Abstract
Cancer immune therapy with checkpoint inhibitors (CPIs) has changed the landscape of treatment for a growing number of indications. These drugs are associated with a specific mechanism of action that has profound implications for both immunology and inflammatory disease. This article looks to set the scene covering the history of CPI therapy to date and outlining the likely future developments.
Keywords: cancer, immunotherapy, drug development, toxicity
Rheumatology key messages
Checkpoint inhibitors (CPIs) are the standard of care for a growing number of cancer indications.
CPI toxicities reflect the tolerance breaking/pro-inflammatory mechanism of action of these drugs.
An exponential growth in use of these drugs over the coming years is expected.
History of cancer immune therapy
Since 2600 bc, the observation, made by Imhotep (an Egyptian polyglot and physician), that intentionally infecting a growing tumour can lead to regression has underwritten a role for the immune system in cancer therapy. William Coley consolidated this in the late 1800s into an Food and Drug Administration (FDA)-approved bacterial inoculate for soft tissue sarcomas. Throughout the 20th century, advancement in molecular biology and refinement of animal models of cancer have permitted a blossoming in our understanding of host immunity and cancer evolution. We now know that the interplay between a nascent cancer and an infiltrating immune response can eliminate the neoplastic cells or begin the process of editing them into a tumour that ultimately escapes immune system control [1].
A successful tumour capitalizes on multiple mechanisms to evade an immune response. Put another way, there are multiple points at which the biology of cancer immune evasion can potentially be capitalized upon for cancer immunotherapy [2]. Examples from clinical translation include inadequate support of tumour-reactive T cells from secreted factors such as cytokines—exemplified by high-dose bolus IL-2, a therapeutic option for melanoma and renal cell carcinoma patients [3, 4], availability of tumour-associated antigens for recognition by tumour infiltrating lymphocytes (TILs) through oncolytic viral therapy (which potentially releases neo-epitopes to antigen-presenting cells in the tumour microenvironment or draining lymph nodes for presentation to TILs) [5] or through adoptive cell therapy with neo-epitope selected TILs [6]. Ultimately the greatest clinical breakthrough to date, in manipulating a hosts immune response against an established tumour, has been realized through checkpoint inhibitor therapy (CPI), monoclonal antibodies directed against inhibitory checkpoints involved in attenuating T cell–mediated immune responses and/or maintaining peripheral tolerance.
CPI therapy: clinical success
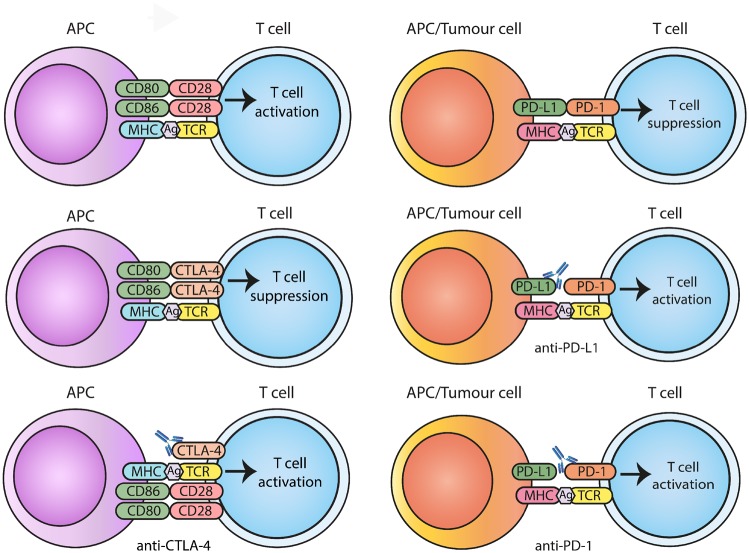
The cytotoxic T-lymphocyte antigen-4 (CTLA-4), a member of the immunoglobulin super-family of receptors, was first identified in mice in the 1980s [7]. It was shown to be an alternative binding partner for the ligands of the co-stimulatory T cell receptor CD28, with T cell inhibitory properties [8, 9]. Inhibition of CTLA-4 on T cells in a murine model of colon cancer established the principal of blockade of inhibitory checkpoints as a potential strategy in cancer immune therapy [10]. CTLA-4 regulates T cells in the early immune response, predominantly in lymph nodes, and acts as a competitive CD28 homologue. It has a higher affinity for B7-1 (CD80), and to a lesser degree B7-2 (CD86), than does CD28 for these ligands [Fig. 1] [11]. T cell receptor signalling rapidly results in upregulation of CTLA-4 on the cell surface [12]. On CD4+ T cells, CTLA-4 down-modulates helper T cell activity and enhances immunosuppression mediated by regulatory T cells [13].
Fig. 1.
Diagram of the interplay between CD28 and CTLA-4 and their shared ligands and PD-1/PD-L1
Blockade of inhibitory checkpoints with specific antibodies leads to release of T cell activation.
Programmed cell death protein 1 (PD-1) was next to be identified in the 1990s [14]. PD-1 is expressed on lymphoid and myeloid immune cells [15]. Its ligands, PD-L1 and PD-L2, are widely expressed in cancers and stromal cell populations [Fig. 1]. It suppresses T cells in peripheral tissues and acts at a later stage in the immune response than CTLA-4. The PD-1/PD-L1 pathway has an important role in the prevention of autoimmunity. PD-1 binds to PD-L1 and PD-L2, leading, in the case of PD-L1, to inhibition of both T cell proliferation and the production of pro-inflammatory cytokines [16]. Building on CTLA-4 and PD-1 biology, there has been a rapid expansion in our understanding of the role of inhibitory checkpoints in cancer immune evasion, with the resultant identification of multiple inhibitory receptors, including lymphocyte activation gene (LAG)-3, VISTA, T cell immunoreceptor with Ig and ITIM domains, T-cell immunoglobulin and mucin-domain containing-3 and OX40 [17]. Following from the observation that CTLA-4 blockade in murine models leads to rejection of established cancer clinical, development of blocking monoclonal antibodies to inhibitory checkpoints and their ligands has blossomed.
Melanoma the ‘immunogenic cancer’
Melanoma is a model cancer for the development of immunotherapy. This is in part due to practicality. Melanoma, both primary and metastatic disease, is relatively accessible to biopsy. As a result, cell line development for preclinical research has resulted in a broad array of available models for immune therapy development. Spontaneous remissions of established melanomas have been documented through time and a good prognosis is associated with autoimmune vitiligo. Both phenomena are attributed to an active endogenous anti-tumour immune response [18]. The discovery of tumour-associated antigens with the potential to activate TILs through endogenous T cell receptors began with antigens identified in melanoma [19]. More recently, the development of platform technologies to enable a detailed understanding of somatic mutational burden in cancers has identified melanoma as the highest scoring tumour by anatomical location [20]. Tumour mutational burden (TMB) is linked to the immunogenicity of a cancer and, in part, to the efficacy of CPIs [21].
In the late 2000s, a meta-analysis of phase 2 advanced melanoma studies described a median survival of 6.2 months (95% CI 5.9–6.5). No meaningful progress had been made for decades through drug development efforts in the disease [22]. Ipilimumab, an anti-CTLA-4 monoclonal antibody, was the first immune CPI to show an overall survival benefit in a randomized phase 3 trial. Melanoma patients were treated with ipilimumab and a vaccine to glycoprotein 100 (gp100), ipilimumab alone or gp100 vaccine alone. The median overall survival was 10.1 months, and 10.0 months in combination with gp100 vaccine, compared with 6.4 months for gp100 alone [23]. These data resulted in the first regulatory approval for a drug for melanoma in decades and heralded the beginning of a new era of cancer therapy. A key observation, borne out in subsequent studies of CPI in melanoma and other diseases, is that within the population who gain benefit from CPI therapy there is a subset who have long-term disease eradication. This ‘long tail on the survival curve’ represents a paradigm shift in outcomes and expectations in the CPI era [24].
The PD-1 targeting monoclonal antibodies pembrolizumab and nivolumab followed ipilimumab into the clinic in 2015 [25, 26]. Targeting PD-1 on cytotoxic T cells resulted in greater efficacy and reduced rates of toxicity compared with CTLA-4 targeting with ipilimumab. The differing immunobiology of CTLA-4 and PD-1 in immune tolerance paved the way for strategies to combine more than one CPI, with the expectation of synergy. The CheckMate 067 study compared ipilimumab monotherapy to nivolumab alone or in combination with ipilimumab [27]. At 4 years of follow-up the median overall survival has not yet been reached for the combination therapy arm. Strikingly, more than half of patients treated with a nivolumab-containing regimen are alive at 4 years [28]. This represents a remarkable change in survival potential for patients with advanced melanoma [22].
In the past year we have seen regulatory approvals for nivolumab and pembrolizumab for the treatment of resected stage 3 melanoma [29, 30]. This is another step change in the number of melanoma patients for whom exposure to CPIs is considered as the standard of care. Introducing immune therapy earlier in the disease process, in a population where a significant proportion are cured already of the cancer, raises the bar further when considering risks associated with treatment.
Beyond melanoma
Melanoma is in reality a relatively rare disease. When we look at the TMB in common cancers, diseases such as lung and bladder cancers do not sit far behind melanoma [20]. The years since 2015 have seen a remarkable number of single-agent CPI clinical trials of PD-1- and PD-L1-targeting antibodies leading to regulatory approvals in thoracic cancers, Merkel cell carcinoma, renal cell carcinoma, urothelial cancers and squamous cell carcinomas of the head and neck and skin, to name but a few [31–38].
For the majority of these and other indications, the impact of CPI monotherapy is not as remarkable as that seen in advanced melanoma. Inevitably this has led to combination strategies moving from concept to impact. In the field of thoracic malignancies, combining anti-PD-1/PD-L1 therapy with platinum-containing chemotherapy is having an increasing impact [35, 39]. Similarly, in renal cell carcinoma and non-small cell lung cancer, the combination of ipilimumab and nivolumab (albeit at different doses than the regimen licensed in melanoma) is translating into routine clinical practice [40, 41].
New indications coupled with trials of combination strategies directed at improving CPI response rates continue to feed an expediential increase in the use of this new class of drugs. Practical considerations such as cost to health care systems, the impact of side effects and the small population who gain meaningful long-term disease control exemplify the need to focus translational strategies on patient selection and management of toxicities to ensure CPI therapy delivers impact and minimizes risk to the populations treated.
Predicting response
Selecting a population of patients more likely to gain benefit from CPIs is desirable. Similarly, identifying futility and helping the selection of populations for trials of combinations to improve impact would reduce the exposure of patients to risk for no return. To date, potential biomarkers for CPI therapy have focused on PD-L1 expression and the TMB.
PD-L1 is widely expressed in the microenvironment of tumours. It is not restricted to tumour cells themselves, but is found in stroma and draining lymph nodes. PD-L1 has been studied as a biomarker in a range of indications. In melanoma, PD-L1 expression is not widely adopted as a biomarker, as responses are seen to PD-1-directed drugs in patients with undetectable levels of PD-L1 in tumour biopsies. In CheckMate 067 for example, patients were stratified by PD-L1 expression of >1% or <1%. Low PD-L1 expression did not predict lack of response to single-agent nivolumab but did define a greater progression-free survival benefit for the combination of ipilimumab and nivolumab. In contrast, the higher PD-L1 subgroup appears to gain less benefit from the addition of ipilimumab to the PD-1 backbone therapy [28]. In thoracic malignancies, the role of PD-L1 testing is less controversial. There is patient selection in place for first-line pembrolizumab based on PD-L1 expression in tumour and stroma [34]. In the second-line setting, the role of PD-L1 selection is much less convincing and licensing is agnostic to PD-L1 expression [32, 38]. The biology of PD-L1 upregulation goes some way towards explaining the lack of consistency/clarity about PD-L1 as a biomarker of efficacy alone. Inflammatory cytokines released by infiltrating immune cells in a tumour microenvironment lead to rapid upregulation of PD-1 in cytotoxic T cells and PD-L1 in the stroma. The timing of biopsy for PD-L1 testing and the ever-changing immune microenvironment in a tumour will influence the degree of expression seen at any one time. This dynamic nature of immune checkpoints makes basing treatment decisions on one biomarker alone a potentially unsatisfactory approach.
The TMB has been show to correlate in part with the likelihood of benefit of CPI therapy in particular tumour groups [20]. Indeed, in a series of 27 different tumour types, a correlation was seen between rates of TMB and response rates with PD-1/PD-L1 therapy [21]. The FDA has licensed pembrolizumab for the treatment of unresectable or metastatic microsatellite instability-high or mismatch repair deficient (dMMR) solid tumours that have progressed following prior treatment [42]. This decision was based on the objective response rate of 39.6% (95% CI 31.7, 47.9) observed in 149 patients with microsatellite instability-high or dMMR tumours across 15 different tumour types. The biology of prediction of benefit based on genetic signatures of high TMB makes sense, however, >60% of microsatellite instability-high/dMMR unstable tumours did not respond to treatment in the pembrolizumab study, again highlighting the unsatisfactory nature of a single biomarker of response for CPI therapy. In non-small cell lung cancer, TMB as a biomarker of ipilimumab and nivolumab efficacy is particularly challenging. Initial interpretation of very immature data appeared to suggest a clear role for the TMB in selecting patients for ipilimumab and nivolumab vs platinum-based chemotherapy, but as data has matured, the relationship has become much less clear and further follow-up is required [40].
Ultimately we do not yet have a biomarker that can predict response to CPI therapy with confidence. Work is ongoing to define cut-off levels for the TMB and to bring together immune signatures that may act as robust guides to the use of these drugs in the clinic.
Toxicity
Ipilimumab and nivolumab as single agents were non-toxic in a preclinical cynomolgus monkey model. In clinical trials, overall adverse event (AE) incidences of ⩾50% are reported. The AEs seen in the studies of CPI therapy are specific and unique to the mechanism of action of these drugs. Side effects are due to immune activation syndromes and to likely self-antigen-specific autoinflammatory and autoimmune immune-related AEs (irAEs). Indeed, irAE rates ⩾45% are consistently reported in clinical trials [43]. There are clear differences between the different CPI targets. Ipilimumab therapy demonstrates a dose-dependent increase in the risk of irAEs that is not seen with PD-1/PD-L1 targeting [44, 45]. Combining CTLA-4 and PD-1 targeting leads to a demonstrably increased risk of irAEs [27, 28, 41]. Targeting the different checkpoints results in overlapping and distinct patterns and rates of irAEs, underpinning the differing immunobiology of the checkpoints immune systems utilize [46]. Furthering our understanding of the immunobiology of irAEs is crucial to improve patients’ outcomes as use expands.
Future directions
Cancer immunotherapy is a multibillion dollar market, which is rapidly expanding. The use of CPI therapy is increasing exponentially as new indications reach regulatory approval. This overview has aimed to cover the journey to date. In so doing, it has highlighted that despite the excitement associated with this new class of drugs, we are still failing to deliver impact in the majority of patients treated. In an attempt to address this, CPI therapy is increasingly being studied in combination with other immunotherapies (including novel immune checkpoints, cell therapies and oncolytic viral therapies), targeted therapies and conventional chemotherapy drugs. In fact, if you search cancer clinical trial databases in 2019, the number of studies under way involving CPIs as combination partners is vast and difficult to fathom from the position of a cancer clinician.
A few approaches have reached advanced stages of development, with optimism of imminent clinical impact. Oncolytic viral therapy, with the licensed virus talimogene laherparepvec (T-VEC), is one such example [47]. In a small phase 1b trial of 21 advanced melanoma patients, combination therapy with pembrolizumab was well tolerated and the response rate was promising [48]. A phase 3 study has completed recruitment, with early results expected in late 2019. Combining targeted therapy approaches with CPI in renal cell carcinoma and melanoma is relatively advanced, with results expected in the next 1–2 years.
Novel checkpoints are under investigation with antibodies in clinical development to co-inhibitory checkpoints and stimulating antibodies to co-stimulatory checkpoints. The most advanced target in clinical development is LAG-3, with antibodies from more than one pharmaceutical company in early to late phase trials across multiple indications [49].
Caution is required however in moving forward with combination strategies in the absence of reliable methods of patient selection/stratification. The recent negative phase 3 study of pembrolizumab ± the indoleamine 2,3-dioxygenase inhibitor epacadostat highlights the risk associated with rapid clinical development of combinations in unselected populations.
Conclusion
Cancer immune therapy with CPIs has changed the face of systemic anticancer therapy for a growing number of indications. The potential for long-term disease control in the context of advanced disease makes this class of therapeutic exciting. However, key challenges remain, including patient selection, cost and lack of efficacy.
Ultimately CPI therapy will continue to increase exponentially, bringing with it a growing burden of irAEs in the clinic and on the wards. Oncology needs to work closely with inflammatory disease specialist teams to ensure we are learning from the increasing prevalence of irAEs and striving to develop evidence-based approaches to their management.
Acknowledgements
SP would like to acknowledge the Guy’s and St Thomas’ NHS Foundation Trust Biomedical Research Centre, the Cancer Research UK City of London Major Centre, the Medical Research Council and John Reece for philanthropic support.
Funding: This paper was published as part of a supplement funded by an educational grant from BMS.
Disclosure statement: SP has received honoraria from Bristol-Myers Squibb, MSD, Roche, GlaxoSmithKline, Amgen and Zelluna. The other author has declared no conflicts of interest.
References
- 1. Dunn GP, Old LJ, Schreiber RD.. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- 2. Blank CU, Haanen JB, Ribas A, Schumacher TN.. Cancer immunology. The “cancer immunogram”. Science 2016;352:658–60. [DOI] [PubMed] [Google Scholar]
- 3. Atkins MB, Lotze MT, Dutcher JP. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105–16. [DOI] [PubMed] [Google Scholar]
- 4. Fyfe G, Fisher RI, Rosenberg SA. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688–96. [DOI] [PubMed] [Google Scholar]
- 5. Hans R, Andtbacka I, Collichio FA. et al. OPTiM: a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol 2013;9(18 Suppl):74–5. [Google Scholar]
- 6. Zacharakis N, Chinnasamy H, Black M. et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 2018;24:724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brunet JF, Denizot F, Luciani MF. et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987;328:267–70. [DOI] [PubMed] [Google Scholar]
- 8. Harper K, Balzano C, Rouvier E. et al. CTLA-4 and CD28 activated lymphocyte molecules are closely related in both mouse and human as to sequence, message expression, gene structure, and chromosomal location. J Immunol 1991;147:1037–44. [PubMed] [Google Scholar]
- 9. Linsley PS, Brady W, Urnes M. et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991;174:561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leach DR, Krummel MF, Allison JP.. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6. [DOI] [PubMed] [Google Scholar]
- 11. Sansom DM. CD28, CTLA-4 and their ligands: who does what and to whom? Immunology 2000;101:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Linsley PS, Bradshaw J, Greene J. et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 1996;4:535–43. [DOI] [PubMed] [Google Scholar]
- 13. Wing K, Onishi Y, Prieto-Martin P. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271–5. [DOI] [PubMed] [Google Scholar]
- 14. Ishida Y, Agata Y, Shibahara K, Honjo T.. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992;11:3887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keir ME, Butte MJ, Freeman GJ, Sharpe AH.. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshall HT, Djamgoz M.. Immuno-oncology: emerging targets and combination therapies. Front Oncol 2018;8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol 2012;23(Suppl 8):viii10–4. [DOI] [PubMed] [Google Scholar]
- 19. Boon T, van der Bruggen P.. Human tumor antigens recognized by T lymphocytes. J Exp Med 1996;183:725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alexandrov LB, Nik-Zainal S, Wedge DC. et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yarchoan M, Hopkins A, Jaffee EM.. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korn EL, Liu PY, Lee SJ. et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527–34. [DOI] [PubMed] [Google Scholar]
- 23. Hodi FS, O’Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schadendorf D, Hodi FS, Robert C. et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robert C, Long GV, Brady B. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 26. Robert C, Schachter J, Long GV. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- 27. Larkin J, Chiarion-Sileni V, Gonzalez R. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hodi FS, Chiarion-Sileni V, Gonzalez R. et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. [DOI] [PubMed] [Google Scholar]
- 29. Eggermont AMM, Blank CU, Mandala M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- 30. Weber J, Mandala M, Del Vecchio M. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 31. Bellmunt J, de Wit R, Vaughn DJ. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brahmer J, Reckamp KL, Baas P. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferris RL, Blumenschein G Jr, Fayette J. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 35. Horn L, Mansfield AS, Szczęsna A. et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- 36. Migden MR, Rischin D, Schmults CD. et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018;379:341–51. [DOI] [PubMed] [Google Scholar]
- 37. Motzer RJ, Escudier B, McDermott DF. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rittmeyer A, Barlesi F, Waterkamp D. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gandhi L, Rodriguez-Abreu D, Gadgeel S. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. [DOI] [PubMed] [Google Scholar]
- 40. Hellmann MD, Ciuleanu TE, Pluzanski A. et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Motzer RJ, Tannir NM, McDermott DF. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marcus L, Lemery SJ, Keegan P, Pazdur R.. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 2019;25:3753. [DOI] [PubMed] [Google Scholar]
- 43. Ochoa de Olza M, Oliva M, Hierro C. et al. Early-drug development in the era of immuno-oncology: are we ready to face the challenges? Ann Oncol 2018;29:1727–40. [DOI] [PubMed] [Google Scholar]
- 44. Brahmer JR, Drake CG, Wollner I. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolchok JD, Neyns B, Linette G. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64. [DOI] [PubMed] [Google Scholar]
- 46. Postow MA, Sidlow R, Hellmann MD.. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 47. Andtbacka RH, Kaufman HL, Collichio F. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 2015;33:2780–8. [DOI] [PubMed] [Google Scholar]
- 48. Ribas A, Dummer R, Puzanov I. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves Anti-PD-1 immunotherapy. Cell 2017;170:1109–19.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lichtenegger FS, Rothe M, Schnorfeil FM. et al. Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol 2018;9:385. [DOI] [PMC free article] [PubMed] [Google Scholar]