Abstract
Immune check point inhibitor (CPI) therapy has revolutionized treatment paradigms for several cancers, but at the cost of triggering a diverse spectrum of immune-mediated injury to non-cancer tissues. The complex biology of these toxicities remains incompletely understood, partly because tissue acquisition from affected areas can be challenging to retrieve, thus hindering development of targeted therapy. Here, we review the literature describing pathology of immune-mediated tissue lesions including gastrointestinal, skin, rheumatic, pulmonary, cardiac, renal and hepatic lesions and highlight key immunological insights.
Keywords: irAE pathology, CPI enterocolitis, CPI skin toxicity, CPI renal injury, CPI rheumatic toxicity, CPI hepatitis, CPI pulmonary toxicity, CPI cardiac toxicity
Rheumatology key messages
Whilst immunopathological mechanisms underpinning irAEs and their classical autoimmune counterparts overlap, important differences also exist.
Autopsy studies are a valuable way to comprehensively evaluate the immunopathological burden of irAEs.
Investigating immune mediated lesions using a high-resolution unbiased immunological platform would advance this evolving field.
Introduction
Whilst immune check point inhibitors (CPIs) induce durable anti-cancer responses in a subset of patients, this comes at the cost of incurring immune mediated toxicities (immune related adverse events [irAEs]). These affect virtually any organ system and can cause significant morbidity, mortality and impaired quality of life. Accordingly, there is a pressing need to understand the etiopathology of irAEs in order to avoid interruption to CPI therapy and offer a targeted approach to their management. Advances in this emerging field are hampered by a paucity of data examining immunopathological aspects of disease. This is partly related to inherent challenges in acquiring tissue from affected areas. In this review, we offer pathological insights into irAEs that have associated histological data (summarized in Table 1).
Table 1.
Summary of histopathological and immunological findings in various CPI-induced toxicities
| Reference | Toxicity | CPI regimen/s | Histological findings | Immunological findings and platform used |
|---|---|---|---|---|
| [2, 6, 9–16, 19, 20, 24] | GI-enterocolitis | Anti- PD-1 monotherapy, anti- CTLA-4 monotherapy and combination regimena | Combined findings: Inflammatory infiltrate in the lamina propria composed of lymphocytes, neutrophils, eosinophils and plasma cells; neutrophilic crypt abscess formation; increased apoptotic activity within the crypt epithelium; crypt epithelial atrophy and crypt dropout. Chronic inflammatory changes including crypt distortion, basal plasmacytosis and paneth cell metaplasia. Granulomas (but uncommon). Lymphocytic colitis and collagenous colitis also described. | Immunohistochemistry and flow cytometry |
| Anti-PD-1: Features of acute colitis; chronic colitis (basal lymphoplasmacytosis and crypt architectural irregularity, paneth cell metaplasia); crypt abscesses; apoptosis; inflammatory infiltrate in the lamina propria composed of lymphocytes, neutrophils, eosinophils and plasma cells. Lymphocytic colitis and collagenous colitis. | Predominance of CD8+ cells | |||
| Anti-CTLA-4: Features of acute colitis; chronic colitis (basal lymphoplasmacytosis and crypt architectural irregularity, paneth cell metaplasia); neutrophilic inflammation only; lymphocytic inflammation only; combined neutrophilic and lymphocytic infiltration; intra-epithelial neutrophilic lymphocytes; cryptitis; crypt abscesses; apoptosis; inflammatory infiltrate in the lamina propria composed of lymphocytes, neutrophils, eosinophils and plasma cells; granulomas. Lymphocytic colitis. | Predominance of CD4+ cells with high TNFα expression. | |||
| Significantly increased expression of the major Th-1 and Th-17 pro-inflammatory cytokines IFN-γ and IL-17A. | ||||
| No decrease in FoxP3+ T regulatory cells. | ||||
| Combination regimen:b Lymphocytic colitis. | n/a | |||
| [28–39, 41, 49, 50] | Skin | Anti -PD-1/PD-L1 monotherapy, anti- CTLA-4 monotherapy and combination regimen | Combined findings: wide range depending on skin lesion but includes:
|
Immunohistochemistry |
Anti-PD-1 or anti-CTLA-4 monotherapy (combined results, with majority on anti-CTLA-4):
| ||||
| Anti-PD-1/L1: histological patterns consistent with TEN; bullous pemphigoid; cutaneous sarcoid; lichenoid dermatosis (including histologic features supporting a lichenoid drug eruption such as parakeratosis, spongiosis, periadnexal/perivascular inflammation, and eosinophils); psoriasiform; neutrophilic dermatoses; eczema; vitiligo; actinic keratosis; seborrhoeic keratosis; folliculitis; Grover’s disease; granulomatous dermatitis. | In TEN: predominance of CD8+ cells, and an increase of programmed death ligand 1 (PD‐L1) expression in both lymphocytes and keratinocytes. | |||
| Anti-CTLA-4: histological patterns consistent with TEN; cutaneous sarcoid; lichenoid dermatosis (more common in anti-PD-1\L1 monotherapy), psoriasiform; neutrophilic dermatoses; vitiligo; dermatitis herpetiformis; superficial perivascular lymphocytic dermatitis; Grover’s disease; granulomatous dermatitis. | n/a | |||
| Combination regimen:b Cutaneous sarcoid; bullous erythema multiforme. | n/a | |||
| [54, 60] | Rheumatic | Anti- PD-1 monotherapy, anti-CTLA-4 monotherapy and combination regimen |
|
Immunohistochemistry (sicca syndrome and myositis), TCR repertoire analysis (myositis only) |
| Sicca syndrome: | ||||
Anti-PD-1/anti-PD-L1 and combination regimen treated patients:
| ||||
| Anti-PD-1:b | Myositis: | |||
| Anti-CTLA-4:b | ||||
Anti-PD-1 and combination regimen treated patients:
| ||||
| Combination regimen:b | ||||
| [71, 72] | Renal | Anti PD-1 monotherapy, anti-CTLA-4 monotherapy and combination regimen | Combined findings: Tubulointerstitial inflammation is most common finding. | Immunohistochemistry |
| Acute interstitial nephritis characterized by diffuse interstitial inflammation and focal severe tubulitis, with a lymphocytic infiltration, and some eosinophils and plasma cells. Granulomatous features also described. | Anti-PD-1 monotherapy, anti-CTLA-4 monotherapy and combination regimen: | |||
| Predominantly a CD4+ infiltrate. | ||||
| 1 patient with thrombotic microangiopathy. | ||||
| Anti-PD-1:b | ||||
| Anti-CTLA-4:b | ||||
| Combination regimen:b | ||||
| [77, 78] | Hepatic | Anti-CTLA-4 or anti-PD-1/PD-L1 | Combined findings: main subtypes are an acute hepatitis pattern with panlobular hepatitis, perivenular infiltrate with endothelialitis or biliary pattern with bile ductular proliferation, mild mixed portal inflammation with little lobular necroinflammation. | Immunohistochemistry |
| Anti-PD-1/L1: no granulomatous inflammation, and infrequent occurrence of central vein endotheliitis. | Similar proportions CD4+ and CD8+ infiltrates in the portal tract, with CD8+ cells dominating lobular infiltrates. | |||
| Anti-CTLA-4: granulomatous inflammation, associated with severe lobular necrotic and inflammatory activity and central vein endotheliitis. | Predominance of CD8+ cells in portal tracts and lobules. | |||
| Combination regimen: n/a | n/a | |||
| [80, 82–84] | Pulmonary | Anti-PD-1/PD-L1 | Range of findings including cellular interstitial pneumonitis, organizing pneumonia, diffuse alveolar damage and non-caseating granulomas. | Immunohistochemistry and mass cytometry (for sarcoid lung lesions)Sarcoid lung lesions
|
| [64, 85] | Cardiac | Anti-PD-1 monotherapy, anti-CTLA-4 monotherapy and combination regimen | Combined findings: patchy to florid T-cell-predominant lymphocytic infiltrate with absence of granulomas or giant cells. | Immunohistochemistry, TCR repertoire analysis, whole transcriptome sequencing |
| Anti-PD-1/L1:b | n/a | |||
| Anti-CTLA-4:b | n/a | |||
| Combination regimen: patchy lymphocytic infiltrate within the myocardium also involving the cardiac sinus and atrioventricular nodes | Abundant CD4+ and CD8+ T cells, as well as CD68+ cells. Absence of CD20+ B cells. | |||
| TCR repertoire analysis of two patients showed clonal expansion of infiltrating T cells in tumour and cardiac muscle. | ||||
| Whole transcriptome sequencing showed increased expression of inflammatory T-cell cytokines and expression of muscle-specific transcripts in the tumour. |
Combination regimen refers to concurrent use of anti-CTLA-4 and anti-PD-1.
Limited individual data for this regimen – literature often reports findings from a cohort of patients on range of CPI.
TEN: toxic epidermal necrolysis; N/A: not available.
Autopsy studies permit sampling of tissue that would not otherwise be easily accessible (e.g. thyroid, brain), and have been described in this context. Koelzer et al. performed an autopsy study of a young patient treated with anti-CTLA-4 and anti-PD-1 therapy, who developed clinically apparent CPI pneumonitis. Intriguingly, two distinct lung pathologies were found, as well as aseptic meningoencephalitis and myocarditis, suggesting that clinically or radiographically apparent irAE may only represent a small proportion of irAEs that actually occur [1].
Gastrointestinal toxicities
CPI-induced enterocolitis is one of the most common reasons for CPI discontinuation and treatment-related death. The relative ease of tissue sampling of the GI tract via endoscopy offers valuable insights into the immunopathological aspects of this toxicity, as well as in advancing mechanistic insights into CPI therapy.
Inflammation has been described along the entire gastrointestinal (GI) tract, from the oesophagus to the colon, with a predilection for the colon- particularly the left side [2–7]. Notably, this may be influenced by sampling bias, as the left side of the colon is more accessible via flexible sigmoidoscopy, whereas right-sided colonic biopsies can only be sampled during colonoscopy (which is more time consuming, costly and requires oral bowel preparation, and hence has additional logistical challenges). Endoscopic findings broadly resemble aspects of inflammatory bowel disease (IBD) including oedema, loss of vascular pattern (in lower GI tract), erythema, erosions, ulcers and mucosal friability, including frank luminal bleeding [2–7]. Necrotising gastritis has also been described [7]. Continuous, confluent inflammation starting from the distal colon and mimicking ulcerative colitis (UC) is typical, but diffuse patchy lesions with normal-looking intervening colonic mucosa, reminiscent of Crohn’s disease (CD) is also seen [3, 6].
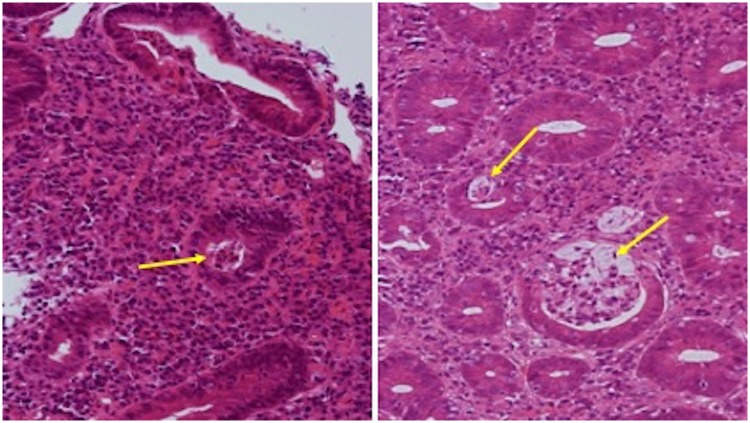
Histologically, there is a wide spectrum of disease, which does not appear to correlate with the type of CPI agent used or whether patients are on immunosuppressive therapy prior to biopsy [2, 8]. The most common findings include an inflammatory infiltrate in the lamina propria, composed of lymphocytes, neutrophils, eosinophils and plasma cells [2, 6, 9–11]. Neutrophilic infiltration of the intra-epithelial compartment, and neutrophilic crypt abscess formation are also common [2, 6, 9–11] (Fig. 1). Increased apoptotic activity within the crypt epithelium, reminiscent of graft vs host disease is a finding in up to around half of cases. Crypt epithelial atrophy and crypt dropout is also reported [11]. Granulomas, resembling those seen in CD are very infrequent [12, 13]. Occasionally, features of chronic inflammation including crypt distortion, basal plasmacytosis and Paneth cell metaplasia, which can mimic IBD, have been reported, although the prominent apoptosis and crypt atrophy or dropout seen in CPI-enterocolitis would be unusual in IBD [11, 12, 14]. To date, the temporal relationship between emergence of GI toxicity and chronicity on biopsy is unclear.
Fig. 1.
Histology section from colonic biopsies in CPI enterocolitis patients showing neutrophil infiltration and crypt abscess formation
A microscopic colitis-like pattern of disease is being increasingly described [7, 15, 16]. Classical microscopic colitis encompasses lymphocytic colitis and collagenous colitis, both of which exhibit a normal endoscopic appearance and are differentiated by histology. There is some evidence that compared with classical microscopic colitis, CPI-microscopic colitis induces a more aggressive disease course requiring more intensive immunosuppression and a greater need for hospitalization [15].
Additional findings in the upper GI tract include lymphocytic gastritis (>30 intraepithelial lymphocytes per 100 epithelial cells). In the duodenum, as well as chronic inflammation with a neutrophil, lymphocyte and plasma cell infiltrate, villus blunting and atrophy have also been described [7, 17].
It is worth highlighting that insights into pathology of lesions mainly stem from mucosal biopsies. Because colectomy is a rare event, data examining pathology across the colonic walls is sparse. In one case of anti-PD-1 perforating colitis, multiple ulcerations, transmural inflammation and necrosis were described [18]. In four colectomy specimens from patients with anti-CTLA-4 enterocolitis, all showed extensive acute severe colitis with abrupt transition between ulcerations and normal mucosae [12].
There are only a few studies that have characterized histological and immunological features in parallel, but predictably an abundance of CD3+ T cells (and not B cells) are commonly reported [19, 20]. In one study of nine ipilimumab-treated patients with CPI enterocolitis, colonic mucosal expression of the major T helper-1 (Th-1) and Th-17 pro-inflammatory cytokines IFN-γ and IL-17A, were significantly upregulated (>10-fold and >5-fold, respectively). IL-17 has a critical role in regulating colonic neutrophil recruitment [21], which may account for the neutrophilic infiltrate frequently seen in this disease. Patients with CPI-enterocolitis also have increased levels of IL-17A in blood [22] suggesting the IL-17 axis, like in IBD [23], may be a key driver of inflammation.
Given that CTLA-4 is constitutively expressed by FoxP3+ T regulatory (Treg) cells, it was initially postulated that CPI toxicity is mediated by loss of this subset. However, several studies have shown the converse to be true: immunohistochemistry and flow cytometry analysis suggest mucosal Treg cells are not depleted and are often increased [8, 9, 20, 24] along with the regulatory cytokine IL-10 [19]. Further studies are needed to determine whether CPI therapy induces changes to Tregs on a functional level, as has been demonstrated in murine models [25].
Interestingly, there is a suggestion that anti-CTLA-4 and anti-PD-1-induced enterocolitis have distinct immunological characteristics. In a study investigating the mucosal immunological profile (using immunohistochemistry and flow cytometry) of 17 anti-CTLA-4 and five anti-PD-1 induced enterocolitis patients, colonic mucosal T-helper CD4+ cells with high expression of the proinflammatory cytokine TNFα were enriched in the former, whilst cytotoxic CD8+ T cells were enriched in the latter [26]. Further work is needed to develop this line of enquiry in order to deliver a personalized medicine approach and answer questions about the efficacy of anti-TNF therapy between CPI regimens.
Skin toxicities
Dermatologic irAEs exhibit a myriad of clinical manifestations, but most commonly involve pruritic, and/or maculopapular eruptions. Biopsies are easily acquired from the skin, explaining the relatively higher number of publications reporting on their pathological features. Curry et al. categorises this toxicity into four groups, according to their histological patterns: inflammatory, immunobullous, alterations of epidermal keratinocytes and alterations of epidermal melanocytes [27].
The inflammatory subtype, encompassing dermal hypersensitivity reactions manifesting as maculopapular eruptions, are the most commonly occurring cutaneous irAE. Histologically, inflammatory lesions display a perivascular lymphocytic infiltrate with eosinophils in the superficial dermis (ranging from scattered to florid), overlying epidermal spongiosis and patchy necrotic keratinocytes [28–30]. Typically, these are managed with emollients, topical steroids, and anti‐histamines, and do not always necessitate discontinuation of CPI therapy. Also within this category are the increasingly described lichenoid dermatides, which are associated with anti-PD-1/PD-L1 therapy [31–33]. Skin biopsies show a dense, band-like lymphocytic infiltrate, hyperkeratosis, hypergranulosis, saw-tooth ridge pattern, and dyskeratosis [33]. In one case series of three anti-PD-1 treated patients, immunohistochemical staining showed a predominance of the CD4+ component compared with CD8+ and only 10% of T cells staining positive for PD-1 [32].
More severe mucocutaneous skin reactions such as Stevens-Johnson syndrome or toxic epidermal necrolysis (TEN) have been described but are rare [30, 34–36]. In one case of nivolumab-induced TEN, from initial biopsy of a morbilliform pruritic skin eruption to TEN presentation, there was an increase in the number of CD8+ lymphocytes within the dermal–epidermal junction and an increase of PD-L1 expression in both lymphocytes and keratinocytes [34].
Autoimmune blistering eruptions associated with immune checkpoint blockade were initially thought to be rare but are being increasingly reported [37–41]. Bullous pemphigoid (BP), a disease characterized by formation of sub-epidermal blisters as a result of auto-antibodies against hemidesmosomal antigens, has been described in this context. Interestingly, it has only been reported with the anti-PD-1 or anti-PD-L1 regimens. Histology and direct immunofluorescence show the pathognomonic features seen in both classic and drug-induced BP, of subepidermal cleft and linear deposition of IgG and C3 at the blister roof of the dermoepidermal junction [37–39]. In one patient where serum ELISA was used as part of the diagnostic work up, auto-antibodies to BP180 (typically seen in classic BP) were also found [39]. Patients tend to respond to corticosteroid therapy but compared with patients with other drug-induced BP, they appear to have a prolonged course, possibly reflecting continued in vivo immune activation.
Lesions associated with altered keratinocytes are uncommon and include Grover's disease (biopsies show acantholytic dyskeratosis) and prurigo nodularis (histology data not available) [27, 42, 43]. On the other hand, lesions associated with altered melanocytes are more commonly reported, including vitiligo, tumoral melanosis (pigmented lesions that clinically resemble melanoma, but histologically show dense aggregates of melanin‐laden benign macrophages and no malignant cells) and regression of melanocytic naevi [28, 30, 44–48].
Additionally, there is the emergence of a new CPI-dermatologic toxicity which does not fit into the categories above: cutaneous interstitial granulomatous dermatitis. This manifests as non-pruritic, often annular plaques involving the inner aspects of the arms, thighs and intertriginous areas. Histologically, granulomatous infiltrates of interstitial lymphocytes and histiocytes are accompanied by fragmentation of collagen and elastic fibres and overlying vacuolar interface dermatitis [41, 49, 50].
There are at least several case reports of anti-CTLA-4 treated and anti-PD-1 treated patients developing pulmonary sarcoidosis with cutaneous involvement. Dermatologic lesions are either papular or nodular and have been described on the forearms and face with biopsy confirming non-caseating granulomas [51–53].
Characterizing the immunological properties of skin lesions may inform a more targeted therapeutic approach, but data is lacking, especially high-resolution immunophenotyping. In one study, immunohistochemistry of 10 biopsies from nine melanoma patients who developed skin irAEs from either anti-CTLA-4 or anti-PD-1 monotherapy were assessed. Predictably, lymphocytes were mainly CD3+ T cells (isolated CD20+ B cells were also present), with a predominance of CD4+ cells over CD8+ cells. CD4+ cells tended to surround the vascular plexus, while CD8+ cells were more loosely scattered in the dermis, sometimes affecting the epidermis (exocytosis). FoxP3+ Treg cells, representing 1–10% of the total lymphocytes were located in the dermis with a perivascular distribution [50].
Rheumatic toxicities
Inflammatory arthritis
Inflammatory arthritis can affect both small and large joints and be oligo or polyarticular. Radiographic and ultrasound findings show joint effusions, synovial thickening and proliferation, and positive colour power doppler on ultrasound in keeping with active inflammation [54–56]. Consistent with this inflammatory phenotype, synovial fluid analyses show increased white cell counts with high neutrophil infiltration, reminiscent of findings in RA [54]. Interestingly, some CPI treated patients undergo sero-conversion, becoming positive for anti-cyclic citrullinated peptide (anti-CCP) antibodies and/or rheumatoid factor [57].
Vasculitis, polymyalgia rheumatica and giant cell arteritis (GCA)
The most common vasculitis associated with CPI therapy are the large vessel vasculitides (including GCA, and aortitis) and vasculitis affecting the nervous system [58]. Of interest, vasculitis is most commonly associated with anti-PD-1 treatment. The PD-1/PD-L1 axis has recently been reported to be deficient in GCA, with low expression reported in affected tissue. Moreover, T-cell infiltration is increased by CPI treatment in a humanized mouse model of granulomatous vasculitis, with increased expression of inflammatory markers [59]. In a case report of CPI-induced GCA, inflammatory infiltrate of the adventitia and muscularis layers in the temporal artery was observed, together with narrowing of the arterial lumen, consistent with intima proliferation and active arteritis [60].
Myositis and myopathy
CPI myositis has been described in several case reports and case series across a range of CPI regimens [61–63] and his associated with myocarditis [61, 63, 64]. In one study, muscle biopsy in all six patients with CPI-myositis (anti-PD-1 monotherapy and combination regimen CPI) showed multifocal necrotic myofibers, sarcolemmal MHC-I, and endomysial inflammation consisting mainly of CD68+ cells (a marker for monocytes/macrophages) expressing PD-L1, and CD8+ cells expressing PD-1 [62]. An abundance of CD4+, CD8+ and CD68+ cells (with absence of CD20+ B cells) was also noted in an autopsy study of two patients [64]. Kimura et al. [61] report a severe case of CPI-induced myositis, myocarditis and myasthenia gravis following one dose of anti-PD-1, with biopsy revealing prominent CD4+ and CD8+ T cells in muscle fibres.
Intriguingly, TCR repertoire analysis performed in the latter two studies both showed clonal expansion in skeletal muscle, suggesting an active antigen-driven adaptive immune response [61, 64].
CPI-induced dermatomyositis appears to be rare [65, 66], displaying classic features of a photosensitive cutaneous rash and elevated serum creatinine kinase. In one case, features of inflammation were seen on muscle biopsy [65], whilst in another, biopsies only showed muscle atrophy [66]. The authors suggested this may be because the biopsy was taken after corticosteroid treatment, although in the majority of irAEs biopsies taken whilst patients are on immunosuppressive therapy still yields positive results.
In a cohort study of 1293 patients who received any CPI agent, 10 had myopathy, with five of these undergoing biopsy. Muscle fibre necrosis was reported in two, and a non-specific myopathic process in the other three patients [67].
Other rheumatic manifestations
CPI-induced sicca syndrome has been described in a few cases [54, 68, 69]. One case series (n = 4), included imaging findings of hypoechoic lesions of the glands with lymphocytic aggregates, resembling those reported in Sjögren syndrome. However, in contrast to classical Sjögren syndrome, none of these patients were positive for Ro or La serum antibodies [54]. Another key difference relates to the predominance of T-cell infiltrates found in CPI-induced sicca compared [69] with B-cell infiltrates in Sjogrens [70]. Taken altogether, this suggests that salivary gland destruction in CPI mediated toxicity is mediated by a distinct pathological mechanism.
Renal toxicities
Only a few studies have reported findings from renal biopsies in patients with CPI-induced kidney injury. Overall, tubulointerstitial inflammation is the most common observation [71, 72] either alone or in combination with other glomerular pathologies. Biopsy-proven acute interstitial nephritis was found in 12/13 patients with CPI-kidney injury (across a range of regimens). Acute interstitial nephritis was characterized by diffuse interstitial inflammation and focal severe tubulitis, with a predominantly CD4+ lymphocytic infiltrate, and some eosinophils and plasma cells. Granulomatous features were present in 3/12 patients [71].
Other pathological findings include granulomatous formations with multinucleated giant cells, lupus nephropathy, thrombotic microangiopathy, nephrotic syndrome focal segmental glomerulosclerosis, minimal-change disease, membranous nephropathy, pauci-immune glomerulonephritis and IgA nephropathy [72]. Renal biopsy of sicca-associated interstitial nephritis demonstrates a T-cell rich infiltrate, suggestive of autoimmune interstitial nephritis, and eosinophils, which are suggestive of a hypersensitivity-like reaction [54].
Administering CPI in renal transplant patients and indeed other recipients of solid organ transplants has been a major concern, with limited data in this subgroup given they are frequently excluded from clinical trials. Similar transplant rejection rates have been reported in both anti-PD-1 and anti-CTLA-4 treated patients [73, 74]. Where available, biopsy findings were consistent with acute… with acute rejection, with a mixture of cellular and antibody mediated rejection.
Hepatic toxicities
Immune-mediated hliver injury most frequently manifests as asymptomatic elevations in liver function tests, particularly the transaminases, although fulminant hepatitis and death has been reported [75]. Hepatitis occurs in ∼5% of patients on CPI monotherapy [11] and up to 30% on combination therapy [76]. Histologically, this may manifest as a predominant injury to hepatocytes (acute hepatitis pattern) or to bile ducts (biliary pattern) [77]. In the former, findings can include panlobular hepatitis, perivenular infiltrate with endothelialitis [77, 78]. In the latter, histological features include bile ductular proliferation and mild mixed portal inflammation with little lobular necroinflammation [77, 78].
Although liver biopsy is infrequently performed it can be useful in discriminating between CPI-induced liver injury, primary autoimmune hepatitis or drug induced liver injury- the diagnosis of which influences management. Eosinophilic infiltration and plasmacotyosis seems to occur less frequently in CPI liver injury, with significantly fewer CD20+ or CD4+ lymphocytes [79].
There appear to be differences in the histological patterns between anti-CTLA-4 and anti-PD-1/PD-L1 therapy. In a case series of 16 patients who underwent liver biopsy for CPI hepatitis, anti-CTLA-4 treated patients (n = 7) had granulomatous inflammation, associated with severe lobular necrotic and inflammatory activity and central vein endotheliitis. Anti-PD-1/PD-L1 treated patients (n = 9) did not have granulomatous inflammation, and central vein endotheliitis was infrequent. Immunostaining suggested that lymphocytes in the portal tracts and lobules were mainly represented by CD8+ T cells in anti-CTLA-4 treated patients, whilst in the other group CD4+ and CD8+ infiltrates were equally represented in the portal tract, with CD8+ cells dominating lobular infiltrates [78].
Pulmonary toxicities
Lung biopsy data in patients with CPI pneumonitis are scarce, but where available show a range of findings, reflecting the variation in clinical and imaging findings. This includes cellular interstitial pneumonitis, organizing pneumonia, diffuse alveolar damage as well as no abnormalities in some cases. The interstitial inflammatory infiltrate includes poorly formed granulomas and eosinophils [80].
Sarcoid-like lung lesions have been described with bronchoalveolar lavage showing an increased CD4+ : CD8+ ratio, and bronchial biopsies revealing non-caseating epithelioid granulomas [1, 81, 82]. One study performed immunophenotyping (mass cytometry) on peripheral blood mononuclear cells prior to the first dose of 15 anti-PD-1 treated melanoma patients, of whom two developed sarcoidosis. These were compared with age‐ and sex‐matched healthy controls (n = 15). Analysis demonstrated abnormally high numbers of circulating Th-1/17 T-cells (a subpopulation of human CD4+ T cells that co‐produce IFN‐γ and IL‐17) in five of 15 melanoma patients, including both patients who developed sarcoidosis post CPI [83]. These findings support data that implicates Th-1/17 cells in the pathogenesis of classic sarcoidosis [84].
Cardiac toxicities
Cardiac irAEs are an uncommon but potentially fatal outcome of CPI therapy. In one study where 11 patients with CPI-myocarditis had a cardiac biopsy or autopsy, histological findings included a patchy to florid T-cell-predominant lymphocytic infiltrate, with absence of granulomas or giant cells [85]. Composition of the lymphocyte compartment was not described. Johnson et al. offer further histological and immunological insights, through their autopsy study on two combination-regimen treated (anti-CTLA-4 and anti-PD-1) patients who developed fatal myocarditis [64].
Myocardial and skeletal T-cell infiltrates in both patients showed abundant CD4+ and CD8+ T cells, as well as CD68+ cells. An absence of CD20+ B cells was confirmed with immunofluorescence studies. Immune infiltration was limited to cardiac and skeletal muscle with no other affected tissues, including adjacent smooth muscle. TCR analysis of infiltrating lymphocytes in cardiac skeletal muscle and tumour showed clonal expansion indicating that antigens present in the muscle were identified by the same T-cell clone. Whole transcriptome sequencing of affected tissue demonstrated increased expression of inflammatory T-cell cytokines as well as expression of muscle-specific transcripts in the tumour, further supporting the existence of a common epitope between tumour and striated muscle.
Conclusion
CPI therapy has revolutionized cancer care for many patients but is hindered by off-target immune-mediated injury to non-cancer tissues often causing severe side effects. Importantly, as insights into the immunopathology of these lesions continues to emerge, we are now learning how best to manage these complications, bearing in mind they may be underpinned by pathological mechanisms distinct to their classical autoimmune counterparts.
Interestingly, there is a hint that that the predominant immune cell infiltrating irAE tissue may differ between toxicities as well as between CPI regimens (e.g. CD4+vs CD8+ T cells). However, limitations include the fact that most data arise from low-resolution techniques used to identify only a subset of immune cells. Given the pivotal role of the immune system in initiating emergence of irAEs, there is a need to evaluate these further using higher resolution unbiased techniques. This will harness targeted therapies that effectively alleviate inflammation in non-cancer tissues without impacting anti-cancer immunity. Furthermore, insights into immune-mediated damage following CPI exposure offers a unique opportunity to understand the fundamental biology of checkpoint molecules and their role in immune tolerance and maintenance of tissue homeostasis.
Acknowledgements
Authors N.P. and H.I. acknowledge that their affiliation has now changed. The previous affiliation was: Centre for Inflammation Biology and Cancer Immunology, King's College London, London, UK.
Disclosure statement: N.P. has received advisory fees from AbbVie, Allergan, Debiopharm International, Ferring and Vifor Pharma and lectures fees from Allergan, Falk, Janssen, Tillotts and Takeda. The other authors have declared no conflicts of interest.
Funding: This paper was published as part of a supplement funded by an educational grant from BMS.
References
- 1. Koelzer VH, Rothschild SI, Zihler D. et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. J Immunother Cancer 2016;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foppen MHG, Rozeman EA, van Wilpe S. et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018;3:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abu-Sbeih H, Ali FS, Luo WY. et al. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J Immunother Cancer 2018;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marthey L, Mateus C, Nachury M. et al. Ipilimumab colitis: a GETAID multicentric study. J Crohns Colitis 2014;8:S146-S. [Google Scholar]
- 5. Gonzalez RS, Salaria SN, Bohannon CD. et al. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology 2017;70:558–67. [DOI] [PubMed] [Google Scholar]
- 6. Verschuren EC, van den Eertwegh AJ, Wonders J. et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol 2016;14:836–42. [DOI] [PubMed] [Google Scholar]
- 7. Collins M, Michot JM, Danlos FX. et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol 2017;28:2860–5. [DOI] [PubMed] [Google Scholar]
- 8. Adler BL, Pezhouh MK, Kim A. et al. Histopathological and immunophenotypic features of ipilimumab-associated colitis compared to ulcerative colitis. J Int Med 2018;283:568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonzalez RS, Salaria SN, Bohannon CD. et al. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology 2017;70:558–67. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Abu-Sbeih H, Mao E. et al. Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis 2018;24:1695. [DOI] [PubMed] [Google Scholar]
- 11. Karamchandani DM, Chetty R.. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists’ perspective. J Clin Pathol 2018;71:665–71. [DOI] [PubMed] [Google Scholar]
- 12. Marthey L, Mateus C, Mussini C. et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis 2016;10:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck KE, Blansfield JA, Tran KQ. et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen JH, Pezhouh MK, Lauwers GY, Masia R.. Histopathologic features of colitis due to immunotherapy with anti-PD-1 antibodies. Am J Surg Pathol 2017;41:643–54. [DOI] [PubMed] [Google Scholar]
- 15. Choi K, Abu-Sbeih H, Samdani R. et al. Can immune checkpoint inhibitors induce microscopic colitis or a brand new entity? Inflamm Bowel Dis 2019;25:385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ibraheim H, Spain L, Samani A. et al. PWE-025 microscopic colonic inflammation in immune check point inhibitor-induced diarrhoea/colitis. BMJ Publishing Group, 2018;67:A80. [Google Scholar]
- 17. Fazal MW, Spain L, Ibraheim H. et al. Upper gastrointestinal inflammation in patients with immune-checkpoint inhibitor induced diarrhoea. Gut 2018;67:A66–A7. [Google Scholar]
- 18. Celli R, Kluger HM, Zhang X.. Anti-PD-1 therapy-associated perforating colitis. Case Rep Gastrointest Med 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bamias G, Delladetsima I, Perdiki M. et al. Immunological characteristics of colitis associated with anti-CTLA-4 antibody therapy. Cancer Invest 2017;35:443–55. [DOI] [PubMed] [Google Scholar]
- 20. Lord JD, Hackman RC, Moklebust A. et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Digest Dis Sci 2010;55:1396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powell N, Walker AW, Stolarczyk E. et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012;37:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarhini AA, Zahoor H, Lin Y. et al. Baseline circulating IL-17 predicts toxicity while TGF-beta 1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:UNSP-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Globig A-M, Hennecke N, Martin B. et al. Comprehensive intestinal T helper cell profiling reveals specific accumulation of IFN-γ+ IL-17+ coproducing CD4+ T cells in active inflammatory bowel disease. Inflamm Bowel Dis 2014;20:2321–9. [DOI] [PubMed] [Google Scholar]
- 24. Oble DA, Mino-Kenudson M, Goldsmith J. et al. α-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol 2008;32:1130–7. [DOI] [PubMed] [Google Scholar]
- 25. Read S, Greenwald R, Izcue A. et al. Blockade of CTLA-4 on CD4+ CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006;177:4376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coutzac C, Adam J, Soularue E. et al. Colon immune-related adverse events: anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J Crohns Colitis 2017;11:1238–46. [DOI] [PubMed] [Google Scholar]
- 27. Curry JL, Tetzlaff MT, Nagarajan P. et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol 2017;44:158–76. [DOI] [PubMed] [Google Scholar]
- 28. Sibaud V, Meyer N, Lamant L. et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol 2016;28:254–63. [DOI] [PubMed] [Google Scholar]
- 29. Lacouture ME, Wolchok JD, Yosipovitch G. et al. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J Am Acad Dermatol 2014;71:161–9. [DOI] [PubMed] [Google Scholar]
- 30. Goldinger SM, Stieger P, Meier B. et al. Cytotoxic cutaneous adverse drug reactions during anti-PD-1 therapy. Clin Cancer Res 2016;22:4023–9. [DOI] [PubMed] [Google Scholar]
- 31. Hwang SJE, Carlos G, Wakade D. et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol 2016;74:455–61.e1. [DOI] [PubMed] [Google Scholar]
- 32. Joseph RW, Cappel M, Goedjen B. et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti–PD-1 therapy. Cancer Immunol Res 2015;3:18–22. [DOI] [PubMed] [Google Scholar]
- 33. Tetzlaff MT, Nagarajan P, Chon S. et al. Lichenoid dermatologic toxicity from immune checkpoint blockade therapy: a detailed examination of the clinicopathologic features. Am J Dermatopathol 2017;39:121–9. [DOI] [PubMed] [Google Scholar]
- 34. Vivar KL, Deschaine M, Messina J. et al. Epidermal programmed cell death‐ligand 1 expression in TEN associated with nivolumab therapy. J Cutan Pathol 2017;44:381–4. [DOI] [PubMed] [Google Scholar]
- 35. Hofmann L, Forschner A, Loquai C. et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side- effects of anti-PD-1 therapy. Eur J Cancer 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 36. Saw S, Lee HY, Ng QS.. Pembrolizumab-induced Stevens–Johnson syndrome in non-melanoma patients. Eur J Cancer 2017;81:237–9. [DOI] [PubMed] [Google Scholar]
- 37. Carlos G, Anforth R, Chou S, Clements A, Fernandez-Peñas P.. A case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumab. Melanoma Res 2015;25:265–8. [DOI] [PubMed] [Google Scholar]
- 38. Jour G, Glitza IC, Ellis RM. et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti‐PD‐1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol 2016;43:688–96. [DOI] [PubMed] [Google Scholar]
- 39. Mochel MC, Ming ME, Imadojemu S. et al. Cutaneous autoimmune effects in the setting of therapeutic immune checkpoint inhibition for metastatic melanoma. J Cutan Pathol 2016;43:787–91. [DOI] [PubMed] [Google Scholar]
- 40. Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR.. Rituximab treatment of nivolumab-induced bullous pemphigoid. JAMA Dermatol 2017;153:603–5. [DOI] [PubMed] [Google Scholar]
- 41. Kaunitz GJ, Loss M, Rizvi H. et al. Cutaneous eruptions in patients receiving immune checkpoint blockade. Am J Surg Pathol 2017;41:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uemura M, Fa'ak F, Haymaker C. et al. A case report of Grover's disease from immunotherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J Immunother Cancer 2016;4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voskens CJ, Goldinger SM, Loquai C. et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Staser K, Chen D, Solus J. et al. Extensive tumoral melanosis associated with ipilimumab‐treated melanoma. Br J Dermatol 2016;175:391–3. [DOI] [PubMed] [Google Scholar]
- 45. Abdel-Rahman O, ElHalawani H, Fouad M.. Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: a meta-analysis. Future Oncol 2015;11:2471–84. [DOI] [PubMed] [Google Scholar]
- 46. Mauzo SH, Tetzlaff MT, Nelson K. et al. Regressed melanocytic nevi secondary to pembrolizumab therapy: an emerging melanocytic dermatologic effect from immune checkpoint antibody blockade. Int J Dermatol 2019;58:1045–52. [DOI] [PubMed] [Google Scholar]
- 47. Woodbeck R, Metelitsa AI, Naert KA.. Granulomatous tumoral melanosis associated with pembrolizumab therapy: a mimicker of disease progression in metastatic melanoma. Am J Dermatopathol 2018;40:523–6. [DOI] [PubMed] [Google Scholar]
- 48. Bari O, Cohen PR.. Tumoral melanosis associated with pembrolizumab-treated metastatic melanoma. Cureus 2017;9:e1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trinidad C, Nelson KC, Glitza Oliva IC. et al. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J Cutan Pathol 2018;45:504. [DOI] [PubMed] [Google Scholar]
- 50. Perret RE, Josselin N, Knol AC. et al. Histopathological aspects of cutaneous erythematous‐papular eruptions induced by immune checkpoint inhibitors for the treatment of metastatic melanoma. Int J Dermatol 2017;56:527–33. [DOI] [PubMed] [Google Scholar]
- 51. Eckert A, Schoeffler A, Dalle S. et al. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology 2009;218:69. [DOI] [PubMed] [Google Scholar]
- 52. Reule RB, North JP.. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J Am Acad Dermatol 2013;69:e272–e3. [DOI] [PubMed] [Google Scholar]
- 53. Cotliar J, Querfeld C, Boswell WJ. et al. Pembrolizumab-associated sarcoidosis. JAAD Case Rep 2016;2:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cappelli LC, Gutierrez AK, Baer AN. et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2017;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mooradian MJ, Nasrallah M, Gainor JF, et al. Musculoskeletal rheumatic complications of immune checkpoint inhibitor therapy: a single center experience Semin Arthritis Rheum 2019;Vol. 48:1127–32. [DOI] [PubMed] [Google Scholar]
- 56. Kostine M, Rouxel L, Barnetche T. et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis 2018;77:393–8. [DOI] [PubMed] [Google Scholar]
- 57. Belkhir R, Le Burel S, Dunogeant L. et al. Rheumatoid arthritis and polymyalgia rheumatica occurring after immune checkpoint inhibitor treatment. Ann Rheum Dis 2017;76:1747–50. [DOI] [PubMed] [Google Scholar]
- 58. Daxini A, Cronin K, Sreih AG.. Vasculitis associated with immune checkpoint inhibitors—a systematic review. Clin Rheumatol 2018;37:2579–84. [DOI] [PubMed] [Google Scholar]
- 59. Zhang H, Watanabe R, Berry GJ. et al. Immunoinhibitory checkpoint deficiency in medium and large vessel vasculitis. Proc Natl Acad Sci 2017;114:E970–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goldstein BL, Gedmintas L, Todd DJ.. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol 2014;66:768–9. [DOI] [PubMed] [Google Scholar]
- 61. Kimura T, Fukushima S, Miyashita A. et al. Myasthenic crisis and polymyositis induced by one dose of nivolumab. Cancer Sci 2016;107:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Touat M, Maisonobe T, Knauss S. et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–e94. [DOI] [PubMed] [Google Scholar]
- 63. Anquetil C, Salem J-E, Lebrun-Vignes B. et al. Immune checkpoint inhibitor–associated myositis: expanding the spectrum of cardiac complications of the immunotherapy revolution. Circulation 2018;138:743–5. [DOI] [PubMed] [Google Scholar]
- 64. Johnson DB, Balko JM, Compton ML. et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liewluck T, Kao JC, Mauermann ML.. PD-1 inhibitor-associated myopathies: emerging immune-mediated myopathies. J Immunother 2018;41:208–11. [DOI] [PubMed] [Google Scholar]
- 66. Ali SS, Goddard AL, Luke JJ. et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA Dermatol 2015;151:195–9. [DOI] [PubMed] [Google Scholar]
- 67. Richter MD, Crowson C, Kottschade LA. et al. Rheumatic syndromes associated with immune‐checkpoint inhibitors: a single‐center cohort of sixty-one patients. Arthritis Rheumatol 2019;71:468–75. [DOI] [PubMed] [Google Scholar]
- 68. Smith MH, Bass AR.. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res 2019;71:362–66. [DOI] [PubMed] [Google Scholar]
- 69. Warner BM, Baer AN, Lipson EJ. et al. Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 2019;24:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nocturne G, Mariette X.. B cells in the pathogenesis of primary Sjogren syndrome. Nat Rev Rheumatol 2018;14:133–45. [DOI] [PubMed] [Google Scholar]
- 71. Cortazar FB, Marrone KA, Troxell ML. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mamlouk O, Selamet U, Machado S. et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wanchoo R, Karam S, Uppal NN. et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017;45:160–9. [DOI] [PubMed] [Google Scholar]
- 74. Abdel-Wahab N, Safa H, Abudayyeh A. et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer 2019;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O'day S, Maio M, Chiarion-Sileni V. et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 2010;21:1712–7. [DOI] [PubMed] [Google Scholar]
- 76. Belli C, Zuin M, Mazzarella L. et al. Liver toxicity in the era of immune checkpoint inhibitors: a practical approach. Crit Rev Oncol Hematol 2018;132:125–9. [DOI] [PubMed] [Google Scholar]
- 77. Kim KW, Ramaiya NH, Krajewski KM. et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013;31:1071–7. [DOI] [PubMed] [Google Scholar]
- 78. De Martin E, Michot J-M, Papouin B. et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. [DOI] [PubMed] [Google Scholar]
- 79. Zen Y, Yeh MM.. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965. [DOI] [PubMed] [Google Scholar]
- 80. Chuzi S, Tavora F, Cruz M. et al. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag Res 2017;9:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Montaudie H, Pradelli J, Passeron T, Lacour JP, Leroy S.. Pulmonary sarcoid‐like granulomatosis induced by nivolumab. Br J Dermatol 2017;176:1060–3. [DOI] [PubMed] [Google Scholar]
- 82. Cousin S, Toulmonde M, Kind M. et al. Pulmonary sarcoidosis induced by the anti-PD1 monoclonal antibody pembrolizumab. Ann Oncol 2016;27:1178–9. [DOI] [PubMed] [Google Scholar]
- 83. Lomax AJ, McGuire HM, McNeil C. et al. Immunotherapy‐induced sarcoidosis in patients with melanoma treated with PD‐1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheum Dis 2017;20:1277–85. [DOI] [PubMed] [Google Scholar]
- 84. Ramstein J, Broos CE, Simpson LJ. et al. IFN-γ–producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. Am J Respir Crit Care Med 2016;193:1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mahmood SS, Fradley MG, Cohen JV. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]