrast sensitivity thresholds declined in Sham and OVX animals compared to their respective controls (***p < 0.001). However, we did not observe a significant impact of menopausal status, although OVX animals displayed a weak trend towards lower contrast sensitivity at 8 weeks. Blue asterisks compare Sham control and OHT eyes, red asterisks compare OVX control and OHT eyes, while the black asterisks compare Sham and OVX OHT eyes. All data are presented as boxplots with bars representing the 10th and 90th percentile. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In regards to contrast sensitivity, we did not find an interaction between menopausal status, OHT, and time points (F2, 195 = 0.39, p = 0.68; Fig. 5B). Contrast sensitivity was reduced by 4% (CI: −27% −27%) and 17% (CI: 3%–30%) at four and eight weeks, respectively, in OVX compared to sham-operated rats after OHT. We also did not find a significant interaction between age, OHT, and time for contrast sensitivity (F2, 195 = 1.25, p = 0.29; Supplementary Fig. 2).
3.3. Impact of menopause and age on retinal structure
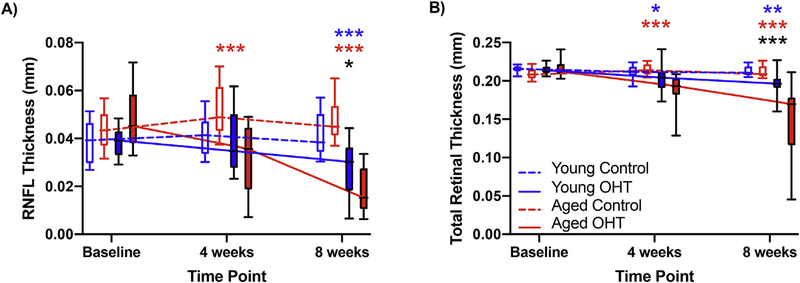
We did not find a significant impact of menopausal status on thinning of the RNFL thickness (F2, 157 = 0.46, p = 0.63) or total retinal thickness over time (F2, 161 = 0.74, p = 0.48; Supplementary 3). However, we did find more RNFL thinning in aged animals compared to young animals in OHT eyes across time (F2, 159 = 5.86, p = 0.003; Fig. 6A). Finally, we found a significant interaction between age, OHT, and time in total retinal thinning (F2, 161 = 11.13, p < 0.001; Fig. 6B).
Fig. 6. Retinal Structural Changes.
Data are shown from young (n =17) and aged (n =21) animals averaged together from Sham and OVX groups A) Both young and aged cohorts had decreased RNFL thickness after eight weeks of OHT. Compared to young animals, the RNFL was thinner in aged animals at eight weeks. B) After inducing OHT, aged animals had more total retinal thinning compared to young animals. However, both young and aged animals had thinning of the retina after four and eight weeks of OHT compared to the control eyes. Results are displayed as the boxplots with bars representing the 10th and 90th percentile. Statistical significance: *p < 0.05, **p < 0.01, and ***p < 0.001. Blue asterisks compare Sham control and OHT eyes, red asterisks compare OVX control and OHT eyes, while the black asterisks compare Sham and OVX OHT eyes. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Visual function correlates with retinal structural changes
In OHT eyes, we examined how changes in visual function outcomes correlated with optic nerve head structural changes. We found that spatial frequency threshold had a moderate correlation to RNFL thickness r88 = 0.51 (CI: 0.34–0.66) and to total retinal thickness r88 = 0.59 (CI: 0.43–0.71). We also found that contrast sensitivity had a moderate correlation to RNFL thickness r88 = 0.52 (CI: 0.34–0.66) and to total retinal thickness r88 = 0.5 (CI: 0.32–0.64). All correlations were statistically significant (p < 0.001; Supplementary Fig. 4).
4. Discussion
Our main objective was to determine how age of menopause onset affects visual function and retinal structure in experimental glaucoma. Based on evidence that early menopause increases a woman’s risk of developing glaucoma, we hypothesized that OVX in young animals with OHT would lead to worse visual function than in aged animals with OHT. Contrary to this original hypothesis, we found that menopause caused a decline in visual function after OHT independent of age. In addition, we hypothesized that after OHT, menopause in young animals would lead to increased thinning of the retina compared to aged menopausal animals. However, our results showed that retinal thinning was dependent on age of animals and independent of menopausal status. Thus, menopause at any age exacerbates visual dysfunction in experimental glaucoma in the OHT model, while menopause did not influence retinal thinning in OHT. We do not have a mechanistic understanding of how menopause worsens visual dysfunction in glaucoma, and suggest that the model presented here will be useful in gaining insights into this important issue.
4.1. Visual function worsens after OVX in OHT eyes
We found a main effect of menopausal status independent of the experimental ages we examined, with OVX having a negative impact on visual function after OHT for both young (3–4 months) and aged (9–10 months) rats. Similar to previous work, (Feng et al., 2013; Sun et al., 2017; Prokai-Tatrai et al., 2013) our results show that OHT reduces spatial frequency threshold and contrast sensitivity. Importantly, we found a significant effect of menopausal status on spatial frequency threshold after OHT but not on contrast sensitivity. Contrast sensitivity may be affected prior to visual field defects in glaucoma patients (Amanullah et al., 2017; Breton et al., 1991). Based on our experimental time points and the rapid decline in visual function in this OHT model, an effect of menopause on contrast sensitivity may not have been detectable here. Assessing the effect of menopausal status on contrast sensitivity in OHT may require more gradual elevations in IOP or examination of earlier time points after inducing OHT.
Several studies in non-glaucomatous humans illustrate that menopause alone lowers spatial frequency and contrast sensitivity (Guaschino et al., 2003; Siesky et al., 2008). However, we did not observe differences between sham and OVX groups at baseline (prior to OHT) visual function measurements. To observe an influence of menopausal status independent of OHT we may need to use more sensitive visual assessments or a longer duration of OVX. Thus, examining alternative methods to assess visual acuity, such as the Morris water maze or measuring the OMR over an extended period of time after OVX, could help determine if menopausal status has a main effect on visual function in the rodent.
4.2. OHT in aged animals increases retinal structural defects
We observed thinning of the RNFL and total retina after inducing OHT in all cohorts, which is consistent with previous studies using animal models of OHT that assessed RNFL thickness (Celebi and Mirza, 2013; Schuman et al., 2007; Gardiner et al., 2012). Abbott et al. examined the impact of age on retinal thickness after three weeks of OHT in rats (Abbott et al., 2014). They reported a 7% thinning of the RNFL in young rats compared to 22% in aged rats after three weeks of OHT (Abbott et al., 2014). While we examined the RNFL at later time points post-OHT (four and eight weeks), we also found that aged rats had a larger degree of RNFL thinning compared to young animals after OHT. In addition, young animals had an 8% decrease in total retinal thickness compared to 38% in aged animals.
However, we did not observe a significant impact of menopausal status on retinal dimensions, suggesting that the effect of OVX are smaller than that of OHT on retinal thickness. A previous study in non-glaucomatous women found that postmenopausal women had local thinning of the RNFL compared to premenopausal women, but after accounting for age, these differences were no longer significant (Atas et al., 2014). Therefore, it is also possible that menopause does not greatly affect the thickness of the retina compared to the effects of age and OHT.
Our finding of greater thinning of the RNFL and total retina in aged animals that was not related to OXV is intriguing because we found menopausal status but not age lead to worse visual function. Previous studies describe the optic nerve head in aged animals as more susceptible to damage due to elevated IOP, (Albon et al., 2000; Burgoyne, 2011) consistent with our findings of greater loss of RNFL and total retinal thickness in aged rats after inducing OHT. It is possible that our visual function measurements are not sensitive enough to detect changes before there are structural manifestations, or that there is sufficient redundancy in the visual pathway that structural change precedes functional change as is seen in human glaucoma (Murphy et al., 2016; Wollstein et al., 2012). Therefore, measuring retinal thickness earlier and more frequently or visual function with more sensitive approaches may reveal a combined impact of age and menopausal status on visual function and retinal structure.
4.3. The relationship between retinal structure and visual function
We found modest correlations between our OMR outcomes and structural changes of the retina. In humans, RNFL thickness has been shown to have a modest correlation (0.40 < r 0.59) with visual field deficits in glaucoma patients (Alasil et al., 2014; Kang et al., 2015; Kim et al., 2014; Wu et al., 2015). Although visual fields are a different measure of visual function than used in our study, we also found a modest correlation between RNFL thickness and total retinal thickness and both spatial frequency threshold and contrast sensitivity. These correlations suggest that visual function measurements in rodents may be informative about the structural health of the retinal ganglion cells and overall retina when it is difficult to determine RNFL thickness.
4.4. Limitations
This study allowed us to assess the impact of surgically-induced menopause on visual function and retinal structural changes longitudinally, but it does have some potential limitations. First, the OVX model of menopause causes a rapid decline in circulating hormones, (Koebele and Bimonte-Nelson, 2016) but does not model the natural decline in hormones associated with menopause. However, it is noteworthy that surgical removal of the ovaries in young women also increases the risk of developing glaucoma, (Vajaranant et al., 2014) suggesting that early hormonal loss is important regardless of whether it occurs naturally or due to surgery. We also did not directly measure estrogen levels in OVX and sham rats. However, OVX is an established and well-characterized model of menopause, (Moalli et al., 2008; Urbankova et al., 2018) and causes the decline of systemic estrogen and progesterone (Koebele and Bimonte-Nelson, 2016). Here, we assayed the success of the OVX surgery in experimental animals by confirming the removal of the ovaries. An additional limitation of the OVX model is the impact on blood pressure, (Lopez-Ruiz et al., 2008) which may affect IOP. However, previous studies have shown that arterial pressure only weakly affects IOP (0.21 mmHg IOP change per 10 mmHg systolic blood pressure change in humans) (Klein et al., 2005). The magnitude of expected arterial pressure changes after OVX in rats is 10–15 mmHg, (Fabricio et al., 2017) which is expected to have a minimal impact on IOP (< 0.4 mmHg). Here, we induced IOP elevations > 5 mmHg; therefore, we believe the impact of OVX on blood pressure was not an influence on our results and conclusions in the present study.
A second potential limitation is the quick onset of OHT in this experimental model of glaucoma (Morrison et al., 1997) which is not typically observed in open-angle glaucoma patients. While rat models of glaucoma display a changes in retina thinning, optic nerve head cupping, visual loss and astrocyte activation, (Prokai-Tatrai et al., 2013; Lye-Barthel et al., 2013; Burgoyne et al., 2005; Yang et al., 2007) none of the current experimental models of glaucoma in rats recapitulate the slow progression observed clinically. Even with this limitation, we still observed an impact of menopausal status on visual function, indicating that menopause alone may be an important aspect to study related to glaucoma progression.
5. Conclusions
The goal of this initial study was to evaluate the impact of menopause on visual function and retinal structure in an animal model of glaucoma. Our key findings were that menopause worsens the decline in visual function after inducing OHT, while not further altering retinal structure within eight weeks. Therefore, menopause may play an independent and important role in visual function loss after OHT. Future studies will aim to determine potential mechanisms of how menopausal status impacts visual function in the context of OHT. Overall, these data support the idea that menopause exacerbates glaucoma progression and motivate further investigation into the impact of menopause in glaucoma.
Supplementary Material
Acknowledgments
We thank Mary Kelley, PhD, at Emory University for her assistance with the statistical analysis. This work was partially funded by the RR&D Service Career Development Award (RX002342; AJF), RR&D Service Career Development Award (RX002928; RA), RR&D Service Research Career Scientist Award (C9257; MTP), and the Georgia Research Alliance (CRE). The opinions expressed herein do not necessarily reflect those of the Department of Veterans Affairs, the National Institutes of Health, or the U.S. Government.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2019.107706.
References
- Abbott CJ, et al. , 2014. Comparison of retinal nerve fiber layer thickness in vivo and axonal transport after chronic intraocular pressure elevation in young versus older rats. PLoS One 9, e114546. 10.1371/journal.pone.0114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasil T, et al. , 2014. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am. J. Ophthalmol. 157, 953–959. 10.1016/j.ajo.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albon J, Purslow PP, Karwatowski WS, Easty DL, 2000. Age related compliance of the lamina cribrosa in human eyes. Br. J. Ophthalmol. 84, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanullah S, et al. , 2017. The relationship between contrast sensitivity and retinal nerve fiber layer thickness in patients with glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol.= Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 255, 2415–2422. 10.1007/s00417-017-3789-4. [DOI] [PubMed] [Google Scholar]
- Atas M, et al. , 2014. Evaluation of the macula, retinal nerve fiber layer and choroid thickness in postmenopausal women and reproductive-age women using spectral-domain optical coherence tomography. Prz Menopauzalny 13, 36–41. 10.5114/pm.2014.41088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton ME, Wilson TW, Wilson R, Spaeth GL, Krupin T, 1991. Temporal contrast sensitivity loss in primary open-angle glaucoma and glaucoma suspects. Investig.Ophthalmol. Vis. Sci. 32, 2931–2941. [PubMed] [Google Scholar]
- Burgoyne CF, 2011. A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma. Exp. Eye Res. 93, 120–132. 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT, 2005. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 24, 39–73. 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Celebi AR, Mirza GE, 2013. Age-related change in retinal nerve fiber layer thickness measured with spectral domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 54, 8095–8103. 10.1167/iovs.13-12634. [DOI] [PubMed] [Google Scholar]
- de Voogd S, et al. , 2008. Estrogen receptors alpha and beta and the risk of open-angle glaucoma: the Rotterdam Study. Arch. Ophthalmol. 126, 110–114. 10.1001/archopht.126.1.110. [DOI] [PubMed] [Google Scholar]
- Douglas RM, et al. , 2005. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 22, 677–684. 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- Evans JD, 1996. Straightforward Statistics for the Behavioral Sciences. Brooks and Cole Publishing. [Google Scholar]
- Fabricio V, et al. , 2017. Resveratrol treatment normalizes the endothelial function and blood pressure in ovariectomized rats. Arq. Bras. Cardiol. 108, 116–121. 10.5935/abc.20170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Chen H, Suyeoka G, Liu X, 2013. A laser-induced mouse model of chronic ocular hypertension to characterize visual defects. J. Vis. Exp. 10.3791/50440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SK, Fortune B, Wang L, Downs JC, Burgoyne CF, 2012. Intraocular pressure magnitude and variability as predictors of rates of structural change in nonhuman primate experimental glaucoma. Exp. Eye Res. 103, 1–8. 10.1016/j.exer.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaschino S, et al. , 2003. Visual function in menopause: the role of hormone replacement therapy. Menopause 10, 53–57. [DOI] [PubMed] [Google Scholar]
- Hulsman CA, et al. , 2001. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am. J. Epidemiol. 154, 138–144. [DOI] [PubMed] [Google Scholar]
- Kang EM, Hong S, Kim CY, Seong GJ, 2015. Relationship between peripapillary retinal nerve fiber layer thickness measured by optical coherence tomography and visual field severity indices. Korean J. Ophthalmol. 29, 263–269. 10.3341/kjo.2015.29.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee HS, Kim NR, Seong GJ, Kim CY, 2014. Relationship between visual acuity and retinal structures measured by spectral domain optical coherence tomography in patients with open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 55, 4801–4811. 10.1167/iovs.13-13052. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Knudtson MD, 2005. Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br. J. Ophthalmol. 89, 284–287. 10.1136/bjo.2004.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebele SV, Bimonte-Nelson HA, 2016. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas 87, 5–17. 10.1016/j.maturitas.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF, 2008. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am. J. Physiol. Heart Circ. Physiol. 295, H466–H474. 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye-Barthel M, Sun D, Jakobs TC, 2013. Morphology of astrocytes in a glaucomatous optic nerve. Investig. Ophthalmol. Vis. Sci. 54, 909–917. 10.1167/iovs.12-10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi F, et al. , 2010. Estrogen receptor beta gene polymorphism and intraocular pressure elevation in female patients with primary open-angle glaucoma. Am. J. Ophthalmol. 149, 826–830. 10.1016/j.ajo.2009.12.030. e821–822. [DOI] [PubMed] [Google Scholar]
- Moalli PA, Debes KM, Meyn LA, Howden NS, Abramowitch SD, 2008. Hormones restore biomechanical properties of the vagina and supportive tissues after surgical menopause in young rats. Am. J. Obstet. Gynecol. 199 10.1016/j.ajog.2008.01.042. 161 e161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Pollack IP, 2003. Glaucoma Science and Practice. Thieme Medical. [Google Scholar]
- Morrison JC, et al. , 1997. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 64, 85–96. 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Morrison JC, Johnson EC, Cepurna W, Jia L, 2005. Understanding mechanisms of pressure-induced optic nerve damage. Prog. Retin. Eye Res. 24, 217–240. 10.1016/j.preteyeres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Murphy MC, et al. , 2016. Retinal structures and visual cortex activity are impaired prior to clinical vision loss in glaucoma. Sci. Rep. 6, 31464 10.1038/srep31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale LR, et al. , 2013. Estrogen pathway polymorphisms in relation to primary open angle glaucoma: an analysis accounting for gender from the United States. Mol. Vis. 19, 1471–1481. [PMC free article] [PubMed] [Google Scholar]
- Prokai-Tatrai K, et al. , 2013. 17beta-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol. Pharm. 10, 3253–3261. 10.1021/mp400313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM, 2004. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investig.Ophthalmol. Vis. Sci. 45, 4611–4616. 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT, 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90, 262–267. 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman JS, et al. , 2007. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Investig.Ophthalmol. Vis. Sci. 48, 3645–3654. 10.1167/iovs.06-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P, 2013. The laboratory rat: relating its age with human’s. Int. J. Prev. Med. 4, 624–630. [PMC free article] [PubMed] [Google Scholar]
- Siesky BA, et al. , 2008. Comparison of visual function and ocular hemodynamics between pre- and post-menopausal women. Eur. J. Ophthalmol. 18, 320–323. [DOI] [PubMed] [Google Scholar]
- Sun D, Moore S, Jakobs TC, 2017. Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J. Exp. Med. 214, 1411–1430. 10.1084/jem.20160412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbankova I, et al. , 2018. First delivery and ovariectomy affect biomechanical and structural properties of the vagina in the ovine model. Int. Urogynecol. J 10.1007/s00192-017-3535-9. [DOI] [PubMed] [Google Scholar]
- Vajaranant TS, Pasquale LR, 2012. Estrogen deficiency accelerates aging of the optic nerve. Menopause 19, 942–947. 10.1097/gme.0b013e3182443137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajaranant TS, et al. , 2014. Risk of glaucoma after early bilateral oophorectomy.Menopause 21, 391–398. 10.1097/GME.0b013e31829fd081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollstein G, et al. , 2012. Retinal nerve fibre layer and visual function loss in glaucoma: the tipping point. Br. J. Ophthalmol. 96, 47–52. 10.1136/bjo.2010.196907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, de Boer JF, Chen L, Chen TC, 2015. Correlation of localized glaucomatous visual field defects and spectral domain optical coherence tomography retinal nerve fiber layer thinning using a modified structure-function map for OCT. Eye 29, 525–533. 10.1038/eye.2014.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Sugihara K, Yamada C, Tsutsumi S, Iwaki Y, 2010. Effect of estrogen on electroretinographic responses in streptozotocin-induced diabetic female rats.Exp. Eye Res. 90, 591–597. 10.1016/j.exer.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Yang H, Downs JC, Bellezza A, Thompson H, Burgoyne CF, 2007. 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping. Investig. Ophthalmol. Vis. Sci. 48, 5068–5084. 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- Zhou X, et al. , 2007. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev. Neurobiol. 67, 603–616. 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.