Abstract
Background:
In children particularly in the developing world, there is a tendency to downplay the role of primary hypertension in their health. In adults, a number of factors have clearly been associated with the incidence of hypertension. Knowledge of the prevalence of hypertension and its associated factors among children in our environment is important and could inform the need for lifestyle changes and routine blood pressure (BP) checks in children so as to reduce BP-related health risks.
Aim:
The aim of this study is to document the prevalence of hypertension and its risk factors among children in Enugu, Nigeria.
Materials and Methods:
Children aged 6–17 years attending the outpatient clinic of a tertiary hospital, were enrolled for the study. Their socioeconomic status (SES), weight, height, BP, and dipstick urinalysis were measured using standardized methods. Adherence to Mediterranean diet was assessed using the Mediterranean Diet Quality Index (KIDMED). The prevalence of hypertension and the influence of these factors on their BP were analyzed.
Results:
Forty-six (9%) of the 491 participants had hypertension. Of these 46 hypertensive children, 72% were females while a significantly higher proportion 57% (P = 0.006), were in the age group 13–17 years. While age, gender, and the presence of protein in urine were significantly associated with hypertension in these children; body mass index, diet, family history of hypertension, and SES were not.
Conclusion:
The prevalence of hypertension in children in this environment is high and appears to be increasing. There is need for routine BP and urinalysis check for all children in our clinics and wards.
Keywords: Children, Enugu, hypertension, prevalence, risk factors
INTRODUCTION
Hypertension has been defined by levels of blood pressure (BP) above which lowering BP will reduce the cardiovascular risk associated with elevated BP and this level has been classically documented at 140/90 mmHg in adults.1 The new 2017 American College of Cardiology/American Heart Association guideline set hypertension stage 1 at >130/80 mmHg rather than 140/90 mmHg as in the European guidelines.2 Factors such as age, height, and gender are important factors in interpreting BP values in children. The most widely used definition of hypertension in children is delineated as BP >95% of expected BP for age, gender, and height.3
Unlike in adults where essential hypertension is very common, both secondary and essential (primary) hypertension are seen in children– primary hypertension being influenced by factors such as birth weight, maturity during birth, heredity, and diet while secondary is influenced by renal abnormalities, coarctation of the aorta, medications, neoplasm, etc.4 Worldwide, the prevalence of hypertension in children ranges between 1% and 5%, with a significant proportion of them underdiagnosed.5 The prevalence of hypertension in children and adolescents in the United States of America is 3.3%, whereas in Europe, prevalence ranging from 2.2% to 22% has been documented.4,6 Across Africa, the prevalence also varies between 0.2% and 24.8%, with a pooled figure of 5.5%.7 In Nigeria, various studies have also demonstrated similar prevalence rates in the neighborhood of 3.5% and 6%.8,12 In 2013 in Enugu, southeast Nigeria, Ujunwa et al.8 reported a prevalence of 5.4%. A similar study in 2014 on pre-school children in Enugu, Nigeria, noted a comparatively low prevalence rate of elevated BP prevalence of 1.9%.13 The authors opined that the prevalence of hypertension in children may still be on the increase.13 A higher prevalence value, however, may be expected when other groups of children are considered since BP increases with growth and development and results in hypertension during the first two decades of life.14 Although the pathogenesis of raised BP in obese children is not widely understood, evidence abounds on other comorbid conditions, which further accentuate the risk of hypertension.15 Aside from the risks associated with it, childhood hypertension is a major killer and one of the most common health concerns in children worldwide. There is also substantive evidence linking it with long-term cardiovascular risk in adulthood.4 Therefore, it is of public health importance.
BP measurement is usually done for adults and has proven to be crucial in the assessment of cardiovascular health.3 This important measurement is not usually done routinely for children, although childhood hypertension detection is a measurement identifying potential future morbidity (essential hypertension) or existing underlying disease (secondary hypertension). In general, screening of children for hypertension is focused on essential hypertension since this is usually asymptomatic in children and may go unnoticed, but later becomes a risk in adulthood. Secondary hypertension comes to the fore with the presentation of the underlying disease. Consequently, childhood essential hypertension has been termed a strong predictor of hypertension in the adult population.14
Interestingly, identifying children with elevated BP and successfully treating them will have an impact on long-term outcomes of cardiovascular disease as well as a sizeable effect on hypertension-related morbidity and mortality. The cost implication of treating adults with raised BP will be addressed when attention is paid to childhood primary hypertension. To further understand the degree of attention that should be given to hypertension in childhood, knowledge of its prevalence and factors associated with it need to be continuously emphasized. It can also inform the need for routine BP check in children and adolescents in the routine children's clinic. This study was undertaken to determine the prevalence and risk factors for hypertension in children attending a tertiary health facility in Enugu, Nigeria.
MATERIALS AND METHODS
The sample size was calculated using the formula for calculating sample size for a cross-sectional study.16 Where z is the standard score corresponding to a confidence level of 1.96, d corresponds to a precision of 0.07 andP corresponds to a prevalence rate of hypertension of 5.4%.8
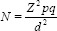
where: N = minimum sample size Z = 1.96; d = total width of the expected confidence interval set at 0.07; P = prevalence from similar study in Enugu (5.4%);8 q = 1-p, i.e., N = 1.96 × 1.96 × 0.54 × 0.46 = 0.95425344/0.0049 = 195. Minimum sample size calculated was 195; however, total sample collected was 500.
After obtaining ethical approval from the Health Ethics Research Committee of the Enugu State University Teaching Hospital (ESUTH) Parklane (ESUTHP/C-MAC/RA/034/075), Enugu, Nigeria, informed consent from the caregiver and assent from older children; children aged 6–17 years attending the outpatient clinic of ESUTH, Enugu, Nigeria, with minor illnesses such as upper respiratory tract infections, skin rash, and children on follow-up who were apparently well, whose parents gave consent for the study were consecutively enrolled for the study over 4 months period (April to July 2017). Children with chronic illnesses, fever, body swelling as well as those with known renal diseases or on steroids; were not included in the study.
An interviewer-administered pro forma was used to collect the biodata, parents' education and occupation and presenting symptoms from eligible children. Socioeconomic status (SES) was ascertained using the socioeconomic classification tool by Oyedeji.17 This tool has five classes (Class I–V). Classes I and II were further classified as upper class; class III as middle class, while Classes IV and V were classified as lower class. Their diet was scored by assessing their adherence to Mediterranean diet using the KIDMED test tool which was developed and validated by Serra-Majem et al.18 The KIDMED questionnaire consists of 16 items, where there are four questions denoting a negative connotation to the Mediterranean diet (consumption of fast food, baked goods, sweets, and skipping breakfast) and 12 questions denoting a positive connotation (consumption of oil, fish, fruits, vegetables, cereals, nuts, pulses, pasta or rice, dairy products, and yoghurt). Questions denoting negative connotations are scored with −1, whereas positive connotation questions are scored with +1. According to the KIDMED index, a score of 0–3 reflects poor adherence to the Mediterranean diet, a score of 4–7 describes average adherence, and a score of 8–12 good adherence.18
The weight was measured using a spring balance scale and stadiometer (SECA model 786 2021 994) was used to measure the height. The BP of the study participants was measured toward the end of the assessment when the child is calm using a Riester sphygmomanometer (Germany, CE 0124; 13094 4 958) with appropriate sized cuff applied on the right arm of each child. An interval of 30 min was allowed and the cuff was completely deflated between readings. The systolic reading was taken as the first Korotkoff sound, and the diastolic pressure was taken as the 5th Korotkoff sound. The determined systolic and diastolic pressures were ascertained using the mean value of the three readings measured by the same person. The children were diagnosed to have hypertension if the BP was ≥95th percentile for age, gender, weight, and height measured in at least three separate occasions.19
The body mass index (BMI) was calculated for each participant and the U.S. BMI-for-age percentile chart for boys and girls were used to group subjects into underweight, normal weight, overweight, and obese. Underweight was taken as BMI <5th percentile, healthy weight as BMI of 5th up to the 85th percentile, overweight as BMI of 85th to <95th percentile, and obese as BMI equal to or >95th percentile for age and gender.20,21
Early morning urine samples were collected for dipstick urinalysis, and the presence of proteinuria was ascertained and recorded in the pro forma. Prevalence of hypertension was analyzed as well as the influence of age, gender, BMI, diet, proteinuria, and socioeconomic class on the BP.
Statistical analysis
All the data obtained was recorded and analyzed using the Statistical Package for the Social Sciences (SPSS®) software for Windows® version 22.0. IBM® Corp., Armonk, NY, USA released 2013. Continuous variables were reported as mean, median, and standard deviation, whereas categorical variables were reported as the number or percentage of participants with a particular characteristic. Test probability value of P < 0.05 was considered statistically significant. Results were presented in tables and prose.
Compliance with ethical standards
This study was funded by the authors. There are no conflicts of interest. Ethical approval for this study was obtained from the Health Research Ethics Committee of ESUTH, Enugu, Nigeria.
RESULTS
Five hundred children were recruited in the study, but 491 of them were analyzed. Nine (1.8%) were not included in the analysis due to incomplete data. Fifty-five percent of the children were females giving a male: female ratio of 1: 1.2. The age ranged from 6 to 17 years and 62.7% were within the school-age of 6–12 years, whereas 62.3% of teenagers (13–17) were females. The prevalence of hypertension was 9% [Figure 1]. Of this 46 hypertensive children, 72% were females while approximately 57% were in the age group 13–17 years.
Figure 1.
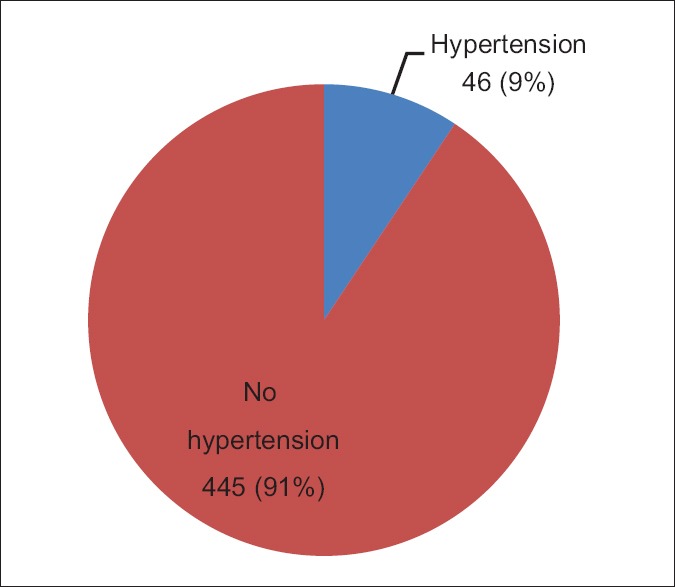
Prevalence of hypertension in children attending the outpatient clinic
Comparing the two age groups (6 12 years, 13–17 years), there was a significantly higher proportion of teenagers with hypertension (14.2%) than school-age children (6.5%) (P = 0.006). No significant difference was found between the mean weight and height of hypertensive and nonhypertensive children in the two age groups [Table 1]. There were more females with hypertension than males (12.1% and 5.9%, respectively). This difference was found to be statistically significant (P = 0.022) [Table 1].
Table 1.
Association between factors of age, sex, and female history of hypertension with hypertension in children
| Hypertension |
OR | 95% CI for OR | P | ||
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| Age (years) | |||||
| 6-12 | 20 (6.5) | 288 (93.5) | 0.419 | 0.227-0.775 | 0.006 |
| 13-17 | 26 (14.2) | 157 (85.8) | |||
| Gender | |||||
| Male | 13 (5.9) | 206 (94.1) | 0.457 | 0.234-0.892 | 0.022 |
| Female | 33 (12.1) | 239 (87.9) | |||
| Family history of hypertension | |||||
| Yes | 25 (12.5) | 175 (87.5) | 1.837 | 0.997-3.382 | 0.051 |
| No | 21 (7.2) | 270 (92.8) | |||
OR – Odds ratio; CI – Confidence interval
Family history of hypertension and socioeconomic status
Positive family history of hypertension was obtained in 40.7% of the children, but this was not significantly associated with raised BP in the children [Table 1]. The socioeconomic classes were grouped into upper (Oyedeji Classes I and II), middle (Oyedeji Class III), and lower (Oyedeji Classes IV and V). Approximately 76% of the children belong to the middle socioeconomic class, whereas 9.4% and 15% belong to upper and lower socioeconomic classes, respectively. No significant difference was found in the prevalence of hypertension in children belonging to the different socioeconomic classes (P = 0.103) [Table 2].
Table 2.
Hypertension and socioeconomic distribution among the children
| Socioeconomic class | Hypertension |
χ2 | P | |
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | |||
| Upper | 16 (6.9) | 217 (93.1) | 4.549 | 0.103 |
| Middle | 19 (10.3) | 165 (89.7) | ||
| Lower | 11 (14.9) | 63 (85.1) | ||
Body mass index
There was no significant association between the BMI and hypertension [Table 3].
Table 3.
Body mass index and hypertension
| BMI (percentiles) | Hypertensio |
OR | 95% CI for OR | P | |
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | ||||
| 5th-85th | 36 (10.1) | 319 (89.9) | |||
| <5th | 4 (4.8) | 79 (95.2) | 0.449 | 0.155-1.298 | 0.139 |
| 85th-95th | 4 (10.8) | 33 (89.2) | 1.074 | 0.360-3.205 | 0.898 |
| >95th | 2 (13.3) | 13 (86.7) | 1.363 | 0.296-6.284 | 0.691 |
OR – Odds ratio; CI – Confidence interval; BMI – Body mass index
Proteinuria
Protein in urine (range trace to ++) was detected in 64 children. There is a significant association of proteinuria with hypertension. Seventeen percent of those with documented proteinuria were hypertensive, whereas only 8.2% of children without protein in urine were hypertensive, P = 0.021 [Table 4].
Table 4.
Hypertension and the presence of protein in urine
| Protein | Hypertension |
χ2, P | |
|---|---|---|---|
| Yes, n (%) | No, n (%) | ||
| Positive | 11 (17.2) | 53 (82.8) | 5.299, 0.021 |
| Negative | 35 (8.2) | 392 (91.8) | |
Diet (KIDMED score)
Using the scoring system for adherence to the Mediterranean diet used Serra-Majem et al.,18 86.6% of the children had medium score, while 4.1% and 9.4% had high and poor scores, respectively. There was no significant association between the scores and hypertension [Figure 2].
Figure 2.
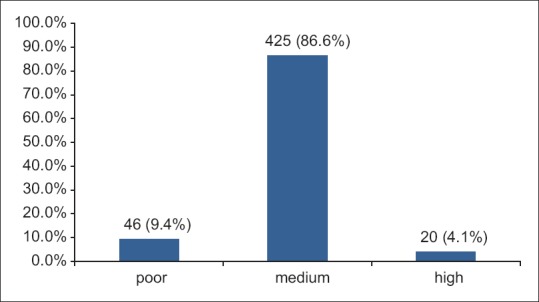
Adherence to mediterranean diet
DISCUSSION
The prevalence rate for hypertension in this study was 9%. In developing countries, particularly African countries, there is a wide variation in the estimated prevalence of hypertension in children and adolescent population ranging from <5% to as high as 20%.22,24 The prevalence of 9% in our study is high compared with prevalence rates in studies done by Okpokowuruk et al.12 in Etoi Uyo (Akwa Ibom State, southern Nigeria) who reported a rate of 3.5% in primary and secondary schools and Umar et al. in Kano, northern Nigeria, 3% in primary school children.25 This study was carried out in ill children (at least those who had reasons to present to the hospital) while the comparative study was carried out among apparently healthy children in schools. In Ghana, West Africa, and in Tunisia in North Africa, closer prevalence rates of 6% and 9.6%, respectively, were obtained by Addo et al. and Harrabi et al.26,27 A study, in the same city of Enugu, among adolescents in a secondary school by Uwaezuoke et al. found a prevalence of 8.2% and as high as 30% among obese students.28 In general, systematic reviews estimates of hypertension in children in Africa and America are 5% and 3.5%, respectively.29,30 The prevalence rate of hypertension in children can be affected by the age of the child (increases with age), the sex (higher in adolescent girls), and BMI (higher in obesity). In our study, 62.3% of 13–17 year-old are females, whereas 56% of 13–17 year-old are females in Akwa Ibom study and the age range for the study population in Kano was 6–14 years. The higher number of adolescent girls and higher age of our children compared with Akwa Ibom and Kano studies, respectively, may partly explain the high prevalence rate of hypertension in our study.
There is a significant association of high BP with gender in our study. Of the 46 children with hypertension 72% are females and 12.1% and 5.9% of females and males, respectively, are hypertensive. A study in Dar es salam, Tanzania, and Fort Worth, Texas found no gender difference in the prevalence of hypertension among school children aged 6–17 years and 8–13 years, respectively.31,32 In Nigeria, while Okpokowuruk et al.12 in Uyo reported no significant association with gender, Uwaezuoke et al.28 in Enugu reported significant association of gender and hypertension (8.2% in females and 3.3% in males) in secondary school students. The finding is also in consonance with that of a prospective study done in Finland by Hu et al.33 Higher risk of obesity in adolescent girls (who constitute 62.3% of our 13–17 year-old), among other factors, may explain the increased prevalence of hypertension in them.
The significant association of hypertension with age (higher in 13–17 years) found in this study is supported by findings in Uyo, Nigeria and Dar es salam, Tanzzania.31 A similar cross-sectional study in a developing setting like Chennai, India, documented a higher prevalence of hypertension among the same age range.34 Documentation of the higher prevalence of hypertension in this age range is not surprising knowing the hormonal and accompanying physiological changes and increasing incidence of obesity during puberty. Urrutia et al.32 in Texas in their study did not find any significant relationship between the ages of children and hypertension, the narrow age range (8–13 years) of the children they studied could explain their findings.
Proteinuria documented in 13% of the study population was significantly associated with hypertension. Gui et al., using a different method, in their study of Turkish school-aged children found no significant association of proteinuria with hypertension but documented increasing prevalence rate of proteinuria with increasing age of the children (0.5%, 3.0%, 16.7% in 6–9, 10–13, and 14–18 year-old, respectively).35 The prevalence of proteinuria in their study was also higher in obese (11.3%) compared with nonobese (6.2%) children. Malla et al. in India, using dipstick, had a prevalence of 6.2% but no significant association with hypertension among 12–14-year-old school children.36 Proteinuria usually benign in the form of transient or orthostatic proteinuria may represent underlying renal disease. Its association with hypertension in this study calls for further study, which could include ruling out renal pathologies such as acute glomerulonephritis in children who have hypertension and proteinuria.
There is no significant association between BMI and hypertension in this study. Other studies in Nigeria found a significant association of hypertension with obesity.12,22,28 However, two of these studies were done among adolescents with a high prevalence of obesity. Only 3% of our study population were obese and 72% had normal BMI. The low prevalence of obesity may explain the finding in our study.
In this study, a positive family history of hypertension was not significantly associated with hypertension in the study population. A significant positive relationship between a family history of hypertension and the presence of hypertension in children was documented by Okoh and Alikor in Port-Harcourt and Mijinyawa et al. in Kano in Nigeria.7,37 A study among adolescents in Latin American population showed a strong link between family history of hypertension and the development of hypertension among adolescents.38 The study was however conducted among obese adolescents in contrast to our study of school-age and adolescents with majority having normal weight. The finding in this study is in corroboration with a study in India by Buch et al.39 and Okpokowuruk et al.12 in Uyo, Nigeria that found no significant relationship between family history of hypertension and hypertension in children. Genetic predisposition, obesity, and environmental factors (psychosocial stressors) contribute to the development of hypertension, especially in adults. The interplay between these factors may explain the difference in the studies.
There is no significant relationship between parental socioeconomic class and the development of hypertension in our study population. This finding was contrary to that of a similar cross-sectional study in Poland, which documented disparities in BPs with respect to SES.40 The finding in the latter appears reasonable as weight and obesity which are strong risk for hypertension; have strong socioeconomic inclinations, and the study was among adolescents who have relatively higher rate of obesity.41 In Indonesia, Julia et al. also found the BP of rural and poor urban children to be significantly higher than that of nonpoor urban children.42 However, Balogun et al., in their study of Nigerian children, aged 8–20 years, found no significant association between parental SES and the BP of the children.43 Although not statistically significant, the table shows that the proportion of children in low socioeconomic class with hypertension (1 in 7) is about two times that of those in upper class (1 in 15). However, the skewed distribution of population observed among the different socioeconomic strata in our study (upper 9.4%, middle 75.6%, and lower 15.1%) may partly explain our findings.
KIDMED scores were not significantly associated with hypertension in this study. In their analysis, Psaltopoulou et al. found adherence to the Mediterranean diet to be inversely associated with high systolic and diastolic BP.44 However, their study was in adults and effect of diet on hypertension might only become evident with increasing age.
CONCLUSION
The prevalence of hypertension among children in our study population is high and appears to be on the increase when compared to figures from earlier studies in the same environment. Age, gender, and proteinuria were significantly associated with hypertension in these children. Sensitization of health workers for routine BP checks in the pediatric clinics and wards, combined with health education of caregivers and older children are necessary interventions in forestalling the morbidity/mortality due to hypertension.
The association between proteinuria and hypertension in children needs to be further investigated. There is a need for a large scale community-based study to further investigate demographic variables that may explain the varied prevalence of hypertension and correlates in different parts of Nigeria.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schiffrin EL, Calhoun DA, Flack JM. Do we need a new definition of hypertension after SPRINT? Am J Hypertens. 2016;29:1127–9. doi: 10.1093/ajh/hpw068. [DOI] [PubMed] [Google Scholar]
- 2.Burnier M, Oparil S, Narkiewicz K, Kjeldsen SE. New 2017 American Heart Association and American College of Cardiology guideline for hypertension in the adults: Major paradigm shifts, but will they help to fight against the hypertension disease burden? Blood Press. 2018;27:62–5. doi: 10.1080/08037051.2018.1430504. [DOI] [PubMed] [Google Scholar]
- 3.Patel N, Walker N. Clinical assessment of hypertension in children. Clin Hypertens. 2016;22:15. doi: 10.1186/s40885-016-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill KD, Li JS. Childhood hypertension: An underappreciated epidemic? Pediatrics. 2016;138:pii: e20162857. doi: 10.1542/peds.2016-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson M, Dana T, Bougatsos C, Blazina I, Norris SL. Screening for hypertension in children and adolescents to prevent cardiovascular disease. Pediatrics. 2013;131:490–525. doi: 10.1542/peds.2012-3523. [DOI] [PubMed] [Google Scholar]
- 6.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European society Of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–920. doi: 10.1097/HJH.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 7.Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: A systematic review and meta-analysis. Lancet Public Health. 2017;2:e375–86. doi: 10.1016/S2468-2667(17)30123-8. [DOI] [PubMed] [Google Scholar]
- 8.Ujunwa FA, Ikefuna AN, Nwokocha AR, Chinawa JM. Hypertension and prehypertension among adolescents in secondary schools in Enugu, South East Nigeria. Ital J Pediatr. 2013;39:70. doi: 10.1186/1824-7288-39-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejike CE, Ugwu CE, Ezeanyika LU. Variations in the prevalence of point (pre) hypertension in a Nigerian school-going adolescent population living in a semi-urban and an urban area. BMC Pediatr. 2010;10:13. doi: 10.1186/1471-2431-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okoh BA, Alikor EA. Childhood hypertension and family history of hypertension in primary school children in Port Harcourt. Niger J Paediatr. 2013;40:184–8. [Google Scholar]
- 11.Okpere AN, Anochie IC, Eke FU. Pattern of blood pressure and hypertension in adolescents in Port Harcourt, Nigeria. West Afr J Med. 2013;32:93–8. [PubMed] [Google Scholar]
- 12.Okpokowuruk FS, Akpan MU, Ikpeme EE. Prevalence of hypertension and prehypertension among children and adolescents in a semi-urban area of uyo metropolis, Nigeria. Pan Afr Med J. 2017;28:303. doi: 10.11604/pamj.2017.28.303.14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odetunde OI, Neboh EE, Chinawa JM, Okafor HU, Odetunde OA, Ezenwosu OU, et al. Elevated arterial blood pressure and body mass index among Nigerian preschool children population. BMC Pediatr. 2014;14:64. doi: 10.1186/1471-2431-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherma A. Hypertension in adolescents. In: Goldstein MA, editor. The Mass General Hospital for Children Adolescent Medicine Handbook. Boston: Springer International Publishing AG; 2017. pp. 119–29. [Google Scholar]
- 15.Gunta SS, Mak RH. Hypertension in children with obesity. World J Hypertens. 2014;4:15–24. [Google Scholar]
- 16.Daniel WW. Biostatistics basic concepts and methodology for the health sciences. In: Wiley HJ, editor. Print book: English: 9th ed., International Student Version. 2010. [Google Scholar]
- 17.Oyedeji G. Socio-economic and cultural background of hospitalized children in Ilesha. Niger Med Pract. 1985;12:111–7. [Google Scholar]
- 18.Serra-Majem L, García-Closas R, Ribas L, Pérez-Rodrigo C, Aranceta J. Food patterns of Spanish schoolchildren and adolescents: The EnKid study. Public Health Nutr. 2001;4:1433–8. doi: 10.1079/phn2001234. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.BMI Percentile Calculator for Child and Teen, English Version. CDC Home. [Last acessed on 2019 May 09]. Available from: http://apps.nccd.cdc.gov/dnpabmi/Calculator.aspx .
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 22.Ejike CE, Ugwu CE, Ezeanyika LU, Olayemi AT. Blood pressure patterns in relation to geographic area of residence: A cross-sectional study of adolescents in Kogi state, Nigeria. BMC Public Health. 2008;8:411. doi: 10.1186/1471-2458-8-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agyemang C, Redekop WK, Owusu-Dabo E, Bruijnzeels MA. Blood pressure patterns in rural, semi-urban and urban children in the Ashanti Region of Ghana, West Africa. BMC Public Health. 2005;5:114. doi: 10.1186/1471-2458-5-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woelk G, Emanuel I, Weiss NS, Psaty BM. Birthweight and blood pressure among children in Harare, Zimbabwe. Arch Dis Child Fetal Neonatal Ed. 1998;79:F119–22. doi: 10.1136/fn.79.2.f119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umar A, Mustafa A, Muuta I. Prevalence of elevated blood pressure among primary school children in Kano Metropolis, Nigeria. Niger J Cardiol. 2016;13:57–61. [Google Scholar]
- 26.Addo J, Amoah AG, Koram KA. The changing patterns of hypertension in Ghana: A study of four rural communities in the Ga district. Ethn Dis. 2006;16:894–9. [PubMed] [Google Scholar]
- 27.Harrabi I, Belarbia A, Gaha R, Essoussi AS, Ghannem H. Epidemiology of hypertension among a population of school children in Sousse, Tunisia. Can J Cardiol. 2006;22:212–6. doi: 10.1016/s0828-282x(06)70898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uwaezuoke SN, Okoli CV, Ubesie AC, Ikefuna AN. Primary hypertension among a population of Nigerian secondary school adolescents: Prevalence and correlation with anthropometric indices: A cross-sectional study. Niger J Clin Pract. 2016;19:649–54. doi: 10.4103/1119-3077.188706. [DOI] [PubMed] [Google Scholar]
- 29.Falkner B. Hypertension in children and adolescents: Epidemiology and natural history. Pediatr Nephrol. 2010;25:1219–24. doi: 10.1007/s00467-009-1200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–96. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 31.Muhihi AJ, Njelekela MA, Mpembeni RN, Muhihi BG, Anaeli A, Chillo O, et al. Elevated blood pressure among primary school children in Dar Es Salaam, Tanzania: Prevalence and risk factors. BMC Pediatr. 2018;18:54. doi: 10.1186/s12887-018-1052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urrutia-Rojas X, Egbuchunam CU, Bae S, Menchaca J, Bayona M, Rivers PA, et al. High blood pressure in school children: Prevalence and risk factors. BMC Pediatr. 2006;6:32. doi: 10.1186/1471-2431-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu G, Barengo NC, Tuomilehto J, Lakka TA, Nissinen A, Jousilahti P, et al. Relationship of physical activity and body mass index to the risk of hypertension: A prospective study in Finland. Hypertension. 2004;43:25–30. doi: 10.1161/01.HYP.0000107400.72456.19. [DOI] [PubMed] [Google Scholar]
- 34.Sundar JS, Adaikalam JMS, Parameswari S, Valarmarthi S, Kalpana S, Shantharam D. Prevalence and determinants of hypertension among urban school children in the age group of 13-17 years in, Chennai, Tamilnadu. Epidemiol. 2013;3:130. [Google Scholar]
- 35.Gui A, Ozer S, Yilmaz R, Sonmegoz E, Kasap T, Takci S, et al. Prevalence of proteinuria in school-aged Turkish children and its association with obesity and hypertension. J Pediatr Res. 2017;4:195–9. [Google Scholar]
- 36.Malla HA, Bhat AM, Shazia B, Rather FA, Najar SM, Wani IA. Prevalence of proteinuria in school children (aged 12-14 years) in Kashmir Valley, India, using dipstick method. Saudi J Kidney Dis Transpl. 2016;27:1006–10. doi: 10.4103/1319-2442.190877. [DOI] [PubMed] [Google Scholar]
- 37.Mijinyawa MS, Iliyasu Z, Borodo MM. Prevalence of hypertension among teenage students in Kano, Nigeria. Niger J Med. 2008;17:173–8. doi: 10.4314/njm.v17i2.37378. [DOI] [PubMed] [Google Scholar]
- 38.Simsolo RB, Romo MM, Rabinovich L, Bonanno M, Grunfeld B. Family history of essential hypertension versus obesity as risk factors for hypertension in adolescents. Am J Hypertens. 1999;12:260–3. doi: 10.1016/s0895-7061(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 39.Buch N, Goyal JP, Kumar N, Parmar I, Shah VB, Charan J, et al. Prevalence of hypertension in school going children of Surat city, Western India. J Cardiovasc Dis Res. 2011;2:228–32. doi: 10.4103/0975-3583.89807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaczmarek M, Stawińska-Witoszyńska B, Krzyżaniak A, Krzywińska-Wiewiorowska M, Siwińska A. Who is at higher risk of hypertension? Socioeconomic status differences in blood pressure among polish adolescents: A population-based Adopolnor study. Eur J Pediatr. 2015;174:1461–73. doi: 10.1007/s00431-015-2554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hothan KA, Alasmari BA, Alkhelaiwi OK, Althagafi KM, Alkhaldi AA, Alfityani AK, et al. Prevalence of hypertension, obesity, hematuria and proteinuria amongst healthy adolescents living in western Saudi Arabia. Saudi Med J. 2016;37:1120–6. doi: 10.15537/smj.2016.10.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julia M, van Weissenbruch MM, Delemarre-van de Waal HA, Surjono A. The influence of socioeconomic status on blood pressure of Indonesian prepubertal children. J Hum Hypertens. 2006;20:546–8. doi: 10.1038/sj.jhh.1002028. [DOI] [PubMed] [Google Scholar]
- 43.Balogun JA, Obajuluwa VA, Olaogun MO, Abereoje OK, Oyeyemi AY, Adeodu OO, et al. Influence of parental socioeconomic status on casual blood pressures of Nigerian school children. Int J Cardiol. 1990;29:63–9. doi: 10.1016/0167-5273(90)90274-9. [DOI] [PubMed] [Google Scholar]
- 44.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: The Greek European prospective investigation into cancer and nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012–8. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]


