ABSTRACT
Aims and objectives
Vancomycin is a drug of choice for various gram-positive bacterial (GPB) infections and is largely prescribed to pediatric intensive care unit (PICU) patients. Despite the different pathophysiology of these patients, limited data are available on pharmacokinetics of vancomycin. There are lack of data for critically ill Indian children; hence, study was conducted to assess the steady-state pharmacokinetics in children admitted to PICU.
Materials and methods
Twelve subjects (seven males, five females) aged 1–12 years were enrolled. Vancomycin (dose of 20 mg/kg per 8 hours) was infused for over 1 hour and steady-state pharmacokinetics was performed on day 3. Vancomycin concentrations were measured by the validated liquid chromatography mass spectrometry method. Pharmacokinetic parameters were calculated using Winnonlin (Version 6.3; Pharsight, St. Louis, MO).
Results
The steady-state mean Cssmax was 40.94 μg/mL (±15.07), and mean AUC0–8 hours was 124.15 μg/mL (±51.27). The mean t1/2 was 4.82 hours (±2.71), Vd was 12.48 L (±4.43), and Cl was 2.08 mL/minute (±0.89). The mean AUC0–24 among 12 subjects was 372.44 μg/mL (±153.82). Among 35 measured trough concentrations, 23 (65.71%) were below, 11 (31.43%) were within, and 1 (2.86%) was above the recommended range.
Conclusion
The pharmacokinetic parameters of vancomycin were comparable with previously reported studies. However, recommended trough levels (10–20 μg/mL) were not achievable with current recommended dosing of 60 mg/kg/day.
How to cite this article
Mali NB, Tullu MS, Wandalkar PP, Deshpande SP, Ingale VC, Deshmukh CT, et al. Steady-state Pharmacokinetics of Vancomycin in Children Admitted to Pediatric Intensive Care Unit of a Tertiary Referral Center. IJCCM 2019;23(11):497–502.
Keywords: Gram-positive bacterial infection, Liquid chromatography mass spectrometry, Pediatric intensive care unit, Pharmacokinetics–pharmacodynamics, Vancomycin
INTRODUCTION
The systemic use of antibiotics is high in PICU patients due to critical illnesses and prevalence of community-acquired/nosocomial infections.1–3 Vancomycin, one of the important systemic antibiotics, is largely used to treat suspected or proven GPB infections. Vancomycin, a large glycopeptide with time-dependent bactericidal activity, was discovered in mid-19th century and till date it is considered as the first-line therapy for methicillin-resistant Staphylococcus aureus (MRSA) infection and other GPB infections.4–7
However, careful use of this antibiotic is imperative due to increasing prevalence of MRSA, rising minimum inhibitory concentrations (MIC) to vancomycin, and vancomycin-resistant enterococci (VRE). Also, there is a relative lack of clinical experience with new antimicrobial molecules vs vancomycin and limited discovery of newer molecules. High rates of vancomycin failure in MRSA infections have been increasingly reported.5,6 Hence, to optimize vancomycin dosing, pharmacokinetics–pharmacodynamics (pk–pd) assessment and therapeutic drug monitoring (TDM) are recommended.8 Vancomycin area under the curve/minimum inhibitory concentration (AUC/MIC) ratio of ≥400 as a pharmacodynamics parameter is recommended for serious GPB infections. To avoid antibiotic resistance, vancomycin trough levels should be achieved between 10 μg/mL and 20 μg/mL. For better clinical outcomes in severe infections due to MRSA, it is recommended that the trough levels should be targeted between 15 μg/mL and 20 μg/mL and most of the pharmacokinetics and TDM data have been generated in adult patients.8–10
Globally, very limited data are available on pharmacokinetics of vancomycin in pediatric patients and there is lack of data, particularly from India. Also, the empirical use of vancomycin at our PICU is high (up to 24%) with lack of TDM and without evidence of pharmacokinetics data.11 Hence, the present study was conducted to assess the steady-state pharmacokinetics of vancomycin in children admitted to PICU.
MATERIALS AND METHODS
Study Design and Duration
A single-center, single-arm, prospective steady-state pharmacokinetics study was conducted over the period of 9 months from December 2015 to August 2016 in children admitted to PICU of a tertiary care medical college and hospital from Western India.
Ethics
The study was initiated after obtaining institutional ethics committee approval.
This study was prospectively registered with clinical trial registry of India with registration number CTRI/2016/01/006527. A written informed consent was obtained from parents/guardians.
Study Subjects
Critically ill subjects of either gender aged 1–12 years with weight ≥7 kg requiring vancomycin therapy (empirically or proven GPB infection) at the dose of 20 mg/kg for every 8 hours were considered for the study. Subjects with creatinine clearance (Crcl) <50 mL/minute at screening and on study day (day 3 of vancomycin treatment) and hypersensitivity to vancomycin hydrochloride or its excipients were excluded.
Study Procedure
Demographics (age, gender, and body weight), serum creatinine, pediatric risk of mortality (PRISM) III score, Gram's stain and culture (for blood or any other appropriate body fluid specimen), antibiotic sensitivity, and MIC for vancomycin were observed prior to initiation of vancomycin therapy.
Vancomycin Treatment
Vancomycin at a dose of 20 mg/kg was infused via an infusion pump over a period of 1 hour for every 8 hours. For the study purpose, vancomycin hydrochloride vials from the same batch, which were available on hospital schedule (free of cost to the subject), were used throughout the study period.
Blood Sampling
Steady-state pharmacokinetics was performed on day 3 of vancomycin treatment. A total of 11 blood samples of 2 mL at each time point were collected in a heparinized tube. The first blood collection was done at 48 hours from the first infusion (i.e., before administration of seventh dose on day 3), which was considered as 0 hour (baseline sample). Following this, blood samples were collected at 1 hour (at the end of infusion), 1.25, 1.5, 2, 2.5, 3, 4, 6 hours, and just before the next dose (8 hours). Additionally, 2 mL of blood was also collected at 72 hours (day 4) before administration of the next dose for the trough level estimation.
Safety Assessment
All adverse events (AEs)/serious adverse events (SAEs) with reference to below definitions were noted during the entire study duration.12
Adverse event: Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An AE can therefore be any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product.
-
Serious adverse event or serious adverse drug reaction (ADR): Any untoward medical occurrence that at any dose:
– Results in death,
– Is life-threatening,
– Requires inpatient hospitalization or prolongation of existing hospitalization,
– Results in persistent or significant disability/incapacity, or
– Is a congenital anomaly/birth defect.
Estimation of Vancomycin by Liquid Chromatography Tandem Mass Spectrometry (LCMS)
A bioanalytical method for the estimation of vancomycin from plasma samples was developed and validated based on the earlier described method and guidelines.13,14 Vancomycin and the internal standard (phenacetin) were extracted by the solid-phase extraction technique using MCX 1 cc, 30 mg cartridges. Separation of components was performed on the C18 column using acetonitrile:ammonium formate:formic acid (60:40:0.1%) as a mobile phase by ultrafast liquid chromatography (Prominence, Shimadzu, Japan). Quantification was performed using the LCMS instrument API 2000 (Applied Biosystems, MDX Sciex, Toronto, Canada) by multiple reaction monitoring transitions as 725 (diprotonated molecule with actual molecular weight of 1449.265 g/mol) to 144.0 and 180.10 to 110.10 for vancomycin and the internal standard, respectively. The spiked plasma drug concentrations were linear over the range of 0.250–100 μg/mL. The intraday and interday precision and accuracy was <15% deviation.
Statistical Analysis
No formal sample size calculation was made for this study and a total of 12 subjects were enrolled. Pharmacokinetic parameters were derived by noncompartment modeling15 using Winnonlin (Version 6.3; Pharsight, St. Louis, MO). The trapezoidal approach was used to estimate AUC and clearance. Area under the curve was performed from 0 hour to 8 hours; hence the AUC0–24 hours was calculated by tripling of AUC0–8.16 The Matzke equation was used to estimate vancomycin clearance for each subject.17
All statistical analysis was done on SPSS software version 20 (IBM, Armonk, NY, USA). Continuous numerical variables (such as Crcl, PRISM III score, and all pharmacokinetics parameters) and categorical variables (such as age, gender, and weight) were assessed for the normality using the Kolmogorov–Smirnov test. Normally distributed data were expressed as mean ± SD, and that not normally distributed were presented as median [range].
RESULTS
Demographics
A total of 18 subjects were approached, from whom 12 were enrolled (6 subjects refused consent). There were seven males and five female with mean age of 5.33 (±3.08) years and the mean weight was 15.58 (±4.50) kg. The mean PRISM III score was 10.58 (±8.27) and mean Crcl was 88.16 (±26.11) mL/minute. A total of 11 subjects received vancomycin for suspected GPB infection and one subject had MRSA with MIC of 1 μg. Demographic details are presented in Table 1.
Table 1.
Demographics of the study population
| ID | Age (years) | Sex | Weight (kg) | PRISM III score | Creatinine clearance | Clinical diagnosis |
|---|---|---|---|---|---|---|
| 1 | 3 | Male | 18 | 0 | 92.58 | Nephrotic syndrome |
| 2 | 3 | Female | 12 | 7 | 64.40 | Post-measles pneumonia |
| 3 | 4 | Male | 14 | 18 | 83.28 | Aplastic anemia |
| 4 | 3 | Male | 11 | 0 | 53.40 | Meningitis |
| 5 | 1 | Female | 8 | 12 | 75.60 | Post-measles bronchopneumonia |
| 6 | 8 | Female | 18 | 9 | 56.70 | Pneumonia |
| 7 | 8 | Female | 23 | 17 | 126.90 | Chronic liver disease |
| 8 | 10 | Male | 21 | 24 | 66.40 | Intracranial abscess |
| 9 | 5 | Male | 20 | 8 | 109.80 | Hepatic encephalopathy with hepatic failure |
| 10 | 8 | Female | 15 | 4 | 118.80 | Bilateral pneumonia |
| 11 | 9 | Male | 15 | 23 | 122.10 | Ulcerative colitis |
| 12 | 2 | Male | 12 | 5 | 88.00 | Left-sided bronchopneumonia with pleural effusion |
| Mean (±SD) | 5.33 (±3.08) | Male = 7, female = 5 | 15.58 (±4.50) | 10.58 (±8.27) | 88.16 (±26.11) |
Pharmacokinetic Parameters
Steady-state pharmacokinetics was performed in all enrolled 12 subjects. The steady-state mean maximum plasma concentration (Cssmax) was 40.94 μg/mL (±15.07) with mean maximum time (Tssmax) of 1 hour and mean minimum plasma concentration (Cssmin) of 7.01 μg/mL (±5.83). The mean half-life (t1/2) was 4.82 hours (±2.71), mean volume of distribution (Vd) was 12.48 L (±4.43,) and mean total body clearance (Cl) was 2.08 mL/minute (±0.89). Mean area under the plasma concentration curve from 0 hour to 8 hours (AUC0–8 hours) was 124.15 μg (±51.27). The mean AUC0–24 among 12 subjects was 372.44 μg/mL (±153.82), and AUC0–24 of >400 μg was achieved in half of study population. All pharmacokinetics parameters of individual subjects are depicted in Table 2, and mean concentrations following the steady state at various time points are shown in Figure 1.
Table 2.
Pharmacokinetics parameters of vancomycin
| Subject ID | Cssmax (μg/mL) | Tssmax (hours) | T1/2 (hours) | AUCss0–8 hours (μg/mL) | Vd (L) | Clearance (mL/minute) | AUC0–24 hours (μg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 45.94 | 1.00 | 2.70 | 138.04 | 8.80 | 2.26 | 414.11 |
| 2 | 54.24 | 1.00 | 4.81 | 175.72 | 6.57 | 0.95 | 527.15 |
| 3 | 45.72 | 1.00 | 3.70 | 153.58 | 7.58 | 1.42 | 460.73 |
| 4 | 18.41 | 1.00 | 3.45 | 54.21 | 15.90 | 3.19 | 162.62 |
| 5 | 18.83 | 1.00 | 5.28 | 43.56 | 20.67 | 2.72 | 130.68 |
| 6 | 28.62 | 1.00 | 3.85 | 82.51 | 19.31 | 3.47 | 247.53 |
| 7 | 59.57 | 1.00 | 8.27 | 208.25 | 12.53 | 1.05 | 624.76 |
| 8 | 61.15 | 1.00 | 2.44 | 141.67 | 10.30 | 2.93 | 425.00 |
| 9 | 52.42 | 1.00 | 11.89 | 181.21 | 13.89 | 0.81 | 543.63 |
| 10 | 41.9 | 1.00 | 4.77 | 108.35 | 13.51 | 1.96 | 325.04 |
| 11 | 38.87 | 1.00 | 3.13 | 110.14 | 9.61 | 2.13 | 330.43 |
| 12 | 25.65 | 1.00 | 3.57 | 92.55 | 11.10 | 2.15 | 277.64 |
| Mean (SD) | 40.94 (±15.07) | 1.00 | 4.82 (±2.71) median [range] = 3.78 [2.44, 11.88] | 124.15 (±51.27) | 12.48 (±4.43) | 2.08 (±0.89) | 372.44 (±153.82) |
Cssmax, maximum concentration at the steady state; Tssmax, maximum time at the steady state; T1/2, elimination half-life; AUCss0–8, steady-state area under the concentration–time curve from 0 hour to 8 hours; Vd, volume of distribution; CL, clearance; AUCss0–24, steady-state area under the concentration–time curve from 0 hour to 24 hours
Fig. 1.
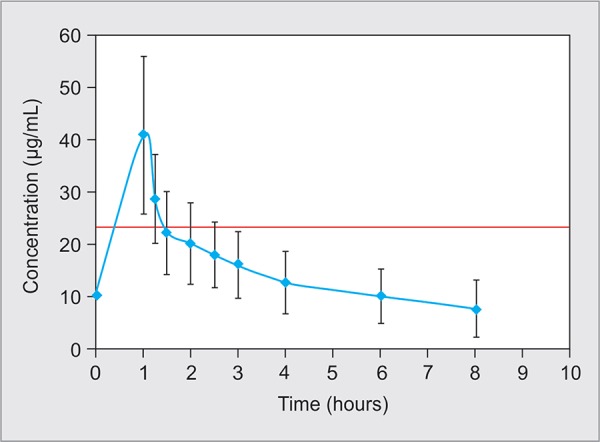
Steady-state concentration plot of vancomycin (n = 12). Data are presented as the mean and standard deviation
Two subjects died post steady-state pharmacokinetics, wherein the cause of death for the first subject (female; age 8 years) was acute hepatic encephalopathy in chronic liver disease and cause of death for the second subject (male; age 9 years) was bronchopneumonia with hypertension in case of ulcerative colitis. None of study subject experienced AE related to vancomycin infusion; however, one subject (female; age 8 years) was shifted to linezolid due to raised creatinine.
Over the study period, a total of 35 trough concentrations were measured among 12 subjects, from which 23 (65.71%) were below the recommended range (10–20 μg/mL), 11 (31.43%) were within the range, whereas 1 (2.86%) was above the recommended value (21.96 μg/mL). All measured trough concentrations have been presented in Table 3.
Table 3.
Vancomycin trough concentrations
| Subject ID | Trough concentrations in μg/mL | ||
|---|---|---|---|
| 48 hours | 56 hours | 72 hours | |
| 1 | 6.95 | 5.41 | No data |
| 2 | 12.85 | 11.23 | 14.45 |
| 3 | 28.04 | 8.17 | 21.96 |
| 4 | 1.76 | 2.95 | 3.02 |
| 5 | 2.72 | 2.02 | 7.71 |
| 6 | 7.01 | 3.79 | 6.41 |
| 7 | 19.42 | 18.47 | 17.54 |
| 8 | 16.02 | 3.40 | 8.14 |
| 9 | 19.63 | 18.22 | 18.03 |
| 10 | 4.72 | 6.47 | 5.33 |
| 11 | 4.92 | 6.82 | 5.09 |
| 12 | 2.52 | 4.56 | 2.88 |
| Mean (SD) | 10.55 (±8.51) | 7.63 (±5.60) | 10.05 (±6.72) |
DISCUSSION
To our knowledge, this is the first Indian study carried out to assess the steady-state pharmacokinetics of vancomycin in critically ill pediatric subjects. It is well known that short duration of vancomycin infusion is strongly associated with ADRs such as red man syndrome;8,18,19 in the present study, all subjects received vancomycin infusion for the duration of 1 hour and it was well tolerated. However, one subject with hepatic dysfunction was discontinued from vancomycin and shifted to linezolid due to raised creatinine.
Large interindividual variability in vancomycin pharmacokinetics has been reported from ill pediatric subjects.20 We also observed variability in major pharmacokinetics parameters; however, it is statistically insignificant (except half-life). The mean Cssmax (40.94 μg/mL ± 15.07) in our population with vancomycin dosing of 60 mg/kg/day aligns with the similar kind of pharmacokinetic study conducted in a South African infant, wherein mean Cssmax (35.5 ± 11.1 μg/mL) was measured with the vancomycin dosing of 10 mg/kg/6 hours.21 However, with same vancomycin dosing (60 mg/kg/day) in cancer PICU subjects, the mean Cssmax was quite less (26 ± 7 μg/mL). This finding could be due to the presence of malignancy since the malignancy was associated with a significantly higher clearance compared to subjects with no history of cancer.22–24
The volume of distribution in our pediatric subjects was high; this finding is quite similar with the published data. Pollard et al. also explained that the increase in the volume of distribution of aminoglycosides is based on the changes in the body compartments due to fluid overload in attempts to maintain hemodynamics.25
It is well known that the vancomycin is eliminated by the kidney; however, in our study we observed that the two subjects with liver disease had longer half-life (8.27 hours and 11.89 hours) compared to subjects with different disease conditions. A study conducted by Harada et al. in subjects with impaired liver disease (with normal renal function) also delineated prolonged half-life in the range of 6.4–11.4 hours.26 A historic study performed by Brown et al. similarly reported prolonged half-life (37.0 ± 74.3) in subjects with hepatic dysfunction and shorter half-life (2.6 ± 1.3) in subjects with normal liver function.27 Our finding aligns with above two studies; however, large-scale population specific study is required to evaluate further conclusion.
We found that 50% of children achieved AUC0–24 ≥400 μg/mL by receiving the dose 60 mg/kg/day of vancomycin and our findings are similar to the study conducted by Chhim et al., where 40% children achieved AUC0–24 ≥400 μg/mL by receiving the same dose.28 The study conducted by Hwang et al. also showed that 54.3% of children achieved AUC0–24 ≥400 μg/mL by receiving the dose of 15 mg/kg every 6 hours.29 In routine clinical practice, measuring AUC/MIC by the traditional method is not practical; hence, steady-state trough concentration is recommended as a surrogate pharmacodynamic parameter for vancomycin therapy.8,10 In the present study, we found that 65.71% of trough concentrations were below the recommended range of 10–20 μg/mL with receipt of the recommended dose of 60 mg/kg/day. These huge subtherapeutic proportions are strongly correlated with previously reported studies.28–30 A prospective study from Iranian children also found that 69% trough concentrations were subtherapeutic with current recommended doses.31 A recent study with recommended vancomycin doses conducted by Buckel et al. found that more than 60% vancomycin courses associated subtherapeutic steady-state trough concentrations.32 To improve adherence to the IDSA/ASHP, Miloslavsky et al. had developed new standardized vancomycin dosing guidelines and implemented intervention in pediatric patients. According to this study, the post-intervention analysis of patients compliant with the guidelines showed an in-control process with a substantial improvement in trough measurements.33
Looking at the current scenario and recommendations from the guidelines and various authors to achieve appropriate trough concentration to get the treatment benefits and to avoid resistance, there is need of individualized vancomycin dosing optimization; however, close observation is highly required since higher doses may induce nephrotoxicity.31,34,35
LIMITATIONS
The main limitation was small sample size with homogeneous population; hence, findings of this study may not be applicable to infants and patients aged >12 years and to subjects with renal insufficiency.
CONCLUSION
The pharmacokinetics profile achieved in our pediatric population is comparable to previously reported studies. However, in hepatic dysfunction subjects, the half-life was prolonged; however, more data should be generated to get further conclusion regarding safety and efficacy of vancomycin in such population. Recommended trough concentrations were not achievable with current recommended dosing since 65.71% of trough concentrations of vancomycin were subtherapeutic. However, large-scale study with standardized vancomycin dosing guidelines along with proper intervention plan is required to derive further conclusion regarding optimization of vancomycin dosing.
What is already known
Vancomycin is commonly used in intensive care units; however, careful use of this antibiotic is necessary.
To optimize vancomycin dosing, pk–pd assessment and TDM are recommended.
For better clinical outcomes, it is recommended that the trough levels should be targeted between 15 μg/mL and 20 μg/mL but most of the pharmacokinetics and TDM data have been generated in adult patients.
What this study adds
The pharmacokinetics profile achieved in our pediatric population is comparable to previously reported studies.
Recommended trough concentrations were not achievable with current recommended dosing since 65.71% of trough concentrations of vancomy cin were subtherapeutic.
A large-scale study is required to derive further conclusion regarding optimization of vancomycin dosing in children.
AUTHORS CONTRIBUTIONS
Nitin Mali, Milind Tullu, Poorwa Wandalkar, Siddharth Deshpande, and Vinod Ingale contributed to the planning of the study, collected/analyzed patient data, conducted literature search, and drafted the manuscript. Chandrahas Deshmukh, Nithya Gogtay, and Urmila Thatte planned the study, helped in literature search, supervised data collection/analysis, and revised the manuscript. Milind Tullu, Vinod Ingale, and Chandrahas Deshmukh were involved in clinical management of the patients. Urmila Thatte will act as a guarantor for the paper.
Acknowledgments
The authors thank Dr Avinash Supe, director (Medical Education and Major Hospitals) and dean of King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College for granting permission to publish this manuscript. The authors also thank Research Society of King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College for providing research grant to perform the present research work. The authors also wish to thank the Indian Council of Medical Research [ICMR] for funding the study via a grant in aid for the Advanced Center in Clinical Pharmacology for evaluating Pharmacokinetic–Pharmacodynamic relationships of anti-infectives
Footnotes
Source of support: Research Society, King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College, Mumbai, granted research fund for this project. The authors also wish to thank the Indian Council of Medical Research [ICMR] for funding the study via a grant in aid for the Advanced Center in Clinical Pharmacology for evaluating Pharmacokinetic–Pharmacodynamic relationships of anti-infectives.
Conflict of interest: None
REFERENCES
- 1.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766–773. doi: 10.1097/01.inf.0000178064.55193.1c. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Kepenekli E, Soysal A, Yalindag-Ozturk N, Ozgur O, Ozcan I, Devrim I, et al. Healthcare-associated infections in pediatric intensive care units in Turkey: a national point-prevalence survey. Jpn J Infect Dis. 2015;68(5):381–386. doi: 10.7883/yoken.JJID.2014.385. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Abbas Q, Haq AU, Kumar R, Ali SA, Hussain K, Shakoor S. Evaluation of antibiotic use in pediatric intensive care unit of a developing country. Indian J Crit Care Med. 2016;20(5):291–294. doi: 10.4103/0972-5229.182197. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyserling HL, Sinkowitz-Cochran RL, Harris JM,, II, Levine GL, Siegel JD, Stover BH, et al. Vancomycin use in hospitalized pediatric patients. Pediatrics. 2003;112(2):e104–e111. doi: 10.1542/peds.112.2.e104. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Vazquez-Guillamet C, Kollef MH. Treatment of Gram-positive infections in critically ill patients. BMC Infect Dis. 2014;14:92. doi: 10.1186/1471-2334-14-92. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select Gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant Pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86(12):1230–1243. doi: 10.4065/mcp.2011.0514. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruniera FR, Ferreira FM, Saviolli LRM, Bacci MR, Feder D, Pedreira MDLG, et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19(4):694–700. [PubMed] [Google Scholar]
- 8.Rybak M, Lomaestro B, Rotschafer JC, Moellering R,, Jr, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98. doi: 10.2146/ajhp080434. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Vandecasteele SJ, De Vriese AS, Tacconelli E. The pharmacokinetics and pharmacodynamics of vancomycin in clinical practice: evidence and uncertainties. J Antimicrob Chemother. 2013;68(4):743–748. doi: 10.1093/jac/dks495. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Mali NB, Deshpande SP, Tullu MS, Deshmukh CT, Gogtay NJ, Thatte UM. A prospective antibacterial utilization study in pediatric intensive care unit of a tertiary referral center. Indian J Crit Care Med. 2018;22(6):422–426. doi: 10.4103/ijccm.IJCCM_365_17. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf
- 13.Food and Drug Administration. Guidance for Industry: Bioanalytical method validation. [Internet]. 2001. https://www.fda.gov/downloads/Drugs/Guidances/ucm368107.pdf https://www.fda.gov/downloads/Drugs/Guidances/ucm368107.pdf Available from:
- 14.Zhang T, Watson DG, Azike C, Tettey JNA, Stearns AT, Binning AR, et al. Determination of vancomycin in serum by liquid chromatography-high resolution full scan mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857(2):352–356. doi: 10.1016/j.jchromb.2007.07.041. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012;929:377–389. doi: 10.1007/978-1-62703-050-2_16. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Blot S, Koulenti D, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study. Crit Care. 2014;18(3):R99. doi: 10.1186/cc13874. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matzke GR, Kovarik JM, Rybak MJ, Boike SC. Evaluation of the vancomycin clearance: creatinine-clearance relationship for predicting vancomycin dosage. Clin Pharm. 1985;4(3):311–315. [PubMed] [Google Scholar]
- 18.Marinho DS, Huf G, Ferreira BL, Castro H, Rodrigues CR, de Sousa VP, et al. The study of vancomycin use and its adverse reactions associated to patients of a Brazilian university hospital. BMC Res Notes. 2011;4:236. doi: 10.1186/1756-0500-4-236. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis AG, Grisi SJ. Adverse effects of vancomycin in children: a review of 22 cases. Sao Paulo Med J. 1997;115(3):1452–1455. doi: 10.1590/S1516-31801997000300010. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Safarnavadeh T, Rezaee S, Dashti-Khavidaki S, Khalili H, Daneshjoo K, Sadrai S, et al. Steady-state pharmacokinetic analysis of vancomycin in Iranian pediatric patients. DARU J Pharm Sci. 2009;17:124–130. [Google Scholar]
- 21.Gous AG, Dance MD, Lipman J, Luyt DK, Mathivha R, Scribante J. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth Intensive Care. 1995;23(6):678–682. doi: 10.1177/0310057X9502300603. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Abdel Hadi O, Al Omar S, Nazer LH, Mubarak S, Le J. Vancomycin pharmacokinetics and predicted dosage requirements in pediatric cancer patients. J Oncol Pharm Pract. 2015;22(3):448–453. doi: 10.1177/1078155215591386. DOI: [DOI] [PubMed] [Google Scholar]
- 23.Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. Pediatr Infect Dis J. 1995;14(8):667–673. doi: 10.1097/00006454-199508000-00004. DOI: [DOI] [PubMed] [Google Scholar]
- 24.Krivoy N, Peleg S, Postovsky S, Ben Arush MW. Pharmacokinetic analysis of vancomycin in steady-state in pediatric cancer patients. Pediatr Hematol Oncol. 1998;15(4):333–338. doi: 10.3109/08880019809014017. DOI: [DOI] [PubMed] [Google Scholar]
- 25.Polard E, Le Bouquin A, Le Corre P, Kérebel C, Trout H, Feuillu A, et al. Non steady state PKS bayasian forecasting and vancomycin pharmacokinetics in ICU adult patients. Ther Drug Monit. 1999;21(4):365–403. doi: 10.1097/00007691-199908000-00003. DOI: [DOI] [PubMed] [Google Scholar]
- 26.Harada H, Miyagawa S, Kawasaki S, Hayashi K, Kitamura H, Katsuyama Y, et al. Study of the pharmacokinetics of vancomycin in patients with impaired liver function. J Infect Chemother. 1999;5:104–107. doi: 10.1007/s101560050018. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Brown N, Ho D, Fong K, Bogerd L, Maksymiuk A, Bolivar R, et al. Effects of hepatic function on vancomycin clinical pharmacology. Antimicrob Agents Chemother. 1983;23(4):603–609. doi: 10.1128/AAC.23.4.603. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhim RF, Arnold SR, Lee KR. Vancomycin dosing practices, trough concentrations, and predicted area under the curve in children with suspected invasive staphylococcal infections. J Pediatric Infect Dis Soc. 2013;2(3):259–262. doi: 10.1093/jpids/pis083. DOI: [DOI] [PubMed] [Google Scholar]
- 29.Hwang D, Chiu NC, Chang L, Peng CC, Huang DTN, Huang FY, et al. Vancomycin dosing and target attainment in children. J Microbiol Immunol Infect. 2017;50(4):494–499. doi: 10.1016/j.jmii.2015.08.027. DOI: [DOI] [PubMed] [Google Scholar]
- 30.Frymoyer A, Guglielmo BJ, Wilson SD, Scarpace SB, Benet LZ, Hersh AL. Impact of a hospitalwide increase in empiric pediatric vancomycin dosing on initial trough concentrations. Pharmacotherapy. 2011;31(9):871–876. doi: 10.1592/phco.31.9.871. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arfa P, Karimi A, Tabatabaei SR, Fahimzad A, Armin S, Sistanizad M. A prospective study to assess vancomycin serum concentrations in pediatric patients with current dosing guidelines. Iran J Pharm Res. 2016;15(1):341–346. [PMC free article] [PubMed] [Google Scholar]
- 32.Buckel WR, Ghobrial S, Tamma PD, Milstone AM, Zhao Y, Hsu AJ. Risk factors for non-therapeutic initial steady-state vancomycin trough concentrations in children and adolescents receiving high empiric doses of intravenous vancomycin. Pediatr Drugs. 2017;19(1):43–51. doi: 10.1007/s40272-016-0202-4. DOI: [DOI] [PubMed] [Google Scholar]
- 33.Miloslavsky M, Galler MF, Moawad I, Actis J, Cummings BM, El Saleeby CM. The impact of pediatric-specific vancomycin dosing guidelines: a quality improvement initiative. Pediatrics. 2017;139(6):e20162423. doi: 10.1542/peds.2016-2423. DOI: [DOI] [PubMed] [Google Scholar]
- 34.Eiland LS, English TM, Eiland EH. Assessment of vancomycin dosing and subsequent serum concentrations in pediatric patients. Ann Pharmacother. 2011;45(5):582–589. doi: 10.1345/aph.1P588. DOI: [DOI] [PubMed] [Google Scholar]
- 35.Durham SH, Simmons ML, Mulherin DW, Foland JA. An evaluation of vancomycin dosing for complicated infections in pediatric patients. Hosp Pediatr. 2015;5(5):276–281. doi: 10.1542/hpeds.2014-0081. DOI: [DOI] [PubMed] [Google Scholar]


