Abstract
Increased versatility of intermolecular radical addition to imino acceptors via photoredox catalysis is reported. Primary and secondary radicals, generated via visible-light photocatalysis from alkyl biscatecholatosilicates with organocatalyst 4CzIPN, add successfully to both aromatic and aliphatic N-acylhydrazones in the presence of MgCl2. With N-benzoylhydrazones, a simple reductive cleavage of the N-N bond of the hydrazine adduct furnishes the free amine. Synthetic utility is exemplified in a synthetic application toward repaglinide, a clinically important hypoglycemic agent.
Graphical Abstract
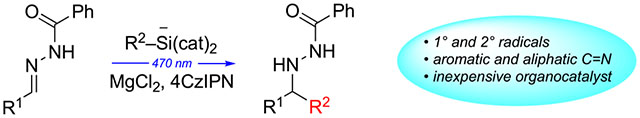
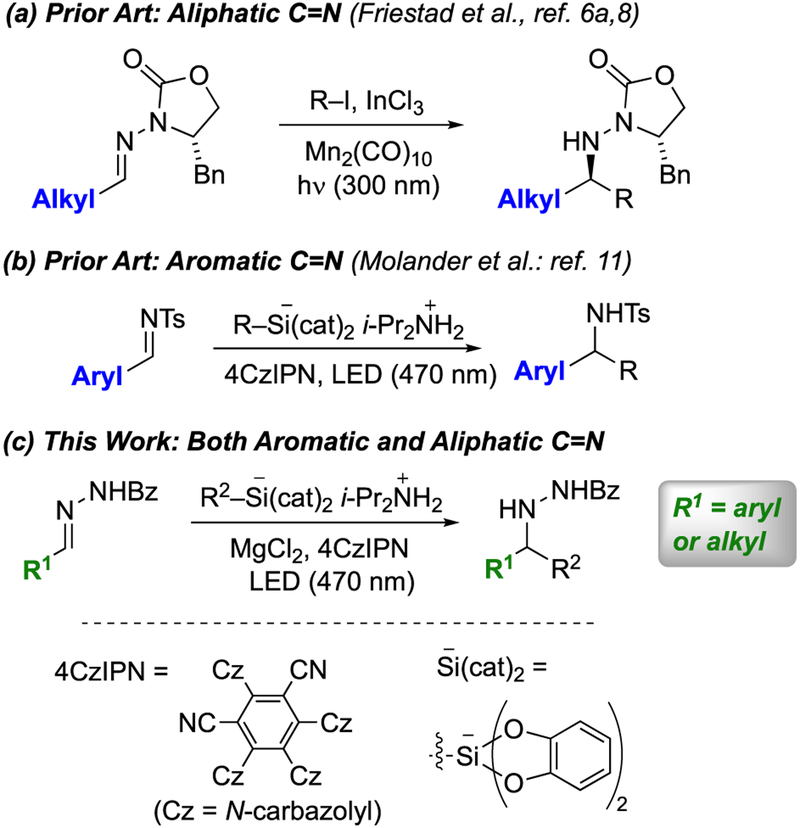
A mines are prevalent in natural products, drugs, and other biologically significant molecules, underlining the importance of developing efficient methods for their synthesis.1 Expanding upon classical imine reduction and addition methods,2 recent developments in this area include transition-metal-catalyzed hydroamination of alkenes and other C-N bond constructions.3 However, imines remain attractive amine precursors because of their ready availability from a wide range of commercial materials and also the versatility for either C-C or C-H bond constructions at the imine carbon to form chiral α-branched amines.1 Often, such reactions have limited applicability to imines from aliphatic aldehydes, as these substrates are subject to competing aza-enolization by deprotonation of the α-carbon.4 We have addressed this issue through the use of radical additions to C=N bonds of hydrazones.5–7 In support of that objective, our group developed a versatile Mn-mediated radical addition to chiral N-acylhydrazones, which enabled the asymmetric synthesis of chiral amines using both primary and secondary radicals (Scheme 1a).8 These reactions are compatible with additional functionality in both radicals and acceptors, facilitating applications in complex molecule synthesis.9,10 While very effective for aliphatic imino acceptors, the Mn-mediated additions were unsuccessful with hydrazones derived from aromatic aldehydes. Conversely, Molander recently reported radical additions to N-sulfonylimines and N-phenylimines that are effective for aromatic acceptors, but incompatible with imines from aliphatic aldehydes.11 These reactions use organosilicate salts as radical precursors in the presence of the organic photoredox catalyst 4CzIPN (Scheme 1b).12 Informed by our prior successes with radical additions to aliphatic N-acylhydrazones, we hypothesized that a combination of N-acylhydrazone radical acceptors with photoredox-catalyzed radical generation would lead to improved versatility in radical additions to C=N bonds. Here, we report a carbon–carbon bond constructive amine synthesis method that (a) takes advantage of the excellent radical acceptor behavior of the N-acylhydrazone functional group, (b) adopts catalytic conditions for radical generation, and (c) expands the versatility of photoredox-catalyzed radical additions to include both aliphatic and aromatic imino acceptors (Scheme 1c).
Scheme 1.
Bridging a Gap in Radical Addition to C=N
Recent developments in photoredox catalysis have impacted a wide variety of radical transformations,13 including radical additions to imino acceptors.14 We planned to exploit N-acylhydrazones such as 1a (Table 1) as radical acceptors, using the known reductive quenching of photoexcited 4CzIPN by alkyl bis-catecholatosilicates to generate alkyl radicals. We envisioned that the proven Lewis acid promoted radical acceptor properties of N-acylhydrazones could expand the versatility of such reactions; hydrazones have not yet been exploited in reductive additions to C=N bonds via photoredox catalysis.11b After radical addition, SET reduction and proton transfer to the intermediate aminyl radical would furnish the desired adduct and also regenerate 4CzIPN.
Table 1.
Optimization of Radical Addition Reaction
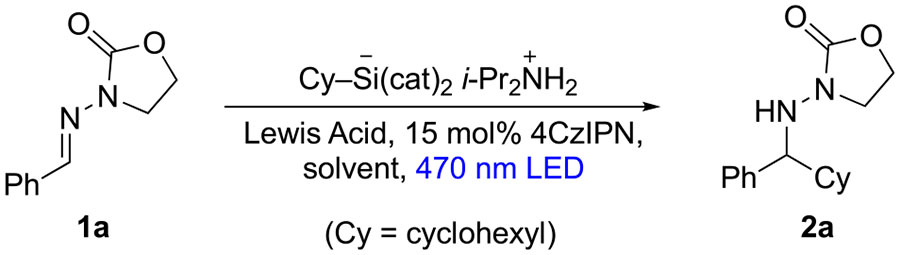 | |||||
|---|---|---|---|---|---|
| entry | Lewis acid (2 equiv) | silicate (equiv) | solvent | time (h) | % conva |
| 1 | none | 2 | DMSO | 24 | 40 |
| 2 | none | 2 | DMSO | 49 | 48 |
| 3 | ZnBr2 | 1 | DMSO | 18 | 27 |
| 4 | Zn(OTf)2 | 1 | DMSO | 18 | 27 |
| 5 | ZnBr2 | 2 | DMSO | 18 | 63 |
| 6 | ZnBr2 | 4 | DMSO | 18 | 64 |
| 7 | ZnBr2 (3 equiv) | 2 | DMSO | 18 | 49 |
| 8 | MgCl2 | 2 | DMSO | 18 | 59 |
| 9 | MgBr2 | 2 | DMSO | 18 | 54 |
| 10 | Mg(OTf)2 | 2 | DMSO | 18 | 31 |
| 11 | ZnBr2 | 2 | DMF | 16 | 28 |
| 12 | MgCl2 | 2 | DMF | 16 | 44 |
| 13 | MgCl2 | 1.2 | EtOH | 23 | 10 |
| 14 | MgCl2 | 2.6 | DMSO | 48 | 86 |
| 15 | MgCl2 | 3 | DMSO | 48 | 22b |
| 16 | MgCl2 | 3 | DMSO | 48 | 68c |
| 17d | MgCl2 | 3 | DMSO | 47 | 88 (60e) |
| 18 | MgCl2 | 2 | DMSO | 24 | 73 |
Determined by 1H NMR integration.
1 mol % of catalyst loading.
5 mol % of catalyst loading.
Conversions in control experiments using conditions of entry 17: Absence of blue LED (0%), absence of 4CzIPN (0%), open to air (67%), replacing silicate with CyBF3K (59%).
Isolated yield.
Toward this end, our initial experiments sought to test whether this photoredox catalysis cycle could be completed with C=N acceptors lacking an anion-stabilizing N-substituent such as sulfonyl; the efficiency of aminyl radical reduction and catalyst turnover was in question. Indeed, N-acylhydrazones are compatible with this redox cycle: In an initial trial at 15 mol % loading of photocatalyst 4CzIPN, cyclohexyl silicate addition to 1a proceeded in DMSO solution with 40% conversion to 2a over 24 h (Table 1, entry 1). Although this result demonstrated successful photocatalyst turnover, the modest conversion did not increase via longer reaction time (entry 2). Next, a variety of Lewis acids (2 equiv) were tested in order to assess the potential for enhanced reactivity via chelation of the N-acylhydrazone8,10a (entries 3–10), with ZnBr2 and MgCl2 offering the most improved results (see the Supporting Information for results with various Lewis acids). Increased Lewis acid loading did not improve reactivity (compare Table 1, entries 5 and 7).
Aside from DMSO, other solvents were screened and found to be inferior; we attribute this to alkyl silicate insolubility problems (entries 11–13). Reaction time, catalyst loading, and silicate stoichiometry were also examined and gratifyingly led to greatly improved conversion (entry 17); these conditions were selected as a starting point to test substrate scope (entry 17).
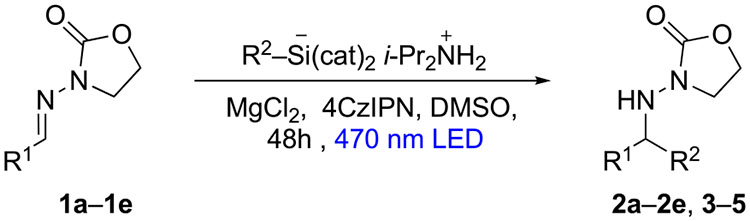
As noted above, one of our main goals for this project was a versatile method tolerant of both aliphatic and aromatic substituents at the C=N bond. Complementing the result with benzaldehyde N-acylhydrazone 1a (Table 2, entry 1), cyclohexyl addition to aliphatic N-acylhydrazones 1b and 1c occurred in modest isolated yields in the presence of unbranched alkyl groups (entries 2 and 3); branching at the α-position of the hydrazone was detrimental to yield (entries 4 and 5). Additions of more reactive primary radicals furnished expected adducts in low yield (entries 6–8), presumably impacted by premature quenching through H-abstraction.
Table 2.
N-Acylhydrazone Compatibility Study with Secondary and Primary Radical Addition
 | ||||
|---|---|---|---|---|
| entry | R1 | product | R2 | yielda (96) |
| 1 | Ph (la) | 2a | Cy | 60 |
| 2 | PhCH2CH2 (lb) | 2b | Cy | 28 |
| 3 | n-C5H11 (lc) | 2c | Cy | 22 |
| 4 | i-Pr (Id) | 2d | Cy | 19 |
| 5 | t-Bu (le) | 2e | Cy | 0 |
| 6 | Ph (la) | 3 | n-Pr | 45 |
| 7 | Ph (la) | 4 | PhCH2CH2 | 21 |
| 8 | PhCH,CH, (lb) | S | i-Bu | 14 |
Used 3 equiv of silicate and 15 mol % of 4CzIPN.
While Table 2 demonstrated some potential for improved versatility, yields were not consistently at a practical level. Fortunately, further scope studies revealed that N-benzoylhydrazones were superior to the hydrazones of Tables 1 and 2. Cyclohexyl addition to N-benzoylhydrazones 6a–6c gave significant improvement to 79–94% isolated yield (Table 3, entries 1–3). When silicate loading was reduced from 3 to 1.5 equiv and catalyst loading was lowered from 15 to 5 mol %, the yield of 7a was only slightly diminished from 79% to 75% (entry 1). Additions to aliphatic N-benzoylhydrazones 6d and 6e also afforded improved yield versus their analogues in Table 2. Importantly, a 15-fold scaleup to 1 g of 6d afforded 60% yield of 7d (entry 4). Cyclohexyl additions to a series of substituted benzaldehyde hydrazones showed tolerance for the presence of electron-donating or -withdrawing effects, and identical yields were obtained when ortho versus para substituents were compared (entries 2, 3, 6, and 7). As before, the effect of branching on the α-carbon for N-benzoylhydrazones was detrimental to radical addition (entry 5).
Table 3.
Addition of Alkyl Radicals to Aliphatic and Aromatic N-Benzoylhydrazones, Including Gram-Scale Reaction
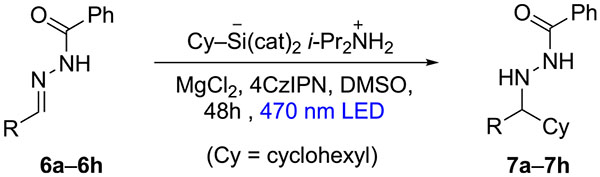 | |||
|---|---|---|---|
| entry | R | product | yield (%) |
| 1 | Ph (6a) | 7a | 79a (75b) |
| 2 | p-tolyl (6b) | 7b | 94a |
| 3 | p-CIC6H4 (6c) | 7c | 84a |
| 4 | PhCH2CH2 (6d) | 7d | 63b(60c) |
| 5 | i-Pr (6e) | 7c | 25b |
| 6 | o-tolyl (6f) | 7f | 94a |
| 7 | o-C1C6H4 (6g) | 7g | 84a |
| 8 | 4-(Me2N)C(sH4 (6h) | 7h | 32a |
0.1–0.3 mmol of 6, 3 equiv of silicate, and 15 mol % of 4CzIPN.
1.5 equiv of silicate and 5 mol % of 4CzIPN.
Gram-scale reaction (4 mmol).
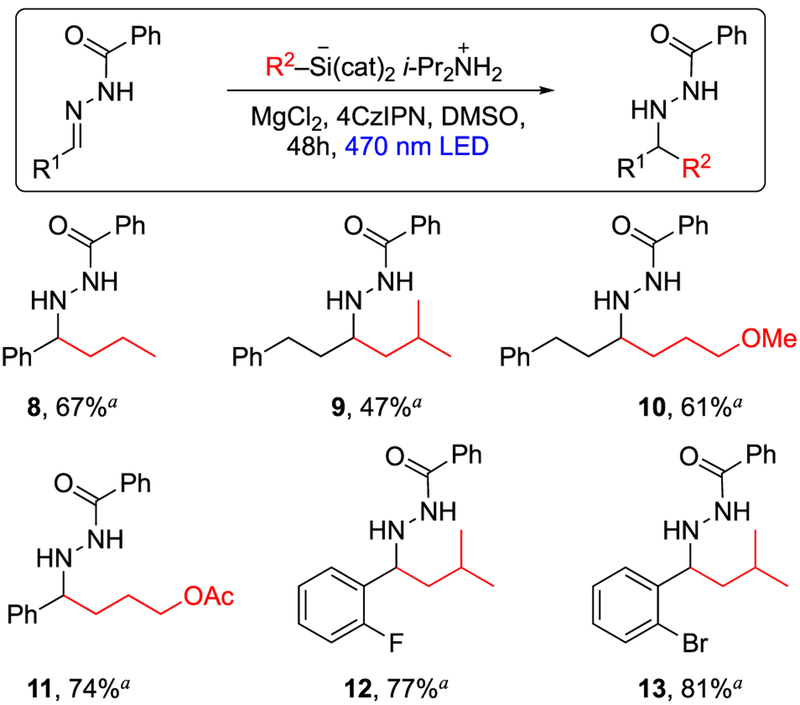
The diversity of alkyl radicals suitable for this transformation was next examined (Scheme 2). Primary alkyl silicates were added to N-benzoylhydrazones using the conditions described previously (Table 3) to afford adducts 8–13. Tolerance of heteroatoms either on the alkyl radical (10 and 11) or on the hydrazone acceptor (7c, 7g, 7h, 12, and 13) suggests that various functional group manipulations (including transitionmetal-catalyzed cross coupling) can be sequenced with these radical additions.
Scheme 2.
Addition of 1° Radicals to N-Benzoylhydrazones
a1.5 eq silicate and 5 mol % 4CzIPN used. bThree eq silicate and 15 mol % 4CzIPN used
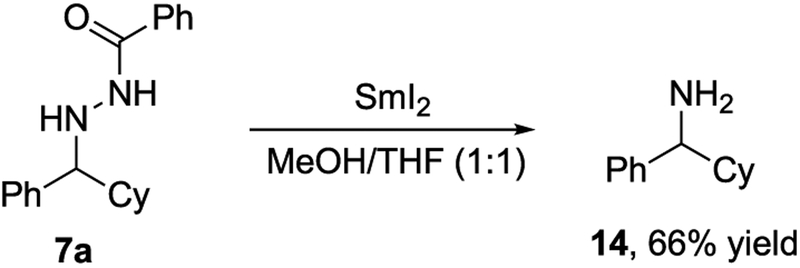
Previously, we have observed that hydrazines bearing N-benzoyl functionality readily undergo N-N bond reduction with SmI2 to liberate the free amine.15 Treatment of adduct 7a with SmI2 yielded free amine 14 in 66% yield (Scheme 3). In combination with the functional group compatibilities of the radical addition, the ease of N-N bond cleavage enhances the potential for applications in syntheses of more complex targets.
Scheme 3.
Accessing a Primary Amine by N-N Cleavage
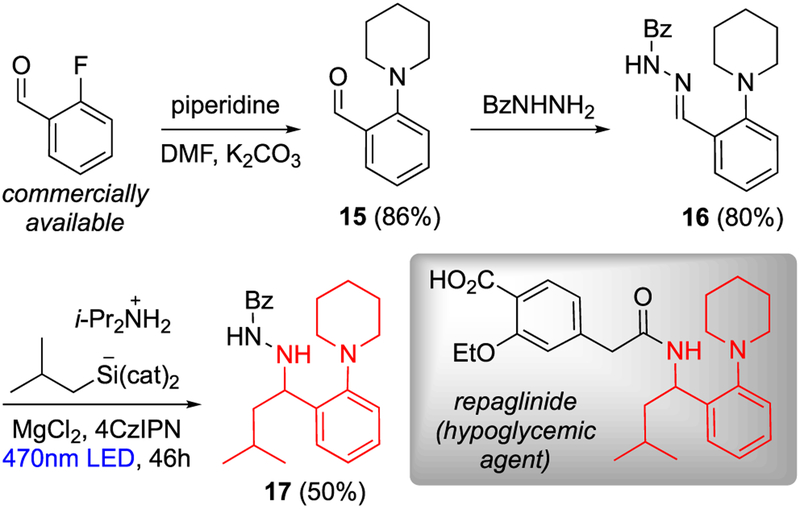
As a demonstration of synthetic utility, we targeted repaglinide, a drug that stimulates insulin production to combat diabetic hyperglycemia.16 Nucleophilic aromatic substitution of commercially available 2-fluorobenzaldehyde with piperidine, followed by condensation with benzoic hydrazide (BzNHNH2), afforded hydrazone 16 in excellent yield. Photoredox-catalyzed isobutyl addition then provided 17, the chiral amine portion of repaglinide (Scheme 4). It is noteworthy that the bulky o-piperidinyl substituent is tolerated in this radical addition.
Scheme 4.
Progress toward Formal Synthesis of Racemic Repaglinide
Amine synthesis has always been a critical undertaking in organic chemistry, given that amines are commonly found in a broad spectrum of compounds of biological importance. The N-acylhydrazone radical acceptors herein allow both aromatic and aliphatic aldehydes to undergo carbon–carbon bond constructive synthesis of amines, facilitating access to a broad range of valuable building blocks for drug discovery. Considering the asymmetric induction strategies we have previously developed for hydrazone radical acceptors,17 further advances in this direction may be anticipated.
Supplementary Material
ACKNOWLEDGMENTS
We thank the NSF (CHE-1362111) for support of our research, University of Iowa training program in Pharmacological Sciences for a predoctoral trainee award to S.C. (T32 GM067795), Ms. Vivian Mitchell and Ms. Mikayla Wymore for their preparation of alkyl silicates, and Mr. Reid Hein for his preparation of compound 1. We also thank Dr. George Crull and Mr. Mike Estenson for assistance with NMR data acquisition and photoreactor construction, respectively.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b03053.
Experimental procedures, spectral data for new compounds, and determination of percent conversion for Table 1 (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Kobayashi S; Ishitani H Catalytic Enantioselective Addition to Imines. Chem. Rev 1999, 99, 1069–1094. [DOI] [PubMed] [Google Scholar]; (b) Friestad GK; Mathies AK Recent developments in asymmetric catalytic addition to CN bonds. Tetrahedron 2007, 63, 2541–2569. [Google Scholar]; (c) Nugent TC; El-Shazly M Chiral Amine Synthesis – Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal 2010, 352, 753–819. [Google Scholar]; (d) Topics In Current Chemistry: Stereoselective Formation of Amines; Li W, Zhang X, Eds.; Springer-Verlag: Berlin, 2014; Vol. 343. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ellman JA; Owens TD; Tang TP N-tert-Butanesulfinyl Imines: Versatile Intermediates for the Asymmetric Synthesis of Amines. Acc. Chem. Res 2002, 35, 984–995. [DOI] [PubMed] [Google Scholar]; (b) Hoffmann S; Nicoletti M; List B Catalytic Asymmetric Reductive Amination of Aldehydes via Dynamic Kinetic Resolution. J. Am. Chem. Soc 2006, 128, 13074–13075. [DOI] [PubMed] [Google Scholar]; (c) Storer RI; Carrera DE; Ni Y; MacMillan DWC Enantioselective Organocatalytic Reductive Amination. J. Am. Chem. Soc 2006, 128, 84–86. [DOI] [PubMed] [Google Scholar]; (d) Louillat M-L; Patureau FW Oxidative C-H amination reactions. Chem. Soc. Rev 2014, 43, 901–910. [DOI] [PubMed] [Google Scholar]
- 3.(a) Müller TE; Hultzsch KC; Yus M; Foubelo F; Tada M Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev 2008, 108, 3795–3892. [DOI] [PubMed] [Google Scholar]; (b) Ruiz-Castillo P; Buchwald SL Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev 2016, 116, 12564–12649. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huang L; Arndt M; Gooßen K; Heydt H; Gooßen LJ Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev 2015, 115, 2596–2697. [DOI] [PubMed] [Google Scholar]
- 4.Friestad GK Addition of Carbanions to Azomethines In Science of Synthesis, Vol. 40a: Compounds with One Saturated Carbon-Heteroatom Bond: Amines and Ammonium Salts; Enders D, Shaumann E, Eds.; Thieme: Stuttgart, 2009; pp 305–342. [Google Scholar]
- 5.Reviews:; (a) Friestad GK Addition of Carbon-Centered Radicals to Imines and Related Compounds. Tetrahedron 2001, 57, 5461–5496. [Google Scholar]; (b) Friestad GK Control of Asymmetry in the Radical Addition Approach to Chiral Amine Synthesis In Topics In Current Chemistry: Stereoselective Formation of Amines; Li W, Zhang X, Eds.; Springer-Verlag: Berlin, 2014; Vol. 343, pp 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Friestad GK Asymmetric Radical Addition to Chiral Hydrazones. In Topics In Current Chemistry: Radicals in Synthesis III; Gansauer A, Heinrich M, Eds.; Springer-Verlag: Berlin, 2012; Vol. 320, pp 61–92. [DOI] [PubMed] [Google Scholar]
- 6.(a) Friestad GK; Qin J Intermolecular Alkyl Radical Addition to Chiral N-Acylhydrazones Mediated by Manganese Carbonyl. J. Am. Chem. Soc 2001, 123, 9922–9923. [DOI] [PubMed] [Google Scholar]; (b) Friestad GK; Shen Y; Ruggles EL Enantioselective Radical Addition to N-Acylhydrazones Mediated by Chiral Lewis Acids. Angew. Chem., Int. Ed 2003, 42, 5061–5063. [DOI] [PubMed] [Google Scholar]; (c) Friestad GK; Massari SE A Silicon Tether Approach for Addition of Functionalized Radicals to Chiral alpha-Hydroxyhydrazones: Diastereoselective Additions of Hydroxymethyl and Vinyl Synthons. J. Org. Chem 2004, 69, 863–875. [DOI] [PubMed] [Google Scholar]; (d) Slater KA; Friestad GK Mn-Mediated Radical-Ionic Annulations of Chiral N-Acylhydrazones. J. Org. Chem 2015, 80, 6432–6440. [DOI] [PubMed] [Google Scholar]
- 7.Selected recent developments in radical addition to imino acceptors:; (a) Fernández-Salas JA; Rodríguez-Fernández MM; Maestro MC; García-Ruano JL Synthesis of Enantiomerically Pure (α-Phenylalkyl)amines with Substituents at the ortho Position through Diastereoselective Radical Alkylation Reaction of Sulfinimines. Eur. J. Org. Chem 2014, 2014, 5265–5272. [Google Scholar]; (b) Fujii S; Konishi T; Matsumoto Y; Yamaoka; Takasu K; Yamada K Radical Aminomethylation of Imines. J. Org. Chem 2014, 79, 8128–8133. [DOI] [PubMed] [Google Scholar]; (c) Prieto A; Melot R; Bouyssi D; Monteiro N C-H Difluoroalkylation of Aldehyde Hydrazones with Functionalized Difluoromethyl Bromides under Copper Catalysis. ACS Catal 2016, 6, 1093–1096. [Google Scholar]; (d) Ni S; Garrido-Castro AF; Merchant RR; de Gruyter JN; Schmitt DC; Mousseau JJ; Gallego GM; Yang S; Collins MR; Qiao JX; Yeung K-S; Langley DR; Poss MA; Scola PM; Qin T; Baran PS A General Amino Acid Synthesis Enabled by Innate Radical Cross-Coupling. Angew. Chem., Int. Ed 2018, 57, 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Matos JLM; Vásquez-Céspedes S; Gu J; Oguma T; Shenvi RA Branch-Selective Addition of Unactivated Olefins into Imines and Aldehydes. J. Am. Chem. Soc 2018, 140, 16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang Y-Y; Bode JW Olefin Amine (OLA) Reagents for the Synthesis of Bridged Bicyclic and Spirocyclic Saturated N-Heterocycles by Catalytic Hydrogen Atom Transfer (HAT) Reactions. J. Am. Chem. Soc 2019, 141, 9739–9745. [DOI] [PubMed] [Google Scholar]
- 8.Friestad GK; Qin J; Suh Y; Marie J-C Mn-Mediated Coupling of Alkyl Iodides and Chiral N-Acylhydrazones: Optimization, Scope, and Evidence for a Radical Mechanism. J. Org. Chem 2006, 71, 7016–7027. [DOI] [PubMed] [Google Scholar]
- 9.Romero KJ; Galliher MS; Pratt DA; Stephenson CRJ Radicals in natural product synthesis. Chem. Soc. Rev 2018, 47, 7851–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Friestad GK; Deveau AM; Marie J-C Stereoselective Mn-Mediated Coupling of Functionalized Iodides and Hydrazones: A Synthetic Entry to the Tubulysin gamma-Amino Acids. Org. Lett 2004, 6, 3249–3252. [DOI] [PubMed] [Google Scholar]; (b) Friestad GK; Jiang T; Mathies AK Aldehyde-Selective Wacker Oxidation in a Thiyl-Mediated Vinyl Group Transfer Route to Daunosamine. Org. Lett 2007, 9, 777–780. [DOI] [PubMed] [Google Scholar]; (c) Friestad GK; Jiang T; Fioroni GM Stereocontrol in Radical Mannich Equivalents for Aminosugar Synthesis: Haloacetal and 2-(Phenylthio)vinyl Tethered Radical Additions to alpha-Hydroxyhydrazones. Tetrahedron 2008, 64, 11549–11557. [Google Scholar]; (d) Friestad GK; Ji A; Baltrusaitis J; Korapala CS; Qin J Scope of Stereoselective Mn-Mediated Radical Addition to Chiral Hydrazones and Application in a Formal Synthesis of Quinine. J. Org. Chem 2012, 77, 3159–3180. [DOI] [PubMed] [Google Scholar]; (e) Friestad GK; Banerjee K; Marié J-C; Mali U; Yao L Stereoselective Access to Tubuphenylalanine and Tubuvaline: Improved Mn-Mediated Radical Additions and Assembly of A Tubulysin Tetrapeptide Analog. J. Antibiot 2016, 69, 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Patel NR; Kelly CB; Siegenfeld AP; Molander GA Mild, Redox-Neutral Alkylation of Imines Enabled by an Organic Photocatalyst. ACS Catal 2017, 7, 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]; For nonreductive photoredox radical processes involving hydrazones, see:; (b) Xu P; Li W; Xie J; Zhu C Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond. Acc. Chem. Res 2018, 51, 484–495. [DOI] [PubMed] [Google Scholar]
- 12.(a) Corcé V; Chamoreau L-M; Derat E; Goddard J-P; Ollivier C; Fensterbank L Silicates as Latent Alkyl Radical Precursors: Visible-Light Photocatalytic Oxidation of Hypervalent Bis-Catecholato Silicon Compounds. Angew. Chem., Int. Ed 2015, 54, 11414–11418. [DOI] [PubMed] [Google Scholar]; (b) Luo J; Zhang J Donor-Acceptor Fluorophores for Visible-Light Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)-C(sp2) Cross-Coupling. ACS Catal 2016, 6, 873–877. [Google Scholar]; 4CzIPN is inexpensively prepared in one step from carbazole and isophthalonitrile.; (c) Uoyama H; Goushi K; Shizu K; Nomura H; Adachi C Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [DOI] [PubMed] [Google Scholar]
- 13.Reviews:; (a) Narayanam JMR; Stephenson CRJ Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev 2011, 40, 102–113. [DOI] [PubMed] [Google Scholar]; (b) Goddard J-P; Ollivier C; Fensterbank L Photoredox Catalysis for the Generation of Carbon Centered Radicals. Acc. Chem. Res 2016, 49, 1924–1936. [DOI] [PubMed] [Google Scholar]; (c) Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]; (d) Shaw MH; Twilton J; MacMillan DWC Photoredox Catalysis in Organic Chemistry. J. Org. Chem 2016, 81, 6898–6926. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sideri IK; Voutyritsa E; Kokotos CG Photoorganocatalysis, small organic molecules and light in the service of organic synthesis: the awakening of a sleeping giant. Org. Biomol. Chem 2018, 16, 4596–4614. [DOI] [PubMed] [Google Scholar]
- 14.(a) Zhang H-H; Yu S Radical Alkylation of Imines with 4-Alkyl-1,4-dihydropyridines Enabled by Photoredox/Brønsted Acid Cocatalysis. J. Org. Chem 2017, 82, 9995–10006. [DOI] [PubMed] [Google Scholar]; (b) Li Y; Zhou K; Wen Z; Cao S; Shen X; Lei M; Gong L Copper(II)-Catalyzed Asymmetric Photoredox Reactions: Enantioselective Alkylation of Imines Driven by Visible Light. J. Am. Chem. Soc 2018, 140, 15850–15858. [DOI] [PubMed] [Google Scholar]; (c) Zheng D; Studer A Photoinitiated Three-Component α-Perfluoroalkyl-β-heteroarylation of Unactivated Alkenes via Electron Catalysis. Org. Lett 2019, 21, 325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Pantaine LRE; Milligan JA; Matsui JK; Kelly CB; Molander GA Photoredox Radical/Polar Crossover Enables Construction of Saturated Nitrogen Heterocycles. Org. Lett 2019, 21, 2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ji P; Zhang Y; Wei Y; Huang H; Hu W; Mariano PA; Wang W Visible-Light-Mediated, Chemo- and Stereoselective Radical Process for the Synthesis of C-Glycoamino Acids. Org. Lett 2019, 21, 3086–3092. [DOI] [PubMed] [Google Scholar]; (f) Wang T; Wang D-H Potassium Alkylpentafluorosilicates, Primary Alkyl Radical Precursors in the C-1 Alkylation of Tetrahydroisoquinolines. Org. Lett 2019, 21, 3981–3985. [DOI] [PubMed] [Google Scholar]; (g) Supranovich VI; Levin VV; Dilman AD Radical Addition to N-Tosylimines via C-H Activation Induced by Decatungstate Photocatalyst. Org. Lett 2019, 21, 4271–4274. [DOI] [PubMed] [Google Scholar]
- 15.Ding H; Friestad GK Trifluoroacetyl-Activated Nitrogen–Nitrogen Bond Cleavage of Hydrazines by Samarium(II) Iodide. Org. Lett 2004, 6, 637–640. [DOI] [PubMed] [Google Scholar]
- 16.(a) Wolffenbuttel BHR; Nijst L; Sels JPJE; Menheere PPCA; Müller, P. G.; Nieuwenhuijzen Kruseman, A. C. Effects of a new oral hypoglycaemic agent, repaglinide, on metabolic control in sulphonylurea-treated patients with NIDDM. Eur. J. Clin. Pharmacol 1993, 45, 113–116. [DOI] [PubMed] [Google Scholar]; (b) Nagarajan P; Rajendiran C; Naidu A; Dubey PK Studies on Diastereofacial Selectivity of a Chiral tert-Butanesulfinimines for the Preparation of (S)-3-Methyl-1-(2-piperidin-1-yl-phenyl)butylamine for the Synthesis of Repaglinide. Asian J. Chem 2013, 25, 9345–9350. [Google Scholar]; (c) Sundaram DTSS; Mitra J; Rajesh C; Islam A; Prabahar KJ; Rao BV; Douglas SP Synthesis of Repaglinide Congeners. Synth. Commun 2015, 45, 2092–2098. [Google Scholar]
- 17.Friestad GK Chiral N-Acylhydrazones: Versatile Imino Acceptors for Asymmetric Amine Synthesis. Eur. J. Org. Chem 2005, 2005, 3157–3172. [DOI] [PubMed] [Google Scholar]; (b) See refs 5 and 6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.